Xylella fastidiosa and Drought Stress in Olive Trees: A Complex Relationship Mediated by Soluble Sugars
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Conditions and Plant Material
2.2. Relative Water Content Measurement
2.3. Carbohydrate Determination
2.4. Total RNA Isolation, cDNA Synthesis and RT-qPCR Analysis
| Name | Sequence 5′-3′ | Reference | GeneBank |
|---|---|---|---|
| Oeβ-Act F | ACTATGAACAGGATCTTGAG | Rossi et al., 2016 [30] | AF545569.1 |
| Oeβ-Act R | GAACCACCACTGAGGACGAT | ||
| OeSUT1F | TCGGTTATGCGGCTGGAT | Sabella et al., 2019 [31] | JN656245.1 |
| OeSUT1R | CAGGCTTTTGTTTTGGTAAATGG | ||
| OeMST2F | GCCAATGTGGACGAGGAGTT | Sabella et al.,2019 [31] | DQ087177.2 |
| OeMST2R | TGCTCCACCTTCCTCGACTCT | ||
| OeSUSY F | GCCTGGACTCTACCGAGTTGTT | Alagna et al., 2016 [32] | unigene02089 |
| OeSUSY R | CACGCATAGGTGTTCCTTGTTC | ||
| OeINV-CW F | AGACAAGGCAGAGACATTCGAC | Alagna et al., 2016 [32] | unigene02494 |
| OeINV-CW R | ATGCATCAGAGCACATGAGAAC | ||
| OeINV-V F | CCAGTCAGCGAAGTGGAAGAAT | Alagna et al., 2016 [32] | unigene01665 |
| OeINV-V R | TGTAACCAGCATCAGCATCAGC | ||
| OeGBSSI F | TGTGCCAAAGTCGACCCTGCCG | Alagna et al., 2016 [32] | unigene00185 |
| OeGBSSI R | TGGTTCACTGCTGGCAGCCCC |
2.5. Statistical Analysis
3. Results
3.1. Evaluation of Xylella fastidiosa Infection and Plant Water Content
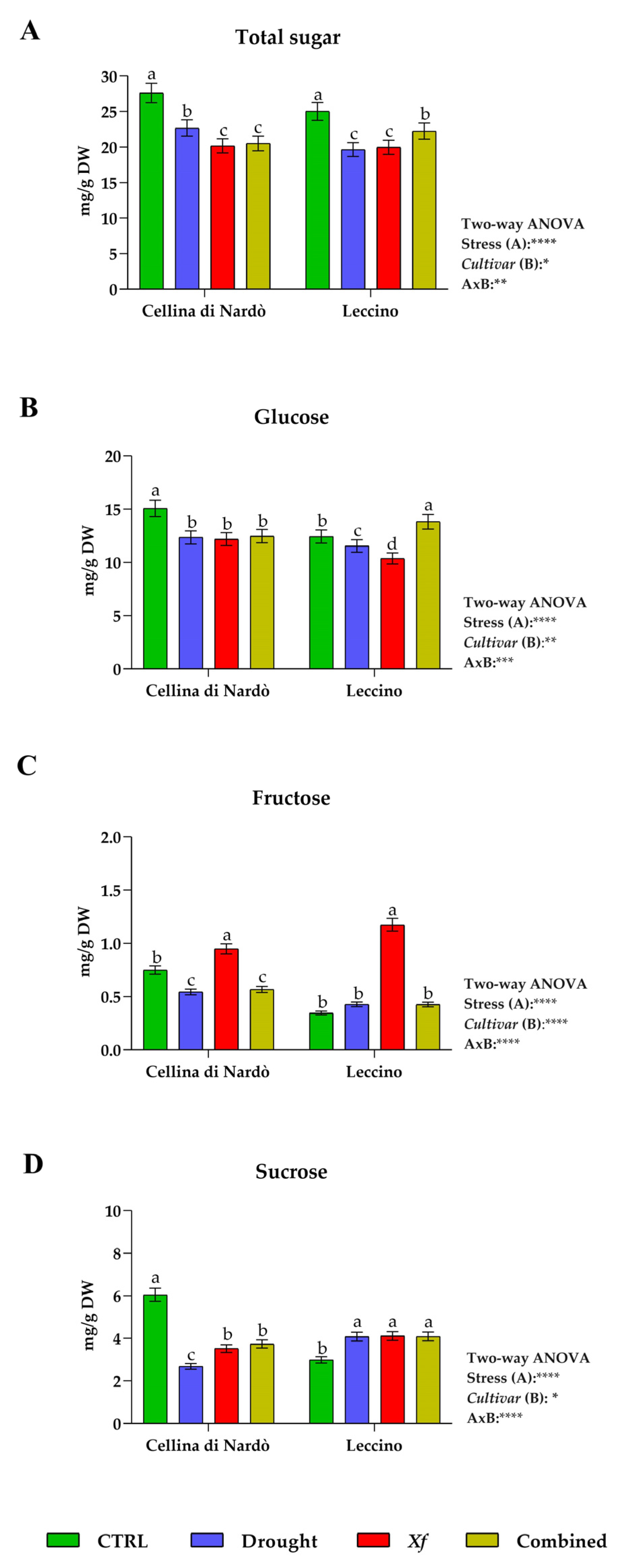
3.2. Determination of Carbohydrate Content
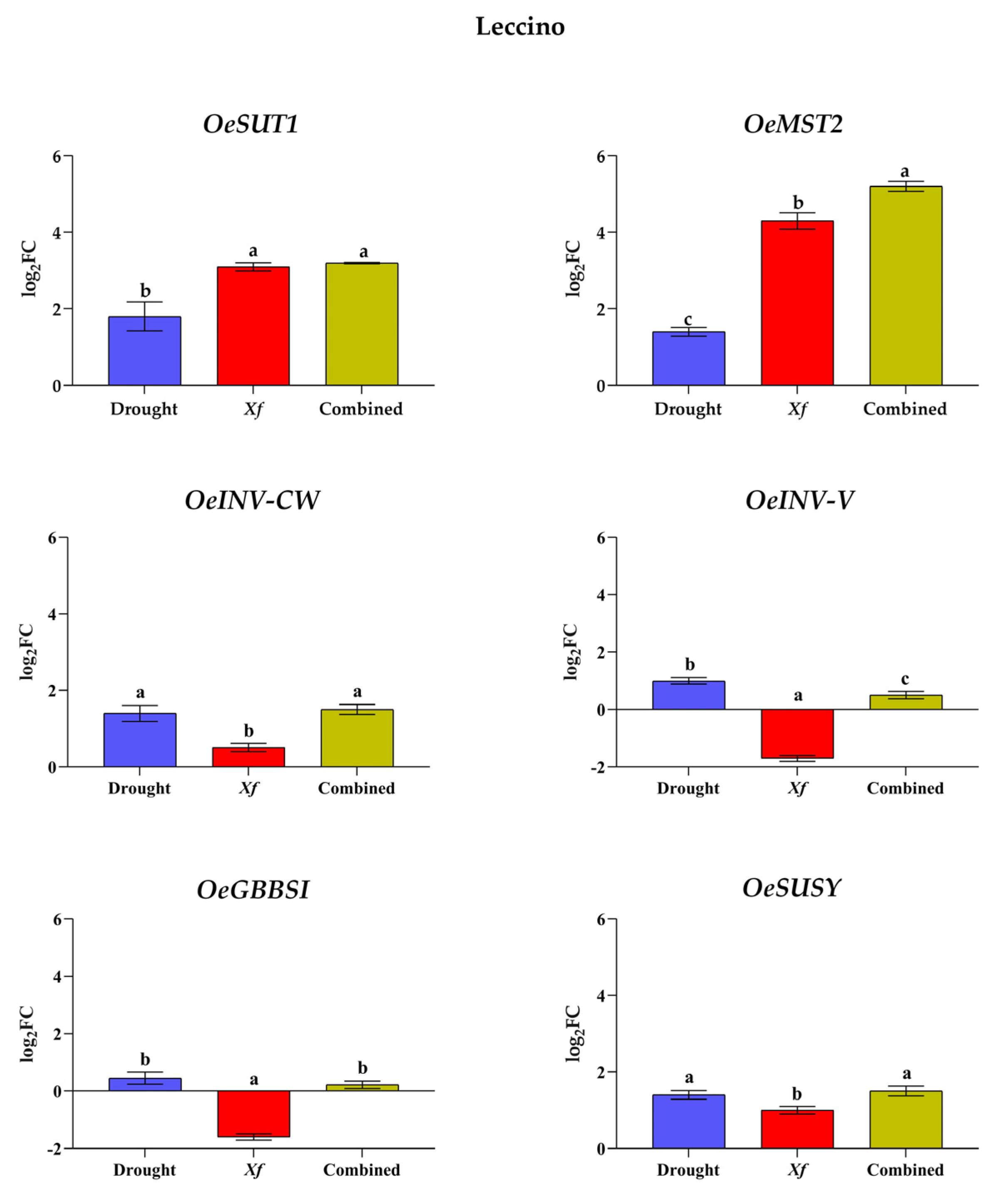
3.3. Expression of the Selected Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Granot, D.; David-Schwartz, R.; Kelly, G. Hexose kinases and their role in sugar-sensing and plant development. Front. Plant Sci. 2013, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- Gangola, M.R.; Bharathi, R.; Ramadoss, B.R. Sugars Play a Critical Role in Abiotic Stress Tolerance in Plants in: Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Wani, S.H., Ed.; Academic Press: London, UK, 2018; pp. 17–38. [Google Scholar] [CrossRef]
- Morkunas, I.; Ratajczak, L. The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol. Plant. 2014, 36, 1607–1619. [Google Scholar] [CrossRef] [Green Version]
- Lawas, L.M.F.; Zuther, E.; Jagadish, S.K.; Hincha, D.K. Molecular mechanisms of combined heat and drought stress resilience in cereals. Curr. Opin. Plant Biol. 2018, 45, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Beckles, D.M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234-235, 80–93. [Google Scholar] [CrossRef]
- Pucciariello, C.; Perata, P. The Oxidative Paradox in Low Oxygen Stress in Plants. Antioxidants 2021, 10, 332. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Ludewig, F.; Cvetkovic, J.; Trentmann, O.; Klemens, P.A.W.; Neuhaus, H.E. In Concert: Orchestrated Changes in Carbohydrate Homeostasis Are Critical for Plant Abiotic Stress Tolerance. Plant Cell Physiol. 2018, 59, 1290–1299. [Google Scholar] [CrossRef]
- Yamada, K.; Osakabe, Y. Sugar compartmentation as an environmental stress adaptation strategy in plants. Semin. Cell Dev. Biol. 2018, 83, 106–114. [Google Scholar] [CrossRef]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (Southern Italy). J. Plant Pathol. 2013, 95, 659–668. [Google Scholar]
- Cardinale, M.; Luvisi, A.; Meyer, J.B.; Sabella, E.; De Bellis, L.; Cruz, A.C.; Ampatzidis, Y.; Cherubini, P. Specific fluorescence in situ hybridization (FISH) test to highlight colonization of xylem vessels by Xylella fastidiosa in naturally infected olive trees (Olea europaea L.). Front. Plant Sci. 2018, 9, 431. [Google Scholar] [CrossRef] [Green Version]
- Giampetruzzi, A.; Morelli, M.; Saponari, M.; Loconsole, G.; Chiumenti, M.; Boscia, D.; Savino, V.N.; Martelli, G.P.; Saldarelli, P. Transcriptome profiling of two olive cultivars in response to infection by the CoDiRO strain of Xylella fastidiosa subsp. pauca. BMC Genom. 2016, 1, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martelli, G.P. The current status of the quick decline syndrome of olive in southern Italy. Phytoparasitica 2016, 44, 1–10. [Google Scholar] [CrossRef]
- Luvisi, A.; Aprile, A.; Sabella, E.; Vergine, M.; Nicolì, F.; Nutricati, E.; Miceli, A.; Negro, C.; De Bellis, L. Xylella fastidiosa subsp. pauca (CoDiRO strain) infection in four olive (Olea europaea L.) cultivars: Profile of phenolic compounds in leaves and progression of leaf scorch symptoms. Phytopathol. Mediterr. 2017, 56, 259–273. [Google Scholar]
- Jlilat, A.; Ragone, R.; Gualano, S.; Santoro, F.; Gallo, V.; Varvaro, L.; Mastrorilli, P.; Saponari, M.; Nigro, F.; D’Onghia, A.M. A non-targeted metabolomics study on Xylella fastidiosa infected olive plants grown under controlled conditions. Sci. Rep. 2021, 11, 1070. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; Vergine, M.; Nicolì, F.; Sabella, E.; Aprile, A.; Negro, C.; Fanelli, V.; Savoia, M.A.; Montilon, V.; Susca, L.; et al. Screening of Olive Biodiversity Defines Genotypes Potentially Resistant to Xylella fastidiosa. Front. Plant Sci 2021, 12, 1734. [Google Scholar] [CrossRef]
- Schneider, K.; van der Werf, W.; Cendoya, M.; Maurits, M.; Navas-Cortes, J.A. Impact of Xylella fastidiosa subspecies pauca in European olives. Proc. Natl. Acad. Sci. USA 2020, 117, 9250–9259. [Google Scholar] [CrossRef] [Green Version]
- Morelli, M.; García-Madero, J.M.; Jos, Á.; Saldarelli, P.; Dongiovanni, C.; Kovacova, M.; Saponari, M.; Baños Arjona, A.; Hackl, E.; Webb, S.; et al. Xylella fastidiosa in Olive: A Review of Control Attempts and Current Management. Microorganisms 2021, 9, 1771. [Google Scholar] [CrossRef]
- Alfio, M.R.; Balacco, G.; Parisi, A.; Totaro, V.; Fidelibus, M.D. Drought index as indicator of salinization of the Salento aquifer (Southern Italy). Water 2020, 12, 1927. [Google Scholar] [CrossRef]
- Marra, F.P.; Marino, G.; Marchese, A.; Caruso, T. Effects of different irrigation regimes on a super-high-density olive grove cv. “Arbequina”: Vegetative growth, productivity and polyphenol content of the oil. Irrig. Sci. 2016, 34, 313–325. [Google Scholar] [CrossRef]
- Harper, S.J.; Ward, L.I.; Clover, G.R.G. Development of LAMP and Real-time PCR Methods for the rapid detection of Xylella fastidiosa for quarantine and field applications. Phytopatology 2010, 100, 1282–1288. [Google Scholar] [CrossRef]
- De Pascali, M.; Vergine, M.; Sabella, E.; Aprile, A.; Nutricati, E.; Nicolì, F.; Buja, I.; Negro, C.; Miceli, A.; Rampino, P.; et al. Molecular Effects of Xylella fastidiosa and Drought Combined Stress in Olive Trees. Plants 2019, 8, 437. [Google Scholar] [CrossRef] [Green Version]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimanting water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- PM 7/24 (4) Xylella fastidiosa. EPPO Bull. 2019, 49, 175–227. [CrossRef] [Green Version]
- Chow, P.S.; Landhausser, S.M. A method for routine of total sugar and starch content in woody plants tissue. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Stein, O.; Granot, D. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, K. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef] [Green Version]
- Slewinski, T.L. Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: A physiological perspective. Mol. Plant. 2011, 4, 641–662. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2011, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Borghi, M.; Francini, M.; Lin, X.; Xie, D.; Sebastiani, L. Salt stress induces differential regulation of the phenylpropanoid pathway in Olea europaea cultivars Frantoio (salt-tolerant) and Leccino (salt-sensitive). J. Plant. Physiol. 2016, 204, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sabella, E.; Aprile, A.; Genga, A.; Siciliano, T.; Nutricati, E.; Nicolì, F.; Vergine, M.; Negr, C.; De Bellis, L.; Luvisi, A. Xylem cavitation susceptibility and refilling mechanisms in olive trees infected by Xylella fastidiosa. Sci. Rep. 2019, 9, 9602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alagna, F.; Cirilli, M.; Galla, G.; Carbone, F.; Daddiego, L.; Facella, P.; Lopez, L.; Colao, C.; Mariotti, R.; Cultrera, N.; et al. Transcript Analysis and Regulative Events during Flower Development in Olive (Olea europaea L.). PLoS ONE 2016, 11, e0152943. [Google Scholar] [CrossRef] [Green Version]
- Semeraro, T.; Gatto, E.; Buccolieri, R.; Vergine, M.; Gao, Z.; De Bellis, L.; Luvisi, A. Changes in olive urban forests infected by Xylella fastidiosa: Impact on microclimate and social health in urban areas. Int. J. Environ. Res. Public Health 2019, 16, 2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, S.; Papadopoulos, M.; Schreiber, U.; Kaiser, W.; Roitsch, T. Complex regulation of gene expression, photosynthesis and sugar levels by pathogen infection in tomato. Physiol. Plant. 2004, 122, 419–428. [Google Scholar] [CrossRef]
- Chen, J.; Ullah, C.; Giddings Vassão, D.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. Sclerotinia sclerotiorum infection triggers changes in primary and secondary metabolism in Arabidopsis thaliana. Phytopathology 2021, 111, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Aked, J.; Hall, J.L. Effect of powdery mildew infection on concentrations of apoplastic sugars in pea leaves. New Phytol. 1993, 123, 283–288. [Google Scholar] [CrossRef]
- Wright, D.P.; Baldwin, B.C.; Shephard, M.C.; Scholes, J.D. Source-sink relationships in wheat leaves infected with powdery mildew: 1. Alterations in carbohydrate metabolism. Physiol. Mol. Plant Pathol. 1995, 47, 237–253. [Google Scholar] [CrossRef]
- Herbers, K.; Takahata, Y.; Melzer, M.; Mock, H.P.; Hajirezaei, M.; Sonnewald, U. Regulation of carbohydrate partitioning during the interaction of potato virus Y with tobacco. Mol. Plant Pathol. 2000, 1, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.; Bundock, N.; Rolfe, S.; Scholes, J. Infection of Arabidopsis thaliana leaves with Albugo candida causes a reprogramming of host metabolism. Mol. Plant Pathol. 2000, 1, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Lecompte, F.; Abro, M.A.; Nicot, P.C. Can plant sugars mediate the effect of nitrogen fertilization on lettuce susceptibility to two necrotrophic pathogens: Botrytis cinerea and Sclerotinia sclerotiorum? Plant Soil. 2013, 369, 387–401. [Google Scholar] [CrossRef]
- Lecompte, F.; Nicot, P.C.; Ripoll, J.; Abro, M.A.; Raimbault, A.K.; Lauri, F.L.; Bertin, N. Reduced susceptibility of tomato stem to the necrotrophic fungus Botrytis cinerea is associated with a specific adjustment of fructose content in the host sugar pool. Ann. Bot. 2017, 119, 931–943. [Google Scholar] [PubMed] [Green Version]
- Hui, D.; Iqbal, J.; Lehmann, K.; Gase, K.; Saluz, H.P.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (lepidoptera, sphingidae) and its natural host Nicotiana attenuata: V. microarray analysis and further characterization of large-scale changes in herbivore-induced mRNAs. Plant Physiol. 2003, 131, 1877–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerli, L.; Stein, M.; Lipka, V.; Schulze-Lefert, P. Somerville, S. Host and non-host pathogens elicit different jasmonate/ethylene responses in Arabidopsis. Plant J. 2004, 40, 633–646. [Google Scholar] [CrossRef] [PubMed]
- de Torres Zabala, M.; Littlejohn, G.; Jayaraman, S.; Studholme, D.; Bailey, T.; Lawson, T.; Tillich, M.; Licht, D.; Bölter, B.; Delfino, L.; et al. Chloroplasts play a central role in plant defence and are targeted by pathogen effectors. Nat. Plants 2015, 1, 15074. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does it Make Any Difference the Fact to Be a C3 or C4 Species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Wind, J.J.; Shi, X.; Zhang, H.; Hanson, J.; Smeekens, S.C.; Teng, S. Fructose sensitivity is suppressed in Arabidopsis by the transcription factor ANAC089 lacking the membrane-bound domain. Proc. Natl. Acad. Sci. USA 2011, 108, 3436–3441. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Xie, J.; Wen, S.; Wu, W.; Tan, L.; Lei, M.; Shi, H.; Zhu, J.K. TPST is involved in fructose regulation of primary root growth in Arabidopsis thaliana. Plant Mol. Biol. 2020, 103, 511–525. [Google Scholar] [CrossRef]
- Bolouri-Moghaddam, M.R.; Le Roy, K.; Xiang, L.; Rolland, F.; Van den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Giardina, T. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front. Plant Sci. 2014, 5, 293. [Google Scholar] [CrossRef]
- Morsy, M.R.; Jouve, L.; Hausman, J.F.; Hoffmann, L.; Stewart, J.M. Alteration of oxidative and carbohydrate metabolism under abiotic stress in two rice (Oryza sativa L.) genotypes contrasting in chilling tolerance. J. Plant Physiol. 2007, 164, 157–167. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Tian, J.; Zhu, Y.; Fan, J. Changes in sucrose metabolism in maize varieties with different cadmium sensitivities under cadmium stress. PLoS ONE 2020, 15, e0243835. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of Sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef] [PubMed]
- Noiraud, N.; Delrot, S.; Lemoine, R. The sucrose transporter of celery: Identification and expression during salt stress. Plant Physiol. 2000, 122, 1447–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dedaldechamp, F.; Allario, T.; Pourtau, N.; Bonnemai, J.L.; Laloi, M.; Coutos-Thevenot, P.; Maurousset, L. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, W.; Zhang, L.; Wu, D.; Liu, S.; Gong, X.; Cui, Z.; Cui, N.; Cao, H.; Rao, L.; Wang, C. Sucrose transporter AtSUC9 Mediated by a low sucrose level is involved in Arabidopsis abiotic stress resistance by regulating sucrose distribution and ABA accumulation. Plant Cell Physiol. 2015, 56, 1574–1587. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.J.; Sun, M.H.; Lu, J.; Kang, H.; You, C.X.; Hao, Y.J. An apple sucrose transporter MdSUT2.2 is a phosphorylation target for protein kinase MdCIPK22 in response to drought. Plant Biotech. J. 2019, 17, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Boldt, K.; Pörs, Y.; Haupt, B.; Bitterlich, M.; Kühn, C.; Grimm, B.; Franken, P. Photochemical processes, carbon assimilation and RNA accumulation of sucrose transporter genes in tomato arbuscular mycorrhiza. J. Plant Physiol. 2011, 5, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.; Youssef-Banora, M.; De Almeida-Engler, J.; Grundler, F.M. The role of callose deposition along plasmodesmata in nematode feeding sites. Mol. Plant Microbe Interact. 2010, 23, 549–557. [Google Scholar] [CrossRef]
- Monfared, H.; Chew, J.K.; Azizi, P.; Xue, G.; Ee, S.; Kadkhodaei, S.; Hedayati, P.; Ismail, I.; Zainal, Z. Overexpression of a rice monosaccharide transporter gene (OsMST6) confers enhanced tolerance to drought and salinity stress in Arabidopsis thaliana. Plant Mol. Biol. Rep. 2020, 1, 14. [Google Scholar] [CrossRef]
- Breia, R.; Conde, A.; Badim, H.; Fortes, A.M.; Geros, H.; Granell, A. Plant SWEETs: From sugar transport to plant-pathogen interaction and more unexpected physiological roles. Plant Physiol. 2021, 186, 836–852. [Google Scholar] [CrossRef]
- Pelah, D.; Wang, W.; Altman, A.; Shoseyov, O.; Bartels, D. Differential accumulation of water stress related proteins, sucrose synthase and soluble sugars in Populus species that differ in their water stress response. Physiol. Plant. 1997, 99, 153–159. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Li, C.; Zhang, Z.; Ma, F.; Li, M. Response of sugar metabolism in apple leaves subjected to short-term drought stress. Plant Physiol. Biochem. 2019, 41, 164–171. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, H.; Wu, J.; Xie, F. Effect of drought stress during soybean R2-R6 growth stages on sucrose metabolism in leaf and seed. Inter. J. Mol. Sci. 2020, 17, 618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Hren, M.; Ravnikar, M.; Brzin, J.; Ermacora, P.; Carraro, L.; Bianco, P.A.; Casati, P.; Borgo, M.; Angelini, E.; Rotter, A.; et al. Induced expression of sucrose synthase and alcohol dehydrogenase I genes in phytoplasma-infected grapevine plants grown in the field. Plant Pathol. 2009, 58, 170–180. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.H.; Tapias, E.C.; Kim, H.K.; Lefeber, A.W.; Erkelens, C.; Verhoeven, J.T.; Brzin, J.; Zel, J.; Verpoorte, R. Metabolic discrimination of Catharanthus roseus leaves infected by phytoplasma using 1H-NMR spectroscopy and multivariate data analysis. Plant Physiol. 2004, 135, 2398–2410. [Google Scholar] [CrossRef] [Green Version]
- Rios, J.A.; Rios, V.S.; Aucique Pérez, C.E.; Cruz, M.F.A.; Morais, L.E.; DaMatta, F.M.; Rodrigues, F.A. Alteration of photosynthetic performance and source–sink relationships in wheat plants infected by Pyricularia oryzae. Plant Pathol. 2017, 66, 1496–1507. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Cruz, J.; Pastenes, C. Water-stress-induced thermotolerance of photosynthesis in bean (Phaseolus vulgaris L.) plants: The possible involvement of lipid composition and xanthophyll cycle pigments. Environ. Exp. Bot. 2012, 77, 127–140. [Google Scholar] [CrossRef]
- Wang, Z.; Quebedeaux, B.; Stutte, G.W. Partitioning of (14C) glucose into sorbitol and other carbohydrates in apple under water stress. Aust. J. Plant Physiol. 1996, 23, 245–251. [Google Scholar] [CrossRef]
- Yi, B.; Zhou, Y.; Gao, M.; Zhang, Z.; Yi, H.; Yang, G.; Xu, W.; Huang, R. Effect of drought stress during flowering stage on starch accumulation and starch synthesis enzymes in sorghum grains. J. Integr. Agric. 2014, 13, 2399–2406. [Google Scholar] [CrossRef]
- Lu, H.; Hu, Y.; Wang, C.; Liu, W.; Ma, G.; Han, Q.; Ma, D. Effects of High Temperature and drought stress on the expression of gene encoding enzymes and the activity of key enzymes involved in starch biosynthesis in wheat grains. Front. Plant Sci. 2019, 10, 1414. [Google Scholar] [CrossRef] [PubMed]
- Pressel, S.; Ligrone, R.; Duckett, J.G. Effects of de- and rehydration on food conducting cells in the moss Polytrichum formosum: A cytological study. Ann. Bot. 2006, 98, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Xiang, L.; Le Roy, K.; Bolouri-Moghaddam, M.R.; Vanhaecke, M.; Lammens, W.; Rolland, F.; Van den Ende, W. Exploring the neutral invertase–oxidative stress defense connection in Arabidopsis thaliana. J. Exp. Bot. 2011, 62, 3849–3862. [Google Scholar] [CrossRef] [Green Version]
- Dahro, B.; Wang, F.; Peng, T.; Liu, J.H. PtrA/NINV, an alkaline/neutral invertase gene of Poncirus trifoliata, confers enhanced tolerance to multiple abiotic stresses by modulating ROS levels and maintaining photosynthetic efficiency. BMC Plant Biol. 2016, 16, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proels, R.K.; Hückelhoven, R. Cell-wall invertases, key enzymes in the modulation of plant metabolism during defence responses. Mol. Plant Pathol. 2014, 15, 858–864. [Google Scholar] [CrossRef]
- Villadsen, D.; Rung, J.H.; Nielsen, T.H. Osmotic stress changes carbohydrate partitioning and fructose-2,6-bisphosphate metabolism in barley leaves. Funct. Plant Biol. 2005, 32, 1033. [Google Scholar] [CrossRef] [PubMed]
- Storr, T.; Hall, J.L. The effect of infection by Erysiphe pisi DC on acid and alkaline invertase activities and aspects of starch biochemistry in leaves of Pisum sativum L. New Phytol. 1992, 121, 535–543. [Google Scholar] [CrossRef]
- Pelleschi, S.; Rocher, J.P.; Prioul, J.L. Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves. Plant Cell Environ. 1997, 20, 493–503. [Google Scholar] [CrossRef]
- Kim, J.Y.; Mahé, A.; Brangeon, J.; Prioul, J.L. A maize vacuolar invertase, IVR2, is induced by water stress. Organ/tissue specificity and diurnal modulation of expression. Plant Physiol. 2000, 124, 71–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albacete, A.; Cantero-Navarro, E.; Großkinsky, D.K.; Arias, C.L.; Balibrea, M.E.; Bru, R. Ectopic overexpression of the cell wall invertase gene CIN1 leads to dehydration avoidance in tomato. J. Exp. Bot. 2015, 66, 863–878. [Google Scholar] [CrossRef]
- Koonjul, P.K.; Minhas, J.S.; Nunes, C.; Sheoran, I.S.; Saini, H.S. Selective transcriptional down-regulation of anther invertases precedes the failure of pollen development in water-stressed wheat. J. Exp. Bot. 2005, 56, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Voegele, R.T.; Wirsel, S.; Möll, U.; Lechner, M.; Mendgen, K. Cloning and characterization of a novel invertase from the obligate biotroph Uromyces fabae and analysis of expression patterns of host and pathogen invertases in the course of infection. Mol. Plant–Microbe Interact. 2006, 19, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M.A.; Feechan, A.; Dry, I.B. Involvement of abscisic acid in the coordinated regulation of a stress-inducible hexose transporter (VvHT5) and a cell wall invertase in grapevine in response to biotrophic fungal infection. Plant Physiol. 2010, 153, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Conrath, U.; Beckers, G.J.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant-Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [Green Version]
- Barradas, C.; Pinto, G.; Correia, B.; Castro, B.; Phillips, A.J.; Alves, A. Drought x disease interaction in Eucalyptus globulus under Neofusicoccum eucalyptorum infection. Plant Pathol. 2018, 67, 87–96. [Google Scholar] [CrossRef]
- Eom, J.S.; Chen, L.Q.; Sosso, D.; Julius, B.T.; Lin, I.W.; Qu, X.Q.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Cho, L.H.; Tun, W.; Jeon, J.S.; An, G. Sucrose signaling in higher plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, K.H.; Sheen, J. Dynamic Nutrient Signaling Networks in Plants. Annu. Rev. Cell Dev. Biol. 2021, 37, 341–367. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [Green Version]
- Bolouri Moghaddam, M.R.; Van den Ende, W. Sweet immunity in the plant circadian regulatory network. J. Exp. Bot. 2013, 64, 1439–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trouvelot, S.; Héloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [Green Version]
- Tarkowski, Ł.P.; Van de Poel, B.; Höfte, M.; Van den Ende, W. Sweet immunity: Inulin boosts resistance of lettuce (Lactuca sativa) against grey mold (Botrytis cinerea) in an ethylene-dependent manner. Int. J. Mol. Sci. 2019, 20, 1052. [Google Scholar] [CrossRef] [Green Version]
- Ramegowda, V.; Senthil-Kumar, M.; Ishiga, Y.; Kaundal, A.; Udayakumar, M.; Mysore, K.S. Drought stress acclimation imparts tolerance to Sclerotinia sclerotiorum and Pseudomonas syringae in Nicotiana benthamiana. Int. J. Mol. Sci. 2013, 14, 9497–9513. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Cv | Ctrl | Drought | Xf | Combined |
|---|---|---|---|---|
| Cellina di Nardò | 97.29% A,a | 87.35% A,b | 52.45% B,c | 33.72% B,d |
| Leccino | 97.72% A,a | 68.19% B,c | 70.97% A,b | 66.85% A,d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pascali, M.; Vergine, M.; Negro, C.; Greco, D.; Vita, F.; Sabella, E.; De Bellis, L.; Luvisi, A. Xylella fastidiosa and Drought Stress in Olive Trees: A Complex Relationship Mediated by Soluble Sugars. Biology 2022, 11, 112. https://doi.org/10.3390/biology11010112
De Pascali M, Vergine M, Negro C, Greco D, Vita F, Sabella E, De Bellis L, Luvisi A. Xylella fastidiosa and Drought Stress in Olive Trees: A Complex Relationship Mediated by Soluble Sugars. Biology. 2022; 11(1):112. https://doi.org/10.3390/biology11010112
Chicago/Turabian StyleDe Pascali, Mariarosaria, Marzia Vergine, Carmine Negro, Davide Greco, Federico Vita, Erika Sabella, Luigi De Bellis, and Andrea Luvisi. 2022. "Xylella fastidiosa and Drought Stress in Olive Trees: A Complex Relationship Mediated by Soluble Sugars" Biology 11, no. 1: 112. https://doi.org/10.3390/biology11010112
APA StyleDe Pascali, M., Vergine, M., Negro, C., Greco, D., Vita, F., Sabella, E., De Bellis, L., & Luvisi, A. (2022). Xylella fastidiosa and Drought Stress in Olive Trees: A Complex Relationship Mediated by Soluble Sugars. Biology, 11(1), 112. https://doi.org/10.3390/biology11010112












