Small Angle X-ray Scattering Sensing Membrane Composition: The Role of Sphingolipids in Membrane-Amyloid β-Peptide Interaction
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Methods
2.1. Sample Preparation
2.1.1. Materials
2.1.2. LUV Preparation
2.1.3. Aβ Preparation
2.1.4. SAXS Method and Analysis
2.1.5. SAXS Data Analysis
3. Results
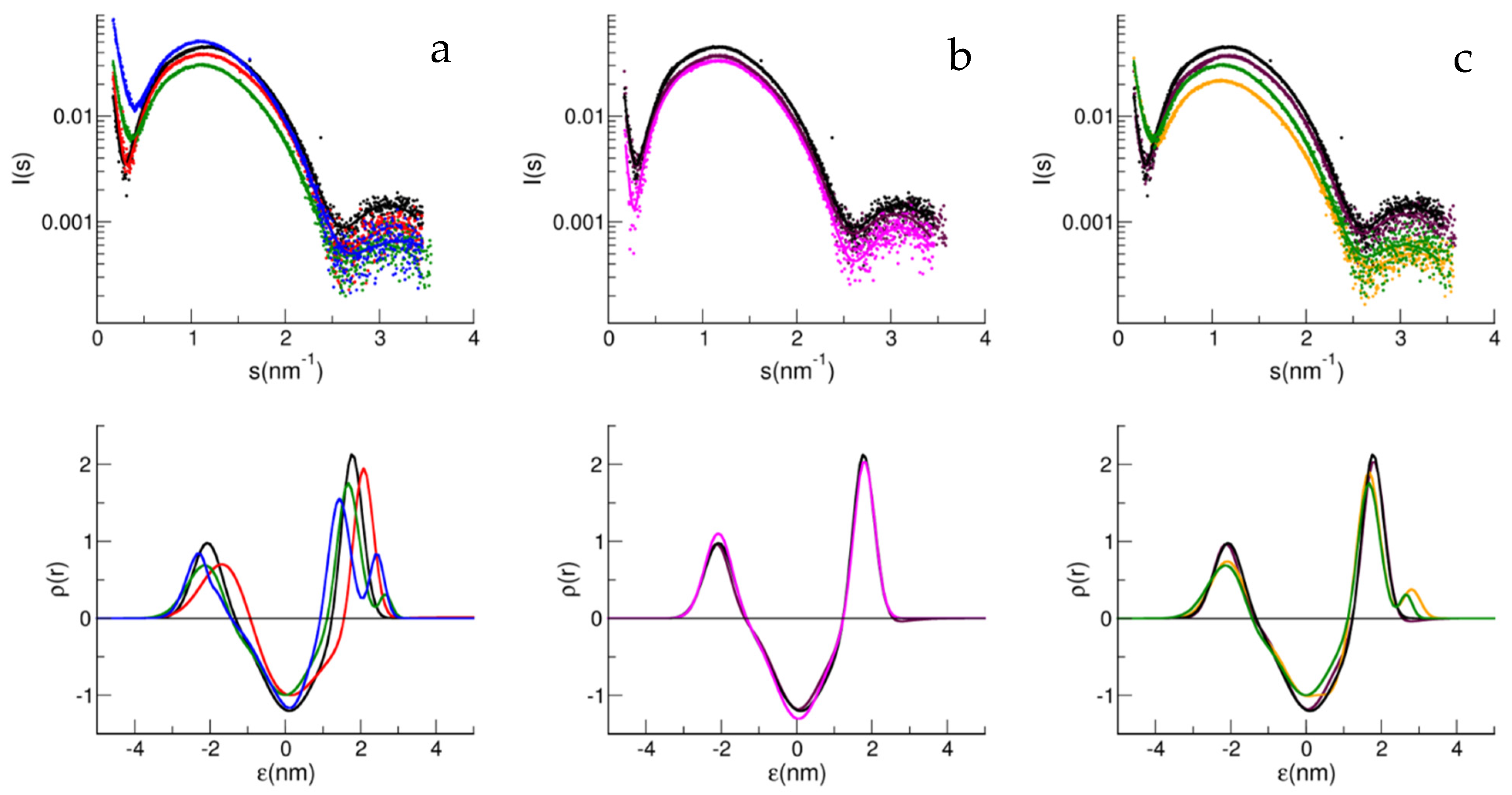
3.1. Characterization of the Liposomes
3.2. Characterization of the Aβ Peptide
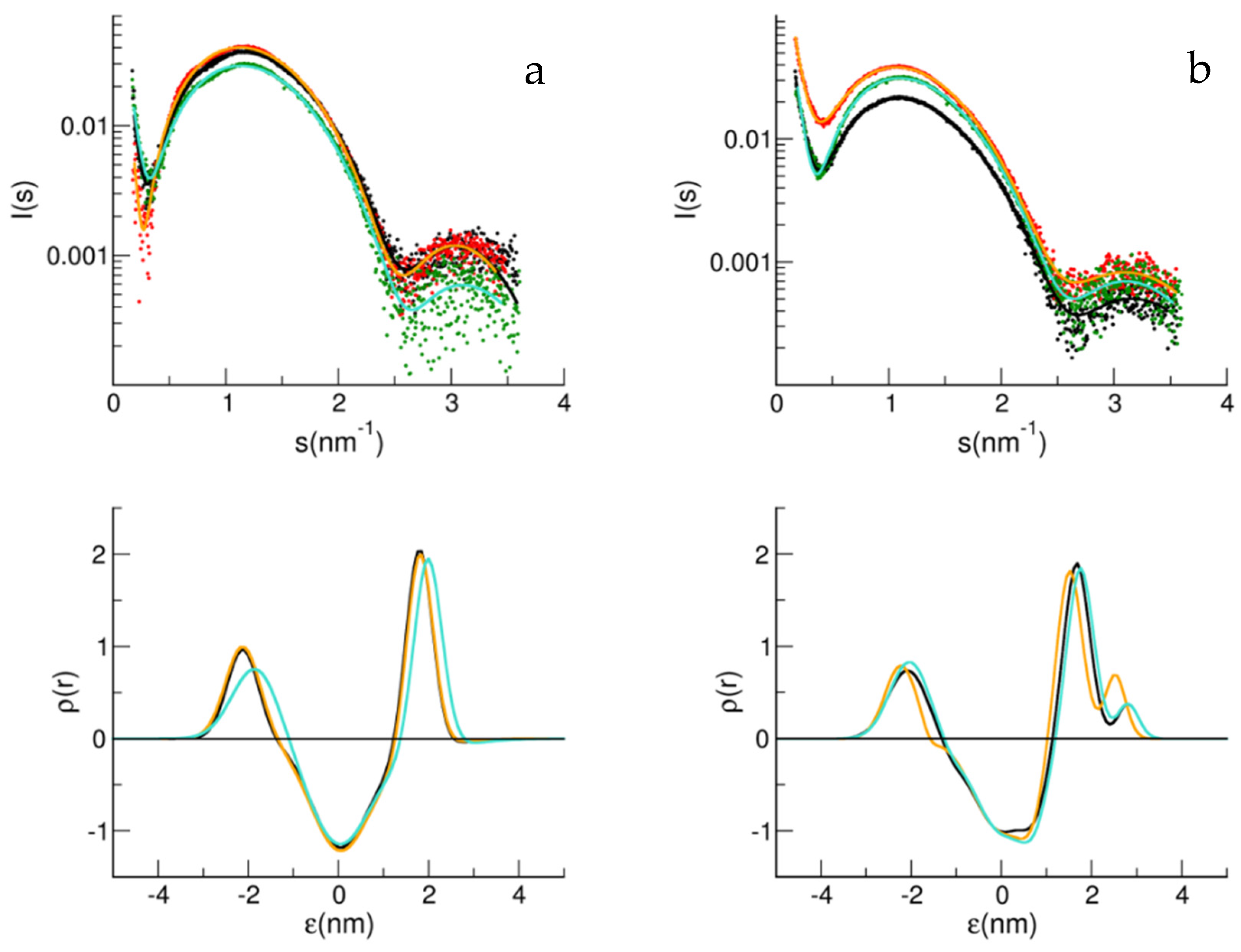
3.3. Aβ Interaction with Different Matrices
Comparison Aβ1–40-Aβ1–42
4. Discussion
4.1. Sphingomyelin
4.2. Mono-Sialo-Gangloside/Sphingomyelin/Cholesterol
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Karran, E.; Mercken, M.; de Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 1998, 95, 6448–6453. [Google Scholar] [CrossRef] [Green Version]
- de Felice, F.G.; Wu, D.; Lambert, M.P.; Fernandez, S.J.; Velasco, P.T.; Lacor, P.N.; Bigio, E.H.; Jerecic, J.; Acton, P.J.; Shughrue, P.J.; et al. Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by Aβ oligomers. Neurobiol. Aging 2008, 29, 1334–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirths, O.; Multhaup, G.; Czech, C.; Blanchard, V.; Moussaoui, S.; Tremp, G.; Pradier, L.; Beyreuther, K.; A Bayer, T. Intraneuronal Aβ accumulation precedes plaque formation in β-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci. Lett. 2001, 306, 116–120. [Google Scholar] [CrossRef]
- LaFerla, F.M.; Green, K.N.; Oddo, S. Intracellular amyloid-β in Alzheimer’s disease. Nat. Rev. Neurosci. 2007, 8, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Canale, C.; Seghezza, S.; Vilasi, S.; Carrotta, R.; Bulone, D.; Diaspro, A.; Biagio, P.L.S.; Dante, S. Different effects of Alzheimer’s peptide Aβ(1–40) oligomers and fibrils on supported lipid membranes. Biophys. Chem. 2013, 182, 23–29. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamaguchi, T.; Fukunaga, S.; Hoshino, M.; Matsuzaki, K. Mechanism of Amyloid β-Protein Aggregation Mediated by GM1 Ganglioside Clusters. Biochemistry 2011, 50, 6433–6440. [Google Scholar] [CrossRef]
- Amaro, M.; Šachl, R.; Aydogan, G.; Mikhalyov, I.I.; Vácha, R.; Hof, M. GM 1 Ganglioside Inhibits β-Amyloid Oligomerization Induced by Sphingomyelin. Angew. Chem. Int. Ed. 2016, 55, 9411–9415. [Google Scholar] [CrossRef] [Green Version]
- Cebecauer, M.; Hof, M.; Amaro, M. Impact of GM1 on Membrane-Mediated Aggregation/Oligomerization of β-Amyloid: Unifying View. Biophys. J. 2017, 113, 1194–1199. [Google Scholar] [CrossRef] [Green Version]
- Brown, R. Sphingolipid organization in biomembranes: What physical studies of model membranes reveal. J. Cell Sci. 1998, 111, 1–9. [Google Scholar] [CrossRef]
- Yanagisawa, K. GM1 Ganglioside-Bound Amyloid-Protein in Alzheimer’s Disease Brain. Neurobiol. Aging 1998, 19, 65–67. [Google Scholar] [CrossRef]
- Nicastro, M.C.; Spigolon, D.; Librizzi, F.; Moran, O.; Ortore, M.G.; Bulone, D.; Biagio, P.L.S.; Carrotta, R. Amyloid β-peptide insertion in liposomes containing GM1-cholesterol domains. Biophys. Chem. 2016, 208, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Carrotta, R.; Arleth, L.; Pedersen, J.S.; Bauer, R. Small-angle X-ray scattering studies of metastable intermediates of?-lactoglobulin isolated after heat-induced aggregation. Biopolymers 2003, 70, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Carrotta, R.; Barthès, J.; Longo, A.; Martorana, V.; Manno, M.; Portale, G.; Biagio, P.L.S. Large size fibrillar bundles of the Alzheimer amyloid β-protein. Eur. Biophys. J. 2007, 36, 701–709. [Google Scholar] [CrossRef]
- Vilasi, S.; Carrotta, R.; Ricci, C.; Rappa, G.C.; Librizzi, F.; Martorana, V.; Ortore, M.G.; Mangione, M.R. Inhibition of Aβ1–42 Fibrillation by Chaperonins: Human Hsp60 Is a Stronger Inhibitor than Its Bacterial Homologue GroEL. ACS Chem. Neurosci. 2019, 10, 3565–3574. [Google Scholar] [CrossRef] [PubMed]
- Fezoui, Y.; Hartley, D.M.; Harper, J.; Khurana, R.; Walsh, D.M.; Condron, M.M.; Selkoe, D.J.; Lansbury, P.T.; Fink, A.L.; Teplow, D.B. An improved method of preparing the amyloid β-protein for fibrillogenesis and neurotoxicity experiments. Amyloid 2000, 7, 166–178. [Google Scholar] [CrossRef]
- Carrotta, R.; Canale, C.; Diaspro, A.; Trapani, A.; Biagio, P.S.; Bulone, D. Inhibiting effect of αs1-casein on Aβ1–40 fibrillogenesis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 124–132. [Google Scholar] [CrossRef]
- Mangione, M.R.; Vilasi, S.; Marino, C.; Librizzi, F.; Canale, C.; Spigolon, D.; Bucchieri, F.; Fucarino, A.; Passantino, R.; Cappello, F.; et al. Hsp60, amateur chaperone in amyloid-beta fibrillogenesis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 2474–2483. [Google Scholar] [CrossRef]
- Ricci, C.; Maccarini, M.; Falus, P.; Librizzi, F.; Mangione, M.R.; Moran, O.; Ortore, M.G.; Schweins, R.; Vilasi, S.; Carrotta, R. Amyloid β-Peptide Interaction with Membranes: Can Chaperones Change the Fate? J. Phys. Chem. B 2018, 123, 631–638. [Google Scholar] [CrossRef]
- Prima, G.; Librizzi, F.; Carrotta, R. Light Scattering as an Easy Tool to Measure Vesicles Weight Concentration. Membranes 2020, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Stepanek, P. Dynamic Light Scattering: The Method and Some Applications; Brown, W., Ed.; Clarendon Press: Oxford, UK, 1993; pp. 177–244. [Google Scholar]
- Guinier, A. X-ray Diffraction in Crystals, Imperfect Crystals, and Amorphous Bodies; Courier Dover Publications: San Francisco, CA, USA; London, UK, 1963. [Google Scholar]
- Brzustowicz, M.R.; Brunger, A.T. X-ray scattering from unilamellar lipid vesicles. J. Appl. Crystallogr. 2005, 38, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Baroni, D.; Zegarra-Moran, O.; Moran, O. Functional and pharmacological induced structural changes of the cystic fibrosis transmembrane conductance regulator in the membrane solved using SAXS. Cell. Mol. Life Sci. 2014, 72, 1363–1375. [Google Scholar] [CrossRef]
- Baroni, D.; Zegarra-Moran, O.; Svensson, A.; Moran, O. Direct interaction of a CFTR potentiator and a CFTR corrector with phospholipid bilayers. Eur. Biophys. J. 2014, 43, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Castorph, S.; Riedel, D.; Arleth, L.; Sztucki, M.; Jahn, R.; Holt, M.; Salditt, T. Structure Parameters of Synaptic Vesicles Quantified by Small-Angle X-ray Scattering. Biophys. J. 2010, 98, 1200–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castorph, S.; Arleth, L.; Sztucki, M.; Vainio, U.; Ghosh, S.K.; Holt, M.; Jahn, R.; Salditt, T. Synaptic Vesicles Studied by SAXS: Derivation and Validation of a Model Form Factor. J. Phys. Conf. Ser. 2010, 247. [Google Scholar] [CrossRef]
- Bouwstra, J.; Gooris, G.; Bras, W.; Talsma, H. Small angle X-ray scattering: Possibilities and limitations in characterization of vesicles. Chem. Phys. Lipids 1993, 64, 83–98. [Google Scholar] [CrossRef]
- Ahyayauch, H.; Raab, M.; Busto, J.V.; Andraka, N.; Arrondo, J.-L.R.; Masserini, M.; Tvaroska, I.; Goñi, F.M. Binding of β-Amyloid (1–42) Peptide to Negatively Charged Phospholipid Membranes in the Liquid-Ordered State: Modeling and Experimental Studies. Biophys. J. 2012, 103, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Habchi, J.; Chia, S.; Galvagnion, C.; Michaels, T.C.T.; Bellaiche, M.M.J.; Ruggeri, F.S.; Sanguanini, M.; Idini, I.; Kumita, J.R.; Sparr, E.; et al. Cholesterol catalyses Aβ42 aggregation through a heterogeneous nucleation pathway in the presence of lipid membranes. Nat. Chem. 2018, 10, 673–683. [Google Scholar] [CrossRef]
- Manna, M.; Mukhopadhyay, C. Binding, Conformational Transition and Dimerization of Amyloid-β Peptide on GM1-Containing Ternary Membrane: Insights from Molecular Dynamics Simulation. PLoS ONE 2013, 8, e71308. [Google Scholar] [CrossRef] [Green Version]
- Feigenson, G.W. Phase Boundaries and Biological Membranes. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 63–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slotte, J.P. The importance of hydrogen bonding in sphingomyelin’s membrane interactions with co-lipids. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 304–310. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.; Fedorov, A.; Prieto, M. Sphingomyelin/Phosphatidylcholine/Cholesterol Phase Diagram: Boundaries and Composition of Lipid Rafts. Biophys. J. 2003, 85, 2406–2416. [Google Scholar] [CrossRef] [Green Version]
- Petruzielo, R.S.; Heberle, F.A.; Drazba, P.; Katsaras, J.; Feigenson, G.W. Phase behavior and domain size in sphingomyelin-containing lipid bilayers. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Johnson, B.; Urbanc, B.; Jenkins, T.; Connell, S.D.A.; Serpell, L.C. Aβ42 oligomers, but not fibrils, simultaneously bind to and cause damage to ganglioside-containing lipid membranes. Biochem. J. 2011, 439, 67–77. [Google Scholar] [CrossRef]
- Chakraborty, D.; Straub, J.E.; Thirumalai, D. Differences in the free energies between the excited states of Aβ40 and Aβ42 monomers encode their aggregation propensities. Proc. Natl. Acad. Sci. USA 2020, 117, 19926–19937. [Google Scholar] [CrossRef]
- Sagle, L.B.; Ruvuna, L.K.; Bingham, J.M.; Liu, C.; Cremer, P.S.; Van Duyne, R.P. Single Plasmonic Nanoparticle Tracking Studies of Solid Supported Bilayers with Ganglioside Lipids. J. Am. Chem. Soc. 2012, 134, 15832–15839. [Google Scholar] [CrossRef] [Green Version]
- Sarmento, M.J.; Ricardo, J.C.; Amaro, M.; Šachl, R. Organization of gangliosides into membrane nanodomains. FEBS Lett. 2020, 594, 3668–3697. [Google Scholar] [CrossRef]
- Fricke, N.; Dimova, R. GM1 Softens POPC Membranes and Induces the Formation of Micron-Sized Domains. Biophys. J. 2016, 111, 1935–1945. [Google Scholar] [CrossRef] [Green Version]


| Name | PC | PS | Chol | GM1 | SM | <M> |
|---|---|---|---|---|---|---|
| BASE (B1) | 78 | 8 | 14 | 0 | 0 | 702 |
| B1 + GM1 2% | 75 | 7 | 16 | 2 | 0 | 718 |
| B1 + SM 5% (B2) | 73 | 7 | 15 | 0 | 5 | 703 |
| B2 + GM1 1% | 72 | 7 | 15 | 1 | 5 | 711 |
| B2 + GM1 3% | 70 | 7 | 15 | 3 | 5 | 727 |
| B2 + GM1 4% | 69 | 7 | 15 | 4 | 5 | 734 |
| B1 + SM 10% (B3) | 68 | 7 | 15 | 0 | 10 | 701 |
| Node1 (nm) | Peak1 (nm) | Node2 (nm) | Peak2 (nm) | rnodes | rpeaks | d (nm) | |
|---|---|---|---|---|---|---|---|
| BASE (B1) | 0.321 | 1.187 | 2.617 | 3.148 | 1.18 | 0.47 | 5.50 |
| +Aβ1–40 | 0.289 | 1.165 | 2.581 | 3.107 | 1.17 | 0.50 | 5.61 |
| +Aβ1–42 | 0.335 | 1.186 | 2.617 | 3.091 | 0.94 | 0.39 | 5.79 |
| B1 + GM1 2% | 0.397 | 1.089 | 2.652 | 3.135 | 1.25 | 0.39 | 6.39 |
| +Aβ1–40 | 0.410 | 1.064 | 2.615 | 3.084 | 1.47 | 0.43 | 6.15 |
| +Aβ1–42 | 0.386 | 1.095 | 2.663 | 3.177 | 1.17 | 0.45 | 6.37 |
| B1 + SM 5% (B2) | 0.311 | 1.172 | 2.626 | 3.114 | 1.16 | 0.46 | 5.57 |
| +Aβ1–40 | 0.279 | 1.149 | 2.610 | 3.083 | 1.15 | 0.54 | 5.59 |
| +Aβ1–42 | 0.301 | 1.168 | 2.605 | 3.134 | 1.16 | 0.46 | 5.55 |
| B2 + GM1 1% | 0.332 | 1.127 | 2.641 | 3.106 | 0.81 | 0.36 | 5.81 |
| +Aβ1–40 | 0.312 | 1.103 | 2.611 | 3.099 | 0.73 | 0.33 | 5.91 |
| +Aβ1–42 | 0.322 | 1.118 | 2.615 | 3.154 | 0.72 | 0.26 | 5.77 |
| B2 + GM1 3% | 0.376 | 1.102 | 2.616 | 3.193 | 1.28 | 0.39 | 6.26 |
| +Aβ1–40 | 0.395 | 1.077 | 2.587 | 3.131 | 1.31 | 0.37 | 6.21 |
| +Aβ1–42 | 0.376 | 1.103 | 2.618 | 3.089 | 1.33 | 0.37 | 6.10 |
| B2 + GM1 4% | 0.405 | 1.097 | 2.637 | 3.101 | 1.6 | 0.54 | 6.06 |
| +Aβ1–40 | 0.409 | 1.066 | 2.622 | 3.077 | 1.86 | 0.52 | 5.95 |
| +Aβ1–42 | 0.400 | 1.089 | 2.619 | 3.067 | 1.95 | 0.67 | 5.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrotta, R.; Mangione, M.R.; Librizzi, F.; Moran, O. Small Angle X-ray Scattering Sensing Membrane Composition: The Role of Sphingolipids in Membrane-Amyloid β-Peptide Interaction. Biology 2022, 11, 26. https://doi.org/10.3390/biology11010026
Carrotta R, Mangione MR, Librizzi F, Moran O. Small Angle X-ray Scattering Sensing Membrane Composition: The Role of Sphingolipids in Membrane-Amyloid β-Peptide Interaction. Biology. 2022; 11(1):26. https://doi.org/10.3390/biology11010026
Chicago/Turabian StyleCarrotta, Rita, Maria Rosalia Mangione, Fabio Librizzi, and Oscar Moran. 2022. "Small Angle X-ray Scattering Sensing Membrane Composition: The Role of Sphingolipids in Membrane-Amyloid β-Peptide Interaction" Biology 11, no. 1: 26. https://doi.org/10.3390/biology11010026
APA StyleCarrotta, R., Mangione, M. R., Librizzi, F., & Moran, O. (2022). Small Angle X-ray Scattering Sensing Membrane Composition: The Role of Sphingolipids in Membrane-Amyloid β-Peptide Interaction. Biology, 11(1), 26. https://doi.org/10.3390/biology11010026







