Melatonin and KNO3 Application Improves Growth, Physiological and Biochemical Characteristics of Maize Seedlings under Waterlogging Stress Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Design, and Location
2.2. Plant Sampling and Measurements
2.2.1. Growth Parameters
2.2.2. Photosynthetic Rate and Chlorophyll Content
2.2.3. Assay of Antioxidant Activity
2.2.4. Determination of H2O2, MDA Content
2.2.5. Soluble Protein and Soluble Sugar Contents Measurement
2.2.6. Determination of Nitrogen Metabolism Enzyme Activities
2.2.7. Determination of the ADH and PDC Enzyme Activities
2.2.8. Statistical Analysis
3. Results
3.1. Effect of Nitrate on Growth Characteristics of Maize Seedlings Treated with Melatonin under Waterlogging Stress Conditions
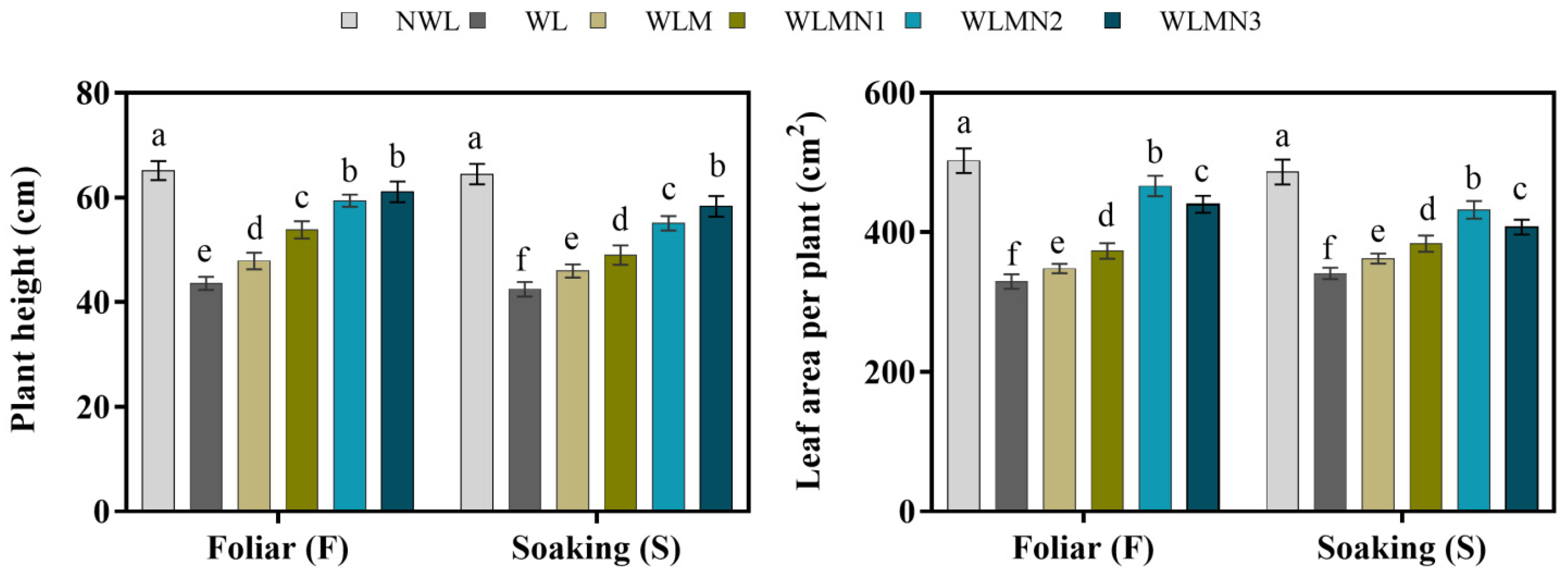
3.2. Effect of Nitrate on Plant Height and Leaf Area per Plant of Maize Seedlings Treated with Melatonin under Waterlogging Stress Conditions
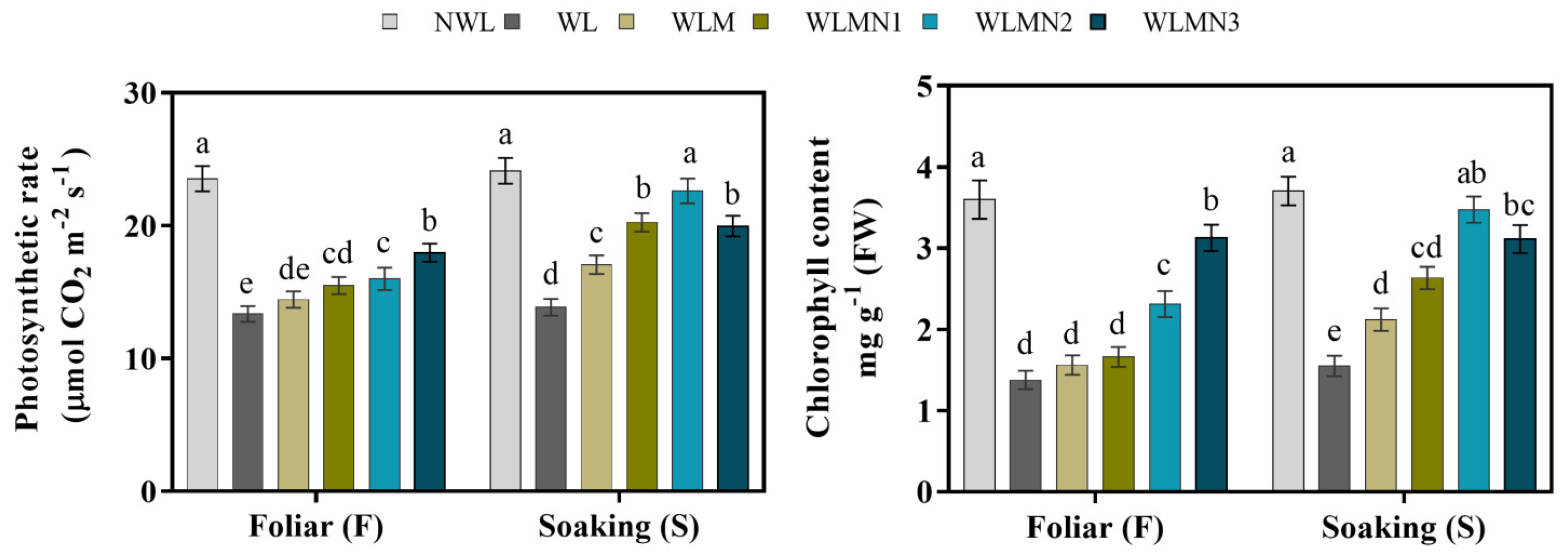
3.3. Effect of Nitrate on Photosynthetic Rate and Chlorophyll Content of Mazie Seedlings Treated with Melatonin under Waterlogging Stress Conditions
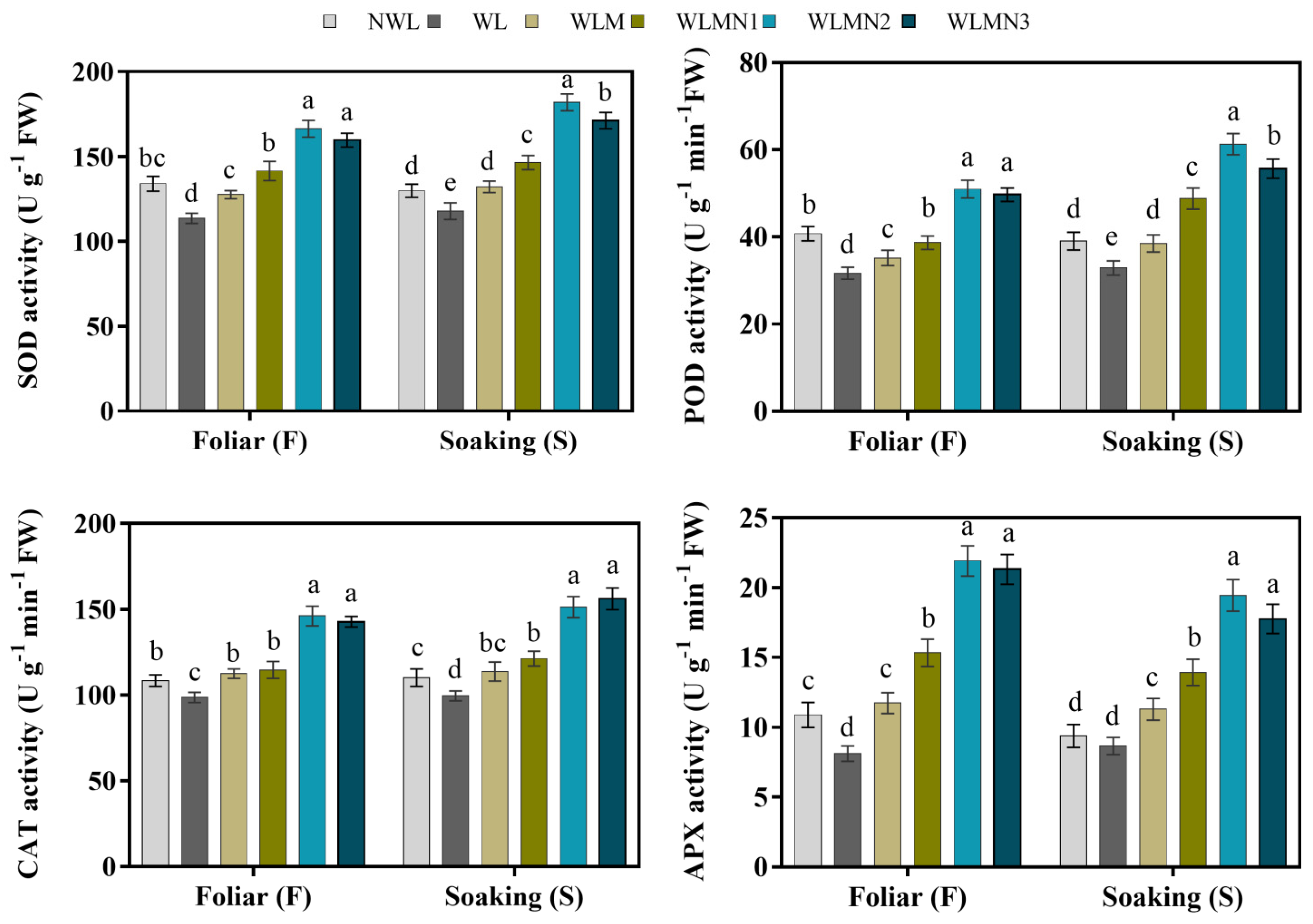
3.4. Effect of Nitrate of on Antioxidant Enzymes Activities of Maize Seedlings Treated with Melatonin under Waterlogging Stress Conditions
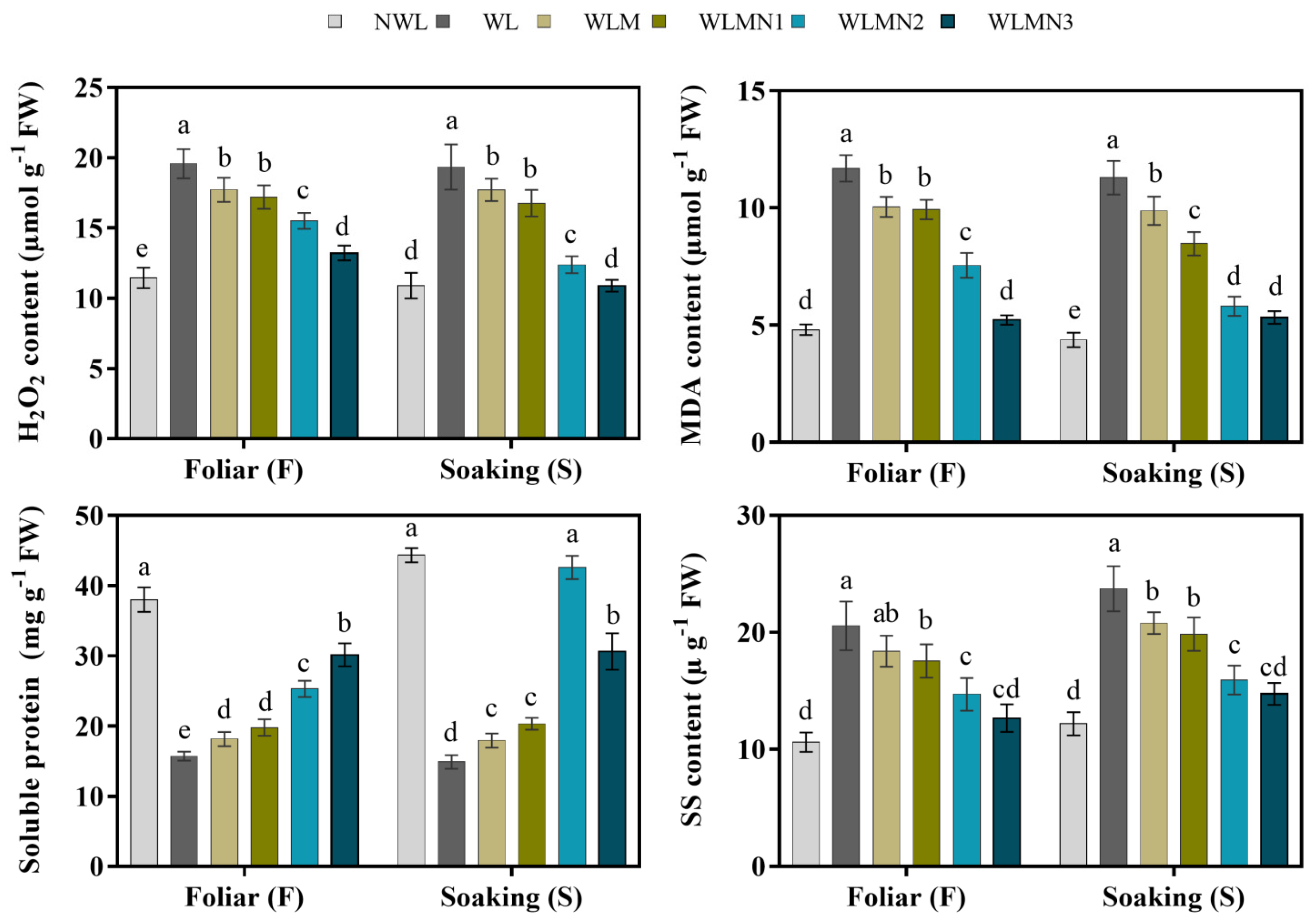
3.5. Effect of Nitrate on H2O2, MDA, Soluble Protein and Soluble Sugar Content of Maize Seedlings Treated with Melatonin under Waterlogging Stress Conditions
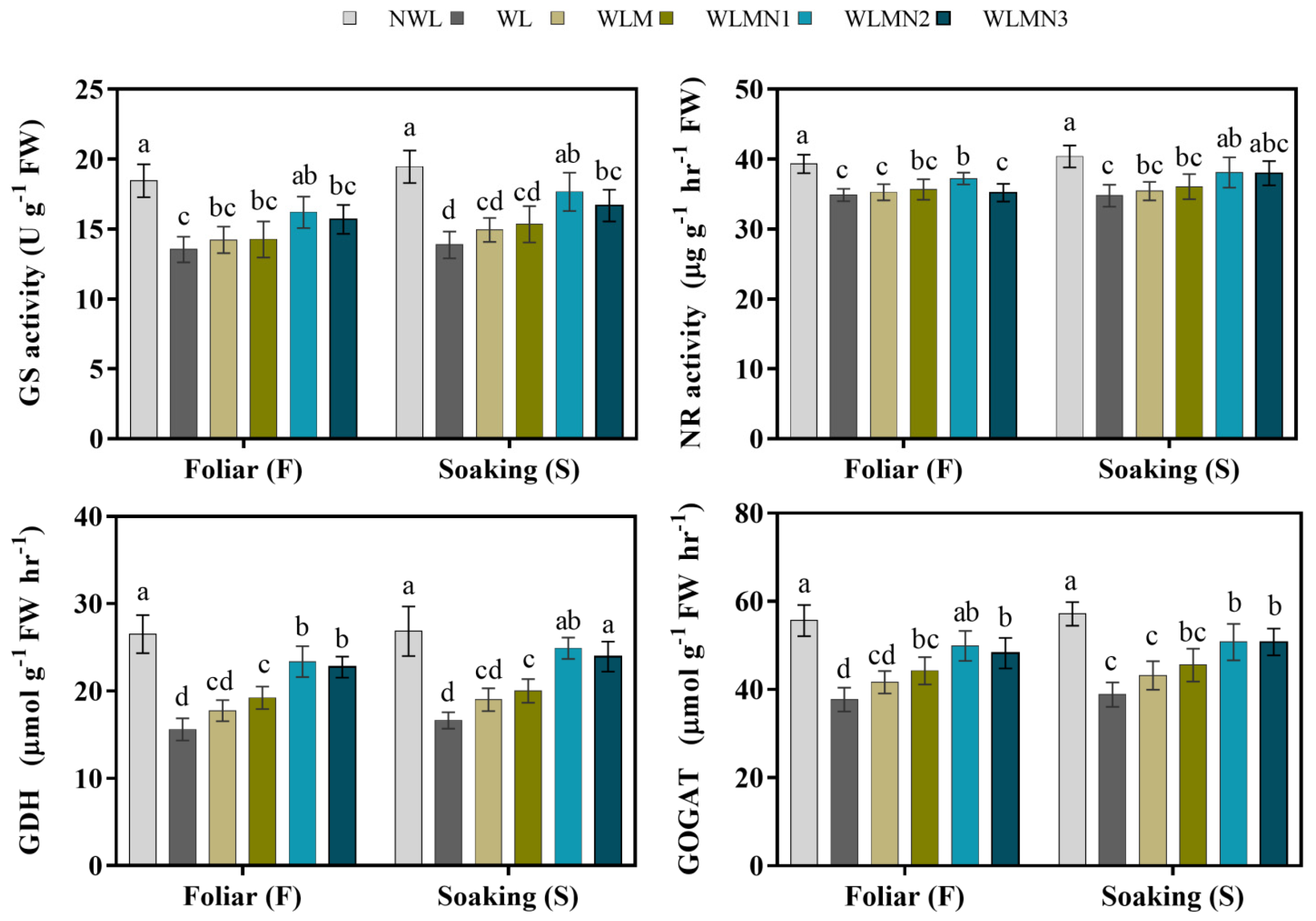
3.6. Effect of Nitrate on Nitrogen Metabolism Enzyme Activities of Maize Seedlings Treated with Melatonin under Waterlogging Stress Conditions
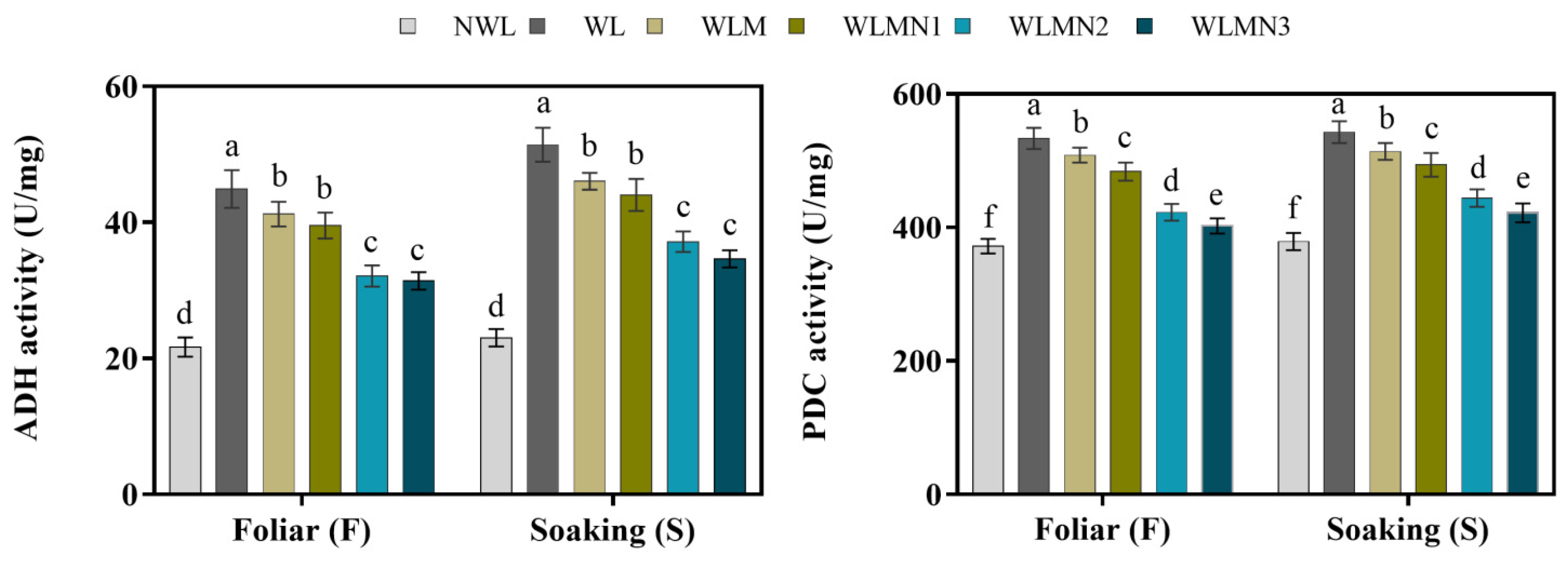
3.7. Effect of Nitrate on Alcohol Dehydrogenase (ADH) and Pyruvate Decarboxylase (PDC) Activities of Maize Seedlings Treated with Melatonin under Waterlogging Stress Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KNO3 | Potassium nitrate |
| H2O2 | Hydrogen peroxide |
| MDA | Malondialdehyde |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| APX | Ascorbate peroxidase |
| N | Nitrogen |
| NaClO | sodium hypochlorite |
| NWL | Control not waterlogging |
| WL | Control waterlogging |
| WLM | WL + 100 µM Melatonin; WLM + 0.25 g KNO3 (WLMN1), WLM + 0.50 g KNO3 (WLMN2), WLM + 0.75 g KNO3 (WLMN3) |
| LSD | Least significant difference |
| DW | Dry weight |
| GS | Glutamine Synthetase |
| NR | Nitrate Reductase |
| GOGAT | Glutamate Synthase |
| GDH | Glutamate Dehydrogenase |
| PDC | pyruvate decarboxylase |
| ADH | Alcohol dehydrogenase |
References
- Goyal, K.; Kaur, K.; Kaur, G. Foliar treatment of potassium nitrate modulates the fermentative and sucrose metabolizing pathways in contrasting maize genotypes under water logging stress. Physiol. Mol. Biol. Plants 2020, 26, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, J.; Tan, D.X.; Wang, N.; Wang, L.; Shan, D.; Kong, J. Melatonin improves waterlogging tolerance of Malus baccata (Linn.) Borkh. seedlings by maintaining aerobic respiration, photosynthesis and ROS migration. Front. Plant Sci. 2017, 8, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabala, S.; Shabala, L.; Barcelo, J.; Poschenrieder, C. Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding. Plant Cell Environ. 2014, 37, 2216–2233. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, Y.; Li, C.; McHugh, A.; Li, Z.; Wu, C. Individual and combined effects of soil waterlogging and compaction on physiological characteristics of wheat in southwestern China. Field Crops Res. 2018, 215, 163–172. [Google Scholar] [CrossRef]
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weits, D.A.; van Dongen, J.T.; Licausi, F. Molecular oxygen as a signaling component in plant development. New Phytol. 2021, 229, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, B.; Zhang, J.; Li, X.; Fan, X.; Dong, S.; Liu, P.; Zhao, B. Effects of waterlogging on the yield and growth of summer maize under field conditions. Can. J. Plant Sci. 2014, 94, 23–31. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, L.; Zhou, Z.; Mei, F.; Qu, L.; Zhou, G. Process of aerenchyma formation and reactive oxygen species induced by waterlogging in wheat seminal roots. Planta 2013, 238, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Jitsuyama, Y. Hypoxia-responsive root hydraulic conductivity influences soybean cultivar-specific waterlogging tolerance. Am. J. Plant Sci. 2017, 8, 770. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Tan, X.; Sun, X.; Zwiazek, J.J. Properties of root water transport in canola (Brassica napus) subjected to waterlogging at the seedling, flowering and podding growth stages. Plant Soil 2020, 454, 431–445. [Google Scholar] [CrossRef]
- Zhang, R.; Yue, Z.; Chen, X.; Wang, Y.; Zhou, Y.; Xu, W.; Huang, R. Foliar applications of urea and melatonin to alleviate waterlogging stress on photosynthesis and antioxidant metabolism in sorghum seedlings. Plant Growth Regul. 2021, 1–10. [Google Scholar] [CrossRef]
- Gu, X.; Xue, L.; Lu, L.; Xiao, J.; Zhang, H. Melatonin Enhances the Waterlogging Tolerance of Prunus persica by Modulating Antioxidant Metabolism and Anaerobic Respiration. J. Plant Growth Regul. 2020, 40, 2178–2190. [Google Scholar] [CrossRef]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, I.; Kamran, M.; Su, W.; Wang, H.; Han, Q. Application of uniconazole improves photosynthetic efficiency of maize by enhancing the antioxidant defense mechanism and delaying leaf senescence in semiarid regions. J. Plant Growth Regul. 2018, 38, 855–869. [Google Scholar] [CrossRef]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Lin, L.; Deng, Q.; Luo, X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Qiao, Y.; Yin, L.; Wang, B.; Ke, Q.; Deng, X.; Wang, S. Melatonin promotes plant growth by increasing nitrogen uptake and assimilation under nitrogen deficient condition in winter wheat. Plant Physiol. Biochem. 2019, 139, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Heffer, P.; Prud’homme, M. Global nitrogen fertilizer demand and supply: Trend, current level and outlook. In Proceedings of the International Nitrogen Initiative Conference, Melbourne, Australia, 4–8 December 2016. [Google Scholar]
- Wang, G.Y.; Hu, Y.X.; Liu, Y.X.; Ahmad, S.; Zhou, X.B. Effects of supplement irrigation and nitrogen application levels on soil carbon–nitrogen content and yield of one-year double cropping maize in subtropical region. Water 2021, 13, 1180. [Google Scholar] [CrossRef]
- Erdal, S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, F.; Sorooshzadeh, A.; Pirdashti, H.; Modarres-Sanavy, S.A.M. Alleviation of waterlogging damage by foliar application of nitrogen compounds and tricyclazole in canola. Aust. J. Crop Sci. 2013, 7, 401–406. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Zheng, X.; He, P.; Wang, L.; Lei, Q.; Feng, C.; Zhou, J.; Li, Q.; Han, Z.; Kong, J. Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. J. Pineal Res. 2014, 57, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cai, J.S.; Li, J.J.; Lu, G.Y.; Li, C.S.; Fu, G.P.; Zhang, X.K.; Liu, Q.Y.; Zou, X.L.; Cheng, Y. Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings. J. Integr. Agric. 2018, 17, 328–335. [Google Scholar]
- Tian, L.; Li, J.; Bi, W.; Zuo, S.; Li, L.; Li, W.; Sun, L. Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) under field conditions. Agric. Water Manag. 2019, 218, 250–258. [Google Scholar] [CrossRef]
- Ahmad, S.; Cui, W.; Kamran, M.; Ahmad, I.; Han, Q. Exogenous Application of Melatonin Induces Tolerance to Salt Stress by Improving the Photosynthetic Efficiency and Antioxidant Defense System of Maize Seedling. J. Plant Growth Regul. 2020, 40, 1270–1283. [Google Scholar] [CrossRef]
- Shi, H.; Chen, Y.; Tan, D.X.; Reiter, R.J.; Chan, Z.; He, C. Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. J. Pineal Res. 2015, 59, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhang, T.; Zhang, P.; Wang, Z.Y. Melatonin attenuates postharvest physiological deterioration of cassava storage roots. J. Pineal Res. 2016, 60, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Khan, A.L.; Shahzad, R.; Kim, Y.H.; Imran, M.; Khan, M.J.; Al-Harrasi, A.; Kim, T.H.; Lee, I.J. Mechanisms of Cr (VI) resistance by endophytic Sphingomonas sp. LK11 and its Cr (VI) phytotoxic mitigating effects in soybean (Glycine max L.). Ecotoxicol. Environ. Saf. 2018, 164, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Li, J.; Sikdar, A.; Hasanuzzaman, M.; Feng, B. Exogenous melatonin modulates the physiological and biochemical mechanisms of drought tolerance in tartary buckwheat (Fagopyrum tataricum (L.) Gaertn). Molecules 2020, 25, 2828. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Ali, S.; Rizwan, M.; Ibrahim, M.; Hussain, A.; Shahid, M.R.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Alleviative role of exogenously applied mannitol in maize cultivars differing in chromium stress tolerance. Environ. Sci. Pollut. Res. 2019, 26, 5111–5121. [Google Scholar] [CrossRef] [PubMed]
- Biswojit, D.; Mubasher, H.; Muhammad, I.; Sangeeta, M.; Min, L.; Liu, S.; Qiu, D. Exogenous melatonin mitigates acid rain stress to tomato plants through modulation of leaf ultrastructure, photosynthesis and antioxidant potential. Molecules 2018, 23, 388. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Li, C. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Qian, Y.; Tan, D.X.; Reiter, R.J.; He, C. Melatonin induces the transcripts of CBF/DREB1s and their involvement in both abiotic and biotic stresses in Arabidopsis. J. Pineal Res. 2015, 59, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, W.; Chen, H.; Zhan, J.; He, C.; Wang, Q. Ammonium nitrogen tolerant Chlorella strain screening and its damaging effects on photosynthesis. Front. Microbiol. 2019, 9, 3250. [Google Scholar] [CrossRef]
- Sahay, S.; Robledo-Arratia, L.; Glowacka, K.; Gupta, M. Root NRT, NiR, AMT, GS, GOGAT and GDH expression levels reveal NO and ABA mediated drought tolerance in Brassica juncea L. Sci. Rep. 2021, 11, 7992. [Google Scholar] [CrossRef]
- Zanandrea, I.; Alves, J.D.; Deuner, S.; de FP Goulart, P.; Henrique, P.d.C.; Silveira, N.M. Tolerance of Sesbania virgata plants to flooding. Aust. J. Bot. 2010, 57, 661–669. [Google Scholar] [CrossRef]
- Gimeno, V.; Díaz-López, L.; Simón-Grao, S.; Martínez, V.; Martínez-Nicolás, J.; García-Sánchez, F. Foliar potassium nitrate application improves the tolerance of Citrus macrophylla L. seedlings to drought conditions. Plant Physiol. Biochem. 2014, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.; Hernández-Ruiz, J. Growth activity, rooting capacity, and tropism: Three auxinic precepts fulfilled by melatonin. Acta Physiol. Plant. 2017, 39, 127. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Zhang, Z.; Liu, N.; Li, D.; Hu, L. Melatonin improved waterlogging tolerance in alfalfa (Medicago sativa) by reprogramming polyamine and ethylene metabolism. Front. Plant Sci. 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Wijaya, L.; Ahmad, P. The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiol. Biochem. 2019, 143, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ren, B.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Comparative proteomic analysis reveals that exogenous 6-benzyladenine (6-BA) improves the defense system activity of waterlogged summer maize. BMC Plant Biol. 2020, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, K.; Zhou, X.; Xi, L.; Wang, Y.; Xu, H.; Pan, T.; Zou, Z. Melatonin alleviates chilling stress in cucumber seedlings by up-regulation of CsZat12 and modulation of polyamine and abscisic acid metabolism. Sci. Rep. 2017, 7, 4998. [Google Scholar] [CrossRef]
- Smethurst, C.F.; Shabala, S. Screening methods for waterlogging tolerance in lucerne: Comparative analysis of waterlogging effects on chlorophyll fluorescence, photosynthesis, biomass and chlorophyll content. Funct. Plant Biol. 2003, 30, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.S.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-induced water stress tolerance in plants: Recent advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X. Melatonin and plants. J. Exp. Bot. 2015, 66, 625–626. [Google Scholar] [CrossRef] [Green Version]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M.; Tekis, S.A. The impact of selenium application on enzymatic and non-enzymatic antioxidant systems in Zea mays roots treated with combined osmotic and heat stress. Arch. Agron. Soil Sci. 2017, 63, 261–275. [Google Scholar] [CrossRef]
- Imran, M.; Latif Khan, A.; Shahzad, R.; Aaqil Khan, M.; Bilal, S.; Khan, A.; Kang, S.M.; Lee, I.J. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants 2021, 13, plab026. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Zeeshan, M.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative effect of melatonin improves drought tolerance by regulating growth, photosynthetic traits and leaf ultrastructure of maize seedlings. BMC Plant Biol. 2021, 21, 368. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.; Egea, J.; Salazar, J.A.; Campoy, J.A. Chilling and heat requirements of Japanese plum cultivars for flowering. Sci. Hortic. 2018, 242, 164–169. [Google Scholar] [CrossRef]
- Sharma, A.; Zheng, B. Melatonin mediated regulation of drought stress: Physiological and molecular aspects. Plants 2019, 8, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, S.; Zheng, S.; Shen, X.; Liu, D. Regulation effects of adding nitrogen on physiological properties and yield of rapeseed after waterlogging during seedling. Soils 2017, 49, 519–526. [Google Scholar]
- Tian, G.; Qi, D.; Zhu, J.; Xu, Y. Effects of nitrogen fertilizer rates and waterlogging on leaf physiological characteristics and grain yield of maize. Arch. Agron. Soil Sci. 2021, 67, 863–875. [Google Scholar] [CrossRef]
- Zhou, Z.; Oosterhuis, D.M. Physiological mechanism of nitrogen mediating cotton (Gossypium hirsutum L.) seedlings growth under water-stress conditions. Am. J. Plant Sci. 2012, 2012, 20019. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.Q.; Chen, B.L.; Liu, R.X.; Zhou, Z.G. Effects of nitrogen application rate on cotton leaf antioxidant enzyme activities and endogenous hormone contents under short-term waterlogging at flowering and boll-forming stage. Chin. J. Appl. Ecol. 2010, 21, 53–60. [Google Scholar]
- Chen, Z.; Cao, X.; Niu, J. Effects of melatonin on morphological characteristics, mineral nutrition, nitrogen metabolism, and energy status in alfalfa under high-nitrate stress. Front. Plant Sci. 2021, 12, 694179. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.; Raven, J.; Lea, P. Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann. Appl. Biol. 2013, 163, 174–199. [Google Scholar] [CrossRef]
- Arora, D.; Bhatla, S.C. Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radic. Biol. Med. 2017, 106, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 2020, 11, 627331. [Google Scholar] [CrossRef]
- Arismendi, M.J.; Almada, R.; Pimentel, P.; Bastias, A.; Salvatierra, A.; Rojas, P.; Hinrichsen, P.; Pinto, M.; Di Genova, A.; Travisany, D. Transcriptome sequencing of Prunus sp. rootstocks roots to identify candidate genes involved in the response to root hypoxia. Tree Genet. Genomes 2015, 11, 11. [Google Scholar] [CrossRef]
| Parameters | Values |
|---|---|
| Soil organic matter | 14.61 g/kg |
| Available nitrogen | 0.88 g/kg |
| Available phosphorus | 48.85 g/kg |
| Available potassium | 96.37 mg/kg |
| Soil pH | 6.83 |
| Water holding capacity | 30.31% |
| Methods | Treatments | Root DW (g/plant) | Stem DW (g/plant) | Shoot DW (g/plant) | Leaf DW (g/plant) | Root Shoot Ratio | Root Length (cm/plant) |
|---|---|---|---|---|---|---|---|
| Foliar Spray | NWL | 0.63 ± 0.01 a | 0.75 ± 0.02 a | 1.70 ± 0.05 a | 0.95 ± 0.06 a | 0.373 | 797.59 ± 25.28 a |
| WL | 0.35 ± 0.03 e | 0.48 ± 0.05 b | 0.98 ± 0.02 e | 0.50 ± 0.03 e | 0.361 | 586.72 ± 24.46 d | |
| WLM | 0.41 ± 0.02 de | 0.53 ± 0.03 b | 1.09 ± 0.03 d | 0.59 ± 0.05 d | 0.374 | 669.73 ± 16.38 c | |
| WLMN1 | 0.44 ± 0.01 cd | 0.54 ± 0.06 b | 1.17 ± 0.04 d | 0.63 ± 0.04 d | 0.379 | 730.08 ± 23.98 b | |
| WLMN2 | 0.50 ± 0.06 b | 0.74 ± 0.05 a | 1.58 ± 0.08 b | 0.83 ± 0.07 b | 0.341 | 749.55 ± 17.35 b | |
| WLMN3 | 0.48 ± 0.05 bc | 0.74 ± 0.06 a | 1.47 ± 0.04 c | 0.73 ± 0.02 c | 0.325 | 744.25 ± 25.04 b | |
| Seed soaking | NWL | 0.63 ± 0.07 a | 0.97 ± 0.05 a | 1.85 ± 0.03 a | 0.88 ± 0.02 a | 0.413 | 811.01 ± 18.76 a |
| WL | 0.35 ± 0.03 d | 0.42 ± 0.06 c | 1.85 ± 0.03 d | 0.49 ± 0.04 d | 0.450 | 562.34 ± 32.51 e | |
| WLM | 0.46 ± 0.03 cd | 0.46 ± 0.05 c | 1.05 ± 0.08 cd | 0.58 ± 0.03 c | 0.444 | 583.20 ± 20.11 de | |
| WLMN1 | 0.50 ± 0.05 c | 0.49 ± 0.04 c | 1.14 ± 0.09 c | 0.66 ± 0.06 c | 0.439 | 618.01 ± 21.75 d | |
| WLMN2 | 0.69 ± 0.03 ab | 0.65 ± 0.10 b | 1.46 ± 0.15 b | 0.81 ± 0.05 ab | 0.479 | 754.95 ± 27.12 b | |
| WLMN3 | 0.61 ± 0.06 b | 0.86 ± 0.11 a | 1.65 ± 0.16 ab | 0.79 ± 0.06 b | 0.374 | 708.47 ± 15.13 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Wang, G.-Y.; Muhammad, I.; Zeeshan, M.; Zhou, X.-B. Melatonin and KNO3 Application Improves Growth, Physiological and Biochemical Characteristics of Maize Seedlings under Waterlogging Stress Conditions. Biology 2022, 11, 99. https://doi.org/10.3390/biology11010099
Ahmad S, Wang G-Y, Muhammad I, Zeeshan M, Zhou X-B. Melatonin and KNO3 Application Improves Growth, Physiological and Biochemical Characteristics of Maize Seedlings under Waterlogging Stress Conditions. Biology. 2022; 11(1):99. https://doi.org/10.3390/biology11010099
Chicago/Turabian StyleAhmad, Shakeel, Guo-Yun Wang, Ihsan Muhammad, Muhammad Zeeshan, and Xun-Bo Zhou. 2022. "Melatonin and KNO3 Application Improves Growth, Physiological and Biochemical Characteristics of Maize Seedlings under Waterlogging Stress Conditions" Biology 11, no. 1: 99. https://doi.org/10.3390/biology11010099
APA StyleAhmad, S., Wang, G.-Y., Muhammad, I., Zeeshan, M., & Zhou, X.-B. (2022). Melatonin and KNO3 Application Improves Growth, Physiological and Biochemical Characteristics of Maize Seedlings under Waterlogging Stress Conditions. Biology, 11(1), 99. https://doi.org/10.3390/biology11010099








