Multispecies Assessment of Anthropogenic Particle Ingestion in a Marine Protected Area
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Biota Samples
2.2.1. Collection, Laboratory Processing and Biological Indices
- Fulton’s condition factor (K) = (total weight (g)/(total length (cm)3) × 100
- Stomach Fullness Index (FI) = stomach content weight (g)/eviscerated weight (g) × 100
- 3.
- Square root of the length–width product index (SLW) = square root of length (cm) × width (cm)
- 4.
- Condition index (CI) = stomach content weight (g)/total weight (g) × 100
- 5.
- Mussels condition Index (MCI) = wet weight of the soft tissue (g)/shell weight (g)
2.2.2. Characterization of Ingested Microparticles
2.3. Data Analysis
2.4. Quality Assurance and Control
3. Results
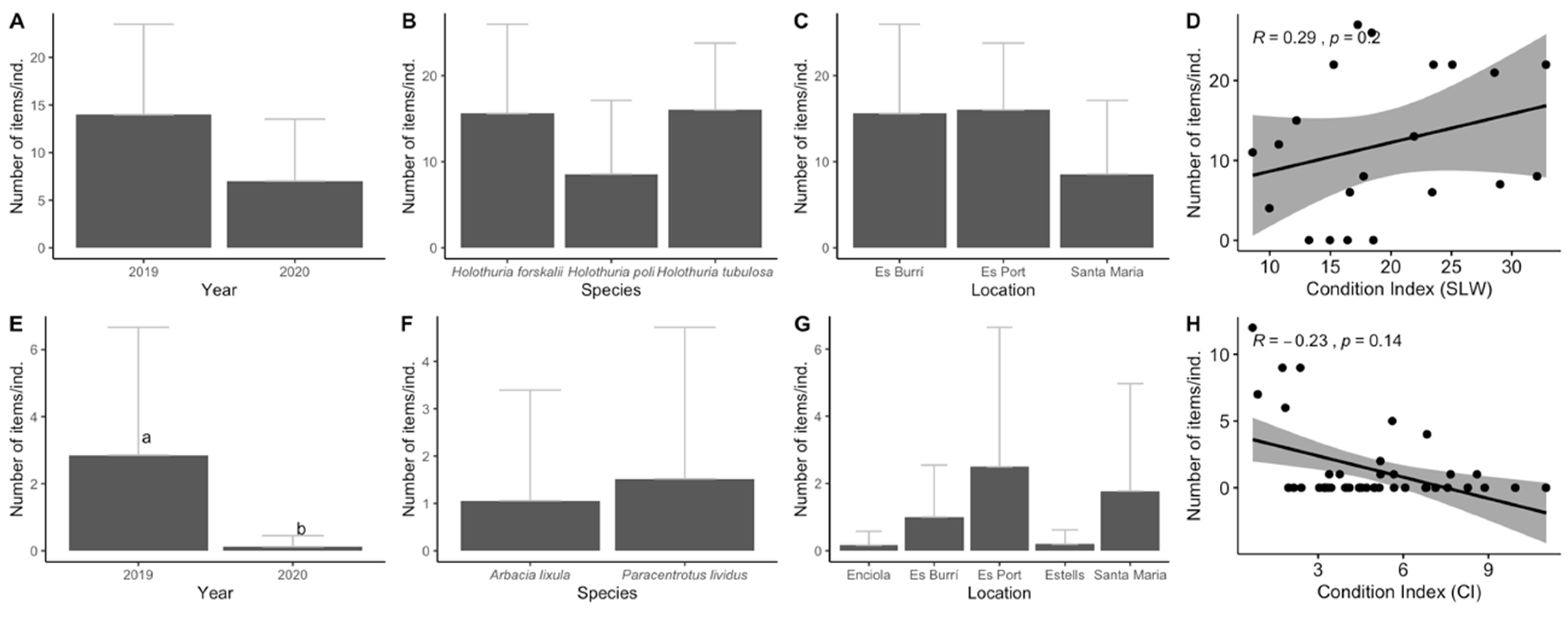
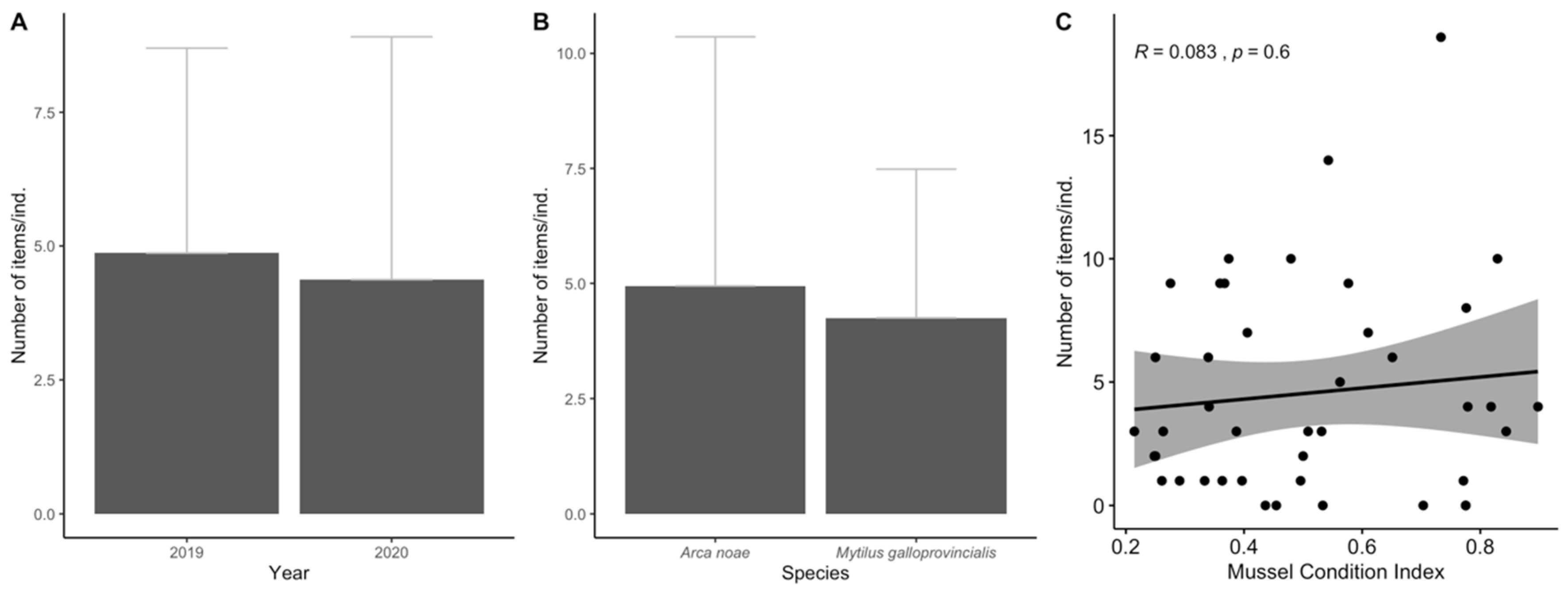
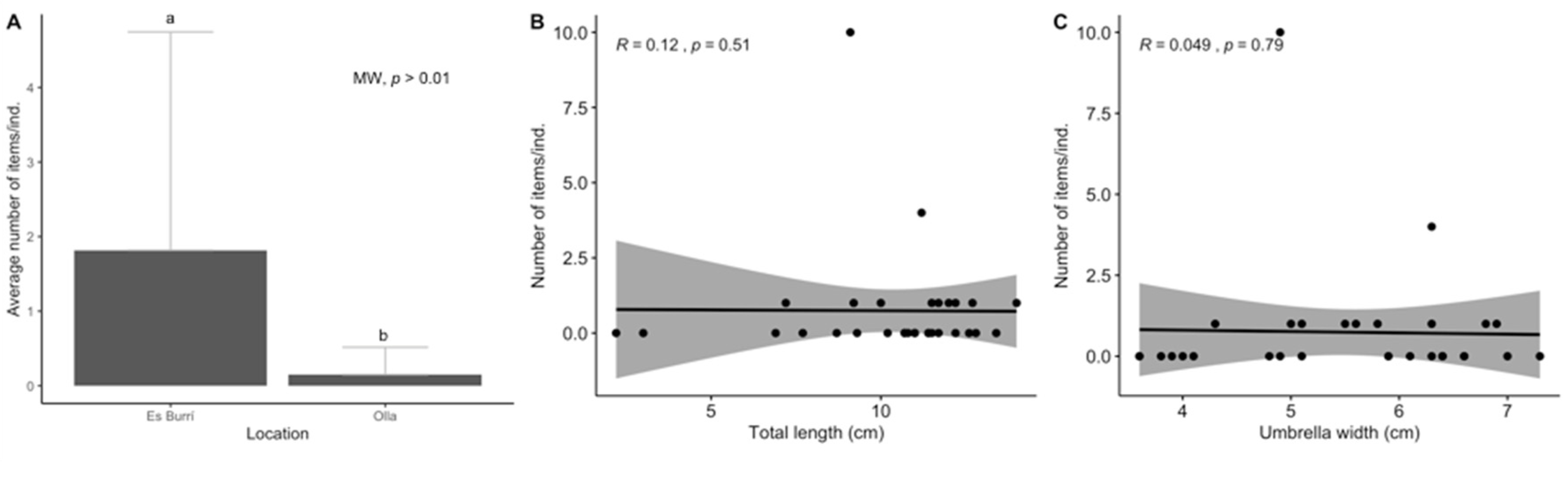
3.1. Biological Parameters
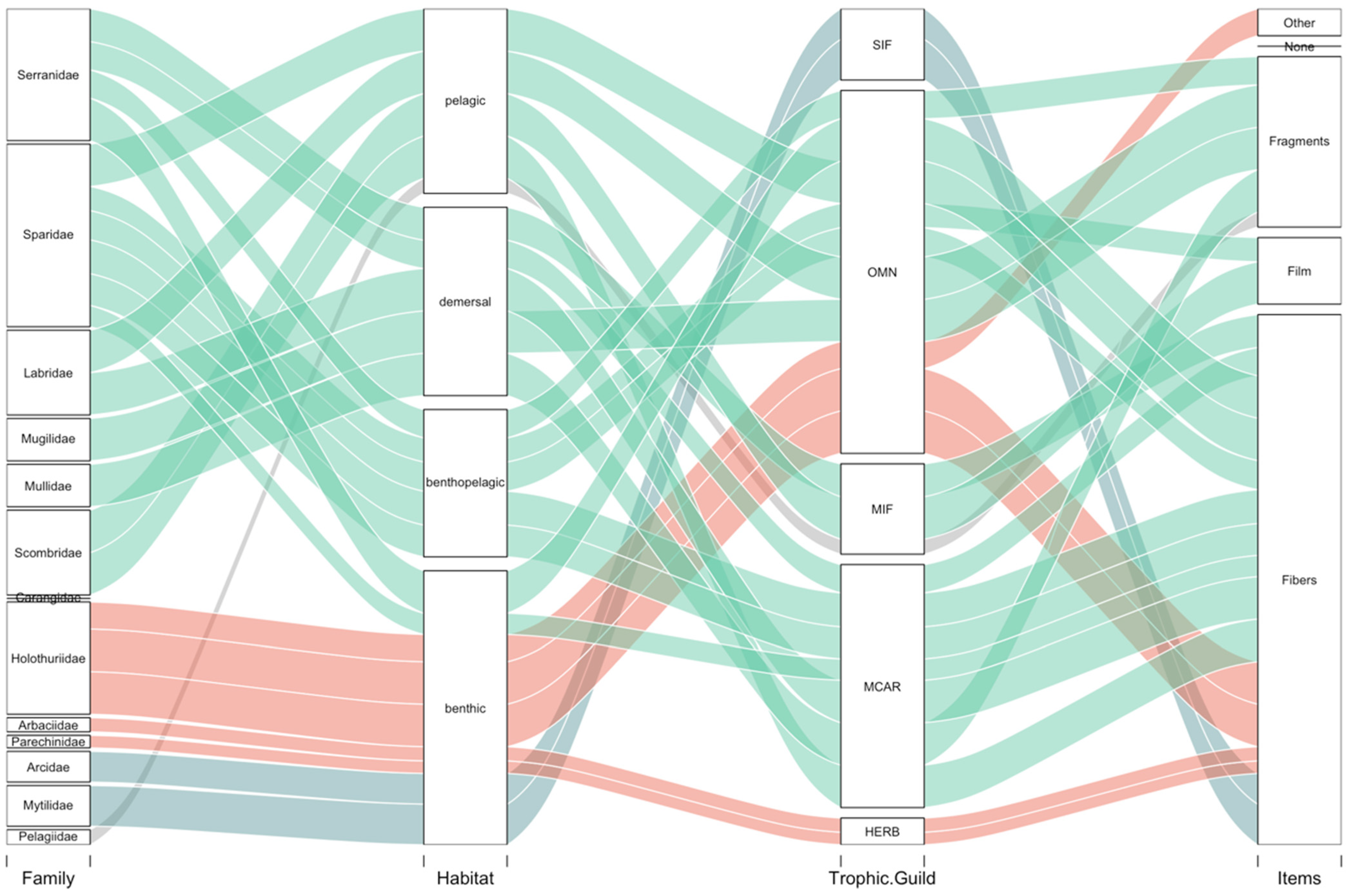
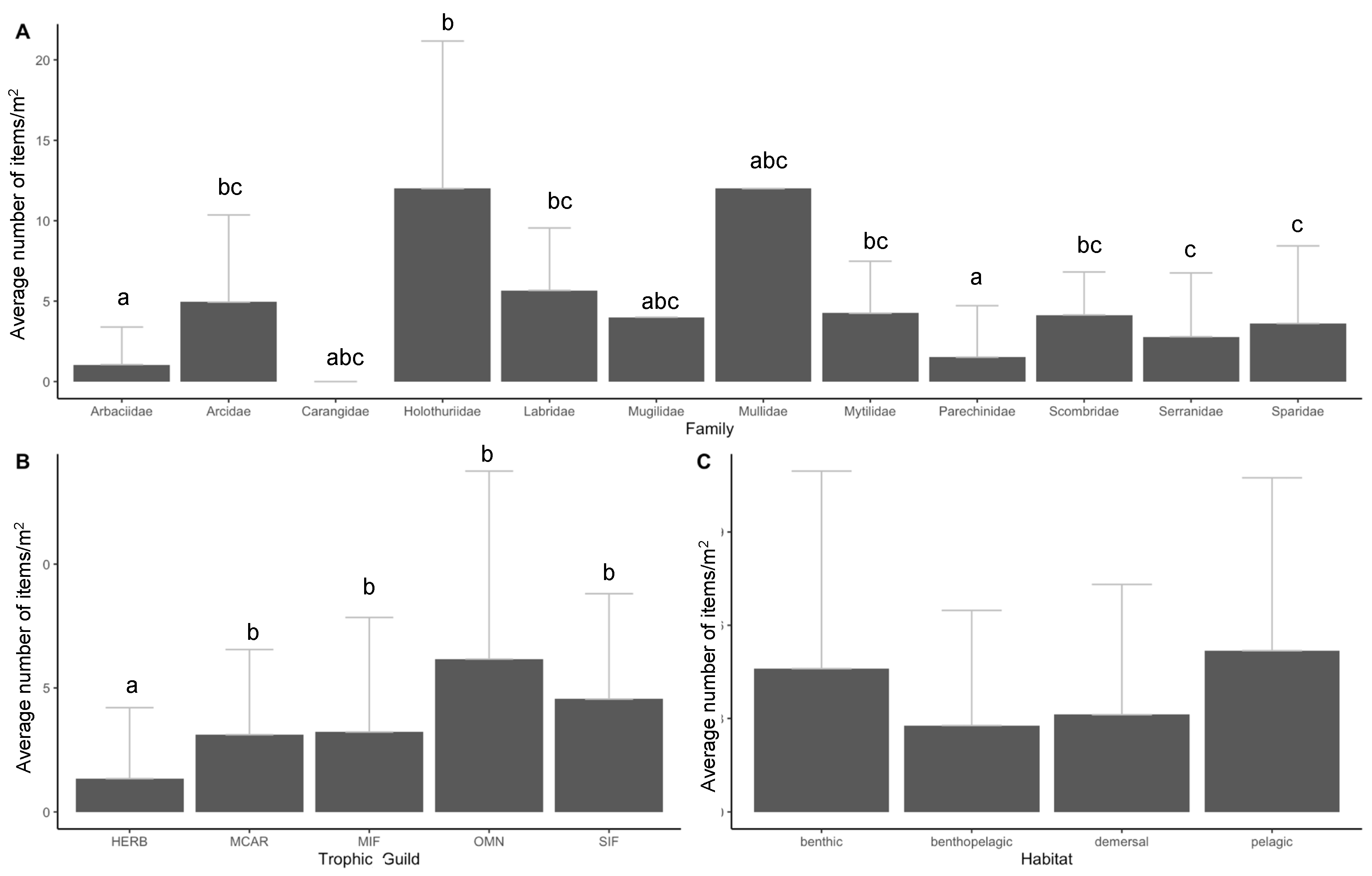
3.2. Ingestion and Characterization
Polymer Characterization
3.3. Data Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collard, F.; Gilbert, B.; Eppe, G.; Roos, L.; Compère, P.; Das, K.; Parmentier, E. Morphology of the Filtration Apparatus of Three Planktivorous Fishes and Relation with Ingested Anthropogenic Particles. Mar. Pollut. Bull. 2017, 116, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Europe Plastics. Plastics—The facts 2020. PlasticEurope 2020, 16, 1–64. [Google Scholar]
- Alomar, C.; Deudero, S.; Compa, M.; Guijarro, B. Exploring the Relation between Plastic Ingestion in Species and Its Presence in Seafloor Bottoms. Mar. Pollut. Bull. 2020, 160, 111641. [Google Scholar] [CrossRef]
- Guerrini, F.; Mari, L.; Casagrandi, R. Modeling Plastics Exposure for the Marine Biota: Risk Maps for Fin Whales in the Pelagos Sanctuary (North-Western Mediterranean). Front. Mar. Sci. 2019, 6, 299. [Google Scholar] [CrossRef]
- Panti, C.; Giannetti, M.; Baini, M.; Rubegni, F.; Minutoli, R.; Fossi, M.C. Occurrence, Relative Abundance and Spatial Distribution of Microplastics and Zooplankton NW of Sardinia in the Pelagos Sanctuary Protected Area, Mediterranean Sea. Environ. Chem. 2015, 12, 618–626. [Google Scholar] [CrossRef]
- Schuyler, Q.A.; Wilcox, C.; Townsend, K.A.; Wedemeyer-strombel, K.R.; Balazs, G.; Sebille, E.V.A.N. Risk Analysis Reveals Global Hotspots for Marine Debris Ingestion by Sea Turtles. Glob. Chang. Biol. 2016, 22, 567–576. [Google Scholar] [CrossRef]
- Wilcox, C.; Van Sebille, E.; Hardesty, B.D. Threat of Plastic Pollution to Seabirds Is Global, Pervasive, and Increasing. Proc. Natl. Acad. Sci. USA 2015, 112, 11899–11904. [Google Scholar] [CrossRef]
- Compa, M.; Alomar, C.; Wilcox, C.; van Sebille, E.; Lebreton, L.; Hardesty, B.D.; Deudero, S. Risk Assessment of Plastic Pollution on Marine Diversity in the Mediterranean Sea. Sci. Total Environ. 2019, 678, 188–196. [Google Scholar] [CrossRef]
- Nadal, M.A.; Alomar, C.; Deudero, S. High Levels of Microplastic Ingestion by the Semipelagic Fish Bogue Boops boops (L.) around the Balearic Islands. Environ. Pollut. 2016, 214, 517–523. [Google Scholar] [CrossRef]
- Renzi, M.; Specchiulli, A.; Blašković, A.; Manzo, C.; Mancinelli, G.; Cilenti, L. Marine Litter in Stomach Content of Small Pelagic Fishes from the Adriatic Sea: Sardines (Sardina pilchardus) and Anchovies (Engraulis encrasicolus). Environ. Sci. Pollut. Res. 2019, 26, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Alomar, C.; Sureda, A.; Capó, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic Ingestion by Mullus Surmuletus Linnaeus, 1758 Fish and Its Potential for Causing Oxidative Stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Alomar, C.; Deudero, S. Evidence of Microplastic Ingestion in the Shark Galeus Melastomus Rafinesque, 1810 in the Continental Shelf off the Western Mediterranean Sea. Environ. Pollut. 2017, 223, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Rios-Fuster, B.; Alomar, C.; Compa, M.; Guijarro, B.; Deudero, S. Anthropogenic Particles Ingestion in Fish Species from Two Areas of the Western Mediterranean Sea. Mar. Pollut. Bull. 2019, 144, 325–333. [Google Scholar] [CrossRef]
- Dantas, N.C.F.M.; Duarte, O.S.; Ferreira, W.C.; Ayala, A.P.; Rezende, C.F.; Feitosa, C.V. Plastic Intake Does Not Depend on Fish Eating Habits: Identification of Microplastics in the Stomach Contents of Fish on an Urban Beach in Brazil. Mar. Pollut. Bull. 2020, 153, 110959. [Google Scholar] [CrossRef]
- Yodzis, P.; Winemiller, K.O. In Search of Operational Trophospecies in a Tropical Aquatic Food Web. Oikos 1999, 87, 327. [Google Scholar] [CrossRef]
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthropogenic Litter; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2015. [Google Scholar]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested Plastic Transfers Hazardous Chemicals to Fish and Induces Hepatic Stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of Known Plastic Packaging-Associated Chemicals and Their Hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef]
- Rios-Fuster, B.; Alomar, C.; Viñas, L.; Campillo, J.A.; Pérez-Fernández, B.; Álvarez, E.; Compa, M.; Deudero, S. Organochlorine Pesticides (OCPs) and Polychlorinated Biphenyls (PCBs) Occurrence in Sparus Aurata Exposed to Microplastic Enriched Diets in Aquaculture Facilities. Mar. Pollut. Bull. 2021, 173, 113030. [Google Scholar] [CrossRef]
- Solomando, A.; Capó, X.; Alomar, C.; Álvarez, E.; Compa, M.; Valencia, J.M.; Pinya, S.; Deudero, S.; Sureda, A. Long-Term Exposure to Microplastics Induces Oxidative Stress and a pro-Inflammatory Response in the Gut of Sparus Aurata Linnaeus, 1758. Environ. Pollut. 2020, 266, 115295. [Google Scholar] [CrossRef]
- Capó, X.; Company, J.J.; Alomar, C.; Compa, M.; Sureda, A.; Grau, A.; Hansjosten, B.; López-Vázquez, J.; Quintana, J.B.; Rodil, R.; et al. Long-Term Exposure to Virgin and Seawater Exposed Microplastic Enriched-Diet Causes Liver Oxidative Stress and Inflammation in Gilthead Seabream Sparus Aurata, Linnaeus 1758. Sci. Total Environ. 2021, 767, 144976. [Google Scholar] [CrossRef] [PubMed]
- Capó, X.; Rubio, M.; Solomando, A.; Alomar, C.; Compa, M.; Sureda, A.; Deudero, S. Microplastic Intake and Enzymatic Responses in Mytilus Galloprovincialis Reared at the Vicinities of an Aquaculture Station. Chemosphere 2021, 280, 130575. [Google Scholar] [CrossRef] [PubMed]
- Rios-Fuster, B.; Arechavala-Lopez, P.; García-Marcos, K.; Alomar, C.; Compa, M.; Álvarez, E.; Julià, M.M.; Solomando Martí, A.; Sureda, A.; Deudero, S. Experimental Evidence of Physiological and Behavioral Effects of Microplastic Ingestion in Sparus Aurata. Aquat. Toxicol. 2021, 231, 105737. [Google Scholar] [CrossRef] [PubMed]
- Santillo, D.; Miller, K.; Johnston, P. Microplastics as Contaminants in Commercially Important Seafood Species. Integr. Environ. Assess. Manag. 2017, 13, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Luna-Jorquera, G.; Thiel, M.; Portflitt-Toro, M.; Dewitte, B. Marine Protected Areas Invaded by Floating Anthropogenic Litter: An Example from the South Pacific. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 245–259. [Google Scholar] [CrossRef]
- Liubartseva, S.; Coppini, G.; Lecci, R. Are Mediterranean Marine Protected Areas Sheltered from Plastic Pollution? Mar. Pollut. Bull. 2019, 140, 579–587. [Google Scholar] [CrossRef]
- Soto-Navarro, J.; Jordá, G.; Compa, M.; Alomar, C.; Fossi, M.C.; Deudero, S. Impact of the Marine Litter Pollution on the Mediterranean Biodiversity: A Risk Assessment Study with Focus on the Marine Protected Areas. Mar. Pollut. Bull. 2021, 165, 112169. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Albouy, C.; Ben Rais Lasram, F.; Cheung, W.W.L.; Christensen, V.; Karpouzi, V.S.; Guilhaumon, F.; Mouillot, D.; Paleczny, M.; et al. The Mediterranean Sea under Siege: Spatial Overlap between Marine Biodiversity, Cumulative Threats and Marine Reserves. Glob. Ecol. Biogeogr. 2012, 21, 465–480. [Google Scholar] [CrossRef]
- Costanzo, L.G.; Marletta, G.; Alongi, G. Assessment of Marine Litter in the Coralligenous Habitat of a Marine Protected Area along the Ionian Coast of Sicily (Central Mediterranean). J. Mar. Sci. Eng. 2020, 8, 656. [Google Scholar] [CrossRef]
- Fossi, M.C.; Romeo, T.; Baini, M.; Panti, C.; Marsili, L.; Campani, T.; Canese, S.; Galgani, F.; Druon, J.-N.; Airoldi, S.; et al. Plastic Debris Occurrence, Convergence Areas and Fin Whales Feeding Ground in the Mediterranean Marine Protected Area Pelagos Sanctuary: A Modeling Approach. Front. Mar. Sci. 2017, 4, 167. [Google Scholar] [CrossRef]
- Dahl, M.; Bergman, S.; Björk, M.; Diaz-Almela, E.; Granberg, M.; Gullström, M.; Leiva-Dueñas, C.; Magnusson, K.; Marco-Méndez, C.; Piñeiro-Juncal, N.; et al. A Temporal Record of Microplastic Pollution in Mediterranean Seagrass Soils. Environ. Pollut. 2021, 273, 116451. [Google Scholar] [CrossRef] [PubMed]
- Alomar, C.; Estarellas, F.; Deudero, S. Microplastics in the Mediterranean Sea: Deposition in Coastal Shallow Sediments, Spatial Variation and Preferential Grain Size. Mar. Environ. Res 2016, 115, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fagiano, V.; Alomar, C.; Compa, M.; Soto-Navarro, J.; Jordá, G.; Deudero, S. Neustonic Microplastics and Zooplankton in Coastal Waters of Cabrera Marine Protected Area (Western Mediterranean Sea). Sci. Total Environ. 2022, 804, 150120. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Orejón, L.F.; Mourre, B.; Sardá, R.; Tintoré, J.; Ramis-Pujol, J. Quarterly Variability of Floating Plastic Debris in the Marine Protected Area of the Menorca Channel (Spain). Environ. Pollut. 2019, 252, 1742–1754. [Google Scholar] [CrossRef]
- Fagiano, V.; Compa, M.; Alomar, C.; García-Marcos, K.; Deudero, S. Marine Plastics in Mediterranean Islands: Evaluating the Distribution and Composition of Plastic Pollution in the Surface Waters along Four Islands of the Western Sea Basin. Environ. Pollut. 2022, 305, 119268. [Google Scholar] [CrossRef] [PubMed]
- Balbín, B.; López-Jurado, J.L.; Flexas, M.M.; Reglero, P.; Vélez-Velchí, P.; González-Pola, C.; Rodríguez, J.M.; García, A.; Alemany, F. Interannual Variability of the Early Summer Circulation around the Balearic Islands: Driving Factors and Potential Effects on the Marine Ecosystem. J. Mar. Syst. 2014, 138, 70–81. [Google Scholar] [CrossRef]
- Fossi, M.; Vlachogianni, T.; Anastasopoulou, A.; Alomar, C.; Baini, M.; Caliani, I.; Campani, T.; Casini, S.; Consoli, P.; Zeri, C.; et al. Toolkit for Monitoring ML and Its Impacts on Biodiversity in Med MPAs. 2019. Available online: https://plasticbustersmpas.interreg-med.eu/ (accessed on 21 October 2019).
- Kurata, N.; Vella, K.; Hamilton, B.; Shivji, M.; Soloviev, A.; Matt, S.; Tartar, A.; Perrie, W. Surfactant-Associated Bacteria in the near-Surface Layer of the Ocean. Sci. Rep. 2016, 6, 19123. [Google Scholar] [CrossRef]
- Poot-Salazar, A.; Hernández-Flores, Á.; Ardisson, P.L. Use of the SLW Index to Calculate Growth Function in the Sea Cucumber Isostichopus Badionotus. Sci. Rep. 2014, 4, 5151. [Google Scholar] [CrossRef]
- Amri, S.; Bensouilah, M.; Ouali, K. Variation of the Condition Index and Sex-Ratio of the Sea Urchin Paracentrotus Lividus in the Southeast Mediterranean. J. Biol. Sci. 2017, 17, 76–83. [Google Scholar] [CrossRef]
- Il Park, K.; Donaghy, L.; Kang, H.S.; Hong, H.K.; Kim, Y.O.; Choi, K.S. Assessment of Immune Parameters of Manila Clam Ruditapes Philippinarum in Different Physiological Conditions Using Flow Cytometry. Ocean Sci. J. 2012, 47, 19–26. [Google Scholar] [CrossRef]
- Herrera, A.; Garrido-Amador, P.; Martínez, I.; Samper, M.D.; López-Martínez, J.; Gómez, M.; Packard, T.T. Novel Methodology to Isolate Microplastics from Vegetal-Rich Samples. Mar. Pollut. Bull. 2018, 129, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Löder, M.G.J.; Kuczera, M.; Mintenig, S.; Lorenz, C.; Gerdts, G. Focal Plane Array Detector-Based Micro-Fourier-Transform Infrared Imaging for the Analysis of Microplastics in Environmental Samples. Environ. Chem. 2015, 12, 563–581. [Google Scholar] [CrossRef]
- D1, B. Polymer Reference Library. Available online: https://jpi-oceans.eu/archive/baseman/main-page.html (accessed on 28 September 2020).
- Macieira, R.; De Oliveira, L.A.D.S.; Cardozo-Ferreira, G.C.; Pimentel, C.R.; Andrades, R.; Gasparini, J.L.R.; Sarti, F.; Chelazzi, D.; Cincinelli, A.; Gomes, L.C.; et al. Microplastic and Articial Cellulose Microfibers Ingestion by Reef Fishes in the Southwestern Atlantic. Mar. Pollut. Bull. 2021, 167, 112371. [Google Scholar] [CrossRef]
- Pinheiro, H.T.; Rocha, L.A.; Macieira, R.M.; Carvalho-Filho, A.; Anderson, A.B.; Bender, M.G.; Di Dario, F.; Ferreira, C.E.L.; Figueiredo-Filho, J.; Francini-Filho, R.; et al. South-Western Atlantic Reef Fishes: Zoogeographical Patterns and Ecological Drivers Reveal a Secondary Biodiversity Centre in the Atlantic Ocean. Divers. Distrib. 2018, 24, 951–965. [Google Scholar] [CrossRef]
- Carlson, D.F.; Aliani, S.; Fredj, E.; Russo, A. Combining Litter Observations with a Regional Ocean Model to Identify Sources and Sinks of Floating Debris in a... Combining Litter Observations with a Regional Ocean Model to Identify Sources and Sinks of Floating Debris in a Semi-Enclosed Basin: The Adriatic Sea. Front. Mar. Sci. 2017, 4, 78. [Google Scholar] [CrossRef]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic Litter Composition of the Turkish Territorial Waters of the Mediterranean Sea, and Its Occurrence in the Gastrointestinal Tract of Fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Savoca, S.; Capillo, G.; Mancuso, M.; Faggio, C.; Panarello, G.; Crupi, R.; Bonsignore, M.; D’Urso, L.; Compagnini, G.; Neri, F.; et al. Detection of Artificial Cellulose Microfibers in Boops Boops from the Northern Coasts of Sicily (Central Mediterranean). Sci. Total Environ. 2019, 691, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Garcia-garin, O.; Vighi, M.; Aguilar, A.; Tsangaris, C.; Digka, N.; Kaberi, H.; Borrell, A. Boops Boops as a Bioindicator of Microplastic Pollution along the Spanish Catalan Coast. Mar. Pollut. Bull. 2019, 149, 110648. [Google Scholar] [CrossRef]
- Sayogo, B.H.; Patria, M.P.; Takarina, N.D. The Density of Microplastic in Sea Cucumber (Holothuria Sp.) and Sediment at Tidung Besar and Bira Besar Island, Jakarta. J. Phys. Conf. Ser. 2020, 1524, 012064. [Google Scholar] [CrossRef]
- Sanchez-vidal, A.; Thompson, R.C.; Canals, M.; De Haan, W.P. The Imprint of Microfibres in Southern European Deep Seas. PLoS ONE 2018, 13, e0207033. [Google Scholar]
- Kane, I.A.; Clare, M.A.; Miramontes, E.; Wogelius, R.; Rothwell, J.J.; Garreau, P.; Pohl, F. Seafloor Microplastic Hotspots Controlle by Deep-Sea Circulation. Science 2020, 368, 1140–1145. [Google Scholar] [CrossRef]
- Compa, M.; Alomar, C.; Morató, M.; Álvarez, E.; Deudero, S. Are the Seafloors of Marine Protected Areas Sinks for Marine Litter? Composition and Spatial Distribution in Cabrera National Park. Sci. Total Environ. 2022, 819, 152915. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, R.; Zhang, T.; Wang, J.; Huang, W.; Li, J.; Xu, J.; Gao, G. Microplastics in Specific Tissues of Wild Sea Urchins along the Coastal Areas of Northern China. Sci. Total Environ. 2020, 728, 138660. [Google Scholar] [CrossRef] [PubMed]
- Hennicke, A.; Macrina, L.; Malcolm-Mckay, A.; Miliou, A. Assessment of Microplastic Accumulation in Wild Paracentrotus Lividus, a Commercially Important Sea Urchin Species, in the Eastern Aegean Sea, Greece. Reg. Stud. Mar. Sci. 2021, 45, 101855. [Google Scholar] [CrossRef]
- Porter, A.; Smith, K.E.; Lewis, C. The Sea Urchin Paracentrotus Lividus as a Bioeroder of Plastic. Sci. Total Environ. 2019, 693, 133621. [Google Scholar] [CrossRef] [PubMed]
- Murano, C.; Agnisola, C.; Caramiello, D.; Castellano, I.; Casotti, R.; Corsi, I.; Palumbo, A. How Sea Urchins Face Microplastics: Uptake, Tissue Distribution and Immune System Response. Environ. Pollut. 2020, 264, 114685. [Google Scholar] [CrossRef]
- Richardson, C.R.; Burritt, D.J.; Allan, B.J.M.; Lamare, M.D. Microplastic Ingestion Induces Asymmetry and Oxidative Stress in Larvae of the Sea Urchin Pseudechinus Huttoni. Mar. Pollut. Bull. 2021, 168, 112369. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Shahadat Hossain, M.; Li, Q.; Kolandhasamy, P.; et al. Using Mussel as a Global Bioindicator of Coastal Microplastic Pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef]
- Fossi, M.C.; Pedà, C.; Compa, M.; Tsangaris, C.; Alomar, C.; Claro, F.; Ioakeimidis, C.; Galgani, F.; Hema, T.; Deudero, S.; et al. Bioindicators for Monitoring Marine Litter Ingestion and Its Impacts on Mediterranean Biodiversity. Environ. Pollut. 2018, 237, 1023–1040. [Google Scholar] [CrossRef]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in Mussels and Fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef]
- Morais, L.M.S.; Sarti, F.; Chelazzi, D.; Cincinelli, A.; Giarrizzo, T.; Martinelli Filho, J.E. The Sea Anemone Bunodosoma Cangicum as a Potential Biomonitor for Microplastics Contamination on the Brazilian Amazon Coast. Environ. Pollut. 2020, 265, 114817. [Google Scholar] [CrossRef]
- Diana, Z.; Sawickij, N.; Rivera, N.A.; Hsu-Kim, H.; Rittschof, D. Plastic Pellets Trigger Feeding Responses in Sea Anemones. Aquat. Toxicol. 2020, 222, 105447. [Google Scholar] [CrossRef]
- Avio, C.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants Bioavailability and Toxicological Risk from Microplastics to Marine Mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ghribi, F.; Richir, J.; Bejaoui, S.; Boussoufa, D.; Marengo, M.; El Cafsi, M.; Gobert, S. Trace Elements and Oxidative Stress in the Ark Shell Arca Noae from a Mediterranean Coastal Lagoon (Bizerte Lagoon, Tunisia): Are There Health Risks Associated with Their Consumption? Environ. Sci. Pollut. Res. 2020, 27, 15607–15623. [Google Scholar] [CrossRef] [PubMed]
- Rapp, J.; Herrera, A.; Bondyale-Juez, D.R.; González-Pleiter, M.; Reinold, S.; Asensio, M.; Martínez, I.; Gómez, M. Microplastic Ingestion in Jellyfish Pelagia Noctiluca (Forsskal, 1775) in the North Atlantic Ocean. Mar. Pollut. Bull. 2021, 166, 112266. [Google Scholar] [CrossRef]
- Liu, C.; Gin, K.Y.H.; Chang, V.W.C. Multi-Biomarker Responses in Green Mussels Exposed to PFCs: Effects at Molecular, Cellular, and Physiological Levels. Environ. Sci. Pollut. Res. 2014, 21, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Abidli, S.; Akkari, N.; Lahbib, Y.; Trigui El Menif, N. First Evaluation of Microplastics in Two Commercial Fish Species from the Lagoons of Bizerte and Ghar El Melh (Northern Tunisia). Reg. Stud. Mar. Sci. 2021, 41, 101581. [Google Scholar] [CrossRef]
- Avio, C.G.; Pittura, L.; d’Errico, G.; Abel, S.; Amorello, S.; Marino, G.; Gorbi, S.; Regoli, F. Distribution and Characterization of Microplastic Particles and Textile Microfibers in Adriatic Food Webs: General Insights for Biomonitoring Strategies. Environ. Pollut. 2020, 258, 113766. [Google Scholar] [CrossRef]
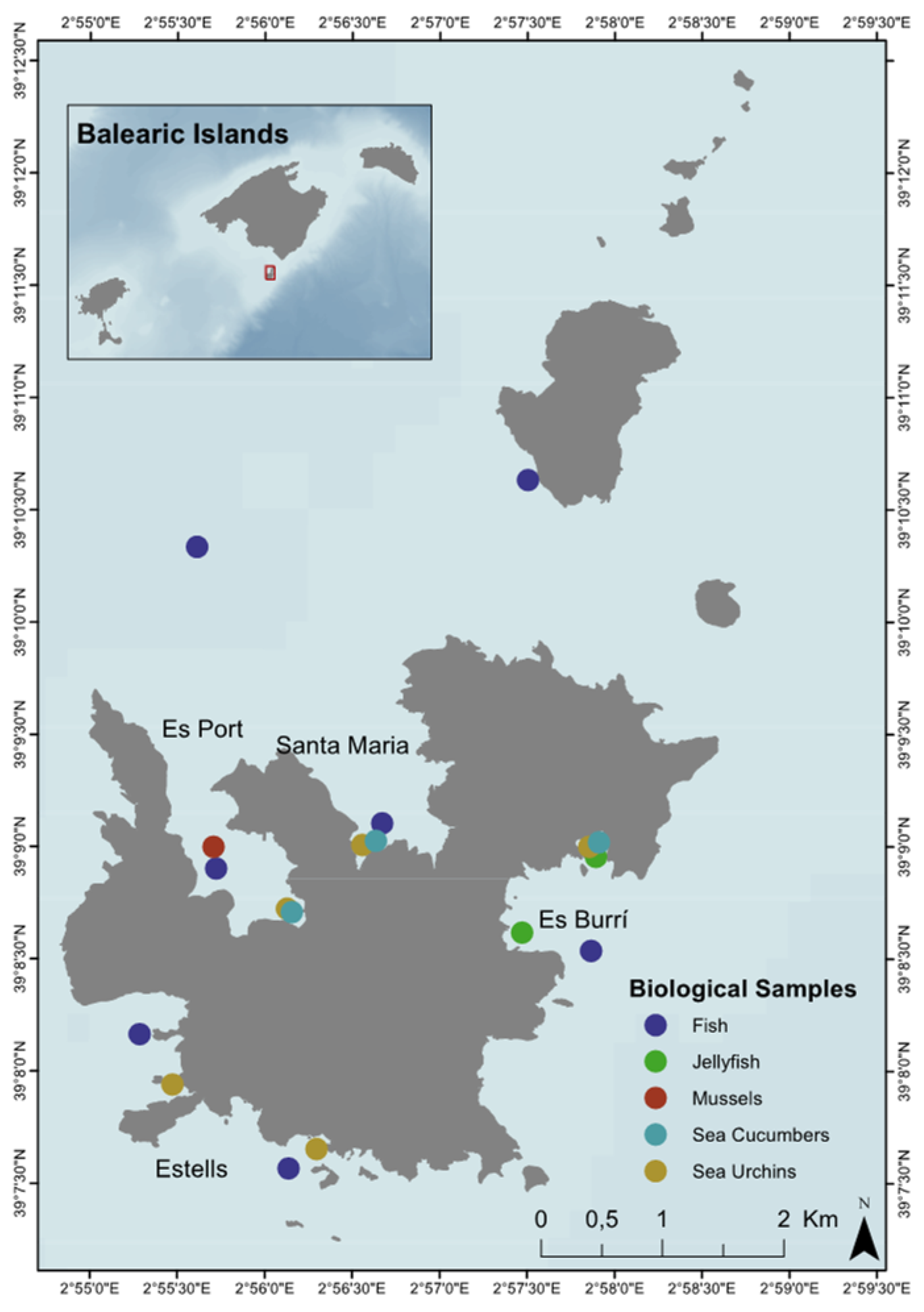
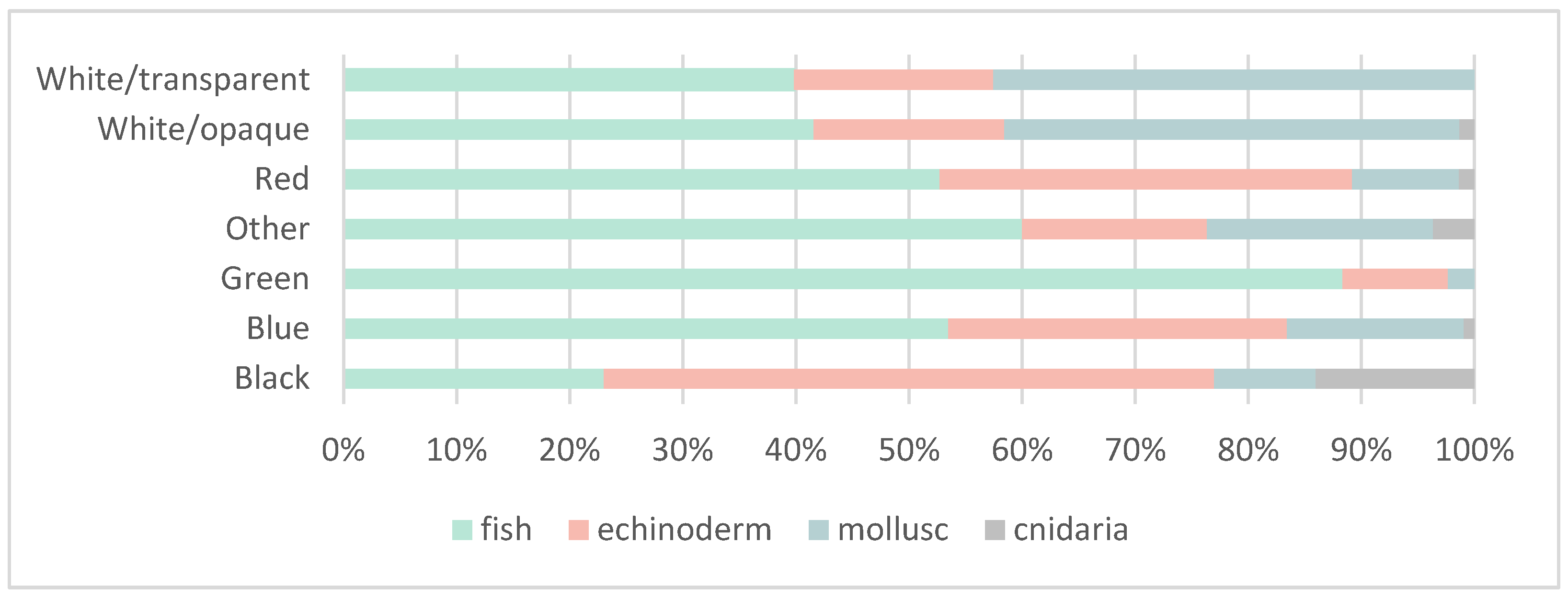
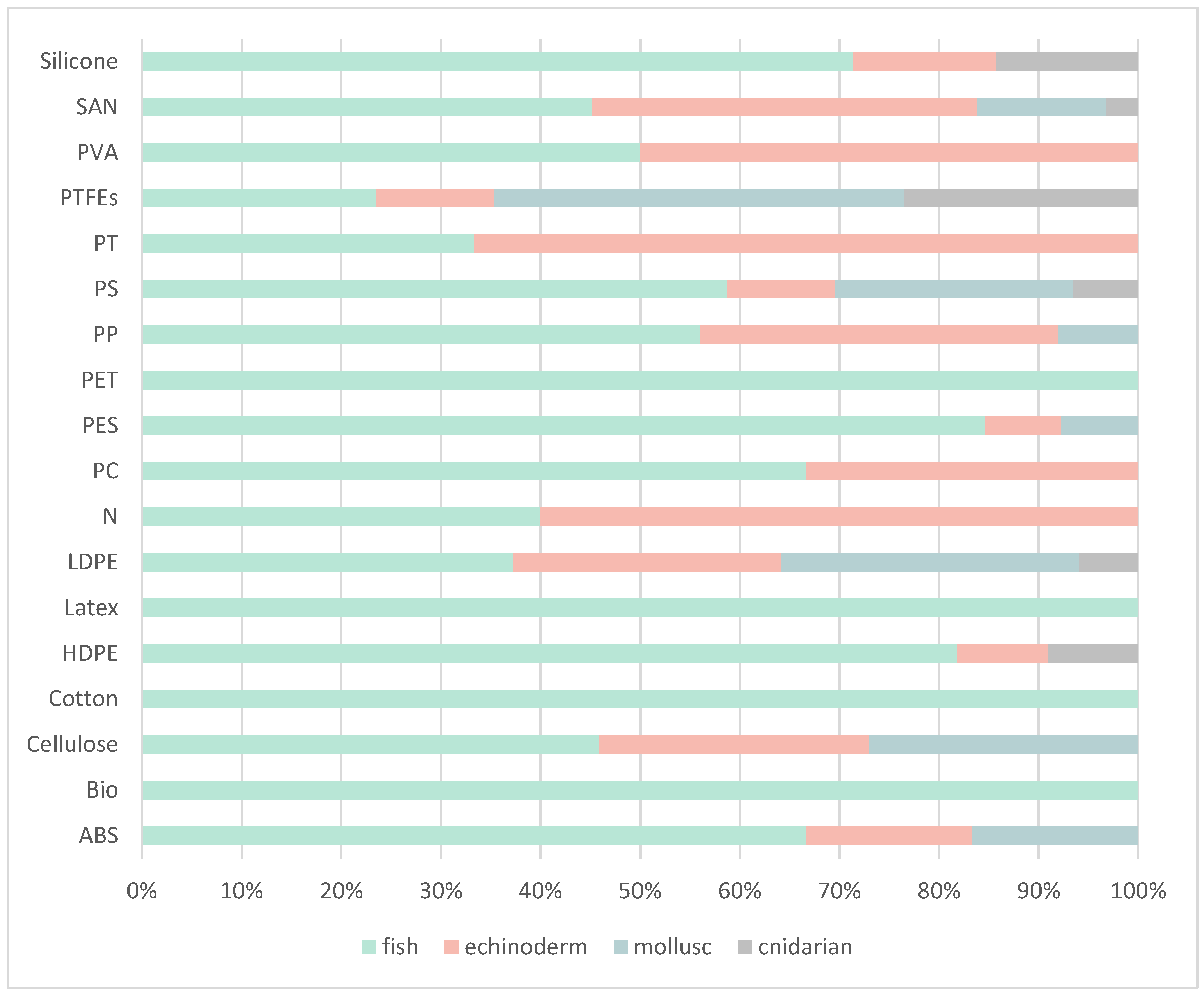
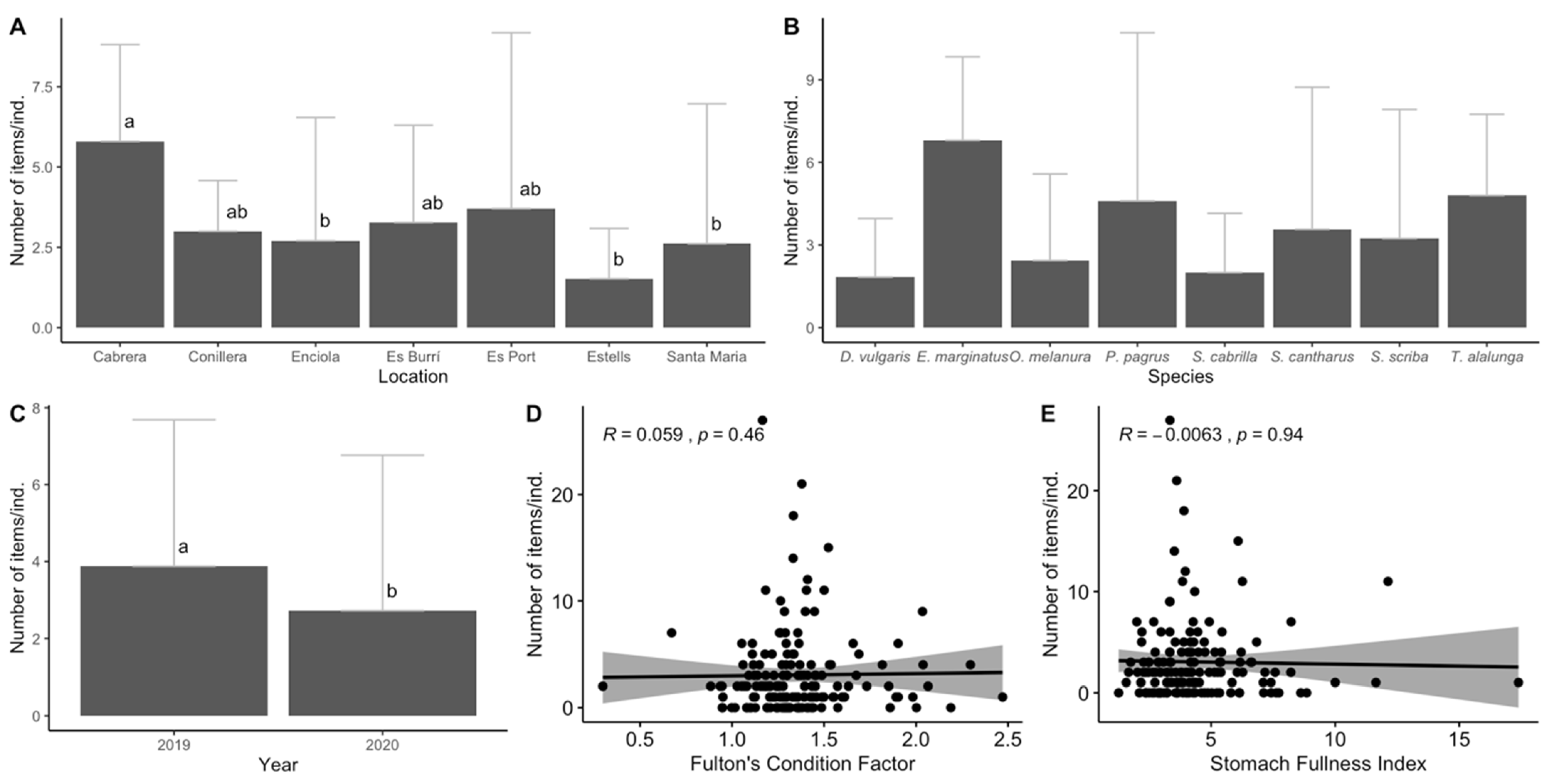
| Type | Family | Species | Number of Individuals | Total Length (cm) | Weight (g) | GIT Weight (g) | FI | FCI |
|---|---|---|---|---|---|---|---|---|
| Fish | Serranidae | Anthias anthias | 1 | 13.6 ± NA | 24.8 ± NA | 0.50 ± NA | 2.0 ± NA | 0.99 ± NA |
| Sparidae | Boops boops | 2 | 25.75 ± 1.62 | 142.8 ± 43.85 | 14.55 ± 3.60 | 11.2 ± 1.09 | 0.82 ± 0.10 | |
| Mugilidae | Chelon auratus | 1 | 37.0 ± NA | 427.20 ± NA | 50.0 ± NA | 4.45 ± NA | 0.99 ± NA | |
| Labridae | Coris julis | 2 | 14.75 ± 2.05 | 32.75 ± 12.5 | 1.1 ± 0.14 | 7.86 ± 2.93 | 1.83 ± 0.02 | |
| Sparidae | Diplodus annularis | 4 | 15.3 ± 0.82 | 65.67 ± 9.28 | 4.43 ± 0.32 | 5.21 ± 1. 86 | 1.58 ± 0.04 | |
| Sparidae | Diplodus vulgaris | 12 | 21.75 ± 2.26 | 167.18 ± 52.58 | 7.83 ± 2.31 | 3.33 ± 1.35 | 1.40 ± 0.28 | |
| Serranidae | Epinephelus marginatus | 5 | 56.7 ± 4.89 | 2584 ± 572.65 | 77.52 ± 17.18 | 4.79 ± 0 | 1.24 ± 0.09 | |
| Labridae | Labrus merula | 1 | 24.8 ± NA | 188.40 ± NA | 8.40 ± NA | 13.72 ± NA | 0.84 ± NA | |
| Mullidae | Mullus surmuletus | 1 | 29.0 ± NA | 296 ± NA | 15.5 ± NA | 5.71 ± NA | 1.21 ± NA | |
| Sparidae | Oblada melanura | 16 | 22.56 ± 2.93 | 167.97 ± 66.43 | 6.9 ± 6.12 | 4.15 ± 2.68 | 1.40 ± 0.24 | |
| Sparidae | Pagrus pagrus | 5 | 17.82 ± 3.57 | 97.22 ± 63.61 | 6.16 ± 5.75 | 6.2 ± 1.60 | 1.59 ± 0.12 | |
| Scombridae | Scomber scombrus | 2 | 34.3 ± 1.83 | 394.5 ± 65.05 | 25.8 ± 1.70 | 7.74 ± 1.78 | 0.97 ± 0.01 | |
| Serranidae | Serranus cabrilla | 33 | 18.89 ± 4.91 | 87.1 ± 55.59 | 2.65 ± 1.95 | 3.28 ± 1.24 | 1.12 ± 0.17 | |
| Serranidae | Serranus scriba | 76 | 16.55 ± 3.12 | 64.82 ± 33.54 | 2.71 ± 3.38 | 4.79 ± 2.35 | 1.48 ± 1.55 | |
| Sparidae | Spondyliosoma cantharus | 7 | 21.58 ± 3.89 | 168.2 ± 70.60 | 7.1 ± 3.38 | 4.63 ± 0.98 | 1.56 ± 0.31 | |
| Scombridae | Thunnus alalunga | 5 | 64.9 ± 2.75 | 5866 ± 666.96 | 175.98 ± 20.01 | 3.33 ± 0 | 2.15 ± 0.23 | |
| Carangidae | Trachurus trachurus | 1 | 35.7 ± NA | 311.9 ± NA | 6.3 ± NA | 2.49 ± NA | 0.69 ± NA | |
| N | Total Length (cm) | Disc Width (cm) | Height (cm) | Weight (g) | Gut Weight (g) | |||
| Echinoderma | Holothuriidae | Holothuria forskalii | 5 | 16.02 ± 2.66 | 4.88 ± 0.49 | 3.38 ± 0.73 | 168.50 ± 36.72 | 30.44 ± 4.67 |
| Holothuriidae | Holothuria poli | 11 | 16.13 ± 4.15 | 3.68 ± 1.01 | 2.97 ± 0.72 | 138.28 ± 47.86 | 22.13 ± 15.86 | |
| Holothuriidae | Holothuria tubulosa | 5 | 27.8 ± 4.15 | 5.56 ± 0.73 | 4.06 ± 0.43 | 390.80 ± 21.85 | 66.22 ± 11.16 | |
| Arbaciidae | Arbacia lixula | 18 | - | 4.79 ± 0.83 | 2.45 ± 0.56 | 49.52 ± 16.72 | 2.09 ± 1.17 | |
| Parechinidae | Paracentrotus lividus | 27 | - | 5.18 ± 0.67 | 3.01 ± 0.58 | 61.93 ± 19.68 | 2.98 ± 1.46 | |
| N | Length (cm) | Width (cm) | Weight (g) | Soft Tissue (g) | Shells (g) | |||
| Molluscs | Arcidae | Arca noae | 18 | 6.11 ± 0.59 | 2.5 ± 0.29 | 24.9 ± 4.45 | 8.41 ± 2.11 | 12.89 ± 2.12 |
| Mytilidae | Mytilus galloprovincialis | 24 | 5.37 ± 1.80 | 3.0 ± 0.98 | 22.01± 16.94 | 3.41 ± 2.34 | 9.04 ± 6.34 | |
| N | Length (cm) | Width (cm) | Height (cm) | Tentacle length (cm) | ||||
| Cnidaria | Pelagiidae | Pelagia noctiluca | 31 | 10.32 ± 2.68 | 5.52 ± 1.11 | 2.1 ± 0.60 | 8.78 ± 1.44 |
| Type | Species | Habitat | Items/ind | % Occurence | Size (mm) | Shape % Items/Ind. | |||
|---|---|---|---|---|---|---|---|---|---|
| (mean ± SD) | mean ± SD | Fibres | Fragment | Film | Other | ||||
| Fish | Anthias anthias | benthic | 6.0 ± NA | 100.0 | 0.065 ± NA | 83.3 | 16.7 | 0.0 | 0.0 |
| Boops boops | pelagic | 14.5 ± 7.8 | 100.0 | 1.00 ± 1.99 | 55.2 | 34.5 | 10.3 | 0.0 | |
| Chelon auratus | pelagic | 5.0 ± NA | 100.0 | 0.30 ± 0.05 | 0.0 | 100.0 | 0.0 | 0.0 | |
| Coris julis | benthic | 1.0 ± 1.4 | 50.0 | 0.34 ± 0.16 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Diplodus annularis | benthopelagic | 3.3 ± 4.0 | 75.0 | 0.34 ± 0.16 | 61.5 | 38.5 | 0.0 | 0.0 | |
| Diplodus vulgaris | benthopelagic | 1.8 ± 2.2 | 66.7 | 1.34 ± 1.69 | 42.9 | 52.4 | 0.0 | 4.8 | |
| Epinephelus marginatus | demersal | 6.8 ± 3.0 | 100.0 | 1.75 ± 1.52 | 76.5 | 23.5 | 0.0 | 0.0 | |
| Labrus merula | demersal | 4.0 ± NA | 100.0 | 0.60 ± 0.16 | 25.0 | 75.0 | 0.0 | 0.0 | |
| Mullus surmuletus | demersal | 12 ± NA | 100.0 | 0.71 ± 0.20 | 25.0 | 75.0 | 0.0 | 0.0 | |
| Oblada melanura | benthopelagic | 2.4 ± 3.2 | 68.8 | 1.99 ± 1.92 | 74.4 | 25.6 | 0.0 | 0.0 | |
| Pagrus pagrus | benthopelagic | 4.6 ± 6.1 | 80.0 | 2.04 ± NA | 91.3 | 8.7 | 0.0 | 0.0 | |
| Scomber scombrus | pelagic | 2.5 ± 0.7 | 100.0 | 2.57 ± 0.89 | 0.0 | 0.0 | 100.0 | 0.0 | |
| Serranus cabrilla | demersal | 2 ± 2.2 | 66.7 | 0.31 ± 0.18 | 86.4 | 13.6 | 0.0 | 0.0 | |
| Serranus scriba | demersal | 3.2 ± 4.7 | 77.6 | 1.31 ± 1.28 | 89.4 | 9.3 | 0.8 | 0.4 | |
| Spondyliosoma cantharus | benthopelagic | 3.6 ± 5.2 | 57.1 | 5.56 ± 4.18 | 16.0 | 4.0 | 80.0 | 0.0 | |
| Thunnus alalunga | pelagic | 4.8 ± 3.0 | 100.0 | 1.59 ± 0.88 | 66.7 | 12.5 | 20.8 | 0.0 | |
| Trachurus trachurus | pelagic | 0 ± NA | 0.0 | - | 0.0 | 0.0 | 0.0 | 0.0 | |
| Echinoderms | Holothuria forskalii | benthic | 15.6 ± 10.4 | 100.0 | 1.70 ± 1.87 | 88.5 | 9.0 | 0.0 | 2.6 |
| Holothuria poli | benthic | 8.6 ± 8.6 | 63.6 | 2.10 ± 1.55 | 42.6 | 13.8 | 0.0 | 43.6 | |
| Holothuria tubulosa | benthic | 16 ± 7.8 | 100.0 | 1.63 ± 1.24 | 87.5 | 6.3 | 3.8 | 2.5 | |
| Arbacia lixula | benthic | 1.1 ± 2.4 | 33.3 | 3.80 ± 2.67 | 89.5 | 10.5 | 0.0 | 0.0 | |
| Paracentrotus lividus | benthic | 1.6 ± 3.3 | 29.6 | 1.87 ± 1.65 | 65.9 | 29.3 | 4.9 | 0.0 | |
| Molluscs | Arca noae | benthic | 5 ± 5.5 | 72.2 | 0.63± 0.57 | 79.8 | 2.2 | 18.0 | 0.0 |
| Mytilus galloprovincialis | benthic | 4.2 ± 3.3 | 95.8 | 1.10 ± 0.97 | 80.0 | 2.0 | 17.0 | 1.0 | |
| Cnidaria | Pelagia noctiluca | pelagic | 4.6 ± 3.9 | 35.5 | - | 39.1 | 56.5 | 0.0 | 4.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Compa, M.; Alomar, C.; López Cortès, M.F.; Rios-Fuster, B.; Morató, M.; Capó, X.; Fagiano, V.; Deudero, S. Multispecies Assessment of Anthropogenic Particle Ingestion in a Marine Protected Area. Biology 2022, 11, 1375. https://doi.org/10.3390/biology11101375
Compa M, Alomar C, López Cortès MF, Rios-Fuster B, Morató M, Capó X, Fagiano V, Deudero S. Multispecies Assessment of Anthropogenic Particle Ingestion in a Marine Protected Area. Biology. 2022; 11(10):1375. https://doi.org/10.3390/biology11101375
Chicago/Turabian StyleCompa, Montserrat, Carme Alomar, María Francesca López Cortès, Beatriz Rios-Fuster, Mercè Morató, Xavier Capó, Valentina Fagiano, and Salud Deudero. 2022. "Multispecies Assessment of Anthropogenic Particle Ingestion in a Marine Protected Area" Biology 11, no. 10: 1375. https://doi.org/10.3390/biology11101375
APA StyleCompa, M., Alomar, C., López Cortès, M. F., Rios-Fuster, B., Morató, M., Capó, X., Fagiano, V., & Deudero, S. (2022). Multispecies Assessment of Anthropogenic Particle Ingestion in a Marine Protected Area. Biology, 11(10), 1375. https://doi.org/10.3390/biology11101375








