Late Miocene Leaves and Endocarps of Choerospondias (Anacardiaceae) from Zhejiang, Eastern China: Implications for Paleogeography and Paleoclimate
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
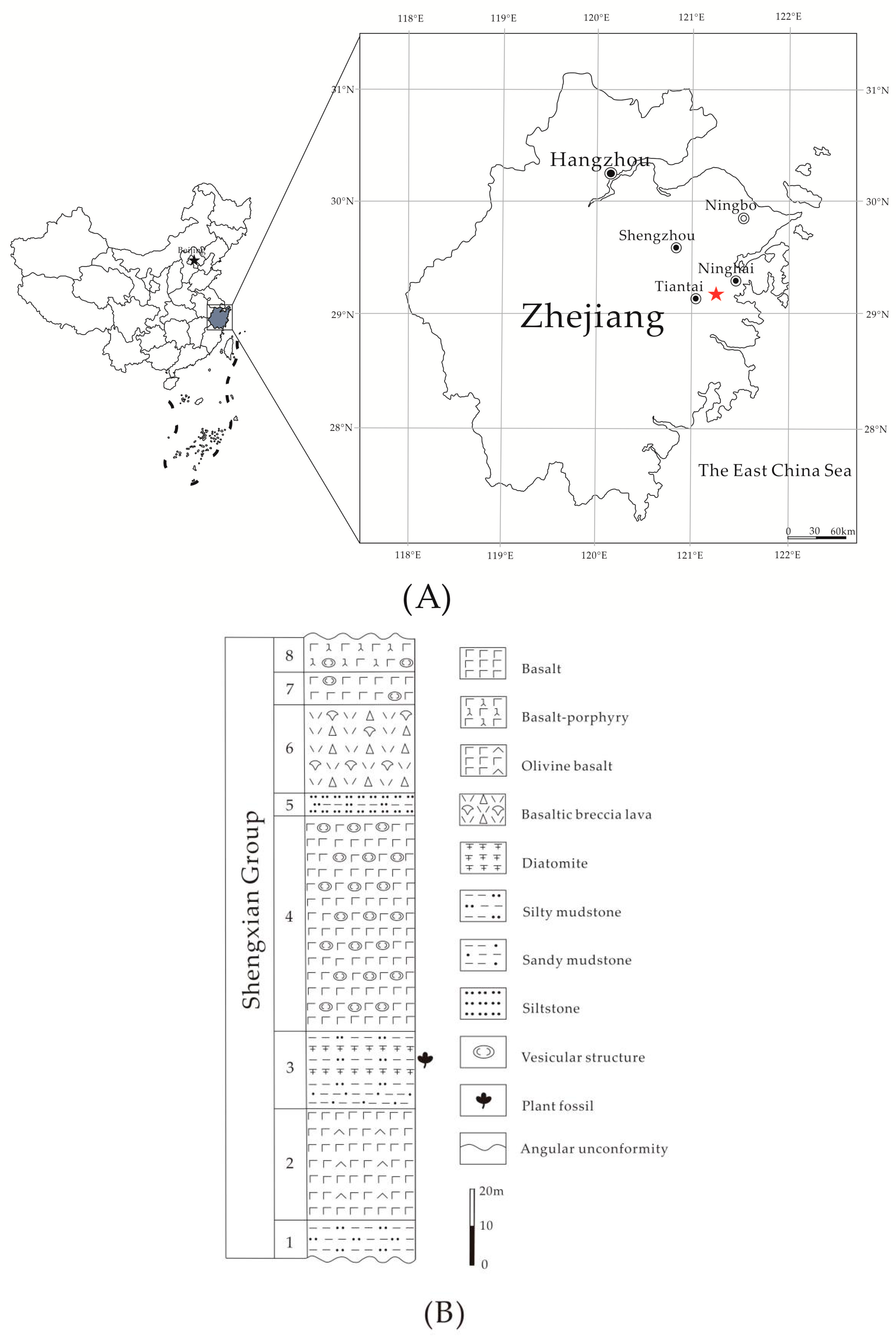
2.1. Geological Setting
2.2. Material Preparation
2.2.1. Fossil Preparation
2.2.2. Extant Leaf Cuticle Treatment
3. Systematic Paleobotany
4. Discussion
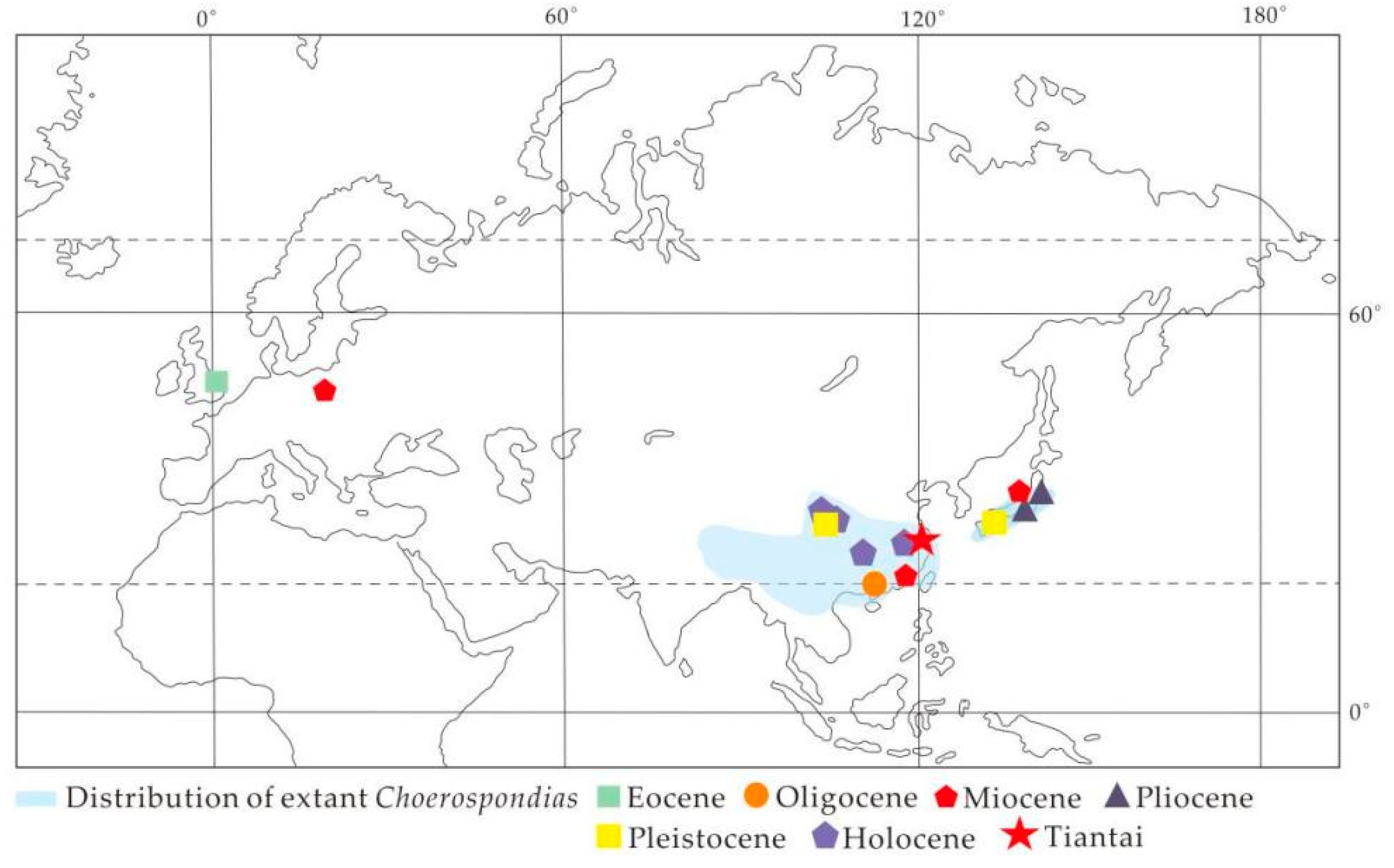
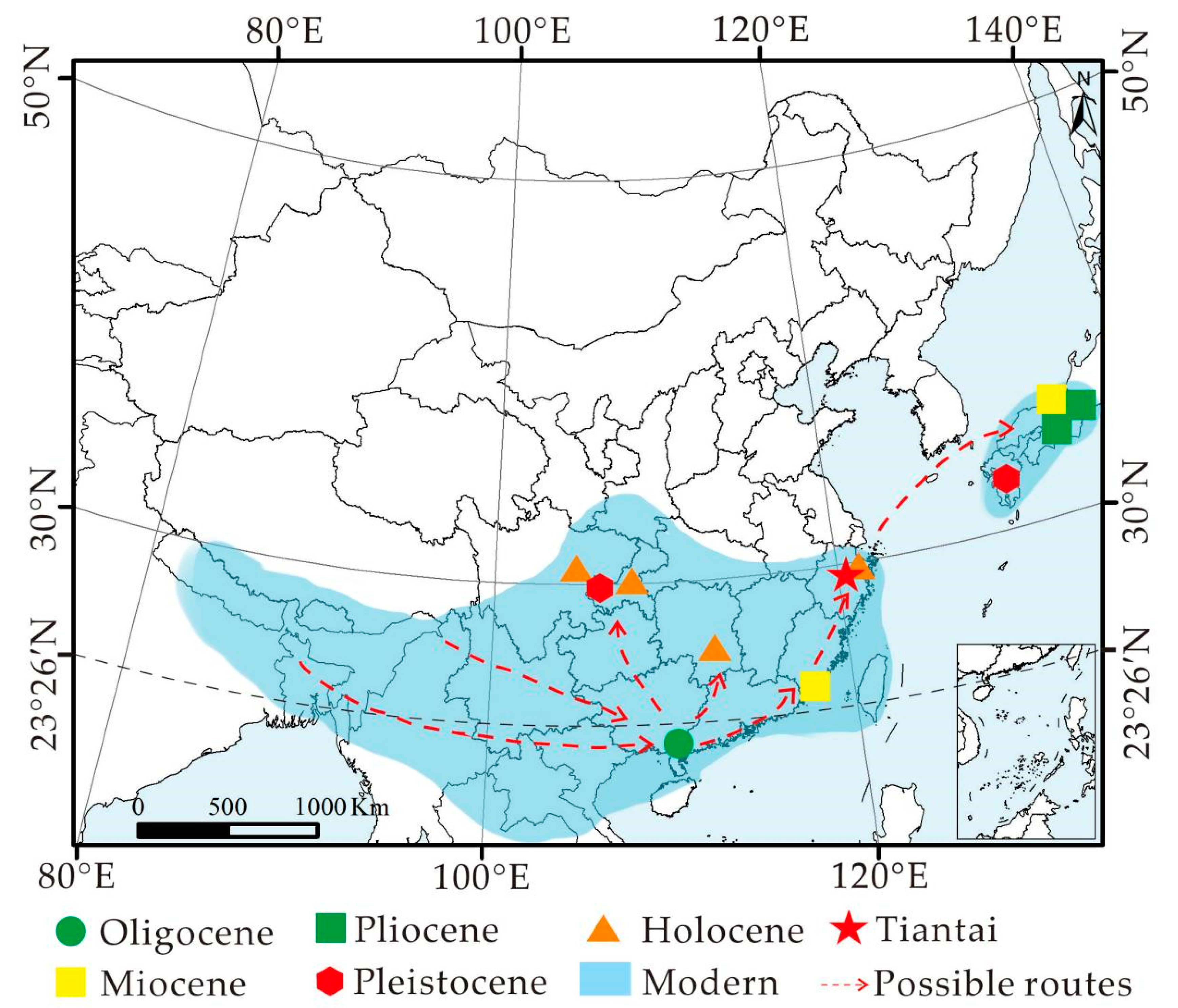
4.1. Paleogeographic History of Choerospondias
4.2. Paleoclimatic Implications of the Current Choerospondias Fossils
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Min, T. Geographical distribution of Anacardiaceae in China and its zonal characteristics. Acta Bot. Yunnanica 1980, 2, 390–401. (In Chinese) [Google Scholar]
- Zheng, M.; Min, T.L. Dicotyledoneae. In Flora Republicae Popularis Sinicae; Science Press: Beijing, China, 1980; Volume 45, pp. 203–281. (In Chinese) [Google Scholar]
- Reid, E.M.; Chandler, M.E.J. The London Clay Flora; British Museum (Natural History): London, UK, 1933; pp. 543–549. [Google Scholar]
- Chandler, M.E.J. The Lower Tertiary Floras of Southern England; British Museum (Natural History): London, UK, 1961. [Google Scholar]
- Manchester, S.R.; Chen, Z.D.; Lu, A.M.; Uemura, K. Eastern Asian endemic seed plant genera and their paleogeographic history throughout the Northern Hemisphere. J. Syst. Evol. 2009, 47, 1–42. [Google Scholar] [CrossRef]
- Kowalski, R. Choerospondias turovensis n. sp., a new Anacardiacean species of the European Neogene identified from the Turów brown coal open-cast mine. Palaeontogr. Abt. B 2010, 284, 1–11. [Google Scholar] [CrossRef]
- Miki, S. On the change of flora in eastern Asia since Tertiary period. The clay or lignite beds flora in Japan with special reference to the Pinus trifolia beds in Central Hondo. Japan. Jour. Bot. 1941, 11, 237–303. [Google Scholar]
- Endo, S. Recent and Fossil Nuts of Choerospondias (Axillaris Roxb), Anascardiaceae, from Japan. Proc. Jpn. Acad. B-Phys. 1948, 24, 4–6. [Google Scholar] [CrossRef][Green Version]
- Momohara, A.; Saito, T. Change of paleovegetation caused by topographic change in and around a sedimentary basin of the Upper Miocene Tokiguchi Porcelain Clay Formation, central Japan. Geosci. Rept. Shimane Univ. 2001, 20, 49–58. [Google Scholar]
- Momohara, A. Pliocene Carya nuts (Juglandaceae) from the Osaka Group, Southwest Japan. J. Phyto. Tax. 1989, 37, 107–112. [Google Scholar]
- Momohara, A.; Mizuno, K. Habitat of plants in the Late Pliocene sedimentary basin on Awaji Island, Central Japan. Jap. J. Hist. Bot. 1999, 6, 49–62. [Google Scholar]
- Fu, Q.; Li, L.; Jin, J.; Liu, X.; Quan, C. Mummified fruits of Choerospondias nanningensis sp. nov. (Anacardiaceae) from the upper Oligocene of a low latitude site in East Asia. J. Syst. Evol. 2017, 55, 477–483. [Google Scholar] [CrossRef]
- Wang, Z.; Herrera, F.; Shu, J.; Yin, S.; Shi, G. A new Choerospondias (Anacardiaceae) endocarp from the middle Miocene of Southeast China and its paleoecological implications. Rev. Palaeobot. Palyno. 2020, 283, 104312. [Google Scholar] [CrossRef]
- Nasu, H.; Gu, H.B.; Momohara, A.; Yasuda, Y. Land-use change for rice and foxtail millet cultivation in the Chengtoushan site, central China, reconstructed from weed seed assemblages. Archaeol. Anthr. Sci. 2012, 4, 1–14. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, Y.; Chen, C. Patterns of human niche construction of the Neolithic Kuahuqiao site. Southeast Cult. 2013, 6, 54–65. (In Chinese) [Google Scholar]
- Jiang, M.; Yan, X.; Zhou, Z.; Zhao, Z. Analysis of plant remains from Jinsha site. Chengdu Archeol. Discov. 2015, 13, 295–313. (In Chinese) [Google Scholar]
- Ma, X.; Bai, J.; Zou, H. Analysis of flotation results from the Yuxi site in Fengdu, Chongqing. Agricult. Archaeol. 2017, 6, 40–46. (In Chinese) [Google Scholar]
- Li, X.; Ma, F.; Xiao, L.; He, W.; Sun, B.; Quan, C.; Yao, Y.Z.; Ren, D.; Wang, X.; Wang, Q.; et al. New records of Podocarpium A. Braun ex Stizenberger (Fabaceae) from the Oligocene to Miocene of China: Reappraisal of the phylogeographical history of the genus. Rev. Palaeobot. Palyno. 2019, 260, 38–50. [Google Scholar] [CrossRef]
- Li, X.C.; Sun, B.N.; Xiao, L.; Ding, S.T.; He, W.L.; Du, B.X. Stratigraphic features of the Zhejiang Neoproterozoic Shengxian Formation and progress in its fossil study. ACTA Geol. Sin. 2014, 2, 145–153. [Google Scholar] [CrossRef]
- Ho, K.S.; Chen, J.C.; Lo, C.H.; Zhao, H.L. 40Ar–39Ar dating and geochemical characteristics of late Cenozoic basaltic rocks from the Zhejiang–Fujian region, SE China: Eruption ages, magma evolution and petrogenesis. Chem. Geol. 2003, 197, 287–318. [Google Scholar] [CrossRef]
- Wang, R.J.; Yang, S.R. Study of Cenozoic basalts and inclusions in Sheng-Xinchang, Zhejiang. Earth Sci. 1987, 12, 241–248. [Google Scholar]
- He, Y.L. Geologic Time and Ecological Environment of the Shengxian Formation Flora from Eastern Zhejiang and Volcanic Activity Influence. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2015. (In Chinese). [Google Scholar]
- RGZP (Regional Geology of Zhejiang Province). Tertiary. In Geological Memoirs. Ser. Vol 1; Bureau of Geology Mineral Resources of Zhejiang Province, Ed.; Geological Publishing House: Beijing, China, 1982; pp. 189–204. [Google Scholar]
- Li, H.M.; Guo, S.X. Middle Cenozoic subvolume. Angiosperms (Part). In Paleontological Atlas of Eastern China (III); Nanjing Institute of Geology and Minerals, Ministry of Geology and Minerals, Eds.; Beijing Geological Press: Beijing, China, 1982; pp. 294–316. [Google Scholar]
- Li, H.M. Neogene floras from eastern Zhejiang, China. In The Evolution of the East Asian Environment, Vol II. Palaeobotany, Palaeozoology and Palaeoanthropology; Whyte, R.O., Ed.; Centre of Asian Studies, University of Hong Kong: Hong Kong, China, 1984; pp. 461–466. [Google Scholar]
- Liu, Y.S.; Zetter, R.; Ferguson, D.K.; Mohr, B.A.R. Discriminating fossil evergreen and deciduous Quercus pollen: A case study from the Miocene of eastern China. Rev. Palaeobot. Palynol. 2007, 145, 289–303. [Google Scholar] [CrossRef]
- BGMRZP (Bureau of Geology and Mineral Resources of Zhejiang Province). Regional Geological Records of Zhejiang Province; Geological Publishing House: Beijing, China, 1989; pp. 1–688. [Google Scholar]
- RGZP (Regional Geology of Zhejiang Province). Lithostratigraphy of Zhejiang Province; China University of Geosciences Press: Wuhan, China, 1996; pp. 1–236. [Google Scholar]
- Liu, Y.S.; Guo, S.; Ferguson, D.K. Catalogue of Cenozoic megafossil plants in China. Palaeontogr. Abt. B 1996, 238, 141–179. [Google Scholar]
- Xiao, L.; An, Y.F.; Li, S.T.; Guo, J.F.; Sun, N.; Yao, X.Y.; Yang, W.T. Changes in carbon isotopes of extant and fossil maples—An indication for paleoenvironmental reconstruction. J. Xian Uni. Sci. Technol. 2017, 5, 688–696. (In Chinese) [Google Scholar] [CrossRef]
- Xiao, L.; Wang, X.; Li, X.M.; Li, X.C.; Jia, H.; Sun, N.; Liang, J.Q.; Wang, Q.; Li, J.X.; Yong, Y.Y. Numerical taxonomy of Miocene Trapa L. fossil fruits from eastern Zhejiang, China. Earth Sci. Front. 2020, 27, 110–123. (In Chinese) [Google Scholar] [CrossRef]
- Hass, H.; Rowe, N.P. Thin sections and wafering. In Fossil Plants and Spores: Modern Techniques; Jones, T.P., Rowe, N.P., Eds.; The Geological Society of London: London, UK, 1990; pp. 76–81. [Google Scholar]
- Liang, J.Q.; Leng, Q.; Höfig, D.F.; Niu, G.; Wang, L.; Royer, D.L.; Burke, K.; Xiao, L.; Zhang, Y.G.; Yang, H. Constraining conifer physiological parameters in leaf gas-exchange models for ancient CO2 reconstruction. Global Planet. Change 2022, 209, 103737. [Google Scholar] [CrossRef]
- Herrera, F.; Mitchell, J.D.; Pell, S.K.; Collinson, M.E.; Daly, D.C.; Manchester, S.R. Fruit morphology and anatomy of the Spondioid Anacardiaceae. Bot. Rev. 2018, 84, 315–393. [Google Scholar] [CrossRef]
- Li, M.T.; Sun, B.N.; Xiao, L.; Ren, W.S.; Li, S.T.; Dai, J. Discovery and paleoclimate reconstruction of the Middle Miocene Betula mioluminifera Hu et Chaney in eastern Zhejiang. Adv. Earth Sci. 2008, 23, 651–658. (In Chinese) [Google Scholar]
- Wang, X.L.; Xiong, C.H.; Sun, B.N.; Wu, J.Y.; Du, B.X. Summary and exploration of experimental techniques for the analysis of plant fossil horizons. ACTA Geol. Sin. 2020, 94, 2476–2486. [Google Scholar] [CrossRef]
- Collinson, M.E. Accumulations of fruits and seeds in three small sedimentary environments in southern England and their palaeoecological implications. Ann. Bot. London 1983, 52, 583–592. [Google Scholar] [CrossRef]
- Uemura, K.; Momohara, A. Plant Megafossils from the Nakatsu Group in the Northern Part of Kanagawa Prefecture; Research Report Kangawa Museum: Kanagawa, Japan, 1991; Volume 6, pp. 143–152. [Google Scholar]
- Tiffney, B.H.; Manchester, S.R. The use of geological and paleontological evidence in evaluating plant phylogeographic hypotheses in the Northern Hemisphere Tertiary. Int. J. Plant Sci. 2001, 162, S3–S17. [Google Scholar] [CrossRef]
- Vickulin, S.V. New London Clay-type Flora from the middle-Russian upland, European Russia. In Proceedings of the Fifth Quadrennial Conference of the International Organisation of Palaeobotany IOPC-V, California, Santa Barbara, CA, USA, 30 June–5 July 1996. [Google Scholar]
- Vickulin, S.V. Palaeogene leaf compressions of myrtaceous affinity from Pasekovo, Middle Russian Upland, southern European Russia. Bot. J. Linn. Soc. 1999, 131, 65–98. [Google Scholar] [CrossRef]
- Sun, J.; Liu, W.; Liu, Z.; Fu, B. Effects of the uplift of the Tibetan Plateau and retreat of Neotethys Ocean on the stepwise aridification of mid-latitude Asian interior. Bull. Chin. Acad. Sci. 2017, 32, 951–958. [Google Scholar] [CrossRef]
- Rögl, F. Palaeogeographic considerations for Mediterranean and Paratethys seaways (Oligocene to Miocene). Ann. Naturh. Mus. 1998, 99, 279–310. [Google Scholar]
- Legendre, S. Evolution of mammalian faunas in Europe during the Eocene and Oligocene. In Eocene-Oligocene Climatic and Biotic Evolution; Prothero, D.A., Berggren, W.A., Eds.; Princeton University Press: Princeton, NJ, USA, 1992; pp. 516–528. [Google Scholar]
- Sun, H.; Li, Z.M. Qinghai-Tibet Plateau uplift and its impact on Tethys flora. Acta Bot. Yunnanica 2003, 18, 852–862. (In Chinese) [Google Scholar]
- Sun, H. Evolution of Arctic-Tertiary Flora in Himalayan-Hengduan Mountains. Acta Bot. Yunnanica. 2002, 06, 671–688. (In Chinese) [Google Scholar]
- Dupont-Nivet, G.; Krijgsman, W.; Langereis, C.G.; Abels, H.A.; Dai, S.; Fang, X. Tibetan plateau aridification linked to global cooling at the Eocene–Oligocene transition. Nature 2007, 445, 635–638. [Google Scholar] [CrossRef]
- Fang, X.M.; Galy, A.; Yang, Y.B.; Zhang, W.L.; Ye, C.C.; Song, C.H. Paleogene global cooling–induced temperature feedback on chemical weathering, as recorded in the northern Tibetan Plateau. Geology 2019, 47, 992–996. [Google Scholar] [CrossRef]
- Thomson, J.R.; Holden, P.B.; Anand, P.; Edwards, N.R.; Porchier, C.A.; Harris, N.B. Tectonic and climatic drivers of Asian monsoon evolution. Nat. Commun. 2021, 12, 4022. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Wang, H.S. Natural Geography of China—Plant Geography; Science Press: Beijing, China, 1983. [Google Scholar]
- Sun, X.; Wang, P. How old is the Asian monsoon system?—Palaeobotanical records from China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 222, 181–222. [Google Scholar] [CrossRef]
- Zhilin, S.G. On the diversity of the flora emerged by the end of the Late Eocene in Kazakhstan. In Modern Problems of Palaeofloristics, Palaeophytogeography and Phytostratigraphy. Proceedings of the International Palaeobotanical Conference, Moscow, Russia, 17–18 May 2005. [Google Scholar]
- Bruch, A.A.; Zhilin, S.G. Early Miocene climate of Central Eurasia—Evidence from Aquitanian floras of Kazakhstan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 248, 32–48. [Google Scholar] [CrossRef]
- Arbuzova, O.N.; Zhilin, S.G. Lignitized endocarps and their impressions of Nyssa sibirica from the lower Miocene of Kazakhstan. Paleontol. J. 1995, 29, 130–136. [Google Scholar]
- Vorobieva, G.A.; Mats, V.D.; Shimaraeva, M.K. Late Cenozoic paleoclimates in the Baikal region. Russ. Geol. Geophys. 1995, 36, 80–93. [Google Scholar]
- Akhmetiev, M.A. Ecological crises of the Paleogene and Neogene in extratropical Eurasia and their putative causes. Paleontol. J. 1996, 30, 738–748. [Google Scholar]
- Kordikova, E.G. Insectivora (Mammalia) from the Lower Miocene of the Aktau Mountains, south-eastern Kazakhstan. Senckenb. Lethaea 2000, 80, 67–79. [Google Scholar] [CrossRef]
- Lepage, B.A. A new species of Tsuga (Pinaceae) from the middle Eocene of Axel Heiberg Island, Canada, and an assessment of the evolution and biogeographical history of the genus. Bot. J. Linn. Soc. 2003, 141, 257–296. [Google Scholar] [CrossRef]
- Tao, J.; Whyte, P.; Aigner, J.S.; Jablonski, N.G.; Taylor, G.; Walker, D.; Wang, P. The Paleogene flora and palaeoclimate of Liuqu Formation in Xizang. Ctr. Asian Stu. Occ. Monogra. 1988, 77, 520–522. [Google Scholar]
- Miller, K.G.; Kominz, M.A.; Browning, J.V.; Wright, J.D.; Mountain, G.S.; Katz, M.E.; Sugarman, P.J.; Cramer, B.S.; Christie-Blick, N.; Pekar, S.F. The Phanerozoic record of global sea-level change. Science 2005, 310, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Houben, A.J.P.; Van Mourik, C.A.; Montanari, A.; Coccioni, R.; Brinkhuis, H. The Eocene–Oligocene transition: Changes in sea level, temperature or both? Palaeogeogr. Palaeoclim. Palaeoecol. 2012, 335–336, 75–83. [Google Scholar] [CrossRef]
- Royden, L.H.; Burchfiel, B.C.; Van der Hilst, R.D. The geological evolution of the Tibetan Plateau. Science 2008, 321, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Van Hinsbergen, D.J.J.; Lippert, P.C.; Dupont-Nivet, G.; McQuarrie, N.; Doubrovine, P.V.; Spakman, W.; Torsvik, T.H. Greater India Basin hypothesis and a two-stage Cenozoic collision between India and Asia. Proc. Natl. Acad. Sci. USA 2012, 109, 7659–7664. [Google Scholar] [CrossRef]
- Hou, Z.; Li, S. Tethyan changes shaped aquatic diversification. Biol. Rev. 2018, 93, 874–896. [Google Scholar] [CrossRef]
- Unger, F. The fossil flora of Kumi on the island of Euboea. Mem. Imperial Acad. Sci. 1867, 27, 1–66. [Google Scholar]
- Ian Milne, R. Northern hemisphere plant disjunctions: A window on tertiary land bridges and climate change? Ann. Bot. London 2006, 98, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Bowerbank, J.S. A history of the Fossil Fruits and Seeds of the London Clay; John Van Voorst: London, UK, 1840. [Google Scholar]
- Chaney, R.W. Tertiary centers and migration routes. Ecol. Monogr. 1947, 17, 139–148. [Google Scholar] [CrossRef]
- Davis, A.G.; Elliott, G.F. The palaeogeography of the London Clay sea. Proc. Geol. Assoc. 1957, 68, 255–277. [Google Scholar] [CrossRef]
- Harley, M.M. A summary of fossil records for Arecaceae. Bot. J. Linn. Soc. 2006, 151, 39–67. [Google Scholar] [CrossRef]
- Milne, R.I.; Abbott, R.J. The origin and evolution of Tertiary relict floras. Adv. Bot. Res. 2002, 38, 281–314. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Mosbrugger, V.; Utescher, T.; Dilcher, D.L. Cenozoic continental climatic evolution of Central Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 14964–14969. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.H.S. The Tibetan Plateau in relation to the vegetation of China. Ann. Mo. Bot. Gard. 1983, 70, 564–570. [Google Scholar] [CrossRef]
- Jia, C.; He, D.; Lu, J. Episodes and geodynamic setting of Himalayan movement in China. Oil Gas Geol. 2004, 25, 121–125. [Google Scholar]
- Fang, X. Stages of uplift of the Tibetan Plateau. Sci. Tech. Rev. 2017, 35, 42–50. (In Chinese) [Google Scholar]
- Wang, C.; Zhao, X.; Liu, Z.; Lippert, P.C.; Graham, S.A.; Coe, R.S.; Yi, H.; Zhu, L.; Liu, S.; Li, Y. Constraints on the early uplift history of the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2008, 105, 4987–4992. [Google Scholar] [CrossRef]
- Wang, P. Neogene stratigraphy and paleoenvironments of China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1990, 77, 315–334. [Google Scholar] [CrossRef]
- Li, J.; Fang, X. Uplift of the Tibetan Plateau and environmental changes. Chin. Sci. Bull. 1999, 44, 2117–2124. [Google Scholar] [CrossRef]
- Gavin, D.G.; Fitzpatrick, M.C.; Gugger, P.F.; Heath, K.D.; Rodríguez-Sánchez, F.; Dobrowski, S.Z.; Hampe, A.; Hu, F.S.; Michael, B.; Ashcroft, P.J.; et al. Climate refugia: Joint inference from fossil records, species distribution models and phylogeography. New Phytol. 2014, 204, 37–54. [Google Scholar] [CrossRef]
- Pell, S.K. Molecular Systematics of the Cashew Family (Anacardiaceae). Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2004. [Google Scholar]
- Pell, S.K.; Mitchell, J.D.; Miller, A.J.; Lobova, T.A. Anacardiaceae. In The Families and Genera of Vascular Plants. Flowering Plants Eudicots Sapindales, Cucurbitales, Myrtaceae; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 10, pp. 7–50. [Google Scholar]
- Min, T.; Barfod, A. Anacardiaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China, 2008; Volume 11, pp. 335–345. [Google Scholar]
- Ye, X.M.; Chen, F.S.; Sun, R.X.; Wu, N.S.; Liu, B.; Song, Y.L. Prediction of Potential Suitable Distribution Areas for Choerospondias axillaris based on MaxEnt Model. J. Jiangxi Agr. Uni. 2019, 41, 440–446. (In Chinese) [Google Scholar]
- He, G.P.; Chen, Y.T.; Sun, Y.X.; Zhang, J.Z.; Sun, H.J.; Zhuo, R.Y. Study on variations of seedling traits in Choerospondias axillaris geographic provenances. For. Res. 2003, 2, 177–182. (In Chinese) [Google Scholar]
- Li, C.S.; Wang, Y.F.; Sun, Q.G. Climate Analysis of Endemic Species—A Novel Method for Quantitative Analysis of Global Climate Change Since Tertiary. Acta Bot. Sin. 2001, 2, 217–220. (In Chinese) [Google Scholar]
- Fang, J.; Wang, Z.; Tang, Z. Atlas of Woody Plants in China: Distribution and Climate; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 1. [Google Scholar]
- Yang, Y.; Wang, W.M.; Shu, J.W.; Chen, W. Miocene palynoflora from Shengxian Formation, Zhejiang Province, southeast China and its palaeovegetational and palaeoenvironmental implications. Rev. Palaeobot. Palyno. 2018, 259, 185–197. [Google Scholar] [CrossRef]
- Li, X.C. The Late Cenozoic Floras from Eastern Zhejiang Province and Their Paleoclimatic Reconstruction. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2010. (In Chinese). [Google Scholar]
- Ding, S.T. Reconstruction of Palevegetation and Paleoclimate of the Late Miocene Flora in Tiantai, Zhejiang, China. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2011. (In Chinese). [Google Scholar]
- Hua, Y.F. Multiple Reconstruction of Miocene Paleoclimate Based on Plant Fossils in Tiantai, Eastern Zhejiang. Master’s Thesis, Lanzhou University, Lanzhou, China, 2020. (In Chinese). [Google Scholar] [CrossRef]
- Herbert, T.D.; Lawrence, K.T.; Tzanova, A.; Peterson, L.C.; Caballero-Gill, R.; Kelly, C.S. Late Miocene global cooling and the rise of modern ecosystems. Nat. Geosci. 2016, 9, 843–847. [Google Scholar] [CrossRef]
- Grimm, G.W.; Denk, T. Reliability and resolution of the coexistence approach-A revalidation using modern-day data. Rev. Palaeobot. Palyn. 2012, 172, 33–47. [Google Scholar] [CrossRef]
- Grimm, G.W.; Potts, A. Fallacies and fantasies: The theoretical underpinnings of the coexistence approach for palaeoclimate reconstruction. Clim. Past 2016, 12, 611–622. [Google Scholar] [CrossRef]
- Grimm, G.W.; Bouchal, J.M.; Denk, T.; Potts, A. Fables and foibles: A critical analysis of thePalaeoflora database and the Coexistence Approach for palaeoclimate reconstruction. Rev. Palaeobot. Palyn. 2016, 233, 611–622. [Google Scholar] [CrossRef]
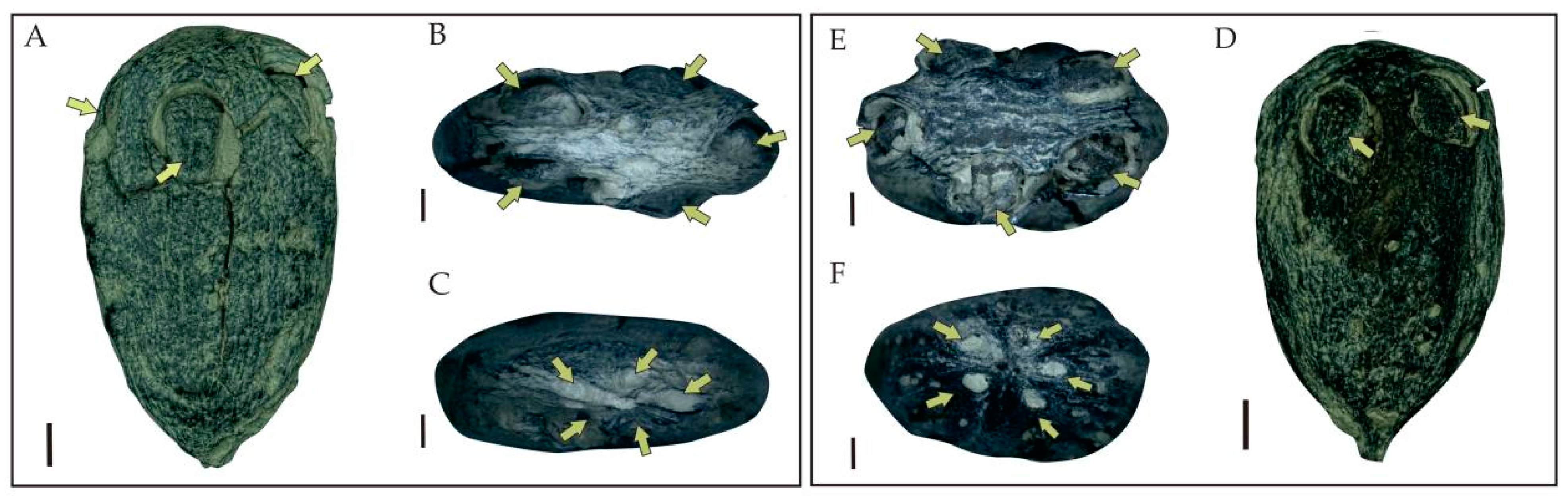
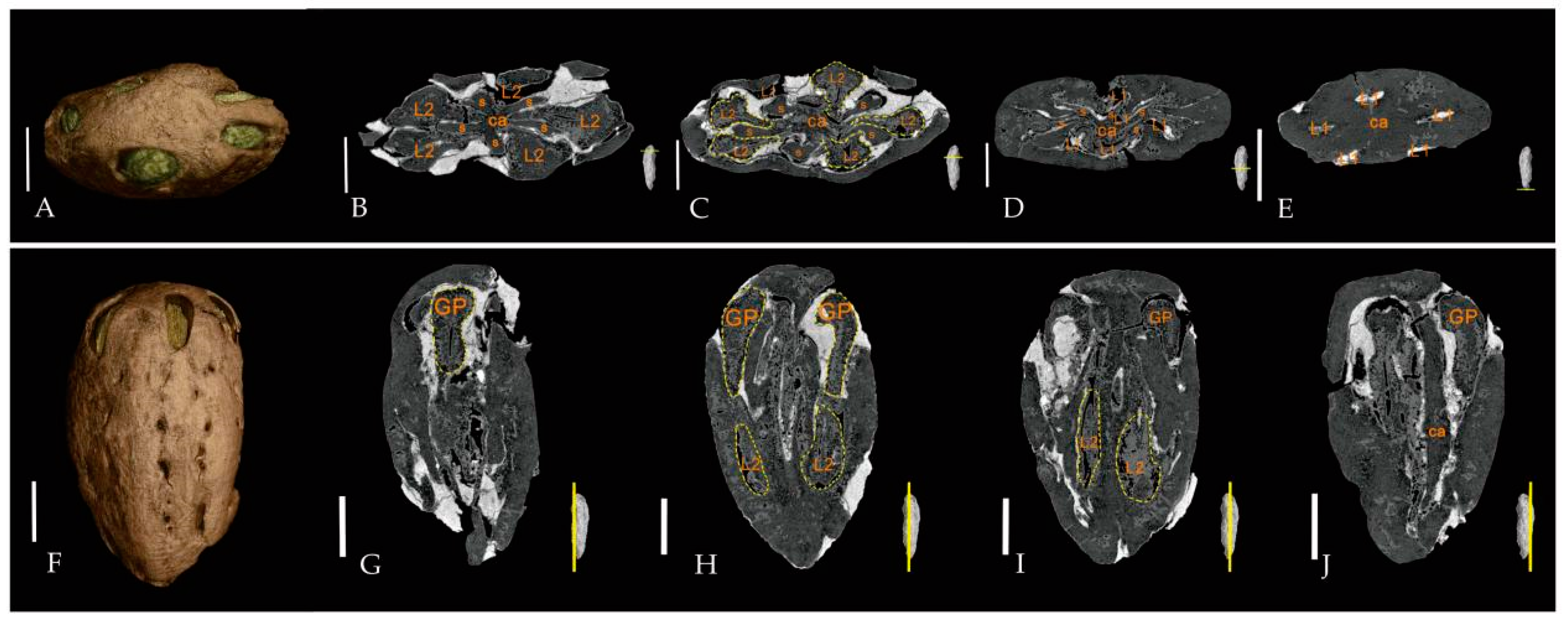
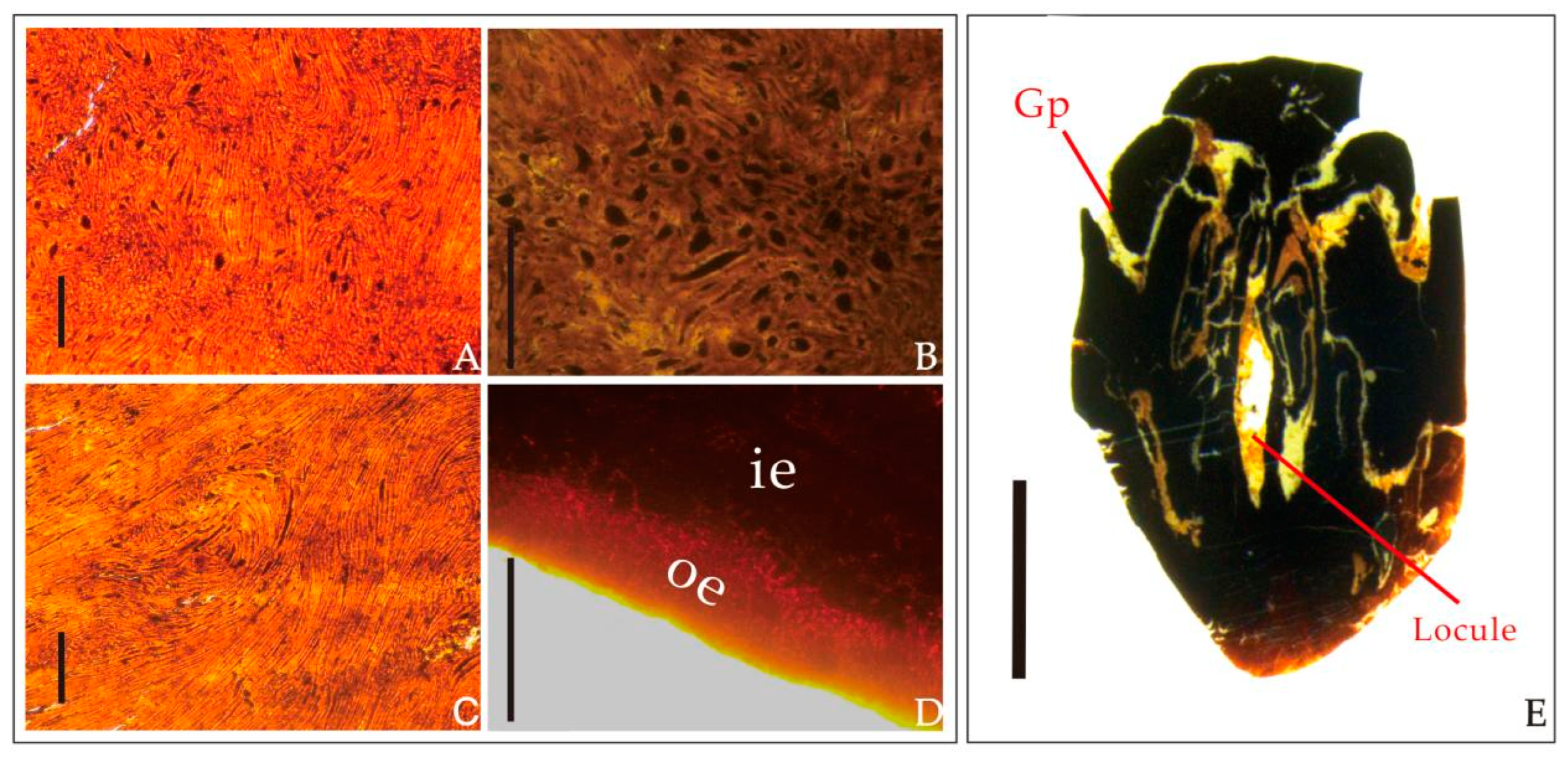
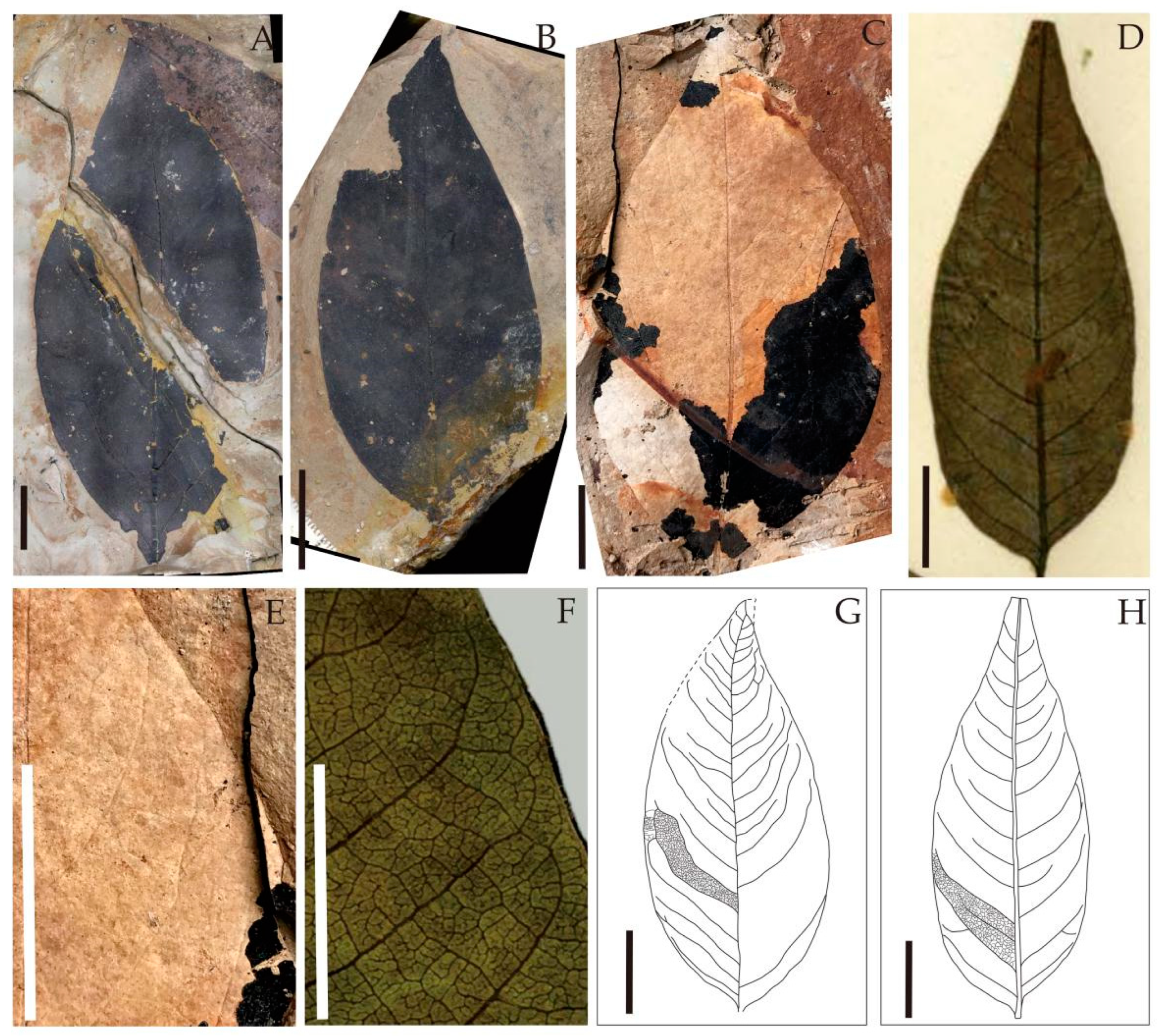
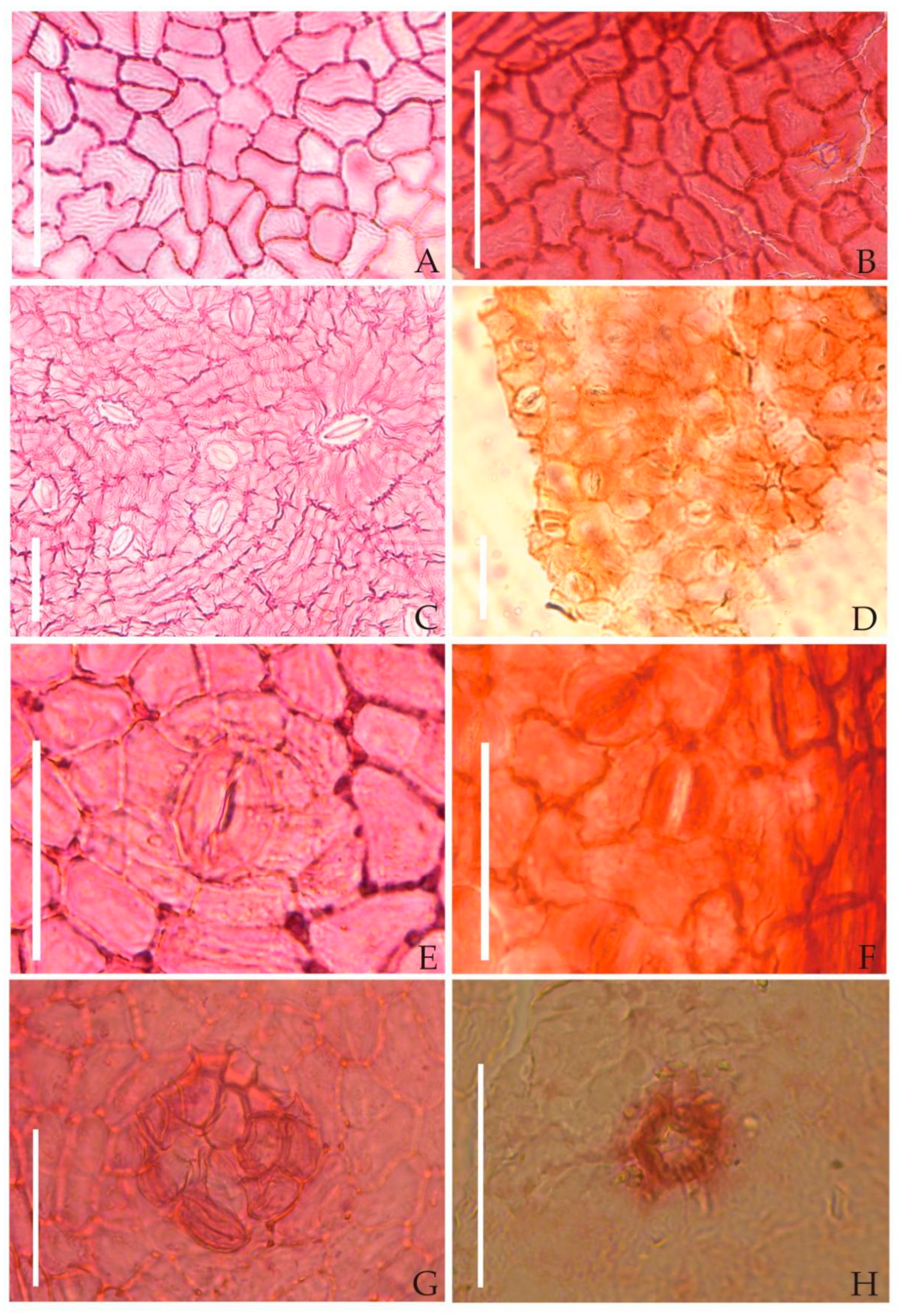
| Species | Endocarp Shape | Length (mm) | Width (mm) | Outer Surface of the Endocarp | Number of Locules Per Fruit | Locality | Age | Source |
|---|---|---|---|---|---|---|---|---|
| C. axillaris | Obovoid | 21–25 | 14–17 | Longitudinal ridges and small pits | Predominantly 5, occasionally 3, 4, or 6 | -- | Modern | [34] |
| C. sheppeyensis | Obovoid | 12–13 | 11–12.5 | Longitudinal rows of pits | 5 | Sheppey, England | Early Eocene | [3,4,37] |
| C. turovensis | Elongate obovoid | 9.5–12 | 5–9 | Longitudinal rows of pits | 5 or 6 | Turow, Poland | Middle Miocene | [6] |
| C. fujianensis | Obovoid to ovoid | 15.7–21.4 | 15.7–20.5 | Longitudinal grooves and scattered pits | 5 or 7 | Fujian, China | Middle Miocene | [13] |
| C. nanningensis | Obovoid | 15–21 | 13–17 | Obscure ridges and few pits | Predominantly 5, occasionally 3, 4, or 6 | Nanning, China | Late Oligocene | [12] |
| C. axillaris (Roxb.) | Obovoid | 21–25 | 14–18 | Pits arranged over the entire surface | 5 | Honshu, Japan; Kyushu, Japan | Late Miocene/Pleistocene | [8] |
| C. sp. cf. C. axillaris | Ovoid | 45 | 23 | no description | 5 | Honshu, Japan | Pliocene | [38] |
| C. tiantaiensis sp. nov. | Obovoid | 16.4–20 | 9.8–11.7 | Scattered, obvious pits; prominent bottom | 5 | Zhejiang, China | late Miocene | (This study) |
| Type | Fossil Choerospondias | Extant Choerospondias | |
|---|---|---|---|
| Leaf morphological characteristics | Leaf shape | Ovate to ovate-lanceolate, long acuminate apex, slightly convex base, broadly cuneate, asymmetrical at the basal insertion, petiole not preserved, leaf base angle about 100~119° | Ovate, ovate-lanceolate, long acuminate apex, broadly cuneate or subrounded base |
| Leaf size | 5–8.5 cm long, 2–3.6 cm wide | 4–12 cm long, 2–4.5 cm wide | |
| Leaf edge | Untoothed margin | Untoothed margin or serrate in young plants | |
| Venation of leaves | Lateral veins arranged pinnately, 10 pairs, opposite or alternate; leaf apices at an angle of about 53°; simple brochidodromous, lateral veins at a more uniform angle with midrib, about 40–60° | Lateral veins pinnately arranged, Simple brochidodromous, 8~12 lateral veins on each side, lateral veins with midrib angle about 50–60° | |
| Epidermal characteristics | Upper epidermis | Irregularly polygonal epidermal cells, varying from quadrilateral to hexagonal; shallowly undulated anticlinal wall | Polygonal epidermal cells; anticlinal walls slightly curved, with a few stomatal apparatuses |
| Lower epidermis | Irregularly polygonal epidermal cells with shallowly undulated anticlinal walls, a trichome base, and multicellular roots | Polygonal epidermal cells with trichome bases, multicellular trichome roots, and radially arranged cells at the base of the trichome | |
| Stomatal apparatus | Cyclocytic stomatal apparatus, about 15–35 μm long and 10–30 μm wide; stomatal apparatus stellate distribution | The transition between anomocytic and cyclocytic, about 35–65 μm long and 24–50 μm wide | |
| Approach | MAT/°C | MAP/mm | Source |
|---|---|---|---|
| Coexistence approach | 16.3–20 | 1160.9–1653.5 | [89] |
| Palynoflora coexistence approach | 17.0–18.5 | 979–1722 | [88] |
| Overlapping distribution analysis | 14.5–18.0 | 825.9–1470.2 | [90] |
| CLAMP method | 15.89 | - | [90] |
| Cell aspect ratio method | 23 | - | [91] |
| Alkenone unsaturation method | 21–28 | - | [92] |
| Climatic factors for extant Choerospondias (CAES) | 5.7–24.7 (12.3–25.5 *) | 669–2435 (950–2700 *) | [87] |
| Nowaday climate of Tiantai | 16.7 | 1391.5 | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Wu, Z.; Guo, L.; Li, X.; Ji, D.; Xia, X.; Wang, J.; Liang, J.; Sun, N. Late Miocene Leaves and Endocarps of Choerospondias (Anacardiaceae) from Zhejiang, Eastern China: Implications for Paleogeography and Paleoclimate. Biology 2022, 11, 1399. https://doi.org/10.3390/biology11101399
Xiao L, Wu Z, Guo L, Li X, Ji D, Xia X, Wang J, Liang J, Sun N. Late Miocene Leaves and Endocarps of Choerospondias (Anacardiaceae) from Zhejiang, Eastern China: Implications for Paleogeography and Paleoclimate. Biology. 2022; 11(10):1399. https://doi.org/10.3390/biology11101399
Chicago/Turabian StyleXiao, Liang, Zeling Wu, Liyan Guo, Xiangchuan Li, Deshuang Ji, Xiaoyuan Xia, Jianan Wang, Jiaqi Liang, and Nan Sun. 2022. "Late Miocene Leaves and Endocarps of Choerospondias (Anacardiaceae) from Zhejiang, Eastern China: Implications for Paleogeography and Paleoclimate" Biology 11, no. 10: 1399. https://doi.org/10.3390/biology11101399
APA StyleXiao, L., Wu, Z., Guo, L., Li, X., Ji, D., Xia, X., Wang, J., Liang, J., & Sun, N. (2022). Late Miocene Leaves and Endocarps of Choerospondias (Anacardiaceae) from Zhejiang, Eastern China: Implications for Paleogeography and Paleoclimate. Biology, 11(10), 1399. https://doi.org/10.3390/biology11101399






