Proteins in Wonderland: The Magical World of Pressure
Abstract
:Simple Summary
Abstract
1. Introduction
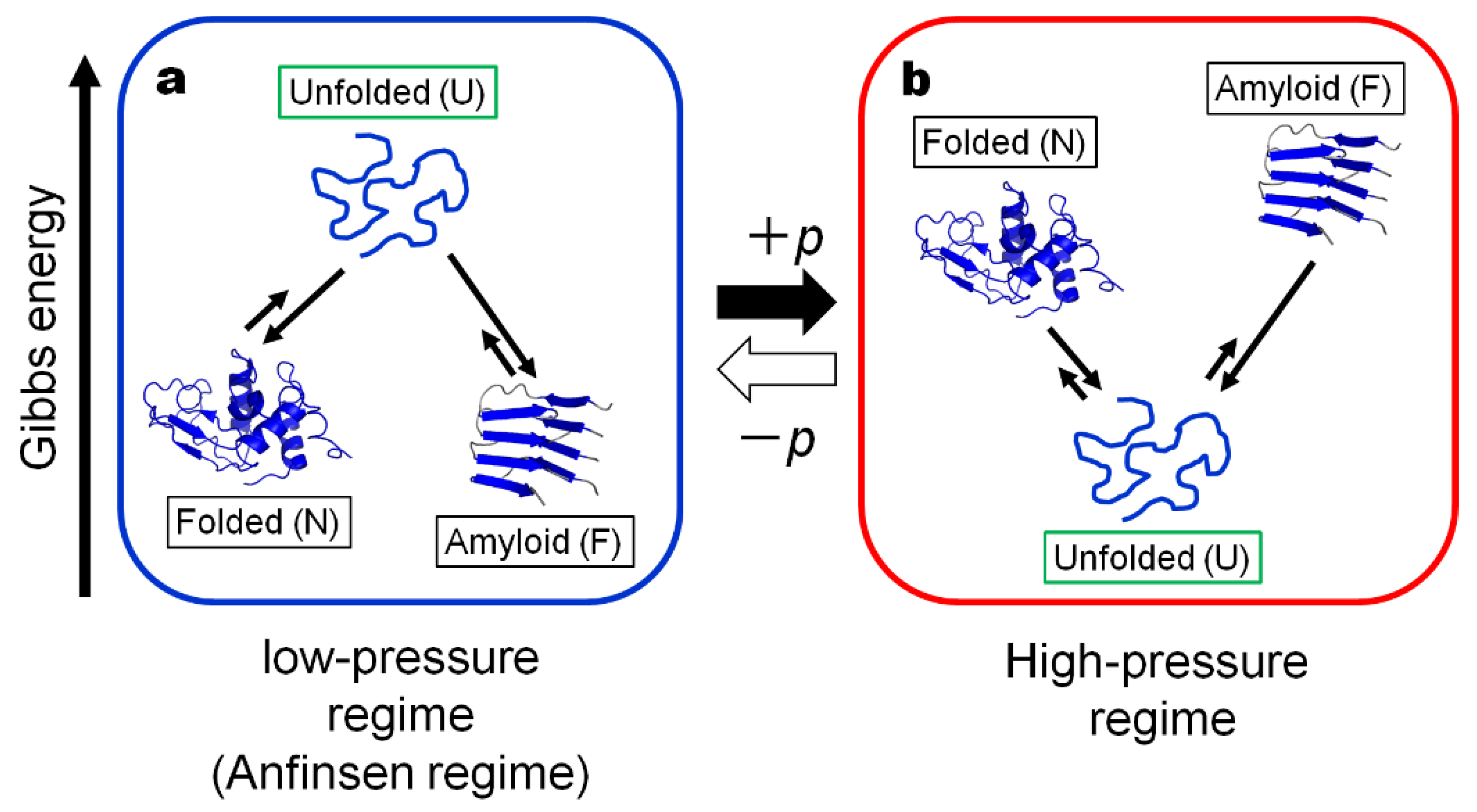
2. Thermodynamic Consideration
2.1. In the “Anfinsen Regime” or the Low-Pressure Regime
2.2. In the “High-Pressure Regime”
3. Materials and Methods
4. Results
4.1. Experimental Demonstration: Turning Amyloid Fibrils “F” Back into the Folded State “N” in Hen Lysozyme
4.2. The Experimental Result
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anfinsen, C.B. Principles that govern the folding of protein chains. Science 1973, 181, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Camilo, M.; Derek, P.T.; Sina, A.; Alastair, G.B.S.; Boris, W. How many species are there on earth and in the ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef] [Green Version]
- Hirata, F.; Sugita, M.; Yoshida, M.; Akasaka, K. Perspective: Structural fluctuation of protein and Anfinsen’s thermodynamic hypothesis. J. Chem. Phys. 2018, 14, 020901. [Google Scholar] [CrossRef] [Green Version]
- Hui Bon Hoa, G.; Douzou, P.; Dahan, N.; Balny, C. High-pressure spectrometry at sub-zero temperatures. Anal. Biochem. 1982, 120, 125–135. [Google Scholar] [CrossRef]
- Hui Bon Hoa, G.; McLean, M.A.; Sligar, S.G. High pressure, a tool for exploring heme protein active sites. Biochim. Biophys. Acta 2002, 1595, 297–308. [Google Scholar] [CrossRef]
- Davydov, D.R.; Hui Bon Hoa, G.; Peterson, J.A. Dynamics of protein-bound water in the heme domain of P450BM3 studied by high-pressure spectroscopy: Cobarrison with P450cam and P450 2B4. Biochemistry 1999, 38, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.; Jonas, A. High pressure NMR spectroscopy of proteins and membranes. Ann. Rev. Biophys. Biomol. Struct. 1994, 23, 287–318. [Google Scholar] [CrossRef]
- Akasaka, K. Exploring the entire conformational space of proteins by high-pressure NMR. Pure Appl. Chem. 2003, 75, 927–936. [Google Scholar] [CrossRef] [Green Version]
- Akasaka, K. Probing conformational fluctuation of proteins by pressure perturbation. Chem Rev. 2006, 106, 1814–1835. [Google Scholar] [CrossRef]
- Kalbitzer, H.R. High pressure NMR methods for characterizing functional sub-states of proteins. Subcell. Biochem. 2015, 72, 179–197. [Google Scholar]
- Akasaka, K. Protein Studies by High Pressure NMR. In Experimental Approaches of NMR Spectroscopy; The Nuclear Magnetic Resonance Society of Japan; Springer: Singapore, 2018; pp. 3–36. [Google Scholar]
- Dubois, C.; Herrada, I.; Barthe, P.; Roumestand, C. Combining High-Pressure Perturbation with NMR Spectroscopy for a Structural and Dynamical Characterization of Protein Folding Pathways. Molecules 2020, 25, 5551. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef]
- Bemporad, F.; Chiti, F. Misfolded Oligomers: Experimental Approaches, Mechanism of Formation, and Structure-Toxicity Relationships. Chem. Biol. 2012, 19, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, A.W.P.; Debelouchina, G.T.; Bayro, M.J.; Clare, D.K.; Caporini, M.A.; Bajaj, V.S.; Jaroniec, C.P.; Wang, L.; Ladizhansky, V.; Müller, S.A.; et al. Atomic structure and hierarchical assembly of a cross-β amyloid fibril. Proc. Natl. Acad. Sci. USA 2013, 110, 5468–5473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiti, F.; Dobson, C.M. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef]
- Burns, A.; Iliffe, S. Alzheimer’s disease. BMJ 2009, 338, b158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araki, S.; Ando, Y. Transthyretin-related familial amyloidotic poly-neuropathy—Progress in Kumamoto, Japan (1967–2010). Proc. Jpn. Acad. Ser. B 2010, 86, 694–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, A.W.; Aldeghi, M.; Blakeley, M.P.; Ostermann, A.; Mas, P.J.; Moulin, M.; Sanctis, D.; Bowler, M.W.; Dieckmann, C.M.; Mitchell, E.P.; et al. A molecular mechanism for transthyretin amyloidogenesis. Nat. Commun. 2019, 10, 925. [Google Scholar] [CrossRef] [Green Version]
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar] [CrossRef] [Green Version]
- Portales-Castillo, I.; Yee, J.; Tanaka, H.; Fenves, A.Z. Beta-2 Microglobulin Amyloidosis: Past, Present, and Future. Kidney360 2020, 1, 1447–1455. [Google Scholar] [CrossRef]
- Foguel, D.B.; Suarez, M.C.; Ferrão-Gonzales, A.D.; Porto, T.C.R.; Palmieri, L.; Einsiedler, C.M.; Andrade, L.R.; Lashuel, H.A.; Lansbury, P.T.; Kelly, J.W.; et al. Dissocia-tion of amyloid fibrils of alpha-synuclein and transthyretin by pressure reveals their reversible nature and the formation of water-excluded cavities. Proc. Natl. Acad. Sci. USA 2003, 100, 9831–9836. [Google Scholar] [CrossRef] [Green Version]
- Westermark, P.; Sletten, K.; Johansson, B.; Cornwell, G.G. Fibril in senile systemic amyloidosis is derived from normal transthyretin. Proc. Natl. Acad. Sci. USA 1990, 87, 2843–2845. [Google Scholar] [CrossRef] [Green Version]
- Jansens, K.J.A.; Rombouts, I.; Grootaert, C.; Brijs, K.; Camp, J.V.; Meeren, P.V.; Rousseau, F.; Schymkowitz, J.; Delcour, J.A. Rational design of amyloid-like fibrillary structures for tailoring food protein techno-functionality and their potential health implications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 84–105. [Google Scholar] [CrossRef] [Green Version]
- Niraula, T.N.; Haraoka, K.; Ando, Y.; Li, H.; Yamada, H.; Akasaka, K. Decreased Thermodynamic Stability as a Crucial Factor for Familial Amyloidotic Polyneuropathy. J. Mol. Biol. 2002, 320, 333–342. [Google Scholar] [CrossRef]
- Niraula, T.N.; Konno, T.; Li, H.; Yamada, H.; Akasaka, K.; Tachibana, H. Pressure-dissociable reversible assembly of naturally denatured lysozyme is a precursor for amyloid fibrils. Proc. Natl. Acad. Sci. USA 2004, 101, 4089–4093. [Google Scholar] [CrossRef] [Green Version]
- Kamatari, Y.O.; Yokoyama, S.; Tachibana, H.; Akasaka, K. Pressure-jump NMR study of dissociation and association of amyloid protofibrils. J. Mol. Biol. 2005, 349, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H. Basic equation in statics and kinetics of protein polymerization and the mechanism of the formation and dis-sociation of amyloid fibrils revealed by pressure perturbation. Subcell. Biochem. 2015, 72, 279–299. [Google Scholar]
- Akasaka, K.; Abdul Latif, A.R.; Nakamura, A.; Matsuo, K.; Tachibana, H.; Gekko, K. Amyloid protofibril is highly voluminous and compressible. Biochemistry 2007, 46, 10444–10450. [Google Scholar] [CrossRef]
- Sasaki, K.; Nakatsuka, K.; Hayashi, I.; Akasaka, K. Efficient conversion of intact hen lysozyme into amyloid fibrils by seeding. J. Biol. Macromol. 2008, 8, 11–18. [Google Scholar]
- Akasaka, K.; Maeno, A.; Murayama, T.; Tachibana, H.; Fujita, Y.; Yamanaka, H.; Nishida, N.; Atarashi, R. Pressure-assisted dissociation and degradation of “proteinase K-resistant” fibrils prepared by seeding with scrapie infected hamster prion protein. Prion 2014, 8, 314–318. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, N.; Mitsui, T.; Sakamoto, M.; Mahara, A.; Yoshimura, K.; Arata, J.; Jinno, C.; Kakudo, N.; Kusumoto, K.; Yamaoka, T. A novel treatment for giant congenital melanocytic nevi combining inactivated autologous nevus tissue by high hydrostatic pressure and a cultured epidermal autograft: First-in-human, open, prospective clinical trial. Plast. Reconstr. Surg. 2021, 9, 71e–76e. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akasaka, K.; Maeno, A. Proteins in Wonderland: The Magical World of Pressure. Biology 2022, 11, 6. https://doi.org/10.3390/biology11010006
Akasaka K, Maeno A. Proteins in Wonderland: The Magical World of Pressure. Biology. 2022; 11(1):6. https://doi.org/10.3390/biology11010006
Chicago/Turabian StyleAkasaka, Kazuyuki, and Akihiro Maeno. 2022. "Proteins in Wonderland: The Magical World of Pressure" Biology 11, no. 1: 6. https://doi.org/10.3390/biology11010006
APA StyleAkasaka, K., & Maeno, A. (2022). Proteins in Wonderland: The Magical World of Pressure. Biology, 11(1), 6. https://doi.org/10.3390/biology11010006





