Predicting Current Potential Distribution and the Range Dynamics of Pomacea canaliculata in China under Global Climate Change
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
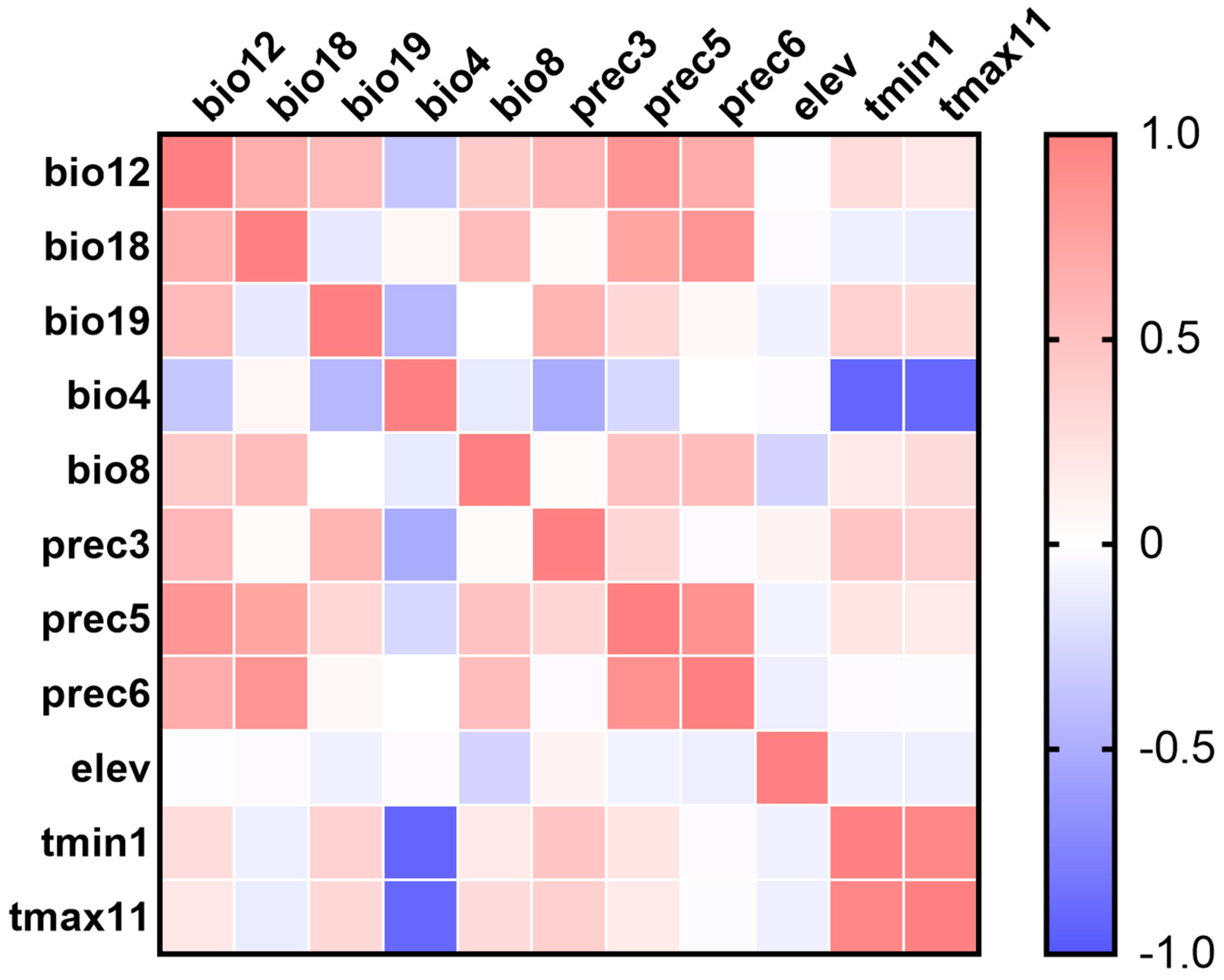
2.1. Environmental Variables
2.2. Occurrence and Analysis of Species
2.3. Change in Potential Distribution and Centroids
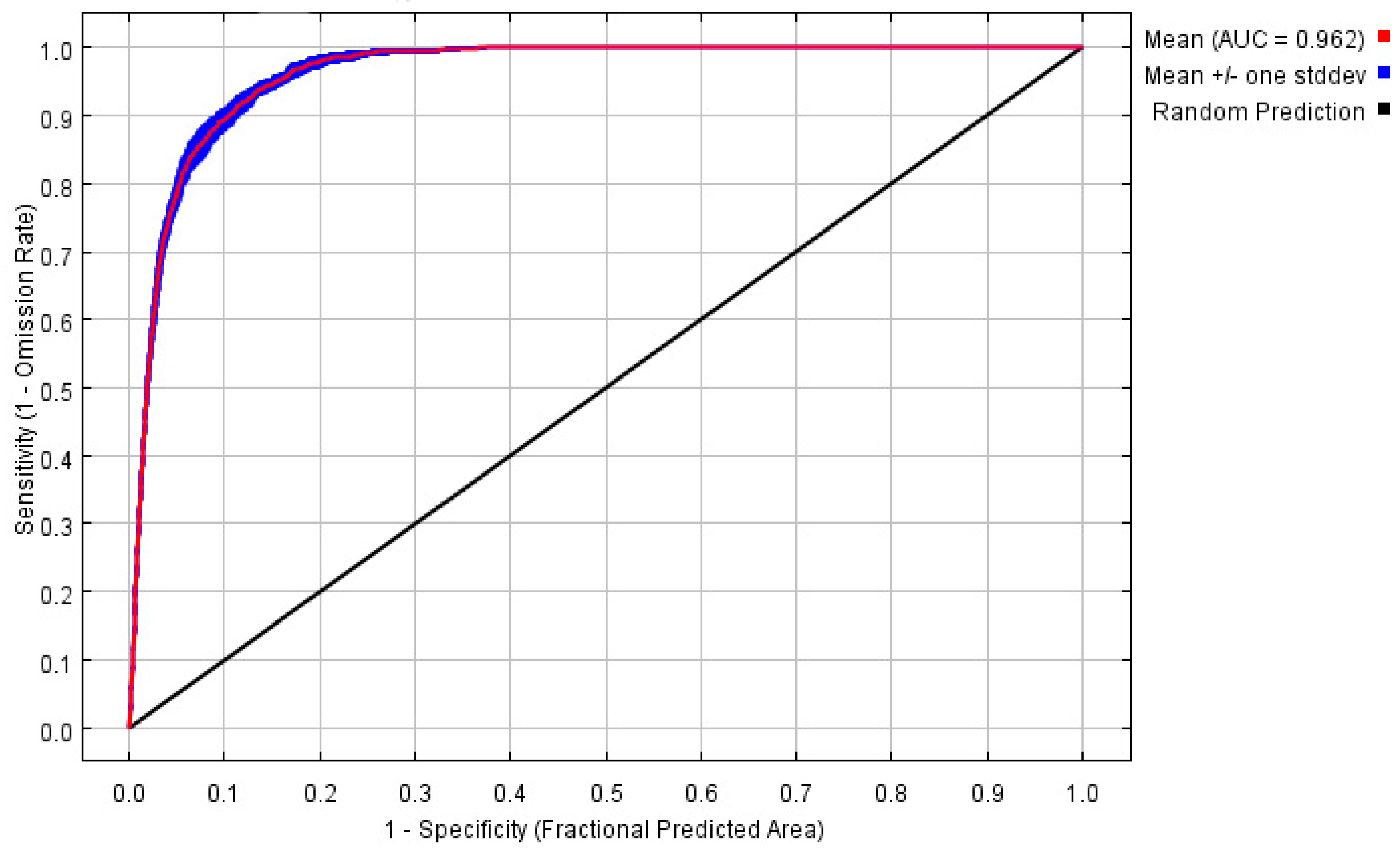
2.4. Optimization and Evaluation of Model
3. Results
3.1. Environmental Variables and Model Optimization
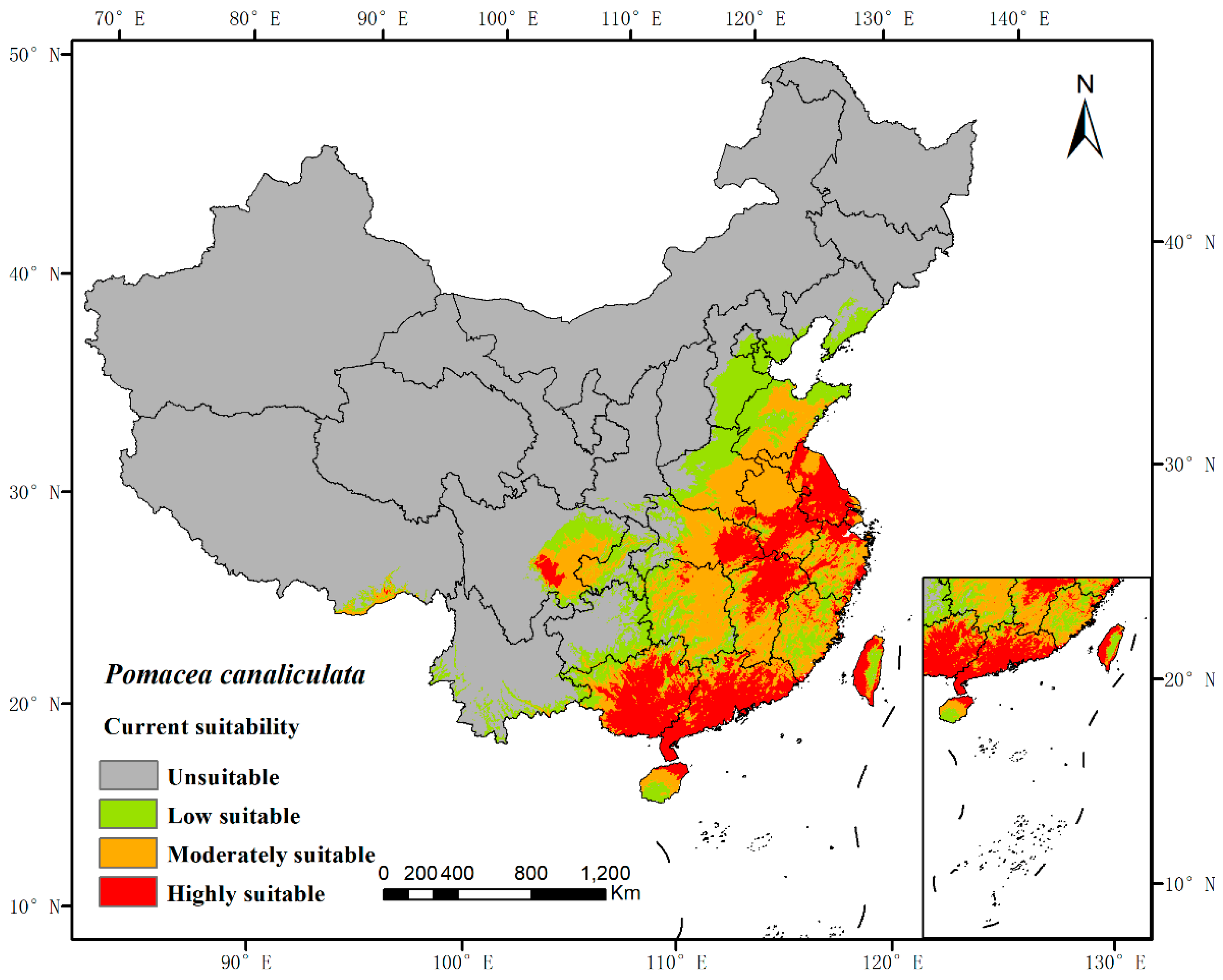
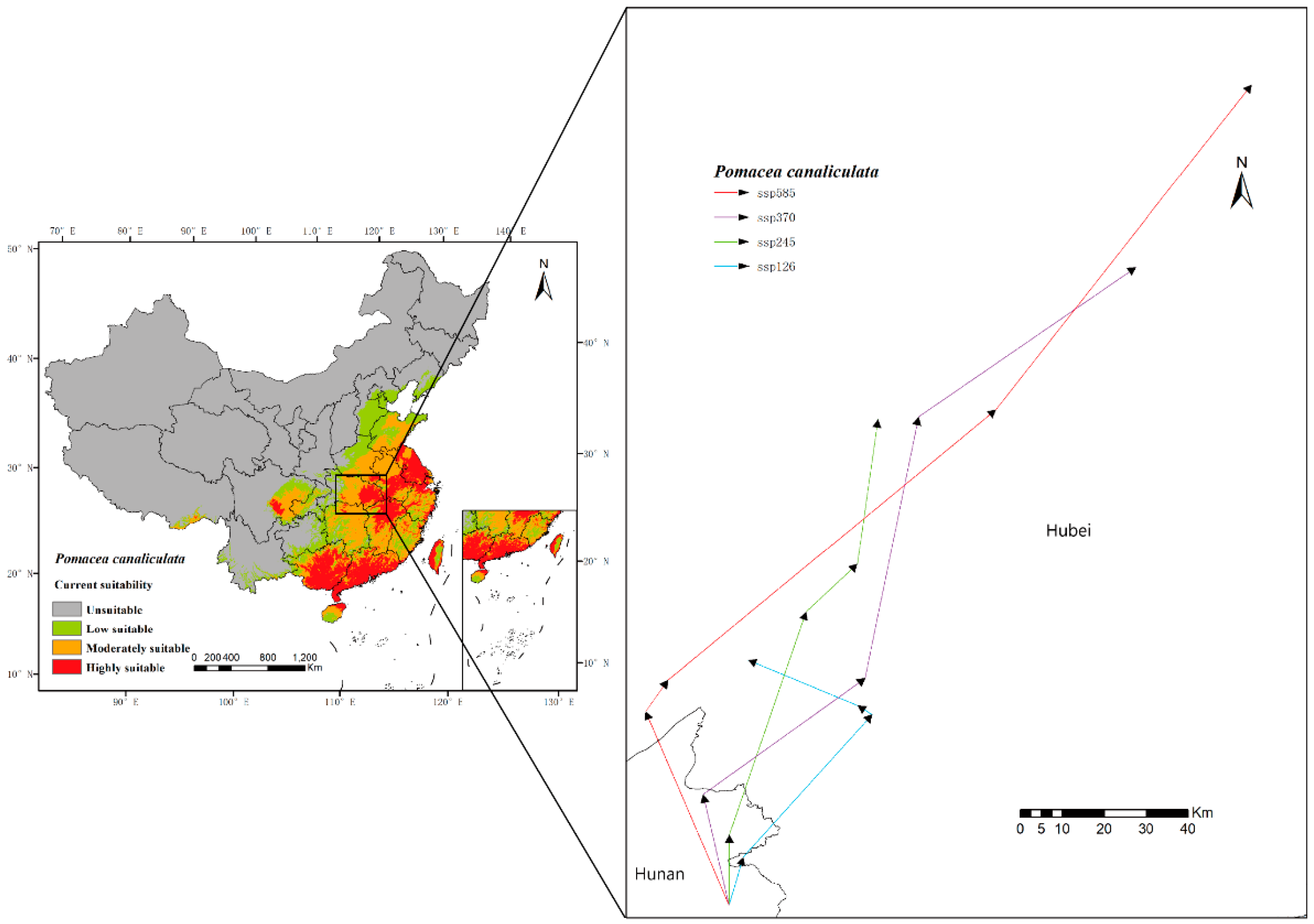
3.2. Current Prediction of P. canaliculata
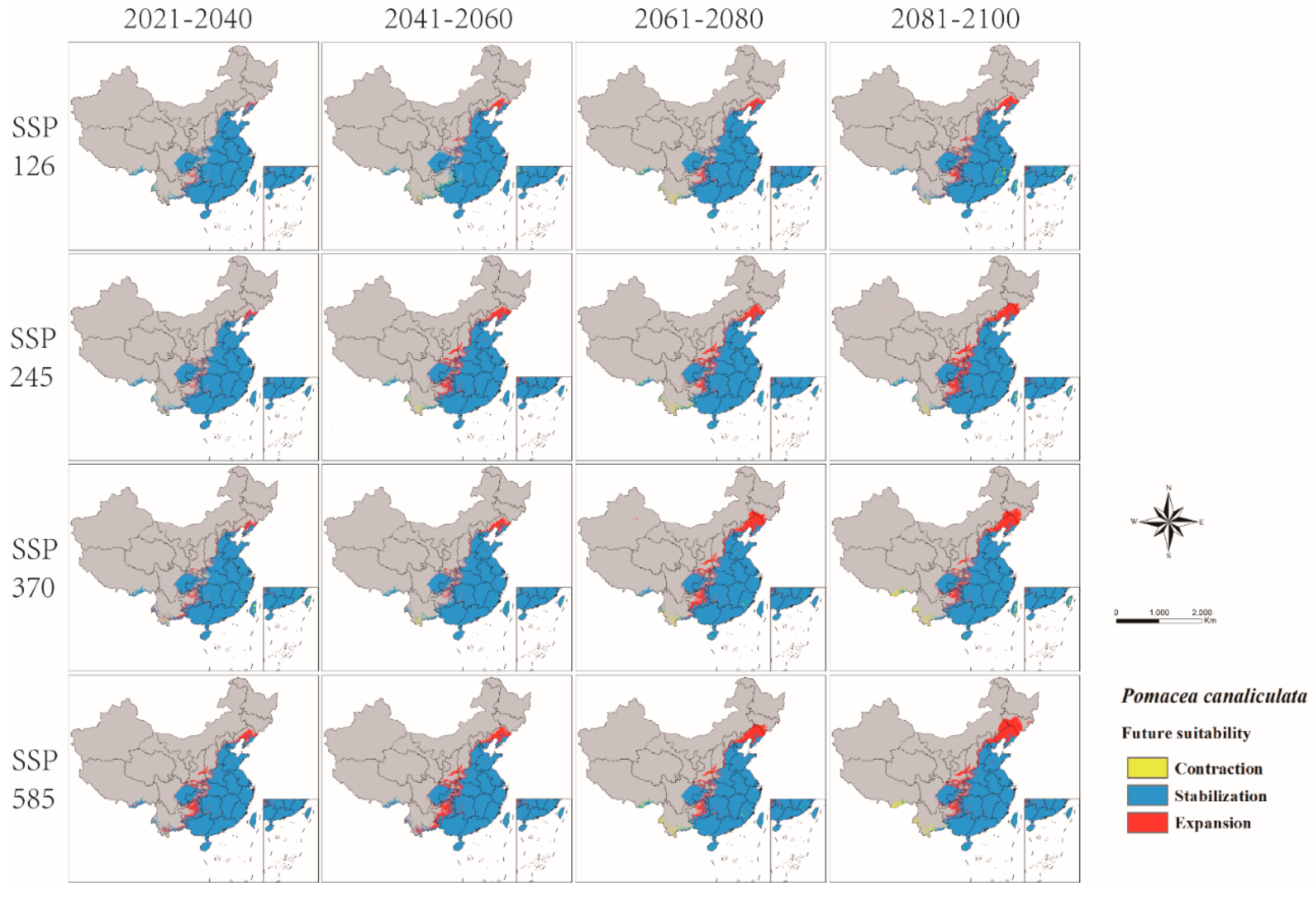
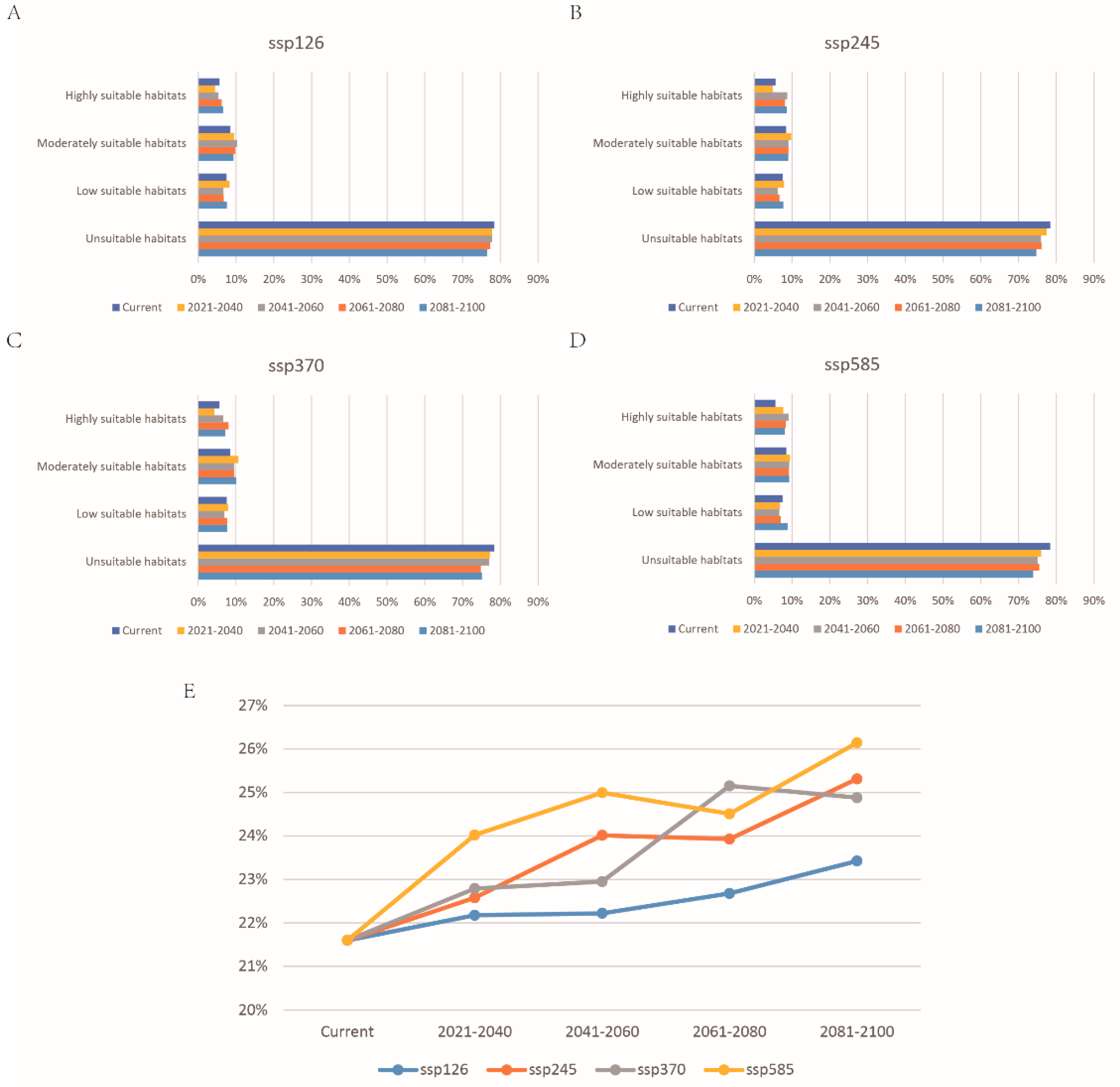
3.3. Future Prediction of P. canaliculata
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaric, I.; Heger, T.; Castro Monzon, F.; Jeschke, J.M.; Kowarik, I.; McConkey, K.R.; Pysek, P.; Sagouis, A.; Essl, F. Crypticity in Biological Invasions. Trends Ecol. Evol. 2019, 34, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Luque, G.M.; Bellard, C.; Bertelsmeier, C.; Bonnaud, E.; Genovesi, P.; Simberloff, D.; Courchamp, F. The 100th of the world’s worst invasive alien species. Biol. Invasions 2014, 16, 981–985. [Google Scholar] [CrossRef]
- Chao-Qun, Z.; Jian-Rong, D. Progress of research on biologically invasive medical molluscs in China. Chin. J. Schistosomiasis Control 2019, 31, 441–445. [Google Scholar] [CrossRef]
- Zhu, G.L.; Tang, Y.Y.; Limpanont, Y.; Wu, Z.D.; Li, J.; Lv, Z.Y. Zoonotic parasites carried by invasive alien species in China. Infect. Dis. Poverty 2019, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Mu, H.; Ip, J.C.H.; Li, R.; Xu, T.; Accorsi, A.; Sanchez Alvarado, A.; Ross, E.; Lan, Y.; Sun, Y.; et al. Signatures of divergence, invasiveness, and terrestrialization revealed by four apple snail genomes. Mol. Biol. Evol. 2019, 36, 1507–1520. [Google Scholar] [CrossRef] [Green Version]
- Yam, R.S.; Fan, Y.T.; Wang, T.T. Importance of macrophyte quality in determining life-history traits of the apple snails pomacea canaliculata: Implications for bottom-up management of an invasive herbivorous pest in constructed wetlands. Int. J. Environ. Res. Public Health 2016, 13, 248. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Liu, S.W.; He, C.; Yu, X.P. Distribution and the origin of invasive apple snails, Pomacea canaliculata and P. maculata (Gastropoda: Ampullariidae) in China. Sci. Rep. 2018, 8, 1185. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zhang, J.E.; Xia, J.; Yang, J.; Guo, J.; Deng, Z.; Luo, M. Comparative characterization of the complete mitochondrial genomes of the three apple snails (Gastropoda: Ampullariidae) and the phylogenetic analyses. Int. J. Mol. Sci. 2018, 19, 3646. [Google Scholar] [CrossRef] [Green Version]
- Sin, T.S. Damage potential of the golden apple snail Pomacea canaliculata (Lamarck) in irrigated rice and its control by cultural approaches. Int. J. Pest Manag. 2003, 49, 49–55. [Google Scholar] [CrossRef]
- Naylor, R. Invasions in agriculture: Assessing the cost of the golden apple snail in Asia. AMBIO 1996, 25, 443–448. [Google Scholar] [CrossRef]
- Horgan, F.G.; Stuart, A.M.; Kudavidanage, E.P. Impact of invasive apple snails on the functioning and services of natural andmanaged wetlands. Acta Oecologica 2014, 54, 90–100. [Google Scholar] [CrossRef]
- Mu, H.; Sun, J.; Cheung, S.G.; Fang, L.; Zhou, H.; Luan, T.; Zhang, H.; Wong, C.K.C.; Qiu, J.W. Comparative proteomics and codon substitution analysis reveal mechanisms of differential resistance to hypoxia in congeneric snails. J. Proteom. 2018, 172, 36–48. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zheng, G.; Dong, S.; Liu, G.; Yu, X. Molecular cloning, characterization and expression analysis of HSP60, HSP70 and HSP90 in the golden apple snail, Pomacea canaliculata. Fish Shellfish Immunol. 2014, 41, 643–653. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Ren, Y.; Wang, H.; Li, S.; Jiang, F.; Yin, L.; Qiao, X.; Zhang, G.; Qian, W.; et al. The genome of the golden apple snail Pomacea canaliculata provides insight into stress tolerance and invasive adaptation. Gigascience 2018, 7, giy101. [Google Scholar] [CrossRef]
- Ottaviani, E.; Accorsi, A.; Rigillo, G.; Malagoli, D.; Blom, J.M.; Tascedda, F. Epigenetic modification in neurons of the mollusc Pomacea canaliculata after immune challenge. Brain Res. 2013, 1537, 18–26. [Google Scholar] [CrossRef]
- Komalamisra, C.; Nuamtanong, S.; Dekumyoy, P. Pila ampullacea and Pomacea canaliculata, as new paratenic hosts of Gnathostoma spinigerum. Southeast Asian J. Trop. Med. Public Health 2009, 40, 243–246. [Google Scholar] [CrossRef]
- Cawas, J.R.; Quisao, C.J.T.; Castillo, D.S.C.; Pornobi, K.O. Prevalence of Angiostrongylus cantonensis among different species of snails in the village of Bagong Sikat Munoz, Nueva Ecija, Philippines and its associated risk factors for zoonotic transmission. J. Parasit. Dis. 2020, 44, 388–394. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Liu, H.X.; Hu, L.; Yang, K.; Steinmann, P.; Chen, Z.; Wang, L.Y.; Utzinger, J.; Zhou, X.N. Invasive snails and an emerging infectious disease: Results from the first national survey on Angiostrongylus cantonensis in China. PLoS Negl. Trop. Dis. 2009, 3, e368. [Google Scholar] [CrossRef] [Green Version]
- Lv, S.; Zhang, Y.; Steinmann, P.; Zhou, X.N. Emerging angiostrongyliasis in Mainland China. Emerg. Infect. Dis. 2008, 14, 161–164. [Google Scholar] [CrossRef]
- Lamsal, P.; Kumar, L.; Aryal, A.; Atreya, K. Invasive alien plant species dynamics in the Himalayan region under climate change. AMBIO 2018, 47, 697–710. [Google Scholar] [CrossRef]
- Tang, J.; Li, J.; Lu, H.; Lu, F.; Lu, B. Potential distribution of an invasive pest, Euplatypus parallelus, in China as predicted by Maxent. Pest. Manag. Sci. 2019, 75, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. Kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meza-Lopez, M.M.; Siemann, E. Warming alone increased exotic snail reproduction and together with eutrophication influenced snail growth in native wetlands but did not impact plants. Sci. Total Environ. 2020, 704, 135271. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Steinmann, P.; Yang, G.-J.; Yang, K.; Zhou, X.-N.; Utzinger, J. The emergence of angiostrongyliasis in the People’s Republic of China: The interplay between invasive snails, climate change and transmission dynamics. Freshw. Biol. 2011, 56, 717–734. [Google Scholar] [CrossRef]
- Ito, K. Environmental factors influencing overwintering success of the golden apple snail, Pomacea canaliculata (Gastropoda: Ampullariidae), in the northernmost population of Japan. Appl. Entomol. Zool. 2002, 37, 655–661. [Google Scholar] [CrossRef] [Green Version]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Liu, X. The Beijing climate center climate system model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Chen, X.; Dai, Y.; Hu, G. Climate sensitivity and feedbacks of BCC-CSM to idealized CO2 forcing from CMIP5 to CMIP6. J. Meteorol. Res. 2020, 34, 865–878. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, Z.; Li, L. Biases and improvements in three dynamical downscaling climate simulations over China. Clim. Dyn. 2016, 47, 3235–3251. [Google Scholar] [CrossRef] [Green Version]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G.; Pearson, R. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Freeman, E.A.; Moisen, G.G. A comparison of the performance of threshold criteria for binary classification in terms of predicted prevalence and kappa—ScienceDirect. Ecol. Model. 2008, 217, 48–58. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Yu, X.P. A new species of apple snail in the Genus Pomacea (Gastropoda: Caenogastropoda: Ampullariidae). Zool. Stud. 2019, 58, e13. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, S.; He, C.; Cowie, R.H.; Yu, X.; Hayes, K.A. Invisible apple snail invasions: Importance of continued vigilance and rigorous taxonomic assessments. Pest. Manag. Sci. 2019, 75, 1277–1286. [Google Scholar] [CrossRef]
- Cao, Y.; Dewalt, R.E.; Robinson, J.L.; Tweddale, T.; Hinz, L.; Pessino, M. Using Maxent to model the historic distributions of stonefly species in Illinois streams: The effects of regularization and threshold selections. Ecol. Modell. 2013, 259, 30–39. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schrder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1336–1379. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Cieplok, A.; Spyra, A. The roles of spatial and environmental variables in the appearance of a globally invasive Physa acuta in water bodies created due to human activity. Sci. Total Environ. 2020, 744, 140928. [Google Scholar] [CrossRef]
- Ibanez, I.; Diez, J.M.; Miller, L.P.; Olden, J.D.; Sorte, C.J.; Blumenthal, D.M.; Bradley, B.A.; D’Antonio, C.M.; Dukes, J.S.; Early, R.I.; et al. Integrated assessment of biological invasions. Ecol. Appl. 2014, 24, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Matsukura, K.; Tsumuki, H.; Izumi, Y.; Wada, T. Physiological response to low temperature in the freshwater apple snail, Pomacea canaliculata (Gastropoda: Ampullariidae). J. Exp. Biol. 2009, 212, 2558–2563. [Google Scholar] [CrossRef] [Green Version]
- Gilioli, G.; Pasquali, S.; Martin, P.R.; Carlsson, N.; Mariani, L. A temperature-dependent physiologically based model for the invasive apple snail Pomacea canaliculata. Int. J. Biometeorol. 2017, 61, 1899–1911. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Sun, Z.; Wang, Z.; Peng, Y. A comparative transcriptomics approach to analyzing the differences in cold resistance in Pomacea canaliculata between Guangdong and Hunan. J. Immunol. Res. 2020, 2020, 8025140. [Google Scholar] [CrossRef]
- Byers, J.E.; McDowell, W.G.; Dodd, S.R.; Haynie, R.S.; Pintor, L.M.; Wilde, S.B. Climate and pH predict the potential range of the invasive apple snail (Pomacea insularum) in the southeastern United States. PLoS ONE 2013, 8, e56812. [Google Scholar] [CrossRef]
- Lei, J.; Chen, L.; Li, H. Using ensemble forecasting to examine how climate change promotes worldwide invasion of the golden apple snail (Pomacea canaliculata). Environ. Monit. Assess. 2017, 189, 404. [Google Scholar] [CrossRef]
- Mu, H.; Sun, J.; Fang, L.; Luan, T.; Williams, G.A.; Cheung, S.G.; Wong, C.K.; Qiu, J.W. Genetic basis of differential heat resistance between two species of congeneric freshwater snails: Insights from quantitative proteomics and base substitution rate analysis. J. Proteome Res. 2015, 14, 4296–4308. [Google Scholar] [CrossRef]
- Pasiecznik, N.M.; Harris, P.J.C.; Smith, S.J. Identifying Tropical Prosopis Species: A Field Guide; HDRA Publishing: Coventry, UK, 2003. [Google Scholar]
- Riahi, K.; van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, A.J.; Li, K.; Qin, J.; Li, H. Effects of glyphosate-based herbicides on survival, development and growth of invasive snail (Pomacea canaliculata). Aquat. Toxicol. 2017, 193, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, F.S.; Peso-Aguiar, M.C.; Assuncao-Albuquerque, M.J. Distribution, feeding behavior and control strategies of the exotic land snail Achatina fulica (Gastropoda: Pulmonata) in the northeast of Brazil. Braz. J. Biol. 2008, 68, 837–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rae, R.; Verdun, C.; Grewal, P.S.; Robertson, J.F.; Wilson, M.J. Biological control of terrestrial molluscs using Phasmarhabditis hermaphrodita—Progress and prospects. Pest. Manag. Sci. 2007, 63, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
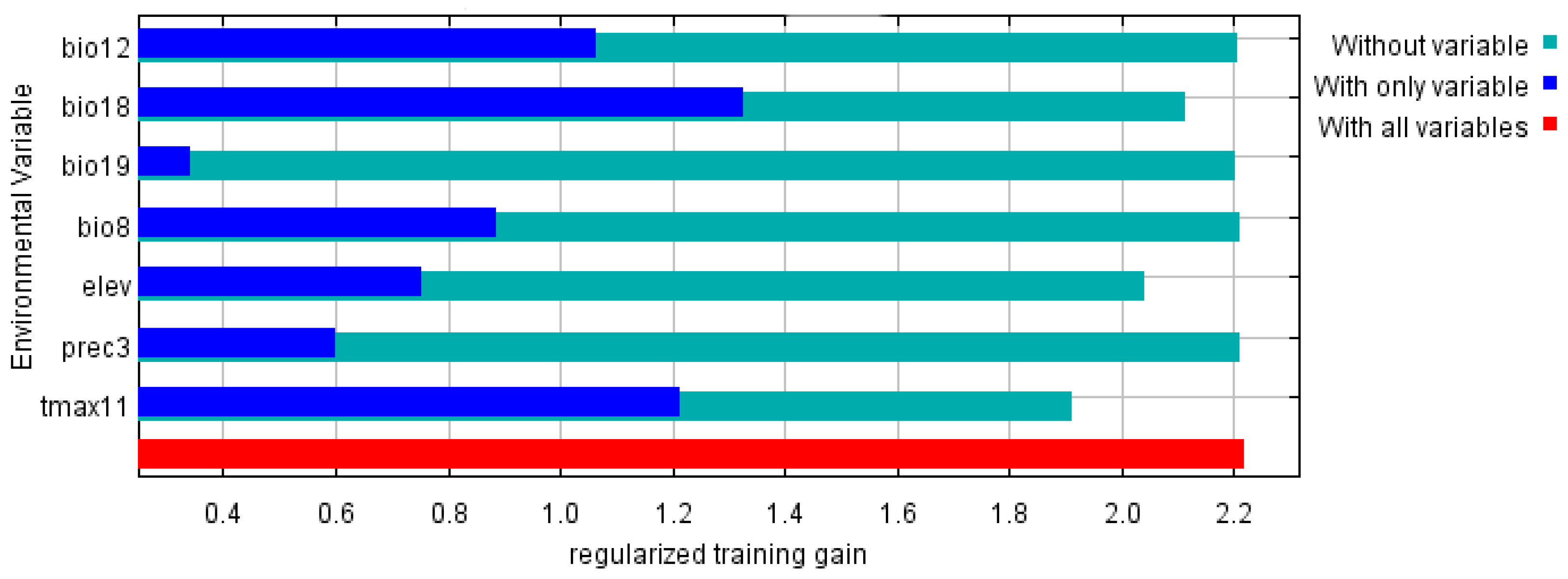
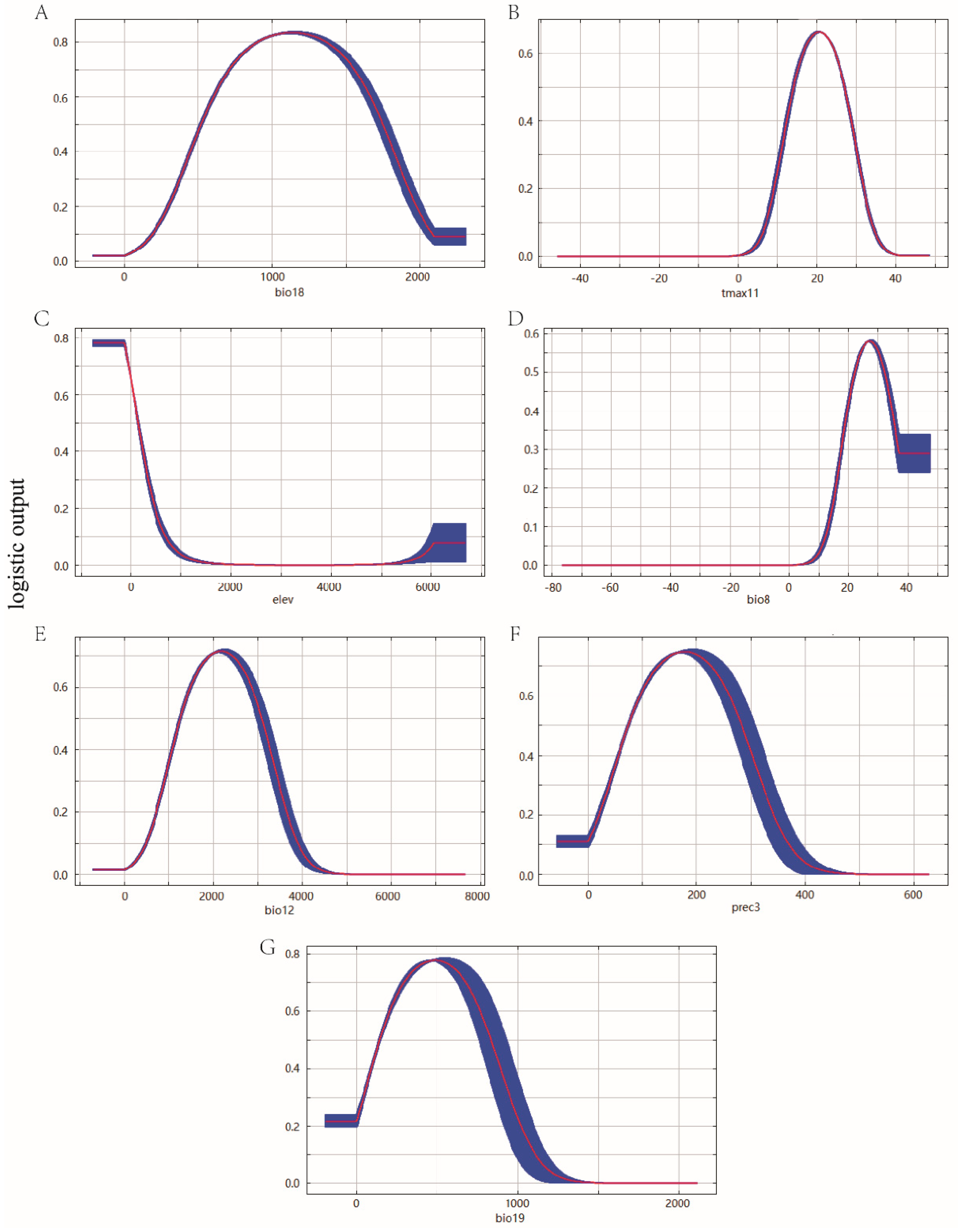
| Code | Environmental Variables | Percent Contribution | Permutation Importance |
|---|---|---|---|
| Bio18 | Precipitation of warmest quarter | 42.4 | 9.6 |
| Tmax11 | Maximum temperature of November | 29.6 | 53.4 |
| Elev | Elevation | 17.6 | 19.2 |
| Bio8 | Mean temperature of wettest quarter | 5.5 | 15.1 |
| Bio12 | Annual precipitation | 1.9 | 0.5 |
| Prec3 | Precipitation of March | 1.5 | 0.8 |
| Bio19 | Precipitation of coldest quarter | 1.4 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; He, Q.; Pan, X.; Liu, Q.; Wu, Y.; Li, X. Predicting Current Potential Distribution and the Range Dynamics of Pomacea canaliculata in China under Global Climate Change. Biology 2022, 11, 110. https://doi.org/10.3390/biology11010110
Yin Y, He Q, Pan X, Liu Q, Wu Y, Li X. Predicting Current Potential Distribution and the Range Dynamics of Pomacea canaliculata in China under Global Climate Change. Biology. 2022; 11(1):110. https://doi.org/10.3390/biology11010110
Chicago/Turabian StyleYin, Yingxuan, Qing He, Xiaowen Pan, Qiyong Liu, Yinjuan Wu, and Xuerong Li. 2022. "Predicting Current Potential Distribution and the Range Dynamics of Pomacea canaliculata in China under Global Climate Change" Biology 11, no. 1: 110. https://doi.org/10.3390/biology11010110
APA StyleYin, Y., He, Q., Pan, X., Liu, Q., Wu, Y., & Li, X. (2022). Predicting Current Potential Distribution and the Range Dynamics of Pomacea canaliculata in China under Global Climate Change. Biology, 11(1), 110. https://doi.org/10.3390/biology11010110






