Exhaustive Exercise Increases Spontaneous but Not fMLP-Induced Production of Reactive Oxygen Species by Circulating Phagocytes in Amateur Sportsmen
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Population
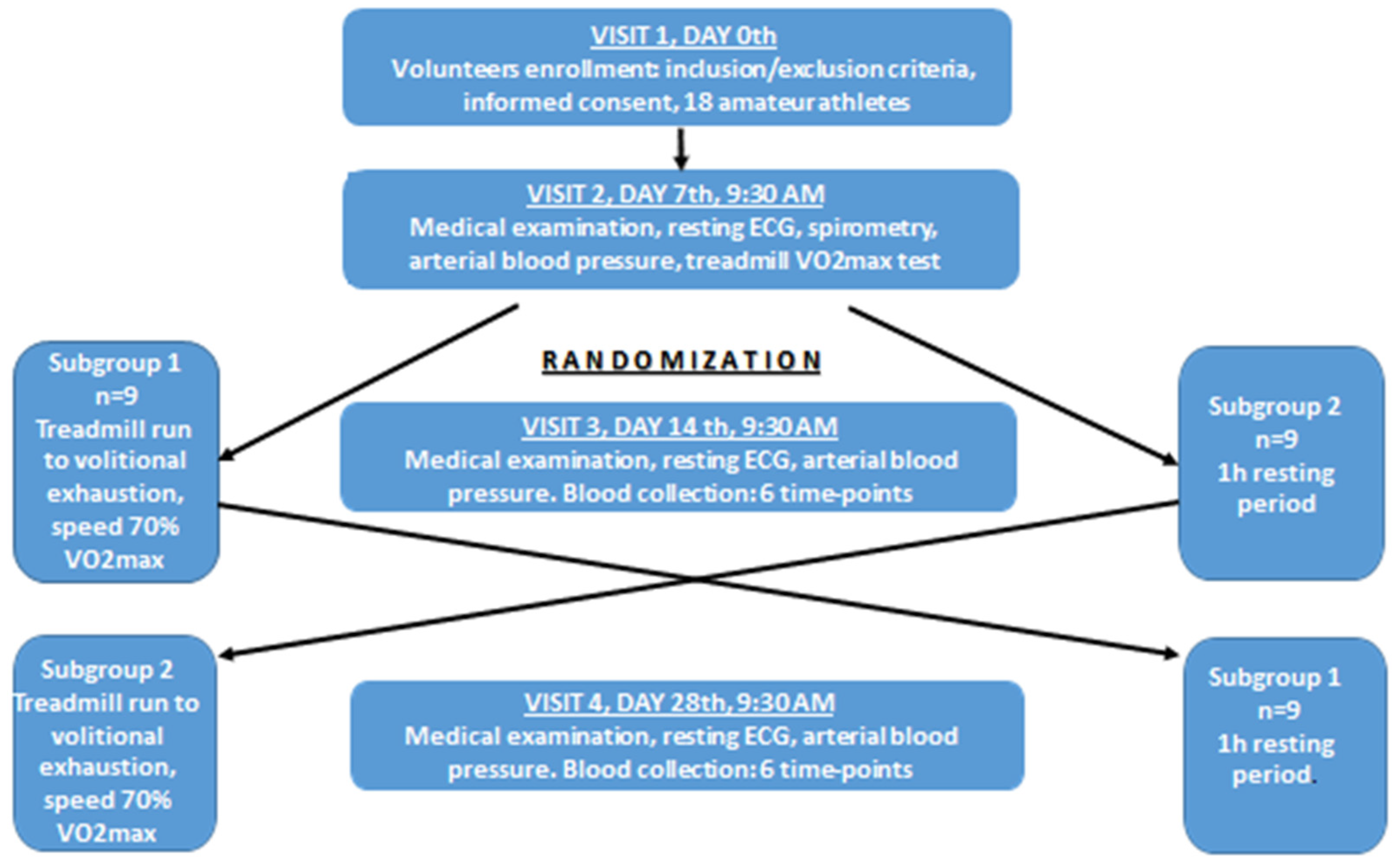
2.2. Study Protocol
2.3. Additional Control Experiment
2.4. Determination of VO2max and Execution of Exhaustive Treadmill Run
2.5. Chemicals and Solutions
2.6. Measurement of the Luminol-Enhanced Whole Blood Chemiluminescence
2.7. Other Determinations
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Studied Amateur Athletes and Exhaustive Treadmill Run
3.2. Tremendous Suppression of Absolute rLBCL and fMLP-LBCL in Patients with Blood Malignancy at the Time When No Phagocytes Were Present in the Circulating Blood
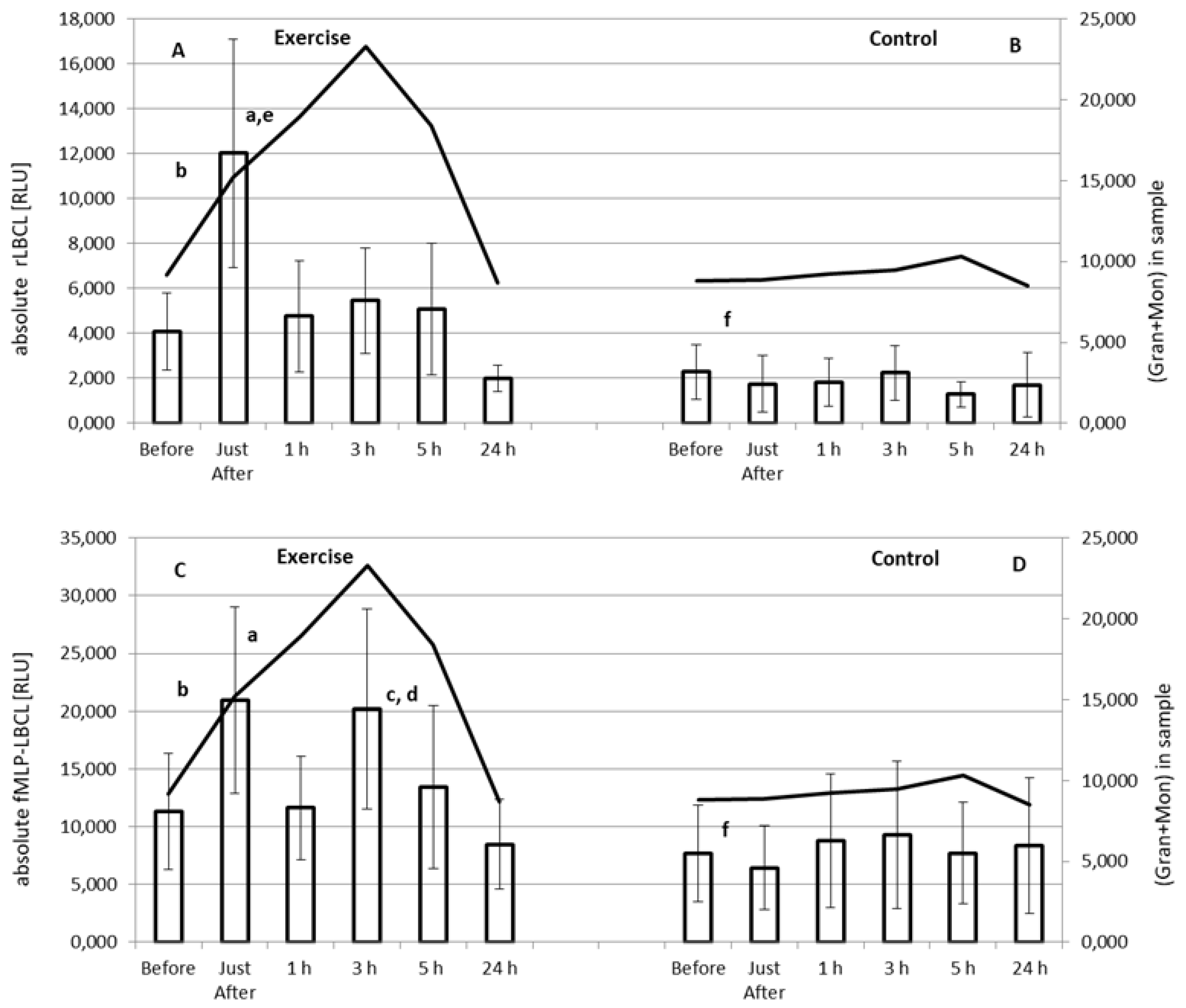
3.3. Effect of Exercise on Absolute rLBCL and fMLP-LBCL
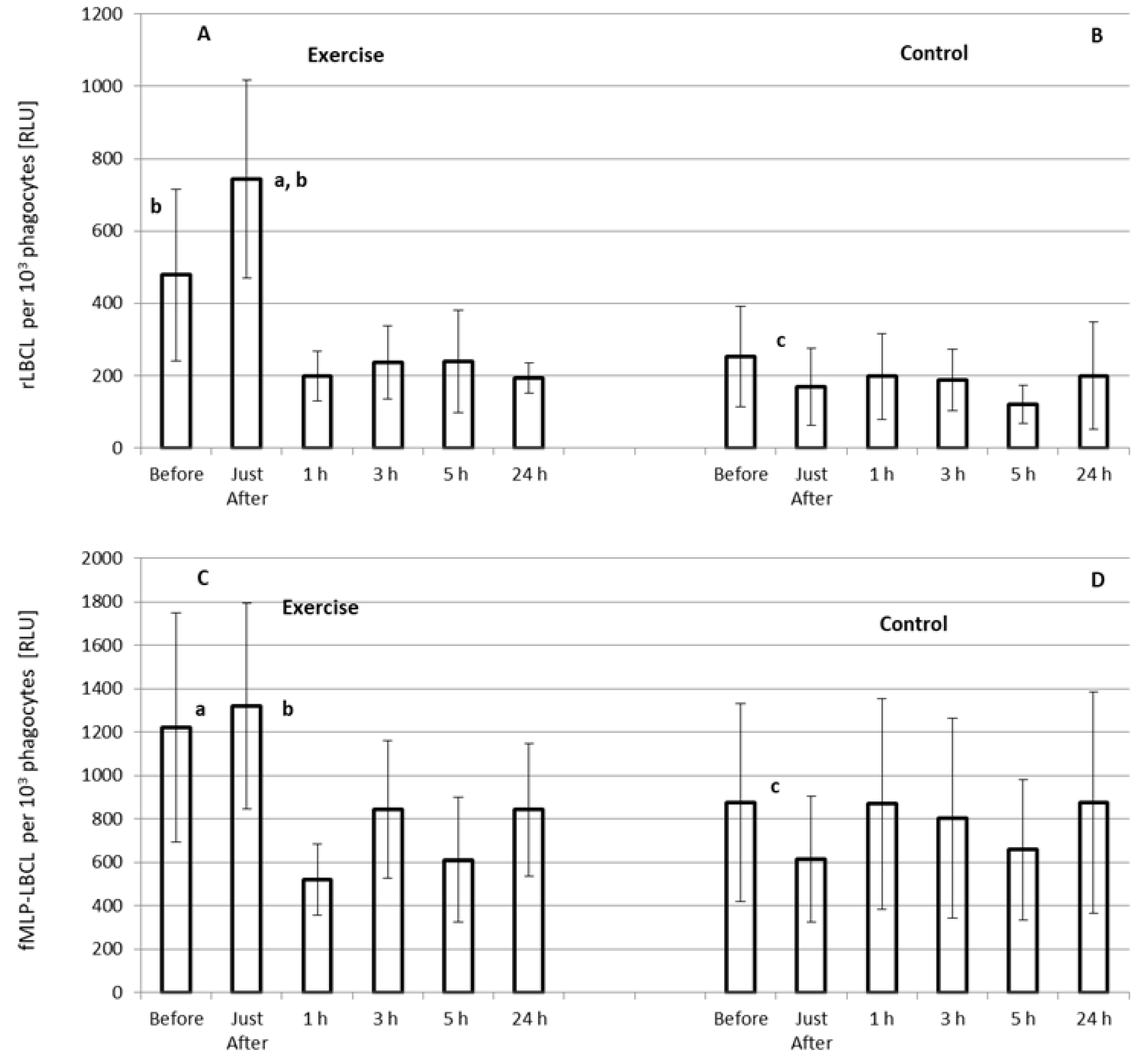
3.4. Effect of Exercise on rLBCL and fMLP-LBCL Expressed as Light Emission per 103 Phagocytes (Normalized per Phagocyte Count)
3.5. Correlations between Absolute LBCL (a-rLBCL, a-fMLP-LBCL) and Selected Bout Characteristics and Clinical Variables
4. Discussion
4.1. Plausible Source of Enhanced Spontaneous ROS Generation in Circulating Blood just after Exhaustive Exercise
4.2. Effect of Exhaustive Exercise on fMLP-Induced ROS Generation in Circulating Blood
4.3. Correlations between LBCL and Selected Variables
4.4. Clinical Significance of Elevated Post-Exercise Spontaneous ROS Generation by Circulating Phagocytes
4.5. Strengths and Weaknesses of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| fMLP | N-formyl-L-methionyl-L-leucyl-L-phenylalanine |
| VO2max | maximal oxygen consumption |
| LBCL | Luminol-enhanced whole blood chemiluminescence |
| a-rLBCL | absolute resting (spontaneous) LBCL |
| rLBCL | resting (spontaneous) LBCL normalized per phagocyte count |
| a-fMLP-LBCL | absolute fMLP-induced LBCL |
| fMLP-LBCL | fMLP-induced LBCL normalized per phagocyte count |
| ROS | reactive oxygen species |
| Gran | granulocytes |
| Mon | monocytes |
| NETs | neutrophil extracellular traps |
| cf-nDNA | circulating cell free nuclear DNA |
| PMA | phorbol 12-myristate 13-acetate |
| OZ | opsonized zymosan |
| RLU | relative light units |
| CK | creatine kinase |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
References
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar] [PubMed]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar]
- Smith, L.L. Overtraining, Excessive Exercise, and Altered Immunity: Is this a T helper-1 versus T helper-2 lymphocyte response? Sports Med. 2003, 33, 347–364. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a Competitive Marathon Race on Systemic Cytokine and Neutrophil Responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Miura, S.; Yoshioka, H.; Mori, Y.; Kometani, T. Changes of thioredoxin, oxidative stress markers, inflammation and muscle/renal damage following intensive endurance exercise. Exerc. Immunol. Rev. 2015, 21, 130–142. [Google Scholar] [PubMed]
- Breitbach, S.; Tug, S.; Simon, P. Circulating Cell-Free DNA: An up-coming molecular marker in exercise physiology. Sports Med. 2012, 42, 565–586. [Google Scholar] [CrossRef]
- Stawski, R.; Walczak, K.; Perdas, E.; Prymont-Przymińska, A.; Zwolińska, A.; Kosielski, P.; Budlewski, T.; Padula, G.; Jerczynska, H.; Nowak, D. Increased Circulating H3 Histone in Response to Repeated Bouts of Exercise Does Not Associate with Parallel Alterations of Cell-Free DNA. Biology 2021, 10, 181. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Stawski, R.; Walczak, K.; Perdas, E.; Wlodarczyk, A.; Sarniak, A.; Kosielski, P.; Meissner, P.; Budlewski, T.; Padula, G.; Nowak, D. Decreased integrity of exercise-induced plasma cell free nuclear DNA—Negative association with the increased oxidants production by circulating phagocytes. Sci. Rep. 2019, 9, 15970. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Suzuki, K.; Kudo, S.; Totsuka, M.; Simoyama, T.; Nakaji, S.; Sugawara, K. Effect of exhaustive exercise on human neutrophils in athletes. Luminescence 2000, 15, 15–20. [Google Scholar] [CrossRef]
- Morozov, V.I.; Pryatkin, S.A.; Kalinski, M.I.; Rogozkin, V.A. Effect of exercise to exhaustion on myeloperoxidase and lysozyme release from blood neutrophils. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 89, 257–262. [Google Scholar] [CrossRef]
- Walker, G.J.; Dziubak, A.; Houghton, L.; Prendergast, C.; Lim, L.; Bishop, N.C. The effect of caffeine ingestion on human neutrophil oxidative burst responses following time-trial cycling. J. Sports Sci. 2008, 26, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Yamai, K.; Takahashi, I.; Kojima, A.; Yamamoto, Y.; Tanabe, M.; Totsuka, M.; Nakaji, S.; Sugawara, N.; Matsuzaka, M. The effects of a two-hour judo training session on the neutrophil immune functions in university judoists. Luminescence 2008, 23, 49–53. [Google Scholar] [CrossRef]
- Robson, P.; Blannin, A.; Walsh, N.; Castell, L.; Gleeson, M. Effects of Exercise Intensity, Duration and Recovery onin vitroNeutrophil Function in Male Athletes. Int. J. Sports Med. 1999, 20, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Quindry, J.C.; Stone, W.L.; King, J.; Broeder, C.E. The Effects of Acute Exercise on Neutrophils and Plasma Oxidative Stress. Med. Sci. Sports Exerc. 2003, 35, 1139–1145. [Google Scholar] [CrossRef]
- Suzuki, K.; Sato, H.; Kikuchi, T.; Abe, T.; Nakaji, S.; Sugawara, K.; Totsuka, M.; Sato, K.; Yamaya, K. Capacity of circulating neutrophils to produce reactive oxygen species after exhaustive exercise. J. Appl. Physiol. 1996, 81, 1213–1222. [Google Scholar] [CrossRef]
- Peake, J.; Wilson, G.; Hordern, M.; Suzuki, K.; Yamaya, K.; Nosaka, K.; Mackinnon, L.; Coombes, J. Changes in neutrophil surface receptor expression, degranulation, and respiratory burst activity after moderate- and high-intensity exercise. J. Appl. Physiol. 2004, 97, 612–618. [Google Scholar] [CrossRef]
- Smith, J.A.; Gray, A.B.; Pyne, D.B.; Baker, M.S.; Telford, R.D.; Weidemann, M.J. Moderate exercise triggers both priming and activation of neutrophil subpopulations. Am. J. Physiol. Integr. Comp. Physiol. 1996, 270, R838–R845. [Google Scholar] [CrossRef] [PubMed]
- Hack, V.; Strobel, G.; Rau, J.-P.; Weicker, H. The effect of maximal exercise on the activity of neutrophil granulocytes in highly trained athletes in a moderate training period. Graefe’s Arch. Clin. Exp. Ophthalmol. 1992, 65, 520–524. [Google Scholar] [CrossRef]
- Ella, K.; Mocsai, A.; Káldi, K. Circadian regulation of neutrophils: Control by a cell-autonomous clock or systemic factors? Eur. J. Clin. Investig. 2018, 48, e12965. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, P.; Hamouda, W.; Garg, R.; Aljada, A.; Ghanim, H.; Dandona, P. Glucose Challenge Stimulates Reactive Oxygen Species (ROS) Generation by Leucocytes. J. Clin. Endocrinol. Metab. 2000, 85, 2970–2973. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, P.; Ghanim, H.; Hamouda, W.; Aljada, A.; Garg, R.; Dandona, P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am. J. Clin. Nutr. 2002, 75, 767–772. [Google Scholar] [CrossRef] [Green Version]
- Pallister, I.; Topley, N. Chemiluminescence: Comparison of Whole Blood with Isolated Polymorphonuclear Leukocytes after Major Trauma. J. Trauma: Inj. Infect. Crit. Care 2004, 57, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Potargowicz, E.; Banach, M.; Łuczyńska, M.; Stolarek, R.; Białasiewicz, P.; Kasielski, M.; Ciałkowska-Rysz, A.; Nowak, D. Increased whole blood chemiluminescence in patients with chronic renal failure independent of hemodialysis treatment. Arch. Immunol. Ther. Exp. 2006, 54, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Bialasiewicz, P.; Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Krol, M.; Markowski, J.; Rutkowski, K.P.; Nowak, D. Sour Cherries but Not Apples Added to the Regular Diet Decrease Resting and fMLP-Stimulated Chemiluminescence of Fasting Whole Blood in Healthy Subjects. J. Am. Coll. Nutr. 2018, 37, 24–33. [Google Scholar] [CrossRef]
- Bialasiewicz, P.; Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Krol, M.; Glusac, J.; Nowak, P.; Markowski, J.; Rutkowski, K.P.; et al. Addition of Strawberries to the Usual Diet Decreases Resting Chemiluminescence of Fasting Blood in Healthy Subjects—Possible Health-Promoting Effect of These Fruits Consumption. J. Am. Coll. Nutr. 2014, 33, 274–287. [Google Scholar] [CrossRef]
- Nowak, P.; Nowak, M.; Nowak, D. Reduction of Oxidative Stress in Human Body via Inhibitory Effect of Plant Phenolics on Circulating Neutrophils; Results of In Vitro and In Vivo Studies. In Plant Antioxidants and Health; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–28. [Google Scholar] [CrossRef]
- Nowak, D.; Bialasiewicz, P.; Antczak, A.; Krol, M.; Piasecka, G. Changes of Intracellular Free Calcium Concentration in Human Polymorphonuclear Leukocytes after Repeated Stimulations with N-Formyl-Methionyl-Leucyl-Phenylalanine. Immunobiology 1995, 192, 343–352. [Google Scholar] [CrossRef]
- Lubkin, D.T.; Bishawi, M.; Barbas, A.S.; Brennan, T.V.; Kirk, A.D. Extracellular Mitochondrial DNA and N-Formyl Peptides in Trauma and Critical Illness: A Systematic Review. Crit. Care Med. 2018, 46, 2018–2028. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Hoppenbrouwers, T.; Autar, A.S.A.; Sultan, A.R.; Abraham, T.E.; Van Cappellen, W.A.; Houtsmuller, A.B.; Van Wamel, W.J.B.; Van Beusekom, H.M.M.; Van Neck, J.W.; De Maat, M.P.M. In Vitro induction of NETosis: Comprehensive live imaging comparison and systematic review. PLoS ONE 2017, 12, e0176472. [Google Scholar] [CrossRef] [Green Version]
- Hazan-Halevy, I.; Seger, R.; Levy, R. The requirement of both extracellular regulated kinase and p38 mitogen-activated protein kinase for stimulation of cytosolic phospholipase A(2) activity by either FcgammaRIIA or FcgammaRIIIB in human neutrophils. A possible role for Pyk2 but not for the Grb2-Sos-Shc complex. J. Biol. Chem. 2000, 275, 12416–12423. [Google Scholar] [PubMed] [Green Version]
- Lieberman, M.M.; Sachanandani, D.M.; Pinney, C.A. Comparative study of neutrophil activation by chemiluminescence and flow cytometry. Clin. Diagn. Lab. Immunol. 1996, 3, 654–662. [Google Scholar] [CrossRef] [Green Version]
- Stawski, R.; Walczak, K.; Kosielski, P.; Meissner, P.; Budlewski, T.; Padula, G.; Nowak, D. Repeated bouts of exhaustive exercise increase circulating cell free nuclear and mitochondrial DNA without development of tolerance in healthy men. PLoS ONE 2017, 12, e0178216. [Google Scholar] [CrossRef]
- Howley, E.T.; Bassett, D.R.; Welch, H.G. Criteria for maximal oxygen uptake: Review and commentary. Med. Sci. Sports Exerc. 1995, 27, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Kukovetz, E.M.; Bratschitsch, G.; Hofer, H.P.; Egger, G.; Schaur, R.J. Influence of Age on the Release of Reactive Oxygen Species by Phagocytes as Measured by a Whole Blood Chemiluminescence Assay. Free Radic. Biol. Med. 1997, 22, 433–438. [Google Scholar] [CrossRef]
- Ristola, M.; Repo, H. Luminol-enhanced chemiluminescence of whole blood. Statistical analysis, and comparison of the responses of different subjects. APMIS 1989, 97, 503–512. [Google Scholar] [CrossRef]
- Dadfar, E.; Jacobson, S.H.; Lundahl, J. Newly recruited human monocytes have a preserved responsiveness towards bacterial peptides in terms of CD11b up-regulation and intracellular hydrogen peroxide production. Clin. Exp. Immunol. 2007, 148, 573–582. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.; Eckmann, C.M.; Koenderman, L.; Verhoeven, A.J.; Roos, D. Eosinophils do respond to fMLP. Blood 1987, 70, 379–383. [Google Scholar] [CrossRef]
- American Thoracic Society. Standardization of Spirometry, 1994 Update. Am. J. Respir. Crit. Care Med. 1995, 152, 1107–1136. [Google Scholar] [CrossRef]
- Chapman, C.B.; Henschel, A.; Minckler, J.; Forsgren, A.; Keys, A. The effect of exercise on renal plasma flow in normal male subjects. J. Clin. Investig. 1948, 27, 639–644. [Google Scholar] [CrossRef]
- Bergeron, R.; Kjær, M.; Simonsen, L.; Bülow, J.; Skovgaard, D.; Howlett, K.; Galbo, H. Splanchnic blood flow and hepatic glucose production in exercising humans: Role of renin-angiotensin system. Am. J. Physiol. Integr. Comp. Physiol. 2001, 281, R1854–R1861. [Google Scholar] [CrossRef] [Green Version]
- Walczak, K.; Stawski, R.; Perdas, E.; Brzezinska, O.; Kosielski, P.; Galczynski, S.; Budlewski, T.; Padula, G.; Nowak, D. Circulating cell free DNA response to exhaustive exercise in average trained men with type I diabetes mellitus. Sci. Rep. 2021, 11, 4639. [Google Scholar] [CrossRef] [PubMed]
- Korabecna, M.; Tesar, V. NETosis provides the link between activation of neutrophils on hemodialysis membrane and comorbidities in dialyzed patients. Agents Actions 2017, 66, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, S.; Nakazawa, D.; Shida, H.; Miyoshi, A.; Kusunoki, Y.; Tomaru, U.; Ishizu, A. NETosis markers: Quest for specific, objective, and quantitative markers. Clin. Chim. Acta 2016, 459, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Beiter, T.; Fragasso, A.; Hartl, D.; Nieß, A.M. Neutrophil Extracellular Traps: A Walk on the Wild Side of Exercise Immunology. Sports Med. 2015, 45, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Breitbach, S.; Sterzing, B.; Magallanes, C.; Tug, S.; Simon, P. Direct measurement of cell-free DNA from serially collected capillary plasma during incremental exercise. J. Appl. Physiol. 2014, 117, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Atamaniuk, J.; Vidotto, C.; Tschan, H.; Bachl, N.; Stuhlmeier, K.M.; Muller, M.M. Increased Concentrations of Cell-Free Plasma DNA after Exhaustive Exercise. Clin. Chem. 2004, 50, 1668–1670. [Google Scholar] [CrossRef]
- Atamaniuk, J.; Stuhlmeier, K.M.; Vidotto, C.; Tschan, H.; Dossenbach-Glaninger, A.; Mueller, M.M. Effects of ultra-marathon on circulating DNA and mRNA expression of pro- and anti-apoptotic genes in mononuclear cells. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 104, 711–717. [Google Scholar] [CrossRef]
- Velders, M.; Treff, G.; Machus, K.; Bosnyák, E.; Steinacker, J.M.; Schumann, U. Exercise is a potent stimulus for enhancing circulating DNase activity. Clin. Biochem. 2014, 47, 471–474. [Google Scholar] [CrossRef] [Green Version]
- Farrera, C.; Fadeel, B. Macrophage Clearance of Neutrophil Extracellular Traps Is a Silent Process. J. Immunol. 2013, 191, 2647–2656. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, V. Neutrophil Extracellular Traps in the Second Decade. J. Innate Immun. 2018, 10, 414–421. [Google Scholar] [CrossRef]
- Vorobjeva, N.; Prikhodko, A.; Galkin, I.; Pletjushkina, O.; Zinovkin, R.; Sud’Ina, G.; Chernyak, B.; Pinegin, B. Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. Eur. J. Cell Biol. 2017, 96, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.; Galkin, I.; Pletjushkina, O.; Golyshev, S.; Zinovkin, R.; Prikhodko, A.; Pinegin, V.; Kondratenko, I.; Pinegin, B.; Chernyak, B. Mitochondrial permeability transition pore is involved in oxidative burst and NETosis of human neutrophils. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165664. [Google Scholar] [CrossRef] [PubMed]
- Granger, V.; Faille, D.; Marani, V.; Noël, B.; Gallais, Y.; Szely, N.; Flament, H.; Pallardy, M.; Chollet-Martin, S.; De Chaisemartin, L. Human blood monocytes are able to form extracellular traps. J. Leukoc. Biol. 2017, 102, 775–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, M.; Lacy, P.; Ueki, S. Eosinophil Extracellular Traps and Inflammatory Pathologies—Untangling the Web! Front. Immunol. 2018, 9, 2763. [Google Scholar] [CrossRef]
- Syu, G.-D.; Chen, H.-I.; Jen, C.J. Severe Exercise and Exercise Training Exert Opposite Effects on Human Neutrophil Apoptosis via Altering the Redox Status. PLoS ONE 2011, 6, e24385. [Google Scholar] [CrossRef] [Green Version]
- Busquets-Cortés, C.; Capó, X.; Martorell, M.; Tur, J.A.; Sureda, A.; Pons, A. Training and acute exercise modulates mitochondrial dynamics in football players’ blood mononuclear cells. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 117, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, H.; Trush, M.A. Detection of mitochondria-derived reactive oxygen species production by the chemilumigenic probes lucigenin and luminol. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1999, 1428, 1–12. [Google Scholar] [CrossRef]
- Jančinová, V.; Drábiková, K.; Nosál, R.; Račková, L.; Majekova, M.; Holomanova, D. The combined luminol/isoluminol chemiluminescence method for differentiating between extracellular and intracellular oxidant production by neutrophils. Redox Rep. 2006, 11, 110–116. [Google Scholar] [CrossRef]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999, 515 Pt 1, 287–291. [Google Scholar] [CrossRef]
- Miralda, I.; Uriarte, S.M.; McLeish, K.R. Multiple Phenotypic Changes Define Neutrophil Priming. Front. Cell. Infect. Microbiol. 2017, 7, 217. [Google Scholar] [CrossRef]
- Yagisawa, M.; Yuo, A.; Kitagawa, S.; Yazaki, Y.; Togawa, A.; Takaku, F. Stimulation and priming of human neutrophils by IL-1 alpha and IL-1 beta: Complete inhibition by IL-1 receptor antagonist and no interaction with other cytokines. Exp. Hematol. 1995, 23, 603–608. [Google Scholar]
- Cabral-Santos, C.; Junior, E.A.D.L.; Fernandes, I.M.D.C.; Pinto, R.Z.; Rosa-Neto, J.C.; Bishop, N.C.; Lira, F.S. Interleukin-10 responses from acute exercise in healthy subjects: A systematic review. J. Cell. Physiol. 2019, 234, 9956–9965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, P.M.-C.; Elbim, C.; Marie, J.-C.; Chiandotto, M.; Gougerot-Pocidalo, M.-A.; El-Benna, J. Anti-inflammatory effect of interleukin-10 on human neutrophil respiratory burst involves inhibition of GM-CSF-induced p47PHOXphosphorylation through a decrease in ERK1/2 activity. FASEB J. 2006, 20, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Silvestrini, A.A.; Silva-Teixeira, D.N.; Nogueira-Machado, J.A. Effect in vitro of gamma interferon and interleukin-10 on generation of oxidizing species by human granulocytes. Inflamm. Res. 1996, 45, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Capsoni, F.; Minonzio, F.; Ongari, A.M.; Carbonelli, V.; Galli, A.; Zanussi, C. Interleukin-10 Down-Regulates Oxidative Metabolism and Antibody-Dependent Cellular Cytotoxicity of Human Neutrophils. Scand. J. Immunol. 1997, 45, 269–275. [Google Scholar] [CrossRef]
- Cabral-Santos, C.; Gerosa-Neto, J.; Inoue, D.; Panissa, V.L.G.; Gobbo, L.A.; Zagatto, A.M.; Campos, E.Z.; Lira, F.S. Similar Anti-Inflammatory Acute Responses from Moderate-Intensity Continuous and High-Intensity Intermittent Exercise. J. Sports Sci. Med. 2015, 14, 849–856. [Google Scholar] [PubMed]
- Suzuki, K.; Totsuka, M.; Nakaji, S.; Yamada, M.; Kudoh, S.; Liu, Q.; Sugawara, K.; Yamaya, K.; Sato, K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol. 1999, 87, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Mizokami, T.; Niihara, H.; Yada, K.; Suzuki, K. Neutrophil Depletion Attenuates Muscle Injury after Exhaustive Exercise. Med. Sci. Sports Exerc. 2016, 48, 1917–1924. [Google Scholar] [CrossRef]
- Strydom, N.; Rankin, S.M. Regulation of Circulating Neutrophil Numbers under Homeostasis and in Disease. J. Innate Immun. 2013, 5, 304–314. [Google Scholar] [CrossRef]
- Blanco, R.A.; Ziegler, T.R.; Carlson, B.A.; Cheng, P.-Y.; Park, Y.; Cotsonis, G.A.; Accardi, C.J.; Jones, D.P. Diurnal variation in glutathione and cysteine redox states in human plasma. Am. J. Clin. Nutr. 2007, 86, 1016–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilking, M.; Ndiaye, M.; Mukhtar, H.; Ahmad, N. Circadian Rhythm Connections to Oxidative Stress: Implications for Human Health. Antioxid. Redox Signal. 2013, 19, 192–208. [Google Scholar] [CrossRef] [Green Version]
- Hammouda, O.; Chtourou, H.; Chahed, H.; Ferchichi, S.; Kallel, C.; Miled, A.; Chamari, K.; Souissi, N. Diurnal Variations of Plasma Homocysteine, Total Antioxidant Status, and Biological Markers of Muscle Injury During Repeated Sprint: Effect on Performance and Muscle Fatigue—A Pilot Study. Chrono-Int. 2011, 28, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Chtourou, H.; Souissi, N. Effect of Time-of-Day on Biochemical Markers in Response to Physical Exercise. J. Strength Cond. Res. 2017, 31, 272–282. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Madrigal-Bujaidar, E.; Álvarez-González, I.; García-Melo, L.F.; Anguiano-Robledo, L.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A. Antioxidant and Adaptative Response Mediated by Nrf2 during Physical Exercise. Antioxidants 2019, 8, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evelson, P.A.; Gambino, G.; Travacio, M.; Jaita, G.; Verona, J.; Maroncelli, C.; Wikinski, R.; Llesuy, S.F.; Brites, F. Higher antioxidant defences in plasma and low density lipoproteins from rugby players. Eur. J. Clin. Investig. 2002, 32, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Brites, F.D.; Evelson, P.A.; Christiansen, M.G.; Nicol, M.F.; Basílico, M.J.; Wikinski, R.W.; Llesuy, S.F. Soccer players under regular training show oxidative stress but an improved plasma antioxidant status. Clin. Sci. 1999, 96, 381–385. [Google Scholar] [CrossRef]
- Wozniak, A.; Drewa, G.; Chesy, G.; Rakowski, A.; Rozwodowska, M.; Olszewska, D. Effect of altitude training on the peroxidation and antioxidant enzymes in sportsmen. Med. Sci. Sports Exerc. 2001, 33, 1109–1113. [Google Scholar] [CrossRef]
- Robertson, J.D.; Maughan, R.J.; Duthie, G.G.; Morrice, P.C. Increased blood antioxidant systems of runners in response to training load. Clin. Sci. 1991, 80, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Ristow, M. Mitohormesis in exercise training. Free Radic. Biol. Med. 2015, 98, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Russomanno, G.; Corbi, G.; Manzo, V.; Ferrara, N.; Rengo, G.; Puca, A.A.; Latte, S.; Carrizzo, A.; Calabrese, M.C.; Andriantsitohaina, R.; et al. The anti-ageing molecule sirt1 mediates beneficial effects of cardiac rehabilitation. Immun. Ageing 2017, 14, 7. [Google Scholar] [CrossRef]
- Ji, L.L.; Kang, C.; Zhang, Y. Exercise-induced hormesis and skeletal muscle health. Free Radic. Biol. Med. 2016, 98, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kashi, D.S.; Shabir, A.; Da Boit, M.; Bailey, S.J.; Higgins, M.F. The Efficacy of Administering Fruit-Derived Polyphenols to Improve Health Biomarkers, Exercise Performance and Related Physiological Responses. Nutrients 2019, 11, 2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, M.D.; Willems, M.E.T. Dietary Anthocyanins: A Review of the Exercise Performance Effects and Related Physiological Responses. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 322–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuzzo, J. Volunteer Bias and Female Participation in Exercise and Sports Science Research. Quest 2021, 73, 82–101. [Google Scholar] [CrossRef]
| Studied Group | Exercise Protocol | Sample and Time of Collection | ROS Measurement | Activator | ROS Generation (Only Significant Changes) | Ref |
|---|---|---|---|---|---|---|
| 11 average trained men | Treadmill run to exhaustion at 70% VO2max | Blood: before and just after exercise | LBCL | fMLP | Increased just after exercise | [9] |
| 8 male cross-country skiers | Treadmill run until exhaustion | Isolated Gran: pre-exercise, 0 h, 1 h, 2 h after exercise | LCL and LgCL | OZ, PMA | Increased PMA- and OZ-induced LCL just after exercise | [10] |
| 10 male rowers | Treadmill run to exhaustion | Blood: pre-exercise, 0 h, 1 h, 3 h, 6 h after exercise | LBCL | OZ | Decreased LBCL at 3 h and 6 h post-exercise | [11] |
| 9 endurance-trained male cyclists | 120 min cycling at 70% VO2max | Blood: pre-exercise, 0 h, 1 h after exercise | PBCL | fMLP | Decreased just after and at 1 h post-exercise | [12] |
| 22 male university judoists | 2 h judo training session at mean HR around 138/min | Blood: before and just after training session | Flow cytometry with hydroethidine probe | OZ | Increased just after exercise | [13] |
| 6 average trained subjects | Exercise at 80% or 55% VO2max to exhaustion | Blood: pre-exercise, 0 h, 1 h, 2.5 h after exercise | Fluorescent label hydroethidine | PMA | Decreased 1 h and 2.5 h after exercise | [14] |
| 9 male subjects | Treadmill maximal exercise test | Blood: pre-exercise, 0 h, 1 h, 2 h post- exercise | LgBCL | PMA | Increased at 2 h post-exercise | [15] |
| 10 male cross-country skiers | Maximal exercise treadmill test to exhaustion | Isolated Gran: pre-exercise, 0 h, 1 h after exercise | LCl and LgCL | OZ | Increased LCL just after exercise | [16] |
| 10 male runners and triathletes | 60 min treadmill run at 60 VO2max and 85 VO2max | Isolated Gran: pre-exercise, 0 h, 1 h after exercise | LCL | PMA and OZ | No effect of both bouts on ROS production | [17] |
| 8 untrained male subjects | 60 min bicycle ergometer exercise at HR around 140/min | Isolated Gran: before and just after exercise | Flow cytometry with dihydrorhodamine-123 probe | PMA and OZ | Increased PMA- stimulated ROS production just after exercise | [18] |
| 10 male long distance runners, 10 triathletes, 10 untrained medical students | Treadmill run to exhaustion | Isolated Gran: pre-exercise, 0 h, 0.5 h, 24 h after exercise | Reduction of ferricytochrome | PMA | Decreased at 0.5 h post-exercise in untrained students, decreased just after exercise in long distance runners and triathlets, increased at 24 h post-exercise in all groups | [19] |
| Demographic/Clinical Variables | Soccer Players | Powerlifters | Whole Group |
|---|---|---|---|
| Number | 10 | 8 | 18 |
| Age (years) | 22 ± 2 (23; 2) | 22 ± 1 (22; 1) | 22 ± 2 (22; 2) |
| Body mass (kg) | 76 ± 10 (78; 14) | 85 ± 12 (81; 11) | 80 ± 12 (78;12) |
| Body mass index (kg/m2) | 23.2 ± 1.7 (23.4; 2.5) | 25.6 ± 2.9 (24.5; 2.6) | 24.3 ±2.6 (23.8; 1.8) |
| VO2max (mL/kg·min) | 49 ± 4 (49; 8) | 50 ± 6 (51; 7) | 49 ± 5 (50; 8) |
| Exercise load (hours/week) | 6.4 ±1.7 (6.3; 3.8) | 8.4 ± 2.6 (8.3; 2.5) | 7.3 ± 2.4 (8.3; 3.7) |
| FVC (%) † | 98 ± 9 (99; 13) | 112 ± 12 a (111; 7) | 104 ± 13 (105; 17) |
| FEV1 (%) † | 97 ± 9 (97; 11) | 108 ± 8 a (109; 7) | 102 ± 10 (101; 14) |
| FEV1/FVC (%) | 82 ± 6 (82; 6) | 81 ± 10 (79; 13) | 82± 8 (82; 6) |
| Hct (%) | 45.9 ± 2.2 (46.6; 3.8) | 45.7 ± 2.7 (45.1; 3.7) | 45.2 ± 2.4 (45.7; 4.0) |
| Hgb (g/dL) | 15.4 ± 0.6 (15.5; 0.7) | 15.3 ± 0.9 (15.1; 0.8) | 15.3 ± 0.7 (15.2; 0.8) |
| RBC (×106/µL) | 5.22 ± 0.26 (5.21; 0.43) | 5.06 ± 0.34 (4.98; 0.37) | 5.15 ± 0.31 (5.12; 0.45) |
| WBC (×103/µL) | 5.96 ± 1.06 (5.90; 0.58) | 5.10 ± 1.66 (4.60; 1.48) | 5.58 ± 1.42 (5.65; 1.55) |
| Lym (×103/µL) | 2.08 ± 0.31 (2.10; 0.55) | 1.84 ± 0.61 (1.65; 0.65) | 1.97 ± 0.48 (1.95; 0.75) |
| Gran (×103/µL) | 3.67 ± 1.02 (3.55; 0.95) | 3.08 ± 1.05 (2.85; 1.33) | 3.14 ± 1.07 (3.25; 1.00) |
| Mon (×103/µL) | 0.21 ± 0.08 (0.20; 0.01) | 0.19 ± 0.06 (0.20; 0.03) | 0.20 ± 0.07 (0.20; 0.01) |
| PLT (×103/µL) | 231 ± 38 (238; 70) | 238 ± 39 (231; 33) | 234 ± 39 (237; 48) |
| Parameter | Exhaustive Treadmill Run | ||
| Soccer Players | Powerlifters | Whole Group | |
| Run distance to exhaustion (km) | 13.0 ± 3.2 (13.6; 5) | 15.0 ± 6.6 (14.0; 8.5) | 13.9 ± 5.1 (13.6; 6.3) |
| Run time (min) | 71 ± 17 (76; 30) | 82 ± 33 (80; 41) | 76 ± 26 (76; 32) |
| Baseline heart rate (beats/min) | 75 ± 7 (75; 6) | 71 ± 8 (70; 2) | 74 ± 8 (74; 7) |
| Heart rate at the end of run (beats/min) | 168 ± 13 (165; 17) | 167 ± 10 (164; 13) | 167 ± 12 (164; 17) |
| % of maximal heart rate at the end of run † | 85 ± 7 (83; 9) | 84 ± 5 (82; 6) | 84 ± 7 (82; 9) |
| Baseline blood pressure (mmHg) S/D | 120/80 ± 9/4 (120/80; 19/4) | 125/78 ± 8/4 (120/80; 15/5) | 122/79 ± 9/4 (120/80; 14/4) |
| Blood pressure after exercise (mmHg) S/D | 137/77 ± 8/5 (140/80; 0/9) | 146/79 ± 11/4 (140/80; 5/0) | 141/78 ± 11/4 (140/80; 0/4) |
| Loss of body mass (kg) | 1.0 ± 0.4 (1.1; 0.4) | 1.4 ± 0.5 (1.5; 0.7) | 1.2 ± 0.5 (1.1; 0.6) |
| Marker | Exhaustive Treadmill Run | |||||
|---|---|---|---|---|---|---|
| Soccer Players | Powerlifters | Whole Group | ||||
| Before | Just After | Before | Just After | Before | Just After | |
| CK (U/L) | 176 ± 67 (142; 48) | 221 ± 89 a (191; 82) | 186 ± 78 (176; 84) | 245 ± 85 a (219; 44) | 180 ± 59 (161; 55) | 233 ± 56 a (219; 67) |
| AST (U/L) | 31 ± 11 (26; 9) | 36 ± 9 (31; 9) | 33 ± 7 (32; 8) | 37 ± 7 (37; 11) | 32 ± 8 (28; 10) | 36 ± 8 (32; 9) |
| ALT (U/L) | 25 ± 11 (19; 8) | 26 ± 12 (20; 6) | 29 ± 7 (30; 9) | 31 ± 8 (33; 13) | 26 ± 10 (22; 13) | 28 ± 9 (27; 14) |
| Lactate (mmol/L) | 1.6 ± 0.3 (1.5; 0.4) | 3.0 ± 1.3 a (3.0; 0.9) | 1.9 ± 0.1 (1.9; 0.1) | 2.9 ± 0.4 a (2.7; 0.4) | 1.7 ± 0.3 (1.8; 0.4) | 3.0 ± 1.1 a (2.8; 0.7) |
| Creatinine (µmol/L) | 86 ± 7 (87; 8) | 105 ± 12 a (105; 14) | 91 ± 12 (86; 11) | 108 ± 14 a (105; 19) | 88 ± 9 (87; 7) | 106 ± 16 a (105; 13) |
| Urea (mmol/L) | 5.6 ± 0.9 (5.5; 1.4) | 6.3 ± 0.9 (5.9; 0.9) | 5.9 ± 1.4 (5.6; 1.1) | 6.8 ± 1.4 (6.2; 1.9) | 5.7 ± 1.1 (5.5; 1.7) | 6.5 ± 1.1 a (6.0; 1.8) |
| Exhaustive Treadmill Run at Speed Corresponding to 70% VO2max | |||||
|---|---|---|---|---|---|
| Before | Just After | 1 h Post | 3 h Post | 5 h Post | 24 h Post |
| 3.25 ± 1.52 a (3.25; 1.28) | 5.35 ± 2.49 c (5.30; 1.58) | 6.57 ± 3.39 c (6.20; 2.63) | 8.03 ± 3.78 b,c (8.75; 3.68) | 6.45 ± 3.31 c (6.90; 4.25) | 3.12 ± 1.34 (3.40; 1.60) |
| Control–1 h without physical exertion instead of treadmill run | |||||
| 3.15 ± 1.31 (3.25; 1.08) | 3.17 ± 1.30 (3.40; 1.18) | 3.30 ± 1.36 (3.50; 1.35) | 3.36 ± 1.33 (3.70; 1.25) | 3.68 ± 1.62 (3.75; 1.58) | 3.09 ± 1.18 (3.40; 0.90) |
| Variable | Patients (n = 5) with Blood Malignancy Treated with Autologous Stem Cell Transplantation | ||
|---|---|---|---|
| Before the Onset of Conditioned Regimen | 3 Days after Infusion of Stem Cells | 14 Days after Infusion of Stem Cells | |
| RBC (×106/µL) | 3.56 ± 0.27 (3.50; 0.04) | 3.32 ± 0.58 (3.13; 0.21) | 3.51 ± 0.49 (3.36; 0.76) |
| WBC (×103/µL) | 5.58 ± 1.89 (5.10; 3.00) | 0.29 ± 0.17 a (0.30; 0.10) | 3.04 ± 1.55 (2.40; 1.50) |
| Phagocytes (Gran + Mon) (×103/µL) | 4.86 ± 1.43 (4.90; 2.00) | 0 ± 0 a (0;0) | 2.13 ± 1.58 (1.30; 1.47) |
| a-rLBCL (RLU) | 5067 ± 839 (5430; 1161) | 260 ± 99 a (270; 67) | 1563 ± 36 (1440; 420) |
| a-fMLP-LBCL (RLU) | 13,724 ± 2375 (14,100; 4370) | 374 ± 207 a (326; 108) | 6137 ± 5098 (3715; 1915) |
| Corelated Variable | Parameters of Absolute Whole Blood Chemiluminescence which Rose in Response to Exercise | ||
|---|---|---|---|
| a-rLBCL Just after Exercise | a-fMLP-LBCL Just after Exercise | a-fMLP-LBCL at 3 h Post-Exercise | |
| Run distance to exhaustion | 0.09 | 0.19 | 0.01 |
| Run time | 0.08 | 0.15 | 0.05 |
| Heart rate at the end of run | −0.27 | −0.18 | −0.09 |
| Loss of body mass | −0.33 | −0.28 | −0.06 |
| Absolute Light Emission | Exhaustive Treadmill Run | |||||
|---|---|---|---|---|---|---|
| Before | Just After | 1 h Post | 3 h Post | 5 h Post | 24 h Post | |
| a-fMLP-LBCL | 0.53 a | 0.40 | 0.64 a | 0.08 | 0.40 | 0.51 a |
| a-rLBCL | 0.40 | 0.29 | 0.08 | −0.02 | 0.46 | 0.40 |
| Control–1 h of resting instead of exhaustive treadmill run | ||||||
| a-fMLP-LBCL | 0.22 | 0.40 | 0.33 | 0.40 | 0.55 a | 0.37 |
| a-rLBCL | 0.38 | 0.28 | 0.29 | 0.56 a | 0.18 | 0.16 |
| Absolute Light Emission | Exhaustive Treadmill Run | |||||
|---|---|---|---|---|---|---|
| Before | Just After | 1 h Post | 3 h Post | 5 h Post | 24 h Post | |
| a-fMLP-LBCL | 0.52 a | 0.40 | 0.64 a | 0.09 | 0.55 a | 0.50 a |
| a-rLBCL | 0.67 a | 0.27 | 0.08 | 0.04 | 0.52 a | 0.42 |
| Control–1 h of resting instead of exhaustive treadmill run | ||||||
| a-fMLP-LBCL | 0.28 | 0.43 | 0.35 | 0.40 | 0.57 a | 0.34 |
| a-rLBCL | 0.41 | 0.28 | 0.63 a | 0.57 a | 0.39 | 0.16 |
| Absolute Light Emission | Exhaustive Treadmill Run | |||||
|---|---|---|---|---|---|---|
| Before | Just After | 1 h Post | 3 h Post | 5 h Post | 24 h Post | |
| a-fMLP-LBCL | 0.53 a | 0.43 | 0.66 a | 0.30 | 0.41 | 0.61 a |
| a-rLBCL | 0.62 a | 0.43 | 0.07 | 0.18 | 0.50 | 0.42 |
| Control–1 h of resting instead of exhaustive treadmill run | ||||||
| a-fMLP-LBCL | 0.22 | 0.43 | 0.31 | 0.41 | 0.41 | 0.52 a |
| a-rLBCL | 0.34 | 0.12 | 0.21 | 0.30 | −0.07 | −0.09 |
| Time-Point of Blood Sampling | Correlations between a-rLBCL and a-fMLP-LBCL | |
|---|---|---|
| Exhaustive Treadmill Run | Control–without Treadmill Run | |
| Before | 0.50 a | 0.55 a |
| Just after | 0.41 | 0.63 a |
| 1 h post-exercise | 0.58 a | 0.53 a |
| 3 h post-exercise | 0.37 | 0.48 a |
| 5 h post-exercise | 0.60 a | 0.44 |
| 24 h post-exercise | 0.11 | 0.53 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielecki, A.; Bortnik, K.; Galczynski, S.; Padula, G.; Jerczynska, H.; Stawski, R.; Nowak, D. Exhaustive Exercise Increases Spontaneous but Not fMLP-Induced Production of Reactive Oxygen Species by Circulating Phagocytes in Amateur Sportsmen. Biology 2022, 11, 103. https://doi.org/10.3390/biology11010103
Chmielecki A, Bortnik K, Galczynski S, Padula G, Jerczynska H, Stawski R, Nowak D. Exhaustive Exercise Increases Spontaneous but Not fMLP-Induced Production of Reactive Oxygen Species by Circulating Phagocytes in Amateur Sportsmen. Biology. 2022; 11(1):103. https://doi.org/10.3390/biology11010103
Chicago/Turabian StyleChmielecki, Adam, Krzysztof Bortnik, Szymon Galczynski, Gianluca Padula, Hanna Jerczynska, Robert Stawski, and Dariusz Nowak. 2022. "Exhaustive Exercise Increases Spontaneous but Not fMLP-Induced Production of Reactive Oxygen Species by Circulating Phagocytes in Amateur Sportsmen" Biology 11, no. 1: 103. https://doi.org/10.3390/biology11010103
APA StyleChmielecki, A., Bortnik, K., Galczynski, S., Padula, G., Jerczynska, H., Stawski, R., & Nowak, D. (2022). Exhaustive Exercise Increases Spontaneous but Not fMLP-Induced Production of Reactive Oxygen Species by Circulating Phagocytes in Amateur Sportsmen. Biology, 11(1), 103. https://doi.org/10.3390/biology11010103







