Simple Summary
Demographic, genetic factors, and maternal lifestyle could modify and alter the microbial diversity of human milk and infants’ gut. We screened human breast milk and infant stool samples from Egyptian sources for possible novel probiotic strains. Forty-one isolates were submitted to the gene bank database, classified, and identified through physiological and biochemical tests. All samples revealed antibiotic resistance, antibacterial activity, and high probiotic features. Six of the isolates revealed less than 95% Average Nucleotide Identity with deposited sequences in the database. Isolate Lactobacillus delbrueckii ASO 100 exhibited the lowest identity ratio with promising probiotic and antibacterial features, enlightening the high probability of being a new probiotic species.
Abstract
Human milk comprises a diverse array of microbial communities with health-promoting effects, including colonization and development of the infant’s gut. In this study, we characterized the bacterial communities in the Egyptian mother–infant pairs during the first year of life under normal breastfeeding conditions. Out of one hundred isolates, forty-one were chosen for their potential probiotic properties. The selected isolates were profiled in terms of morphological and biochemical properties. The taxonomic evidence of these isolates was investigated based on 16S rRNA gene sequence and phylogenetic trees between the isolates’ sequence and the nearest sequences in the database. The taxonomic and biochemical evidence displayed that the isolates were encompassed in three genera: Lactobacillus, Enterococcus, and Lactococcus. The Lactobacillus was the most common genus in human milk and feces samples with a high incidence of its different species (Lacticaseibacillus paracasei, Lactobacillus delbrueckii, Lactiplantibacillus plantarum, Lactobacillus gasseri, and Lacticaseibacillus casei). Interestingly, BlastN and Jalview alignment results evidenced a low identity ratio of six isolates (less than 95%) with database sequences. This divergence was supported by the unique physiological, biochemical, and probiotic features of these isolates. The isolate L. delbrueckii, ASO 100 exhibited the lowest identity ratio with brilliant probiotic and antibacterial features suggesting the high probability of being a new species. Nine isolates were chosen and subjected to probiotic tests and ultrastructural analysis; these isolates exhibited antibiotic resistance and antibacterial activity with high probiotic characteristics, and high potentiality to be used as prophylactic and therapeutic agents in controlling intestinal pathogens.
1. Introduction
The American Academy of Pediatrics evidenced the prophylactic and therapeutic roles of human milk in educating infants’ immune systems and providing protection against many infectious diseases such as gastrointestinal, respiratory, inflammatory bowel, and allergic diseases [1,2]. These protective effects of breast milk are due to the orchestrated action of several bioactive molecules, such as oligosaccharides, fatty acids, immunoglobulins, cytokines, immune cells, lactoferrin, immunomodulating factors, and healthy microbial communities [3].
Breast milk is the second integral source of infant microbes after the birth canal in vaginally born infants [4]. It has been predicted that an infant takes approximately 105–107 commensal bacteria every day via consuming 800 mL of breast milk. Human breast milk contributes a distinctive role in the initiation, development, and composition of the neonatal gut microbiota. The human milk microbiome has a diverse array of bacterial species, including beneficial, commensal, and potentially probiotic bacteria [5].
Probiotics are live bacteria that deliver health benefits to the host when consumed at adequate levels as described by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO) [6]. Probiotic bacteria have many beneficial characteristics such as the ability to colonize and dominate in the neonatal gut, ability to resist stomach acid and bile salts, adherence to the intestinal mucosa, initiation of anti-inflammatory responses, inhibition of pathogens by the production of antimicrobial constituents, and augmentation of the immune system [7,8,9].
A new era of therapeutics is in perspective in which probiotics and their purified molecules will be employed as a wise, safe alternative to medication and other treatments to control health imbalance and diseases in humans and animals [10]. In this manner, several studies have reported the role of probiotics in the prevention and treatment of inflammatory bowel disease [11,12] food hypersensitivity [13], cardiometabolic disorders [14], and antitumor activity [15].
Previous studies have evidenced the richness of human milk and feces samples with promising novel probiotics. The investigation by Lee et al. [16] reported the presence of novel Lactobacillus gasseri EJL and Bifidobacterium breve JTL strains in the milk and feces samples of Korean Mother-infant pairs. A recent interesting study by Li et al. [17] identified novel LAB bacterial strains belonging to Lactobacillus gasseri, Lactiplantibacillus plantarum, and Lacticaseibacillus rhamnosus from Chinese infants with potential probiotic characteristics against inflammation and oxidative stress-related human diseases.
The microbial diversity of human milk and consequently, infants’ gut is controlled by environmental, demographic and genetic factors, and the maternal lifestyle. Bioprospecting the gut microbiota, and selecting promising probiotic candidates, is of great importance to the new insights of personalized medicine. In the current investigation into future perspectives in treating chronic diseases, we prospect for such healthy probiotics from Egyptian populations characterized by unique immune systems. Targeted bacterial isolates were profiled in terms of morphological, biochemical, and ultrastructural properties. Probiotic tests, antibiotic susceptibility, and antibacterial activity were also considered. The taxonomic evidence of these isolates was demonstrated based on 16S rRNA gene sequence and phylogenetic tree analysis.
2. Materials and Methods
2.1. Isolation, Phenotypic and Biochemical Features of Lactic Acid Bacteria
We recruited healthy mothers and their infants as volunteers from the community of the Faculty of Agriculture, Benha University, Moshtohor, Qalyubia, Egypt. Breast milk and stool samples were collected from 18 mother-baby pairs. Fresh feces from healthy infants between one and twelve months of age were collected during home study visits. Breast milk samples were collected from mothers (25–35 years) in sterile tubes using a manual expression with sterile gloves after cleaning the nipples and areola by wiping with a swab soaked in sterile water.
Ten grams from each fecal sample were diluted in 90 mL of sterile peptone water (0.1 g/L, Merck, Darmstadt, Germany). One ml of fresh breast milk was diluted in sterile peptone water. A series of dilutions of the fecal and milk samples were performed in peptone water, and bacteria in those samples were cultured by deploying the pour plate method on either MRS (pH 6.4) or MRS-cysteine agar (pH 5.5, 0.05%), and M17 agar media to selectively isolate the presumptive lactic acid bacteria (LAB). Plates were incubated at 37 °C for 72 h under anaerobic conditions (in an anaerobe jar using Oxoid AnaeroGen Compact). Single pure colonies were picked up and purified through three successive subcultures on the MRS medium. All the purified isolates were preserved in MRS broth containing 20% (v/v) glycerol as frozen stocks at −40 °C.
Isolated pure cultures were identified as LAB by cell morphology, Gram staining, catalase, and oxidase reaction [18,19,20]. In addition, LAB isolates were tested for gas production from glucose in MRS broth with an inverted Durham tube [21]. The Carbohydrate fermentative profile of the LAB was investigated against a cohort of 17 different sugars [21]. Further biochemical tests were performed to confirm the presumptive LAB isolates. Isolates that showed Gram-positive, catalase, and oxidase negative were selected as presumptive LAB and were further confirmed using the 16S rDNA genome typing.
2.2. Molecular Identification of LAB Isolates by 16S rRNA Sequencing
2.2.1. Genomic DNA Extraction
Genomic DNA was extracted from fresh lactic acid bacterial isolates using QIAamp DNA Micro Kit, Cat. No./ID: 56304, following the manufacturer’s instructions. The concentration and purity of purified DNA were assessed on a BioTek Epoch 2 spectrophotometer (Thermo Scientific, Waltham, MA, USA). DNA integrity was checked on 1% agarose gel electrophoresis followed by visualization using a gel Doc™ EZ imaging system with image lab™ software (Bio-Rad, Hercules, CA, USA).
2.2.2. Amplification of 16S rDNA of Isolates
Universal 16S rRNA primers 27F: 5′-AGAGTTTGGATCMTGGCTCAG-3′ and 1492R: 5′-CGGTTACCTTGTTACGACTT-3′ were utilized for DNA amplification [22,23]. PCR reaction volume of 50 µL contained 0.4 μM of each primer with a concentration of 10 pM, 400 μM of dNTP mix, 5 µL PCR reaction buffer (10×), 2 μM MgCl2, 2.5 units of TAKARA Taq DNA polymerase, 1 μL of template DNA, and the final volume was adjusted with sterilized double water. The PCR program was performed by applying a thermal cycle PCR machine (SensoQuest, Göttingen, Germany) as follows: initial denaturation at 95 °C for 3 min; then 35 cycles of denaturation at 95 °C for 50 s, annealing at 55 °C for 1 min, and extension at 72 °C for 1 min; followed by a final extension at 72 °C for 10 min. Amplified PCR fragments were subjected to 2% agarose gel electrophoresis and stained with ethidium bromide using a GeneRuler™ 1 kb DNA ladder, followed by visualization using a gel Doc™ EZ Imaging System with Image Lab™ Software (Bio-Rad, Hercules, CA, USA).
2.2.3. Sequencing and Phylogenetic Analysis
PCR amplicons were purified according to the instructions of QIAquick PCR Purification Kit. Purified amplicons were sequenced in Macrogen Company in South Korea. To determine closely related bacteria, the 16S rRNA sequences were aligned and compared with known sequences in the NCBI nucleotide database using the BLASTn algorithm. Jalview software [24] (http://www.jalview.org/ accessed on 12 September 2021) was utilized to detect Single-nucleotide polymorphisms (SNPs) and pairwise sequence alignment between each acquired sequence (isolate) and the nearest deposited sequences in the NCBI database. Phylogenetic tree construction was performed to evidence the evolutionary relationship among the isolates and the closest ones in the database using the Maximum Likelihood method based on the Tamura-Nei model with MEGA X software [25].
2.3. Scanning Electron Microscopy
Based on the previous morphological, biochemical, and physiological characterization of the isolates and molecular evidence of the 16S rRNA sequences, nine unconventional representative isolates from different species were chosen and subjected to ultrastructural analysis and probiotic tests.
2.3.1. Growth Conditions
All the strains were in the form of pure frozen cultures and were sub-cultured three times in growth media containing 10% skimmed milk powder (w/v; Merck, Darmstadt, Germany) supplemented with 0.2% yeast extract (w/v; Difco, Beirut, Lebanon). The growth temperature was 37 °C for all strains except those of the cocci which were incubated at 30 °C, the inoculum amount was 3% for the bacilli strains and 2% for all the others. The incubation time was 6 h for the cocci strains and 8 h for the bacilli strains.
2.3.2. Isolate Preparation for SEM
After the third subculture, 0.5 mL were taken from the coagulated media and washed with 0.5 mL of phosphate buffer (Merck, 0.1 mol L−1; pH 7.2). After three washes, each was followed by centrifugation at 2000 rpm for 10 min; the cells were resuspended in 1 mL of the buffer [26]. A small number of cells (approximately 200 µL) were attached to poly-L-lysine coated cover glass and fixed in 2% (v/v; Merck) Glutaraldehyde. Cells were rinsed with 0.1 M sodium cacodylate (Merck; pH 7.4) buffer, post-fixed in 1% OsO4 (Merck), and exposed to thio-carbohydrazide (Merck) as described by [27]. Each sample was fixed on an iron stub and then made electrically conductive by coating it (in a vacuum chamber) with a thin layer of gold for 40 s. The moisture of freeze-dried samples was completely removed by placing the freeze-dried sample in an air-tight desiccator containing silica gel. The weight of samples was periodically measured until constant weight to confirm the complete removal of moisture. At least four images of typical structures at 1500 magnification were recorded using a scanning electron microscope (FEI Company, Eindhoven, The Netherlands) model quanta 250 FEG (field emission gun) attached with EDX unit (energy dispersive x-ray analyses), The images were taken at an excitation voltage of 20 K.V., at different magnifications varying from 400 to 6000 and working distance varying from 13.7–14.2 mm. Only 5000 magnification was shown for the present study.
2.4. Probiotic Characteristics of Isolates
2.4.1. Acidity Resistance
LAB isolates (1 mL of each isolate) were inoculated individually into MRS and M17 broth (10 mL). MRS broth was adjusted to pH 3 and M17 broth was adjusted to pH 6.4 and incubated at 37 °C for 1, 2, and 3 h. Viable counts of the acid-tolerant bacteria were enumerated after incubation aerobically or anaerobically at 37 °C for 48 h [28].
2.4.2. Bile Salt Tolerance
Ox-gall salt media was applied to study the bile tolerance of the LAB isolates [28]. One ml of Activated isolates was inoculated into 10 ml MRS and M17 broth media containing 0.5% of the ox-bile salt. The control comprised MRS and M17 broth without bile salt. The viable bacteria were enumerated after incubation aerobically or anaerobically at 37 °C for 48 h.
2.4.3. Bile Salt Hydrolase Activity Assay
The isolates were tested for bile salt hydrolase on MRS and M17 agar fortified with 0.5% sodium salts of tauro-deoxycholic acid (TDCA) [29]. Activated isolates were inoculated and plated onto MRS and M17 agar containing TDCA. The plates were incubated anaerobically or aerobically at 37 °C for 48 h. Bile salt hydrolase activity was indicated by deoxycholic acid precipitate around the colonies.
2.4.4. Antagonistic Activity
The antagonistic activity of the LAB isolates against three pathogenic bacteria was carried out by the agar diffusion test. The targeted pathogens were activated in tryptic soy broth (TSB). one hundred microliter of the test bacteria were spread onto Muller-Hinton agar plates. Plates were air dried for 15 minutes and discs were impregnated with 30 µL of cell-free filtered supernatants (obtained by centrifugation of the LAB cultures at 5000 rpm for 5 min). The plates were incubated at 37 °C for 24 h, and the diameter of inhibition zones (mm) was measured around the discs [30]. The experiment was performed in triplicate for each LAB isolate. The antagonistic activity was tested against Bacillus subtilis, Staphylococcus aureus, and Escherichia coli.
2.4.5. Antibiotic Susceptibility Testing
Antibiotic resistance of the isolates was tested against five selected antibiotics (Oxoid, UK); Tetracycline (30 μg), Neomycin (30 μg), Vancomycin (30 μg), Kanamycin (30 μg), and Streptomycin (10 μg). Isolates were grown in MRS and M17 broth at 37 °C for 24 h. Overnight isolates were inoculated into MRS and M17 broth and freshly diluted 1: 10 in MRS and M17 broth; 0.1 mL of each diluted isolate was inoculated into MRS and M17 agar kept at 45 °C and poured into Petri plates to solidify. After solidification of the inoculated agar plates, antibiotic discs were placed on the surface of the plates. After 24 h incubation at 37 °C, the diameters of inhibition zones around the discs were measured (mm) according to [31]. Data (average of two determinations) were expressed in terms of resistance (R); moderate susceptibility (MS) and susceptibility (S); according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [32].
2.4.6. Statistical Analysis
All values were expressed as a mean of six replicates ± SE. Differences between isolates were estimated by one-way analysis of variance using SAS software [33]. Differences were significant at p ≤ 0.05. A Duncan multiple ranges test [34] was utilized to evaluate the significant differences among means.
3. Results
3.1. Isolation and Identification of Lactic Acid Bacteria
Out of 100 bacterial isolates, only 41 isolates were chosen as gram-positive, catalase-negative, and non-endospore forming lactic acid bacterial isolates as shown in Table 1. All the cocci isolates were positive for facultative anaerobic or microaerophilic tests. Under microscopic investigation, only 14 isolates were identified as cocci-shaped bacteria and 39 were characterised as rod-shaped bacteria.

Table 1.
Morphological properties of lactic acid bacterial isolates.
3.2. Physiological and Biochemical Characteristics
Physiological and biochemical characteristics for Cocci isolates are presented in Table 2. All Cocci isolates were resistant to growth at 4.0% bile salt and pH 6.8. On another hand, they revealed a negative profile for growth at pH 9.6 and gas production ability. For Growth at different temperatures, they have grown successfully at 40 °C, 10 °C, and negatively at 45 °C. All isolates showed positive results for growth at different NaCl levels (4 and 6.5%), except ASO26 and ASO290 isolates, which were negative for growth at 6.5% NaCl. Positive abilities to hydrolyse arginine, coagulate milk and produce acid from glucose were detected in all studied isolates. Sugar fermentation profiles and the ability to produce acid from lactose, raffinose, salicin, fructose, glucose, and mannose were positive for all strains. However, these isolates displayed a negative ability to produce acid from mannitol, ribose, trehalose, sorbitol, and xylose.

Table 2.
Physiological and biochemical Characteristics of Cocci lactic acid bacteria.
As shown in Table 3, the 25-rod lactic acid bacterial isolates revealed a positive ability to grow at 4.0% bile salt, pH 6.8, and pH 9.6 with a negative ability for gas production. For Growth at different temperatures, they have grown successfully at 45 °C and negatively at 15 °C. All isolates showed positive results for growth at 6.5% NaCl levels. Positive abilities to hydrolyse arginine and coagulate milk were detected in all studied isolates. Sugar fermentation profiles and the ability to produce acid from lactose, galactose, glucose, fructose, maltose, sucrose, mannose, rhamnose, arabinose, and melibiose were positive for all strains. However, these isolates displayed a negative ability to produce acid from ribose, mannitol, ribose, salicin, and sorbitol. Interestingly, all isolates displayed positive profiles for acid production from xylose, raffinose, and trehalose except ASO57, ASO55, ASO5, and ASO100 which showed a negative profile for xylose, raffinose, and trehalose.

Table 3.
Physiological and biochemical characteristics of rod lactic acid bacteria.
3.3. Molecular Identification
The amplified PCR products of the 16S rRNA gene of the selected 41 isolates were sequenced and deposited in the NCBI database. The accession numbers of the isolates and their identity ratio with the closest deposited sequences in the database are shown in Table 4. The Pairwise sequence alignment results revealed the presence of three genera, Lactobacillus, Enterococcus and Lactococcus. A high incidence of Lactobacillus species (L. paracasei, L. delbrueckii, L. plantarum, L. gasseri and L. casei) was found in human milk and feces. Fifteen different isolates belonged to L. paracasei and four isolates belonged to L. delbrueckii. However, only two isolates were detected for each species of L. plantarum and L. gasseri and one isolate belonged to L. casei. In addition, the genus Enterococcus revealed a high incidence with twelve isolates scattered in the species E. faecium, E. faecalis, and E. lactis.

Table 4.
The obtained Accession numbers and Identity ratio with the nearest accession in the database.
Interestingly, BlastN and Jalview alignment results of the six isolates evidenced a low identity ratio ranging from 81.39% to 94.63% for the accession numbers of MZ930471, OK030542, OK033573, OK021544, OK032511, and OK032119. Moreover, 11 isolates showed identity from 95% to 97%, 14 isolates displayed identity from 97% to 98% and only 10 isolates were from 98.14% to 98.95% identity ratio in comparison with the closest similar sequences in the database.
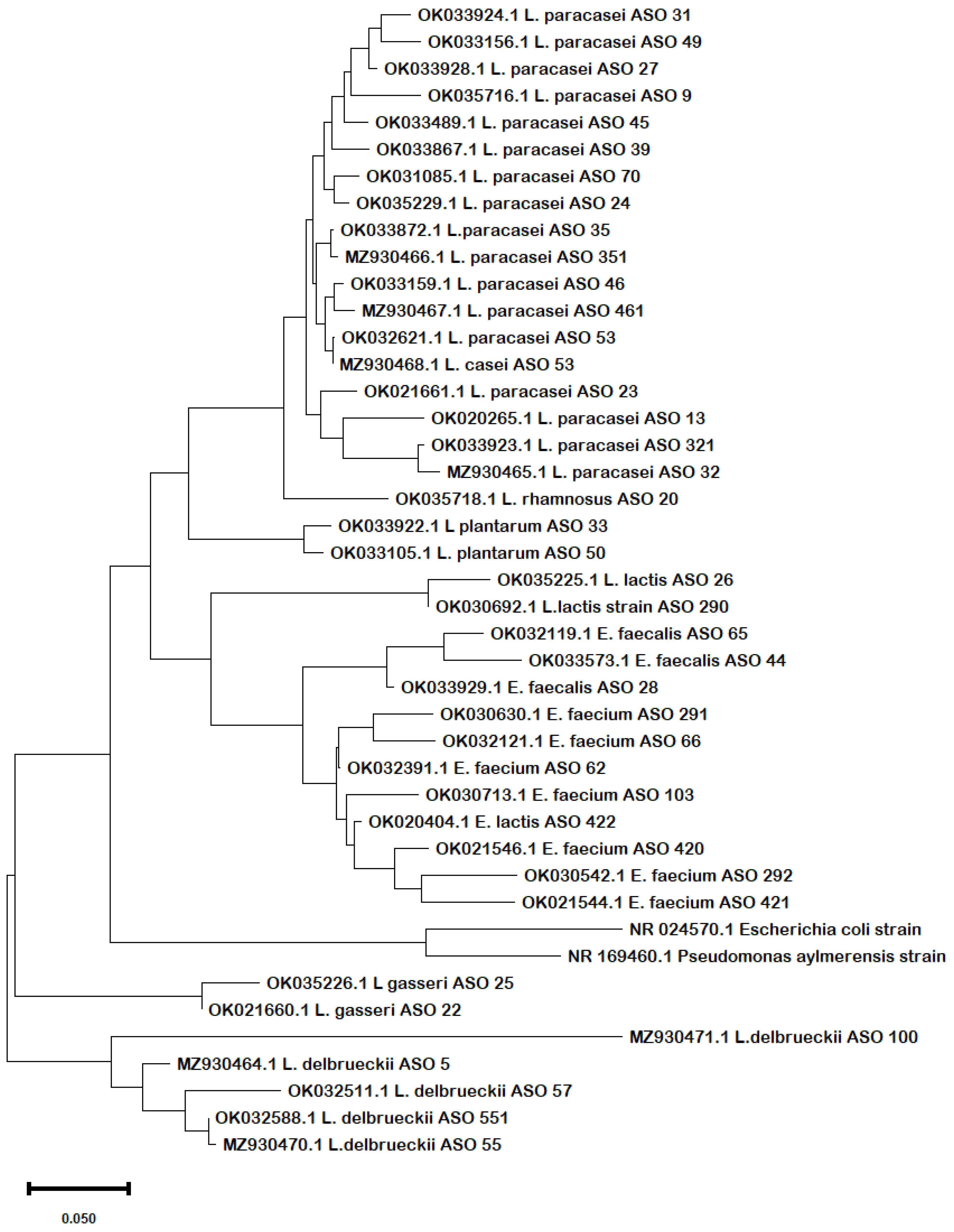
Phylogenetic analysis of the obtained sequences confirmed the same results in terms of the evolutionary relationship among the strains. From the phylogenetic tree (Figure 1), it can be inferred that there was a clear similarity among the different species of Lactobacillus, Lacticaseibacillus, and Lactococcus except for L. delbrueckii and L. gasseri that diverged in different clades. The species of Enterococcus spp. were clustered together, revealing close similarities.
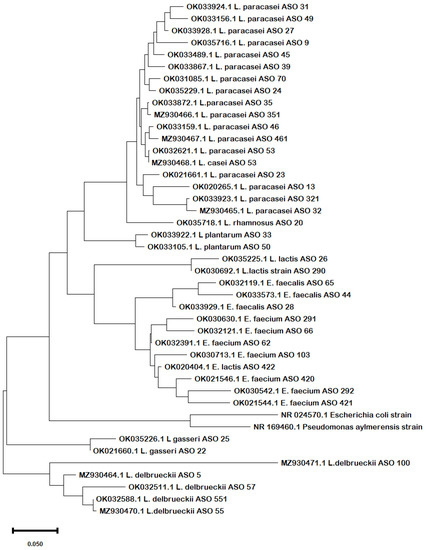
Figure 1.
Phylogenetic tree shows the evolutionary relationships between the 16S rRNA sequences of the obtained concatenated nucleotide sequences of their 16S rRNA. The Maximum Likelihood tree was constructed using the MEGA X software with the Maximum Likelihood algorithm and default setting. The bar length represents 0.05 substitutions per nucleotide site. Branch support was estimated from 1000 bootstrap replicates. E. coli and Pseudomonas 16S rRNA sequences serve as outgroups to root the tree.
3.4. Scanning Electron Microscopy (SEM)
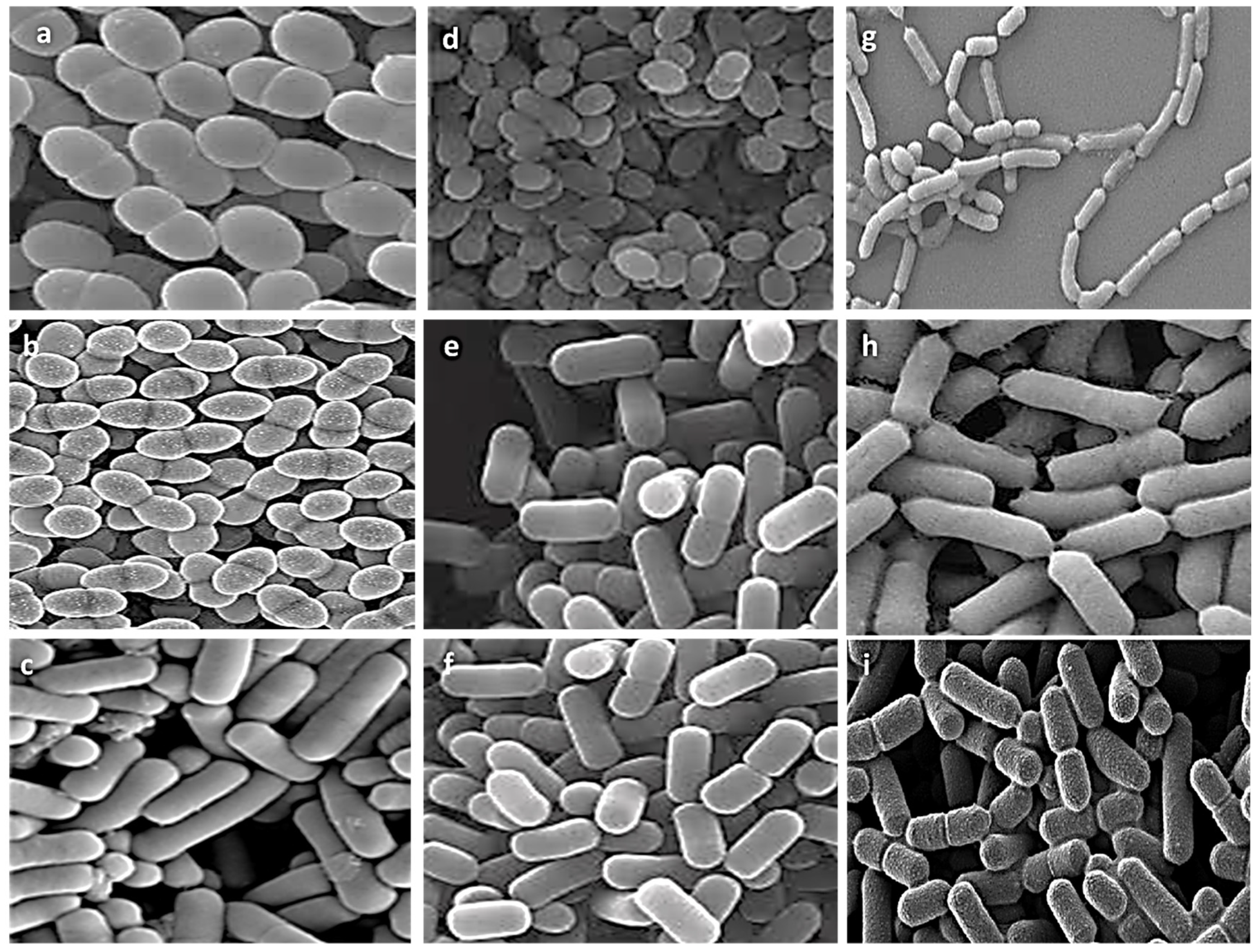
Nine representative isolates from different genera were selected for further experiments based on literature evidence of their probiotic characteristics and the uniqueness of their biochemical, physiological, and 16S rRNA molecular profiles. Morphological ultrastructure features of these isolates are presented in Figure 2. Images of the isolates showed the presence of three cocci isolates (E. faecalis ASO44, E. faecium ASO292, L. lactis ASO26) and six bacilli isolates (L. delbrueckii ASO100, L. plantarum ASO50, L. casei ASO53, L. rhamnosus ASO20, L. gasseri ASO25, L. paracasei ASO32). There were obvious differences in cell shapes and assemblies of all investigated isolates.
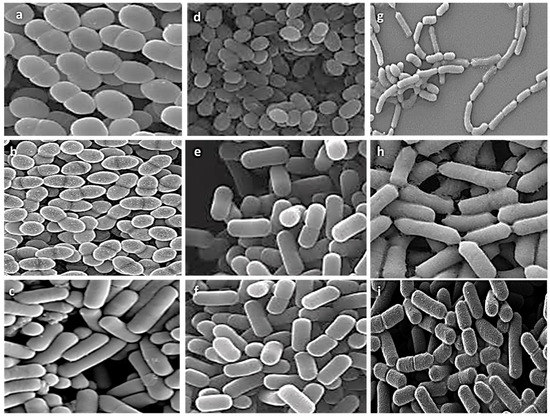
Figure 2.
Scanning electron microscopy visualization of different isolates, (a) E. faecalis ASO44; (b) E. faecium ASO292; (c) L. delbrueckii ASO100; (d) L. lactis ASO26; (e) L. plantarum ASO50; (f) L. case ASO53; (g) L. rhamnosus ASO20; (h) L. gasseri ASO25 (i) L. paracasei ASO32. Images were captured at an excitation voltage of 20 K.V., at 5000 magnification with a working distance of 13.7–14.2 mm.
3.5. Probiotic Characteristics
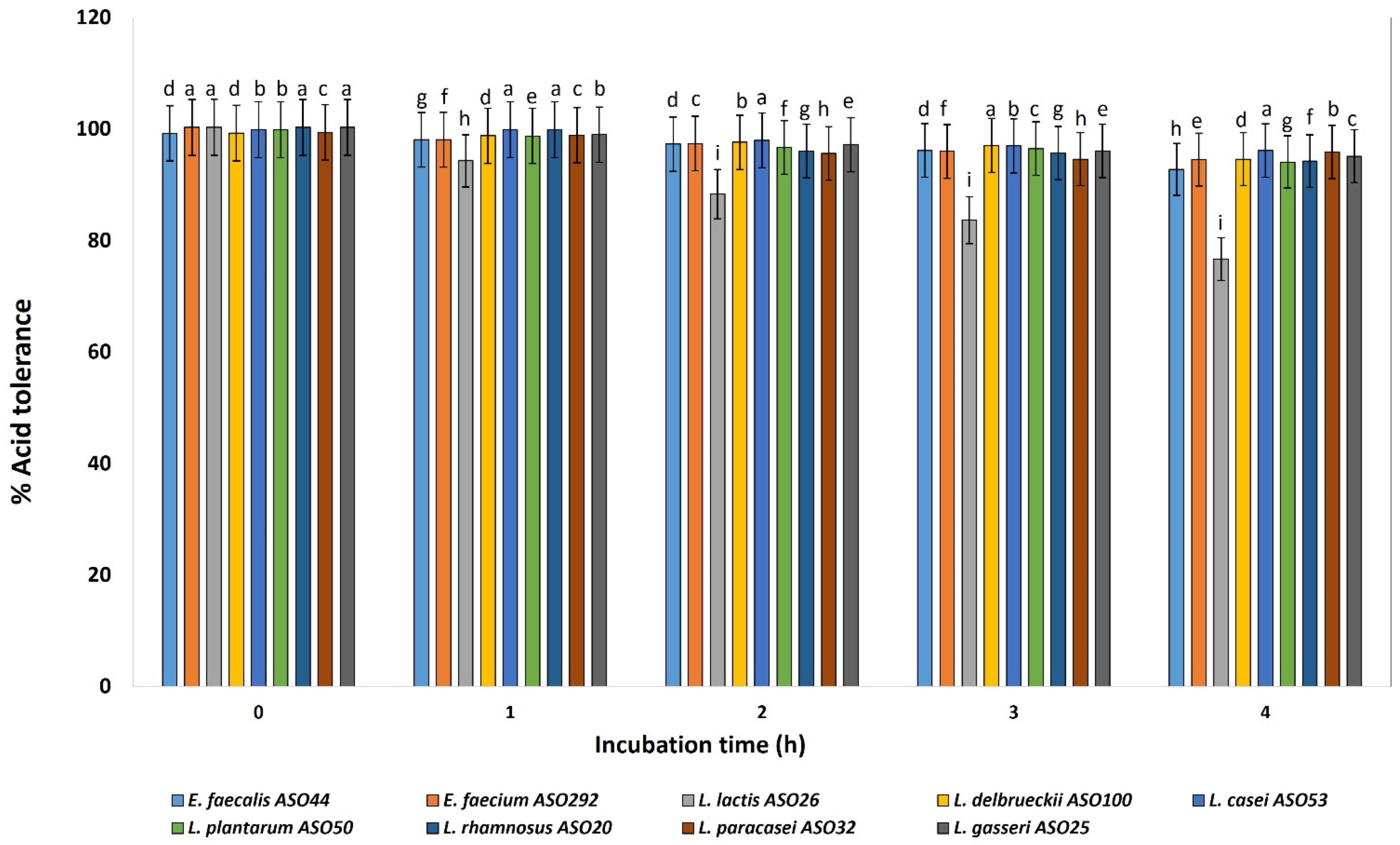
Nine isolates from different species were selected for focusing on their probiotic and ultrastructural characteristics. All investigated isolates revealed acid tolerance and survived very well and were not affected by decreasing the pH value from 6 to 3 for 4 h after incubation (Figure 3). The viable cell count of all isolates remained higher than 6–7 Log CFU mL−1. At pH 3, slight decreases were determined in the cell count of L. lactis ASO 26. Relatively log CFU mL−1 increases were determined for L. delbrueckii ASO100, L. paracasei ASO32, and L. plantarum ASO50, for 4 h at pH 3. According to this test, all isolates were resistant to low pH except L. lactis ASO 26 which was sensitive to low pH.
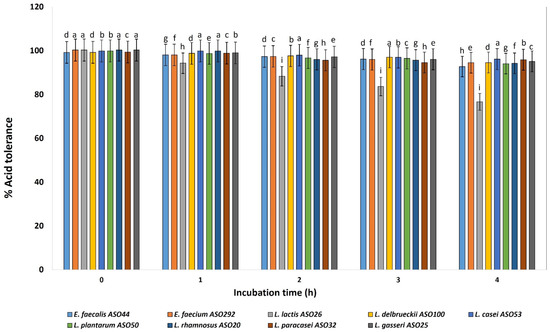
Figure 3.
Percentage of acid tolerance of the isolated lactic acid bacteria during different incubation times of 0, 1, 2, 3 and 4 h. LAB isolates were grown on pH 3.0 for 4 h, and percentages of tolerance were estimated by counting viable bacterial counts which tolerated the acidic medium with relative to neutral pH conditions (pH 6.4). a–i Estimates with the same letters are not significantly different (p > 0.05) among different isolates for the same incubation time.
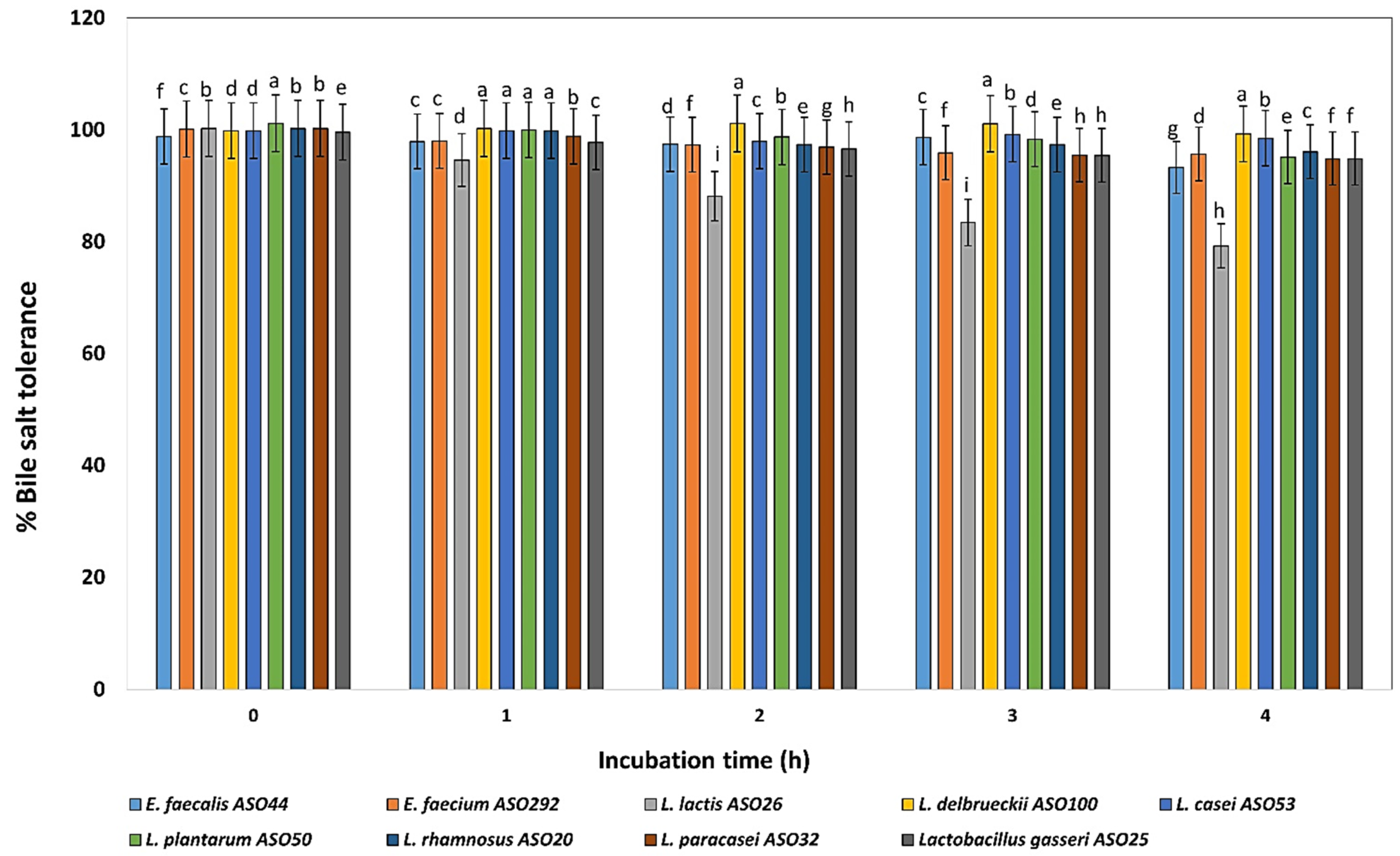
The bile salt (ox-gall bile salts 0.5%) tolerance test revealed that all isolates grew well in the presence of 0.5 % bile salts during incubation at 4 h except, L. lactis ASO 26 which showed declined profile during the incubation time. On another hand, L. delbrueckii ASO100, L. paracasei ASO32, and L. plantarum ASO50 exhibited the highest bile salts tolerance, respectively (Figure 4).
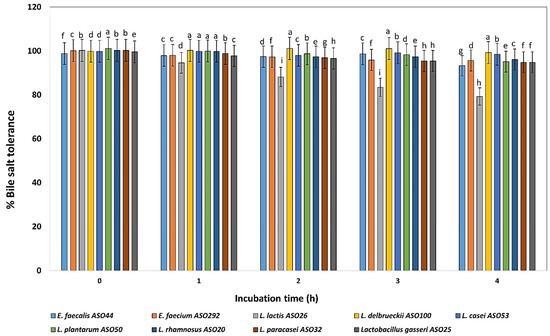
Figure 4.
Percentage of bile tolerance of the isolated lactic acid bacteria during different incubation times of 0, 1, 2, 3, and 4 h. LAB isolates were grown on 0.5% ox-bile salt for 4 h, and percentages of tolerance were estimated by counting viable bacterial counts which tolerated the bile salt with relative to untreated controls (with no bile salts). a–i Estimates with the same letters are not significantly different (p > 0.05) among different isolates for the same incubation time.
All isolates expressed bile salts hydrolase activity and deconjugated ability with taurine or glycine-bile acid or both. This activity showed as a hole around the colonies after growth in agar plate supplemented with 0.5% TDCA or 0.5% GDCA. The isolates L. delbrueckii ASO100 and L. casei ASO53 exhibited the highest bile salts hydrolase activity (Figure 5).
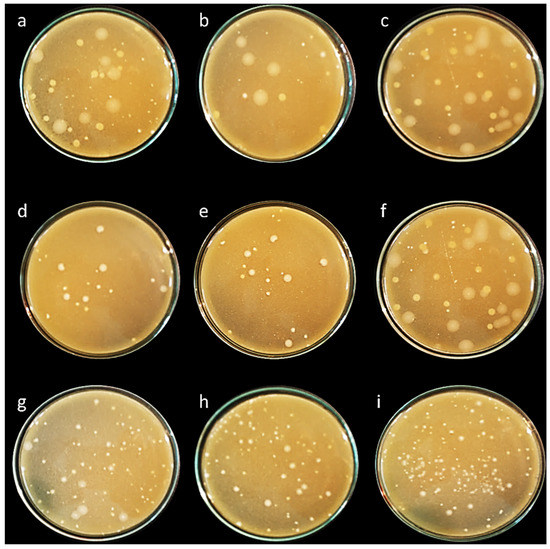
Figure 5.
Bile salt hydrolase’s activity assay of isolated lactic acid bacteria. Activated LAB isolates were inoculated and plated onto MRS and M17 agar containing tauro-deoxycholic acid. The plates were incubated anaerobically or aerobically at 37 °C for 48 h. Bile salt hydrolase activity was indicated by deoxycholic acid precipitate around the colonies. (a) E. faecalis ASO44; (b) E. faecium ASO292; (c) L. delbrueckii ASO100; (d) L. lactis ASO26; (e) L. plantarum ASO50; (f) L. casei ASO53; (g) L. rhamnosus ASO20; (h) L. gasseri ASO25 (i) L. paracasei ASO3.
Regarding antibiotic resistance, all studied isolates were resistant to kanamycin, tetracycline, neomycin, streptomycin, and vancomycin except, L. lactis ASO26, which was sensitive to streptomycin and vancomycin. The isolates L. delbrueckii ASO100 and L. casei ASO53 displayed the highest resistance pattern against kanamycin, tetracycline, neomycin, and streptomycin. However, the isolate L. rhamnosus ASO20 revealed a high resistant profile against vancomycin (Table 5).

Table 5.
Antibiotic resistance of isolated lactic acid bacteria.
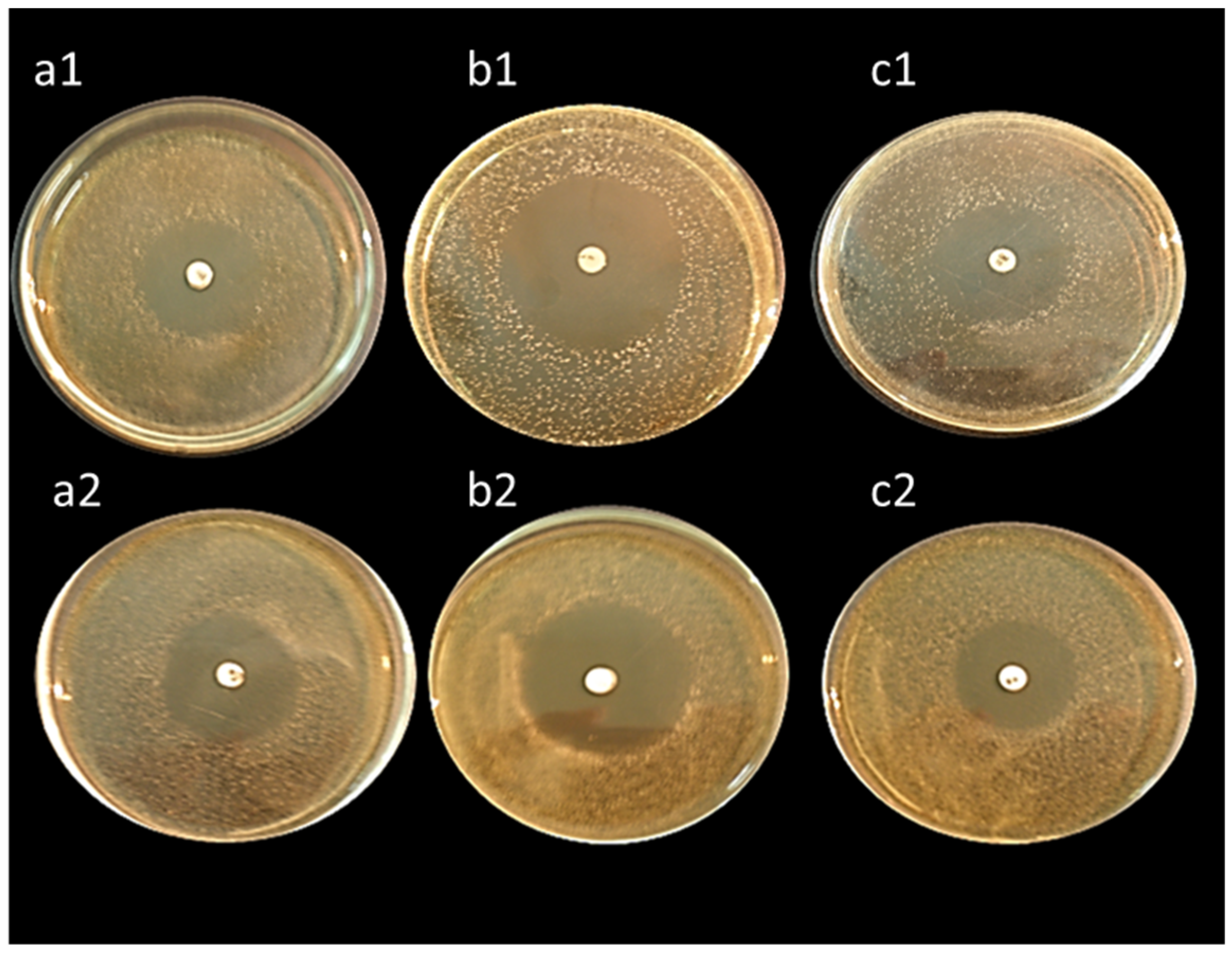
All isolates exhibited high antibacterial activity against Bacillus subtilis, Staphylococcus aureus, and Escherichia coli except, L. lactis ASO26 which did not reveal any antibacterial activity (Table 6). Interestingly, the isolates L. delbrueckii ASO100 and L. rhamnosus ASO20 displayed the highest antibacterial activity (Figure 6).

Table 6.
Effect of antimicrobial activity of some lactic acid bacteria on some pathogenic and spoilage bacteria.
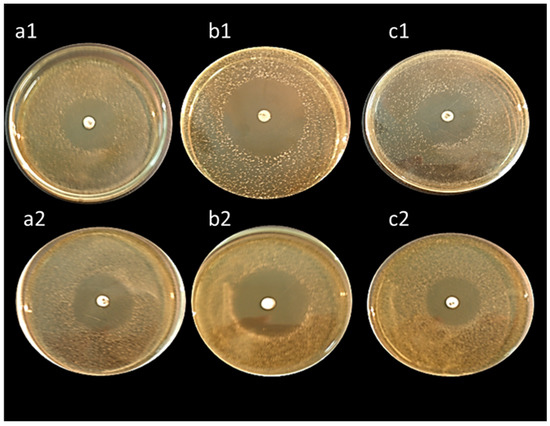
Figure 6.
Antibacterial activity of cell-free supernatants of L. delbrueckii ASO100 (upper three plates) and L. rhamnosus ASO20 (lower three plates) against Bacillus subtilis (a1,a2), Staphylococcus aureus (b1,b2), and Escherichia coli (c1,c2). The plates were firstly inoculated with the tested pathogenic bacteria, then allowed to air dry for 15 min; discs impregnated with cell-free supernatants were spotted on the middle of the plates. Diameters of the formed inhibition zones were measured after incubation at 37 °C for 24 h.
4. Discussion
In this investigation, we seek new potent probiotics from the breast milk of Egyptian mothers and stool samples of their infants. The novel aspects of our study include (1) exploring human milk microbiota diversity from Egyptian samples for the first time and (2) isolating innovative probiotics from Egyptian infants’ feces that are characterized by a unique immune system as prophylactic and therapeutic agents for controlling chronic diseases.
Where lactic acid bacteria colonize gut epithelial cells and withstand pathogens and reactive oxygen species (ROS) associated with gut diseases, it should have the ability to endure harsh conditions in the human body (intestinal juice, low pH, and salivary enzymes) to maintain gut microbiota balance, immune homeostasis and monitor beneficial physiological roles in human health [33,34]. In this manner and based on morphological and physiological characteristics, we selected only gram-positive, catalase-negative, positive microaerophilic and non-endospore forming lactic acid bacterial isolates to be investigated in the current study. Rod isolates revealed a positive ability to grow at 4.0% bile salt, pH 6.8, pH 9.6, 6.5% NaCl, at 45 °C with positive abilities to hydrolyze arginine and coagulate milk in accordance with the investigations of Soni et al. [35] and Lackey et al. [36]. All isolates exhibited high abilities to ferment various carbohydrates and produce acid from lactose, galactose, glucose, fructose, maltose, sucrose, mannose, rhamnose, arabinose, and melibiose. However, these isolates displayed a negative ability to produce acid from ribose, mannitol, ribose, salicin, and sorbitol. These results agreed with the recent report of Li et al. [17] who isolated 27 gram-positive and catalase-negative strains from healthy infant feces and evidenced their negative profile to mannitol and sorbitol. Interestingly, unlike all isolates, ASO57, ASO55, ASO5, and ASO100 showed negative profiles for producing acid from xylose, raffinose, and trehalose reflecting unique profiles and possible different probiotic characteristics.
Pairwise sequence alignment of 16S rRNA sequences revealed the presence of three genera, Lactobacillus, Enterococcus, and Lactococcus. Lactobacillus was the most common in human milk and feces samples with a high incidence of its different species (L. paracasei, L. delbrueckii, L. plantarum, L. gasseri and L. casei); these results were matched with the previous study of Zhang et al. [37] who reported a high incidence of Lactobacillus strains in feces samples of Chinese babies. Interestingly, six of our isolates evidenced low identity ratios ranging from 81.39% to 94.63% (less than 95%) with database sequences. The isolate that revealed the lowest identity ratio (L. delbrueckii, ASO 100) expressed the highest antibiotic resistance, antibacterial and probiotic activity. This isolate could be a new species as reported by Thompson et al. [38] and Badr et al. [39], and strains from different microbial species share less than 95% Average Nucleotide Identity (ANI). The low identity ratio of this isolate specifically was evidenced by chemical, physiological and probiotic features. Hence, this isolate and probably the other five isolates that shared less than 95% Average Nucleotide Identity could be a new probiotic species with novel and unique characteristics. These isolates showed the nearest similarity to E. faecium and L. delbrueckii strains. The previous investigation by Evivie et al. [40] confirmed the medicinal usage of L. delbrueckii isolates as a probiotic against foodborne pathogens. Moreover, recent studies have evidenced the role of E. faecium as a promising probiotic candidate for both human and animal use [41,42,43,44]. Despite 16S rRNA being a very conserved region, six of our isolates revealed huge divergence in that region and this could be explained by the effect of environmental, demographic, genetic factors, and the maternal lifestyle on modifying the microbial diversity of human milk and infants’ gut [45].
Previous investigations evidenced the role of current isolated strains as a promising candidate probiotic for medicinal and industrial usage for humans and animals. An interesting study by Salaris et al. [46] revealed that L. paracasei is a promising candidate probiotic that exhibits prophylactic potential effect against SARS-CoV-2 infection. Moreover, Otaka et al. [47] indicated that L. paracasei was useful to alleviate depressive symptoms, partly through its association with an abundance of actinobacteria in the gut microbiota. Another investigation by Guerra et al. [48] showed that lactobacilli isolates from newborn stools exhibited different probiotic properties such as gastrointestinal tolerance, antibiotic susceptibility, inhibition of pathogen biofilm formation, absence of alfa or gamma-blood hemolysis, and lysozyme sensibility. Besides, Hill et al.’s investigation [49] showed that the L. casei clusters have the potential to be used prophylactically or therapeutically in diseases related to a disturbance to the gut microbiota. Probiotics like L. plantarum are beneficial bacteria that stimulate the digestive system, fight pathogenic microbes, and help the human body to produce vitamins. Many people take L. plantarum probiotic pills to heal or prevent complaints, including seasonal allergies and irritable bowel syndrome [50].
For testing the competing probiotic characteristics of our isolates, we selected nine representative isolates from different genera for further experiments based on literature evidence of their probiotic characteristics and the uniqueness of their biochemical, physiological, and 16S rRNA molecular profiles. We utilized scanning electron microscopy investigation for deep visualization of morphological ultrastructure features of these isolates confirming clear differences in cell shapes and assemblies of all investigated isolates. From the selected isolates there were three cocci isolates (E. faecalis ASO44, E. faecium ASO292, L. lactis ASO26) and six bacilli isolates (L. delbrueckii ASO100, L. plantarum ASO50, L. casei ASO53, L. rhamnosus ASO20, L. gasseri ASO25, L. paracasei ASO32). All isolates revealed aggregation ability confirming their ability to colonize gut epithelial cells.
Owing to market competition and demand, probiotics must be able to endure challenging environments including the acidic environment of the gastrointestinal tract (GIT), intestinal bile salts, and digestive enzymes. Most exogenous microbes die when ingested into the GIT because of the very low pH of the secreted gastric juice (pH of 2.0). It is expected that probiotic strains should be able to adapt and tolerate the acidic nature of the GIT as they pass by to colonize the gut of their host [51,52]. Moreover, pH tolerance is important for the improvement of fermented foods like yogurt and cheese that affect strain sustainability due to their high acidity. Interestingly, all the investigated isolates in this study revealed acid tolerance of the isolated LAB to the pH value from 6 to 3 for 4 h after incubation. LAB are known for their capability to tolerate acidic pH [53]. Our result resembles preceding studies that reported the survival of LAB strains against simulated gastric juice with a pH of 2.0 [54,55].
One of the major requirements for probiotic selection is the ability to survive and grow in the GIT, so they should be able to tolerate the intestinal bile salt. Probiotic physiological alterations, including exopolysaccharide synthesis and carbohydrate fermentation, are related to the resistance to elevated bile salts [56]. The adaptation of probiotics to bile salts is also connected to the structure of membrane proteins and fatty acids as well as the prevention of pathogen adherence to human mucus [57,58]. To compete with pathogens when employed in functional foods, probiotic strains must possess resistance to bile salts. Tolerance of an average level of 0.3% of the bile salt has been estimated in many studies for potential probiotic LAB candidates [59]. Interestingly, our results estimated that, except for the L. lactis ASO 26, all the LAB isolates in this study exhibited tolerance to 0.5% bile salts for a 4 h incubation period.
Regarding antibiotic resistance in probiotics, it is considered a safety concern, as antibiotic resistance encoding genes could transfer among the microorganism community of the gut. The genomic context of the antibiotic resistance determinants of the current study’s probiotic strains is unknown but a future follow-up study will be performed to go through whole genome sequencing of these isolates to ensure that the antibiotic resistance determinants are not present as part of mobile genetic elements. Previous evidence by [60] reported the lack of cytochrome-mediated electron transport in Lactobacillus genera, and the presence of D-Ala-D-lactate in their peptidoglycan, hence, their resistance to different antibiotics including streptomycin, and vancomycin is considered to be intrinsic. Consequently, LAB probiotic strains can be used safely as pills alongside or after antibiotic treatment to restore the gut microbiota homeostasis [61]. In this case, antibiotic resistance possesses a strong advantage in order for probiotics to survive under antibiotic treatment conditions. In the current study, all studied isolates were resistant to kanamycin, tetracycline, neomycin, streptomycin, and vancomycin except, L. lactis ASO26, which was sensitive to streptomycin and vancomycin. The literature evidenced that most LAB species are resistant to kanamycin [62,63]. Remarkably, all isolates were resistant to streptomycin and vancomycin, in harmony with previous reports [64,65].
All tested isolates exhibited high antibacterial activity against Bacillus subtilis, Staphylococcus aureus, and Escherichia coli except, L. lactis ASO26 which did not reveal any antibacterial activity. Our results were in coincidence with Klayraung et al. [66], who investigated the antibacterial activity of lactobacilli isolated from four kinds of traditional fermented foods on Staphylococcus aureus, Salmonella typhi, and Escherichia coli, reporting the high antibacterial potency of LAB against S. aureus, S. typhi, and E. coli. The antibacterial activity of lactic acid bacteria could be explained by their production of a wide variety of different inhibitory substances that prolong the time scale of preservation of the fermented products. The preservative action of LAB in foods results from the formation of metabolites with antimicrobial activity, e.g., organic acids (lactic, acetic, formic, etc.), hydrogen peroxide (in the presence of oxygen), diacetyl, aldehydes (e.g., β-hydroxy-propionaldehyde) and bacteriocins or bactericidal proteins during lactic fermentation, which make them useful in food bio-preservation.
As the newly identified LAB isolates exhibited high acid and bile salt tolerance, antibiotic resistance, and antibacterial activity, they could be applied as effective and competing probiotic pills for modulating intestinal pathogens and human diseases. Interestingly, pairwise sequence alignment results evidenced a low identity ratio of six isolates (less than 95%) with a high probability to be new species. Further research will be assessed to go through whole genomic sequencing of these isolates, especially the isolate L. delbrueckii, ASO 100 that will be subject to complete proteomic analysis to stand for its probiotic determinants, as it revealed the most brilliant probiotic and antibacterial features, along with another in vivo experiment that will be conducted to test the prophylactic and therapeutic ability of this isolate to modulate gut–brain axis microbiota in an Alzheimer’s disease animal model.
5. Conclusions
In this study, we screened human breast milk and infant stool samples from Egyptian sources to hunt for innovative Probiotic isolates. Forty-one isolates were submitted to the gene bank database, classified, and identified through physiological and biochemical tests. The representative samples from the different species revealed antibiotic resistance, antibacterial activity, and high probiotic features. Six of our isolates revealed less than 95% Average Nucleotide Identity with other deposited sequences in the database. The isolate L. delbrueckii, ASO 100 exhibited the lowest identity ratio with promising probiotic and antibacterial features, casting light on its high probability of being a new probiotic species.
Author Contributions
Conceptualization, A.A.A., S.Y.M.M., O.A.B., I.A.A. and S.A.M.; Data curation, A.A.A., S.Y.M.M.; Formal analysis, O.A.B., S.A.M., M.M.A.M., N.E., M.A., B.A.S., I.A.A. and A.E.; Funding acquisition, S.Y.M.M.; Methodology, A.A.A., S.Y.M.M., O.A.B., S.A.M. and M.M.A.M.; Resources, N.E., M.A., B.A.S., I.A.A. and A.E.; Validation, A.A.A., S.Y.M.M. and O.A.B.; Visualization, A.A.A., A.E., S.Y.M.M., O.A.B. and S.A.M.; Writing—original draft, A.A.A., S.Y.M.M., O.A.B. and S.A.M., N.E., M.A., B.A.S., I.A.A. and A.E.; Writing—review & editing, A.A.A., S.Y.M.M., I.A.A., O.A.B. and S.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by institutional fund projects under no (IFP-A-2022-2-5-22). Therefore, the authors gratefully acknowledge technical and financial support from the ministry of education and the University of Hafr Al Batin—Saudi Arabia.
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Faculty of Medicine, Benha University, Egypt. The ethical approval number is BUFTM0304021.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available upon request by corresponding authors.
Acknowledgments
The authors wish to give thanks for the technical and financial support from the Ministry of Education and the University of Hafr Al Batin—Saudi Arabia. The authors gratefully acknowledge all mothers’ volunteers in the community around the Faculty of Agriculture, Benha University for their cooperation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brink, L.R.; Mercer, K.E.; Piccolo, B.D.; Chintapalli, S.V.; Elolimy, A.; Bowlin, A.K.; Matazel, K.S.; Pack, L.; Adams, S.H.; Shankar, K. Neonatal Diet Alters Fecal Microbiota and Metabolome Profiles at Different Ages in Infants Fed Breast Milk or Formula. Am. J. Clin. Nutr. 2020, 111, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Eidelman, A.; Schanler, R. Breastfeeding and the Use of Human Milk; American Academy of Pediatrics: Itasca, IL, USA, 2018. [Google Scholar]
- Medjaoui, I.; Rahmani, B.; Talhi, M.; Mahammi, F.Z.; Moghtit, F.Z.; Mehtar, N.; Gaouar, S.B.S. Isolation and Characterization of Lactic Acid Bacteria from Human Milk and Newborn Feces. J. Pure Appl. Microbiol. 2016, 10, 2613–2620. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K. Association between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Olivares, M.; Díaz-Ropero, M.P.; Martín, R.; Rodríguez, J.M.; Xaus, J. Antimicrobial Potential of Four Lactobacillus Strains Isolated from Breast Milk. J. Appl. Microbiol. 2006, 101, 72–79. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Yousefi, B.; Eslami, M.; Ghasemian, A.; Kokhaei, P.; Salek Farrokhi, A.; Darabi, N. Probiotics Importance and Their Immunomodulatory Properties. J. Cell. Physiol. 2019, 234, 8008–8018. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel Approaches for Co-Encapsulation of Probiotic Bacteria with Bioactive Compounds, Their Health Benefits and Functional Food Product Development: A Review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- Le, B.; Yang, S.H. Efficacy of Lactobacillus Plantarum in Prevention of Inflammatory Bowel Disease. Toxicol. Rep. 2018, 5, 314–317. [Google Scholar] [CrossRef]
- He, D.; Wang, Y.; Lin, J.; Xing, Y.-F.; Zeng, W.; Zhu, W.-M.; Su, N.; Zhang, C.; Lu, Y.; Xing, X.-H. Identification and Characterization of Alcohol-Soluble Components from Wheat Germ-Apple Fermented by Lactobacillus Sp. Capable of Preventing Ulcerative Colitis of Dextran Sodium Sulfate-Induced Mice. J. Funct. Foods 2020, 64, 103642. [Google Scholar] [CrossRef]
- Dos Santos, S.C.; Konstantyner, T.; Cocco, R.R. Effects of Probiotics in the Treatment of Food Hypersensitivity in Children: A Systematic Review. Allergol. Immunopathol. 2020, 48, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, R.G.S.; de Albuquerque, T.M.R.; de Luna Freire, M.O.; Ferreira, G.A.H.; Dos Santos, L.A.C.; Magnani, M.; Cruz, J.C.; Braga, V.A.; de Souza, E.L.; de Brito Alves, J.L. The Probiotic Lactobacillus Fermentum 296 Attenuates Cardiometabolic Disorders in High Fat Diet-Treated Rats. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Mehwish, H.M.; Fang, H.; Padhiar, A.A.; Zeng, X.; Khurshid, M.; He, Z.; Zhao, L. Characterization and Anti-Tumor Activity of Exopolysaccharide Produced by Lactobacillus Kefiri Isolated from Chinese Kefir Grains. J. Funct. Foods 2019, 63, 103588. [Google Scholar] [CrossRef]
- Lee, H.; Lee, C.-K.; Kim, K. Isolation of Novel Strains of Lactobacillus Gasseri EJL and Bifidobacterium Breve JTL from Breast Milk and Infant Feces: A Longitudinal Study of a Mother-Infant Pair. Microbiol. Biotechnol. Lett. 2021, 49, 1–8. [Google Scholar] [CrossRef]
- Li, B.; Pan, L.-L.; Sun, J. Novel Probiotic Lactic Acid Bacteria Were Identified from Healthy Infant Feces and Exhibited Anti-Inflammatory Capacities. Antioxidants 2022, 11, 1246. [Google Scholar] [CrossRef]
- Buck, J.D. Nonstaining (KOH) Method for Determination of Gram Reactions of Marine Bacteria. Appl. Environ. Microbiol. 1982, 44, 992–993. [Google Scholar] [CrossRef]
- Schaad, N.W.; Jones, J.B.; Chun, W. Laboratory Guide for the Identification of Plant Pathogenic Bacteria; American Phytopathological Society (APS Press): Saint Paul, MN, USA, 2001; ISBN 0890542635. [Google Scholar]
- Hadioetomo, R.S. Mikrobiologi Dasar Dalam Praktek Teknik dan ProsedurDasar Laboratorium; Gramedia: Jakarta, Indonesia, 1993. [Google Scholar]
- Bulut, Ç. Isolation and Molecular Characterization of Lactic Acid Bacteria from Cheese; Izmir Institute of Technology: İzmir, Turkey, 2003; ISBN 9798505503249. [Google Scholar]
- Ashry, N.M.; Alaidaroos, B.A.; Mohamed, S.A.; Badr, O.A.M.; El-Saadony, M.T.; Esmael, A. Utilization of Drought-Tolerant Bacterial Strains Isolated from Harsh Soils as a Plant Growth-Promoting Rhizobacteria (PGPR). Saudi. J. Biol. Sci. 2022, 29, 1760–1769. [Google Scholar] [CrossRef]
- Abdelatty, A.M.; Mandouh, M.I.; Mohamed, S.A.; Busato, S.; Badr, O.A.M.; Bionaz, M.; Elolimy, A.A.; Moustafa, M.M.A.; Farid, O.A.A.; Al-Mokaddem, A.K. Azolla Leaf Meal at 5% of the Diet Improves Growth Performance, Intestinal Morphology and P70S6K1 Activation, and Affects Cecal Microbiota in Broiler Chicken. Animal 2021, 15, 100362. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Kokkinosa, A.; Fasseas, C.; Eliopoulos, E.; Kalantzopoulos, G. Cell Size of Various Lactic Acid Bacteria as Determined by Scanning Electron Microscope and Image Analysis. Lait 1998, 78, 491–500. [Google Scholar] [CrossRef]
- Jaya, S. Microstructure Analysis of Dried Yogurt: Effect of Different Drying Methods. Int. J. Food Prop. 2009, 12, 469–481. [Google Scholar] [CrossRef]
- Sabikhi, L.; Babu, R.; Thompkinson, D.K.; Kapila, S. Resistance of Microencapsulated Lactobacillus Acidophilus LA1 to Processing Treatments and Simulated Gut Conditions. Food Bioprocess Technol. 2010, 3, 586–593. [Google Scholar] [CrossRef]
- Moser, S.A.; Savage, D.C. Bile Salt Hydrolase Activity and Resistance to Toxicity of Conjugated Bile Salts Are Unrelated Properties in Lactobacilli. Appl. Environ. Microbiol. 2001, 67, 3476–3480. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Kim, J.-K.; Kim, H.-J.; Kim, W.-Y.; Kim, Y.-B.; Park, Y.-H. Selection of a Potential Probiotic Lactobacillus Strain and Subsequent in Vivo Studies. Antonie Van Leeuwenhoek 2001, 80, 193–199. [Google Scholar] [CrossRef]
- Başyiğit, G.; Kuleaşan, H.; Karahan, A.G. Viability of Human-Derived Probiotic Lactobacilli in Ice Cream Produced with Sucrose and Aspartame. J. Ind. Microbiol. Biotechnol. 2006, 33, 796–800. [Google Scholar] [CrossRef]
- Wayne, P.A. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 20th Informational Supplement; CLSI: Nueva Ecija, Philippines, 2010. [Google Scholar]
- Valladares, R.; Sankar, D.; Li, N.; Williams, E.; Lai, K.-K.; Abdelgeliel, A.S.; Gonzalez, C.F.; Wasserfall, C.H.; Larkin III, J.; Schatz, D. Lactobacillus Johnsonii N6. 2 Mitigates the Development of Type 1 Diabetes in BB-DP Rats. PLoS ONE 2010, 5, e10507. [Google Scholar] [CrossRef]
- Alberda, C.; Gramlich, L.; Meddings, J.; Field, C.; McCargar, L.; Kutsogiannis, D.; Fedorak, R.; Madsen, K. Effects of Probiotic Therapy in Critically Ill Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Clin. Nutr. 2007, 85, 816–823. [Google Scholar] [CrossRef]
- Soni, M.; Shah, H.R.; Patel, S.M. Isolation, Identification and Analysis of Probiotic Characteristics of Lactobacillus Spp. from Regional Yoghurts from Surendranagar District, Gujarat. Asian. J. Dairy Food Res. 2021, 40, 267–272. [Google Scholar] [CrossRef]
- Lackey, K.A.; Williams, J.E.; Meehan, C.L.; Zachek, J.A.; Benda, E.D.; Price, W.J.; Foster, J.A.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W. What’s Normal? Microbiomes in Human Milk and Infant Feces Are Related to Each Other but Vary Geographically: The INSPIRE Study. Front. Nutr. 2019, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, S.; Guidesi, E.; Zonenschain, D.; Sagheddu, V.; Lee, S.; Lim, C.-Y.; Elli, M. Isolation and Characterization of New Probiotic Strains from Chinese Babies. J. Clin. Gastroenterol. 2018, 52, S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.C.; Chimetto, L.; Edwards, R.A.; Swings, J.; Stackebrandt, E.; Thompson, F.L. Microbial Genomic Taxonomy. BMC Genom. 2013, 14, 913. [Google Scholar] [CrossRef] [PubMed]
- Badr, O.A.M.; EL-Shawaf, I.I.S.; El-Garhy, H.A.S.; Moustafa, M.M.A. Isolation and Molecular Identification of Two Novel Cyanobacterial Isolates Obtained from a Stressed Aquatic System. Gene Rep. 2018, 13, 110–114. [Google Scholar] [CrossRef]
- Evivie, S.E.; Abdelazez, A.; Li, B.; Lu, S.; Liu, F.; Huo, G. Lactobacillus Delbrueckii Subsp. Bulgaricus KLDS 1.0207 Exerts Antimicrobial and Cytotoxic Effects in Vitro and Improves Blood Biochemical Parameters in Vivo against Notable Foodborne Pathogens. Front. Microbiol. 2020, 11, 583070. [Google Scholar] [CrossRef]
- Palkovicsné Pézsa, N.; Kovács, D.; Gálfi, P.; Rácz, B.; Farkas, O. Effect of Enterococcus Faecium NCIMB 10415 on Gut Barrier Function, Internal Redox State, Proinflammatory Response and Pathogen Inhibition Properties in Porcine Intestinal Epithelial Cells. Nutrients 2022, 14, 1486. [Google Scholar] [CrossRef]
- Lee, D.; Goh, T.W.; Kang, M.G.; Choi, H.J.; Yeo, S.Y.; Yang, J.; Huh, C.S.; Kim, Y.Y.; Kim, Y. Perspectives and Advances in Probiotics and the Gut Microbiome in Companion Animals. J. Anim. Sci. Technol. 2022, 64, 197. [Google Scholar] [CrossRef]
- Habib, B.; Vaid, S.; Bangotra, R.; Sharma, S.; Bajaj, B.K. Bioprospecting of Probiotic Lactic Acid Bacteria for Cholesterol Lowering and Exopolysaccharide Producing Potential. Biologia 2022, 77, 1931–1951. [Google Scholar] [CrossRef]
- Wajda, Ł.; Ostrowski, A.; Błasiak, E.; Godowska, P. Enterococcus Faecium Isolates Present in Human Breast Milk Might Be Carriers of Multi-Antibiotic Resistance Genes. Bacteria 2022, 1, 66–87. [Google Scholar] [CrossRef]
- Grech, A.; Collins, C.E.; Holmes, A.; Lal, R.; Duncanson, K.; Taylor, R.; Gordon, A. Maternal Exposures and the Infant Gut Microbiome: A Systematic Review with Meta-Analysis. Gut Microbes 2021, 13, 1897210. [Google Scholar] [CrossRef]
- Salaris, C.; Scarpa, M.; Elli, M.; Bertolini, A.; Guglielmetti, S.; Pregliasco, F.; Brun, P.; Castagliuolo, I. Lacticaseibacillus Paracasei DG Enhances the Lactoferrin Anti-SARS-CoV-2 Response in Caco-2 Cells. Gut Microbes 2021, 13, 1961970. [Google Scholar] [CrossRef] [PubMed]
- Otaka, M.; Kikuchi-Hayakawa, H.; Ogura, J.; Ishikawa, H.; Yomogida, Y.; Ota, M.; Hidese, S.; Ishida, I.; Aida, M.; Matsuda, K. Effect of Lacticaseibacillus Paracasei Strain Shirota on Improvement in Depressive Symptoms, and Its Association with Abundance of Actinobacteria in Gut Microbiota. Microorganisms 2021, 9, 1026. [Google Scholar] [CrossRef]
- Guerra, A.F.; Lemos Junior, W.J.F.; dos Santos, G.O.; Andrighetto, C.; Gianomini, A.; Corich, V.; Luchese, R.H. Lactobacillus Paracasei Probiotic Properties and Survivability under Stress-Induced by Processing and Storage of Ice Cream Bar or Ice-Lolly. Ciência Rural. 2018, 48. [Google Scholar] [CrossRef]
- Hill, D.; Sugrue, I.; Tobin, C.; Hill, C.; Stanton, C.; Ross, R.P. The Lactobacillus Casei Group: History and Health Related Applications. Front. Microbiol. 2018, 9, 2107. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, N.; Bottacini, F.; Van Sinderen, D.; Gahan, C.G.M.; Corsetti, A. Comparative Genomics of Lactiplantibacillus Plantarum: Insights into Probiotic Markers in Strains Isolated from the Human Gastrointestinal Tract and Fermented Foods. Front. Microbiol. 2022, 13, 1353. [Google Scholar] [CrossRef]
- Dunne, C.; O’Mahony, L.; Murphy, L.; Thornton, G.; Morrissey, D.; O’Halloran, S.; Feeney, M.; Flynn, S.; Fitzgerald, G.; Daly, C. In Vitro Selection Criteria for Probiotic Bacteria of Human Origin: Correlation with in Vivo Findings. Am. J. Clin. Nutr. 2001, 73, 386s–392s. [Google Scholar] [CrossRef] [PubMed]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, R.-U.; Jahid, I.K. Isolation, Characterization, and Assessment of Lactic Acid Bacteria toward Their Selection as Poultry Probiotics. BMC Microbiol. 2019, 19, 253. [Google Scholar] [CrossRef]
- Pinto, M.G.V.; Franz, C.M.A.P.; Schillinger, U.; Holzapfel, W.H. Lactobacillus Spp. with in Vitro Probiotic Properties from Human Faeces and Traditional Fermented Products. Int. J. Food Microbiol. 2006, 109, 205–214. [Google Scholar] [CrossRef]
- Blajman, J.; Gaziano, C.; Zbrun, M.V.; Soto, L.; Astesana, D.; Berisvil, A.; Scharpen, A.R.; Signorini, M.; Frizzo, L. In Vitro and in Vivo Screening of Native Lactic Acid Bacteria toward Their Selection as a Probiotic in Broiler Chickens. Res. Vet. Sci. 2015, 101, 50–56. [Google Scholar] [CrossRef]
- García-Hernández, Y.; Pérez-Sánchez, T.; Boucourt, R.; Balcázar, J.L.; Nicoli, J.R.; Moreira-Silva, J.; Rodríguez, Z.; Fuertes, H.; Nuñez, O.; Albelo, N. Isolation, Characterization and Evaluation of Probiotic Lactic Acid Bacteria for Potential Use in Animal Production. Res. Vet. Sci. 2016, 108, 125–132. [Google Scholar] [CrossRef]
- Talib, N.; Mohamad, N.E.; Yeap, S.K.; Hussin, Y.; Aziz, M.N.M.; Masarudin, M.J.; Sharifuddin, S.A.; Hui, Y.W.; Ho, C.L.; Alitheen, N.B. Isolation and Characterization of Lactobacillus Spp. from Kefir Samples in Malaysia. Molecules 2019, 24, 2606. [Google Scholar] [CrossRef] [PubMed]
- Ruas-Madiedo, P.; Gueimonde, M.; Arigoni, F.; de los Reyes-Gavilán, C.G.; Margolles, A. Bile Affects the Synthesis of Exopolysaccharides by Bifidobacterium Animalis. Appl. Environ. Microbiol. 2009, 75, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Sánchez, B.; Ruas-Madiedo, P.; De Los Reyes-Gavilán, C.G.; Margolles, A. Cell Envelope Changes in Bifidobacterium Animalis Ssp. Lactis as a Response to Bile. FEMS Microbiol. Lett. 2007, 274, 316–322. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Kalavathy, R.; Sieo, C.C.; Alitheen, N.B.; Liang, J.B.; Jahromi, M.F.; Ho, Y.W. Isolation and Characterization of Lactobacillus Strains as Potential Probiotics for Chickens. Pertanika J. Trop. Agric. Sci. 2014, 37, 141–157. [Google Scholar]
- Gueimonde, M.; Sánchez, B.; G. de los Reyes-Gavilán, C.; Margolles, A. Antibiotic Resistance in Probiotic Bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef]
- Sharma, P.; Tomar, S.K.; Goswami, P.; Sangwan, V.; Singh, R. Antibiotic Resistance among Commercially Available Probiotics. Food Res. Int. 2014, 57, 176–195. [Google Scholar] [CrossRef]
- Bazireh, H.; Shariati, P.; Azimzadeh Jamalkandi, S.; Ahmadi, A.; Boroumand, M.A. Isolation of Novel Probiotic Lactobacillus and Enterococcus Strains from Human Salivary and Fecal Sources. Front. Microbiol. 2020, 11, 597946. [Google Scholar] [CrossRef] [PubMed]
- Gueimonde, M.; Margolles, A.; Clara, G.; Salminen, S. Competitive Exclusion of Enteropathogens from Human Intestinal Mucus by Bifidobacterium Strains with Acquired Resistance to Bile—A Preliminary Study. Int. J. Food Microbiol. 2007, 113, 228–232. [Google Scholar] [CrossRef]
- Argyri, A.A.; Zoumpopoulou, G.; Karatzas, K.-A.G.; Tsakalidou, E.; Nychas, G.-J.E.; Panagou, E.Z.; Tassou, C.C. Selection of Potential Probiotic Lactic Acid Bacteria from Fermented Olives by in Vitro Tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef]
- Temmerman, R.; Pot, B.; Huys, G.; Swings, J. Identification and Antibiotic Susceptibility of Bacterial Isolates from Probiotic Products. Int. J. Food Microbiol. 2003, 81, 1–10. [Google Scholar] [CrossRef]
- Klayraung, S.; Viernstein, H.; Sirithunyalug, J.; Okonogi, S. Probiotic Properties of Lactobacilli Isolated from Thai Traditional Food. Sci. Pharm. 2008, 76, 485–504. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).