Carotegenic Virgibacillus halodenitrificans from Wadi El-Natrun Salt Lakes: Isolation, Optimization, Characterization and Biological Activities of Carotenoids
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection, Screening and Isolation of Halophilic Carotenoid-Producing Bacteria
2.2. Molecular Identification of the Selected Halophilic Carotenoid-Producing Isolate
2.3. Morphological and Physiological Characterization of Selected Isolate
2.4. Pigment Extraction
2.5. Optimization of Pigment Production by Statistical Design of Experiments (DOE)
2.5.1. Screening of Significant Independent Variables Influence the Pigments Production by Plackett–Burman Design (PBD)
2.5.2. Central Composite Design (CCD) Method
2.5.3. Statistical Analysis
2.5.4. Validation of Experimental Model
2.6. Characterization of Extracted Pigment
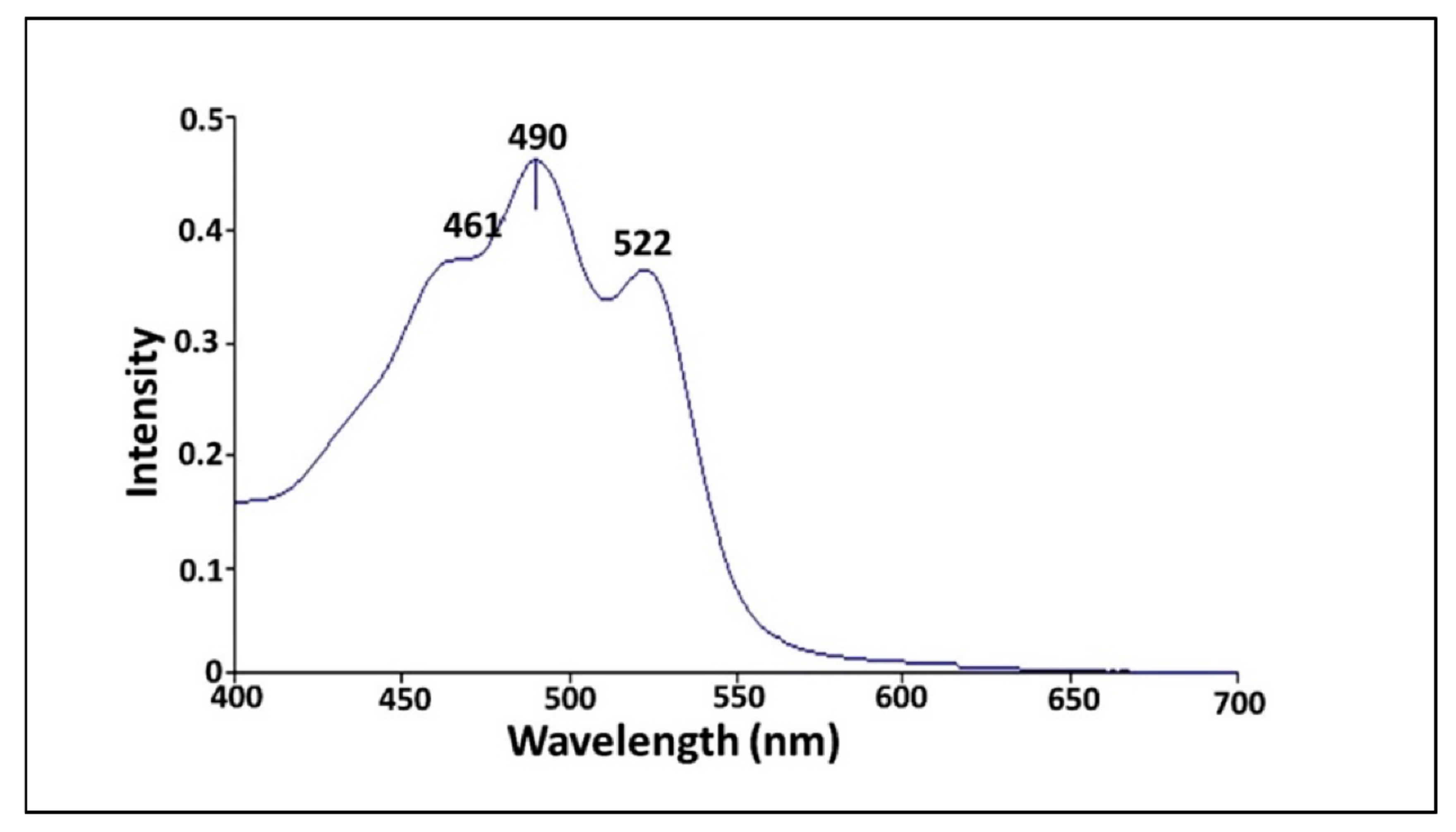
2.6.1. UV-Vis Spectra Absorption
2.6.2. Raman Spectroscopy
2.6.3. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.6.4. Thin-Layer Chromatography
2.6.5. LC–MS
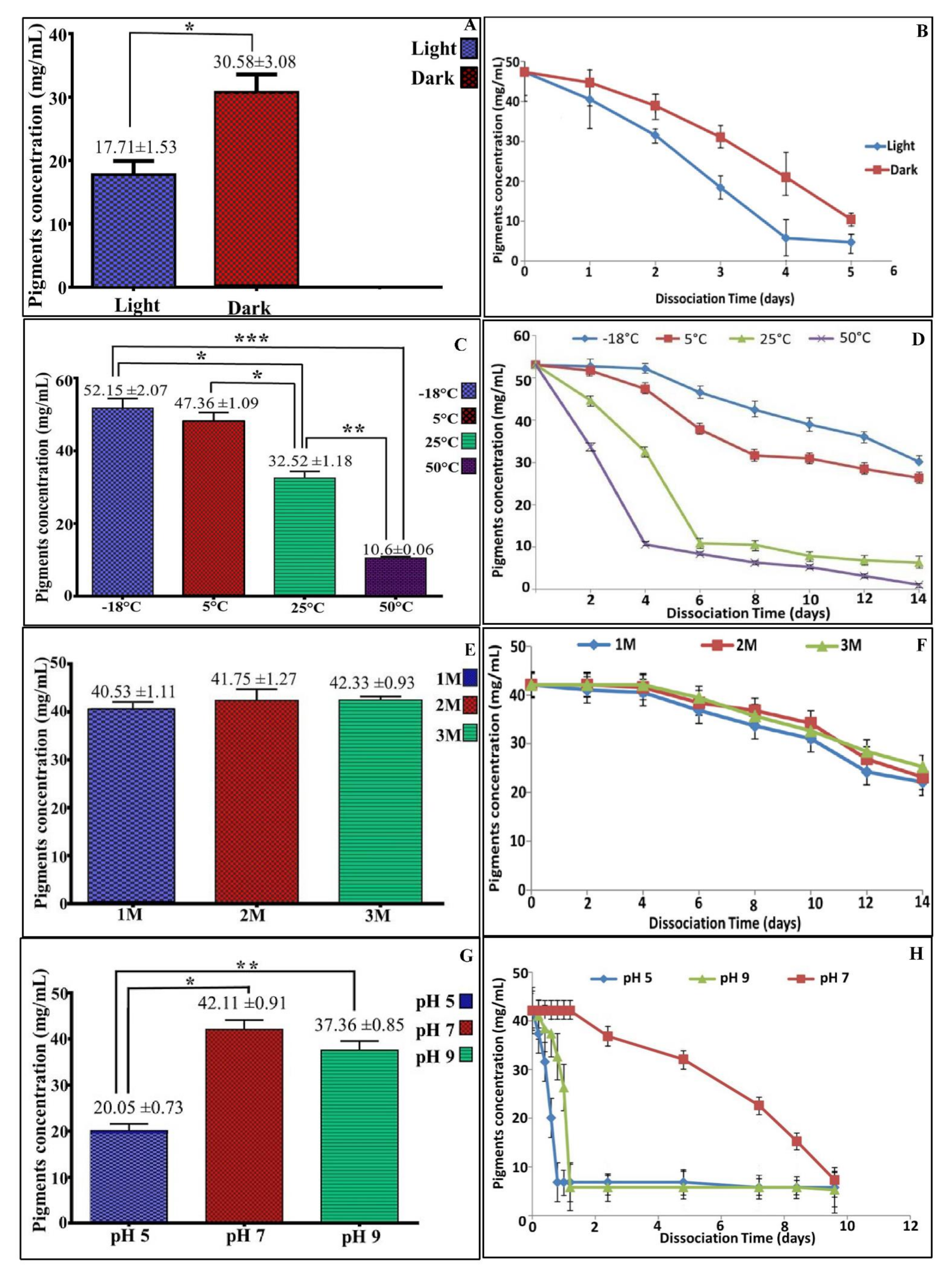
2.7. Stability of Pigment
2.8. Biological Activity of Halophilic Carotenoids
2.8.1. Antimicrobial Activity
2.8.2. Antibiofilm Potency
3. Results
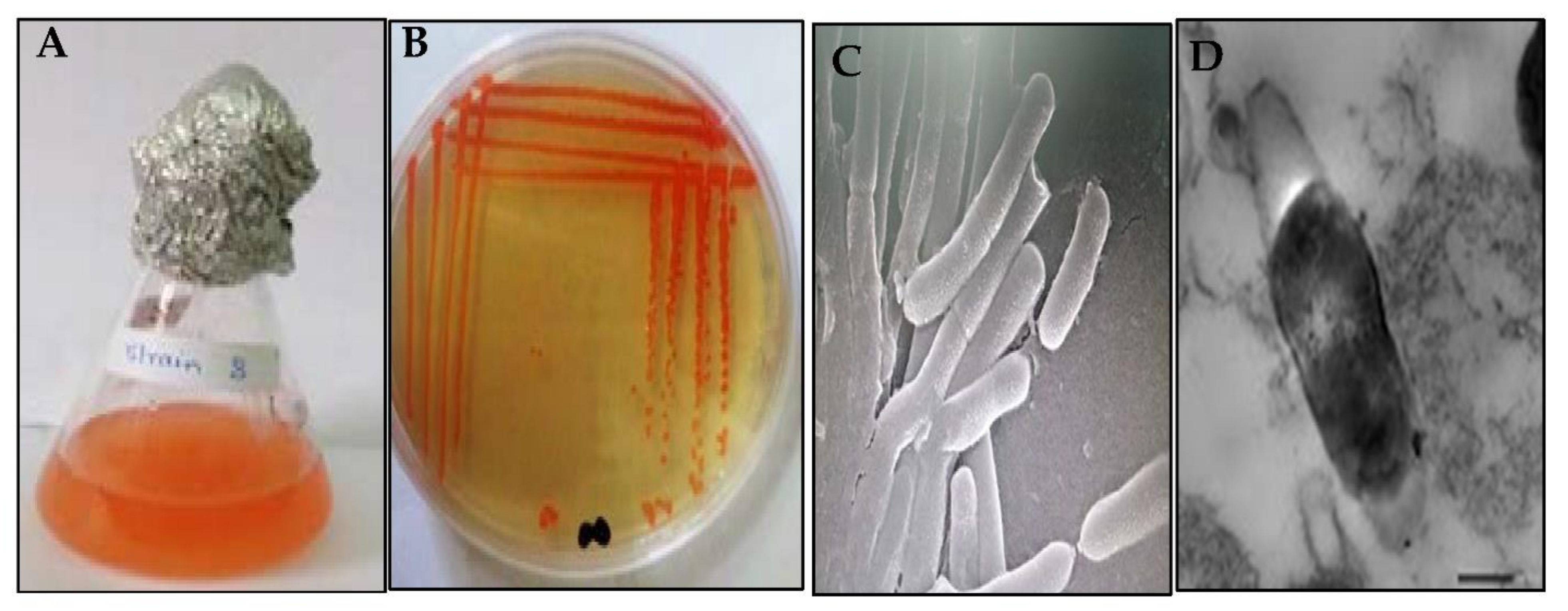
3.1. Screening, Isolation and Molecular Identification of Halophilic Carotenoid-Producing Bacteria
3.2. Phenotypic Characterization
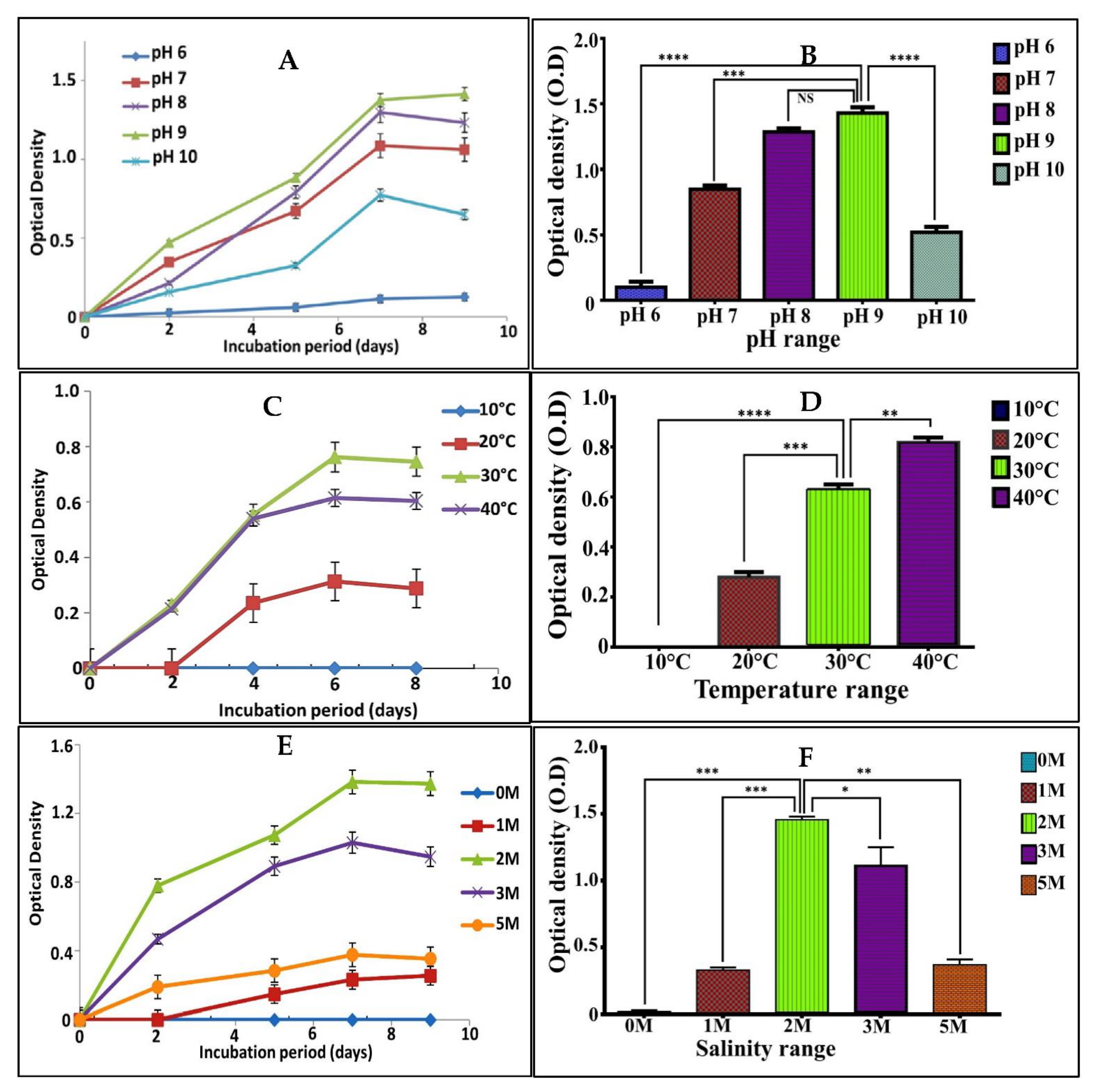
3.3. Physiological Characterization
3.4. Optimization of Pigment Production Using Experimental Design
3.4.1. Screening of Significant Independent Variables Influencing Pigment Production by Plackett–Burman Design (PBD)
| Run Order | KCl | MgSO4 | Y.E. | Peptone | NaCl | Inoculum Size (%) | pH | Volume/Flask (mL) | Experimental Pigment Weight | Predicted Pigment Weight |
|---|---|---|---|---|---|---|---|---|---|---|
| (g/L) | (mg/mL) | |||||||||
| 1 | 2.5 | 40 | 8 | 2.5 | 233.6 | 0.5 | 6 | 50 | 13.05 | 14 |
| 2 | 2.5 | 10 | 8 | 10 | 233.6 | 0.5 | 9 | 150 | 21 | 21.42 |
| 3 | 10 | 10 | 8 | 2.5 | 29.2 | 0.5 | 9 | 150 | 0.57 | 0.157 |
| 4 | 10 | 40 | 8 | 2.5 | 233.6 | 5 | 6 | 150 | 7.89 | 6.94 |
| 5 | 2.5 | 40 | 2 | 2.5 | 29.2 | 5 | 9 | 150 | 0.368 | 0.368 |
| 6 | 2.5 | 10 | 2 | 2.5 | 29.2 | 0.5 | 6 | 50 | 8.63 | 7.68 |
| 7 | 10 | 40 | 2 | 10 | 233.6 | 0.5 | 9 | 50 | 29.47 | 27.3 |
| 8 | 2.5 | 10 | 2 | 10 | 233.6 | 5 | 6 | 150 | 22.31 | 21.89 |
| 9 | 10 | 10 | 2 | 2.5 | 233.6 | 5 | 9 | 50 | 14.31 | 16.42 |
| 10 | 2.5 | 40 | 8 | 10 | 29.2 | 5 | 9 | 50 | 2.21 | 2.94 |
| 11 | 10 | 10 | 8 | 10 | 29.2 | 5 | 6 | 50 | 4.78 | 4.05 |
| 12 | 10 | 40 | 2 | 10 | 29.2 | 0.5 | 6 | 150 | 9.52 | 11.63 |
3.4.2. Central Composite Design (CCD) for Optimization of Pigment Production
3.4.3. Multiple Regression Analysis and ANOVA
3.4.4. Graphical Interpretation of the Response Surface Model

3.4.5. Experimental Verification of Model
3.5. Stability of the Pigment
3.6. Characterization of Pigment
3.6.1. UV-Vis Spectrophotometer
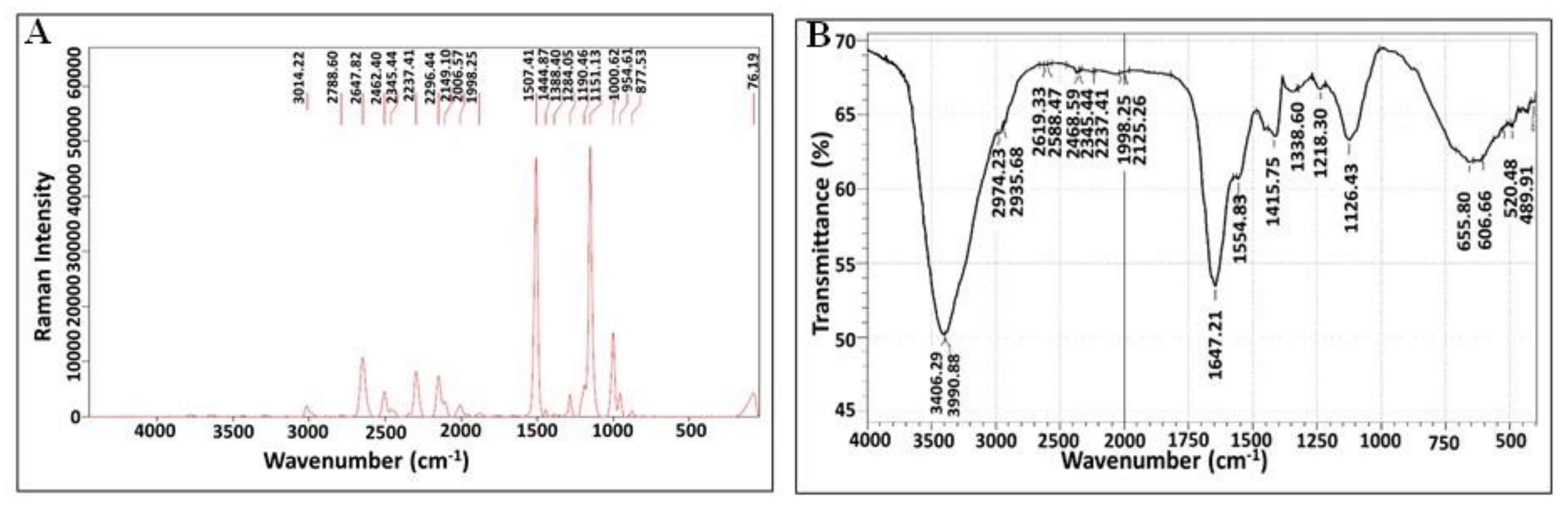
3.6.2. Raman Spectroscopy and Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3.6.3. Thin-Layer Chromatography (TLC) and Liquid Chromatography–Mass Spectrometry (LC–MS)
3.7. Biological Activity of Halophilic Carotenoids
3.7.1. Antimicrobial Activity
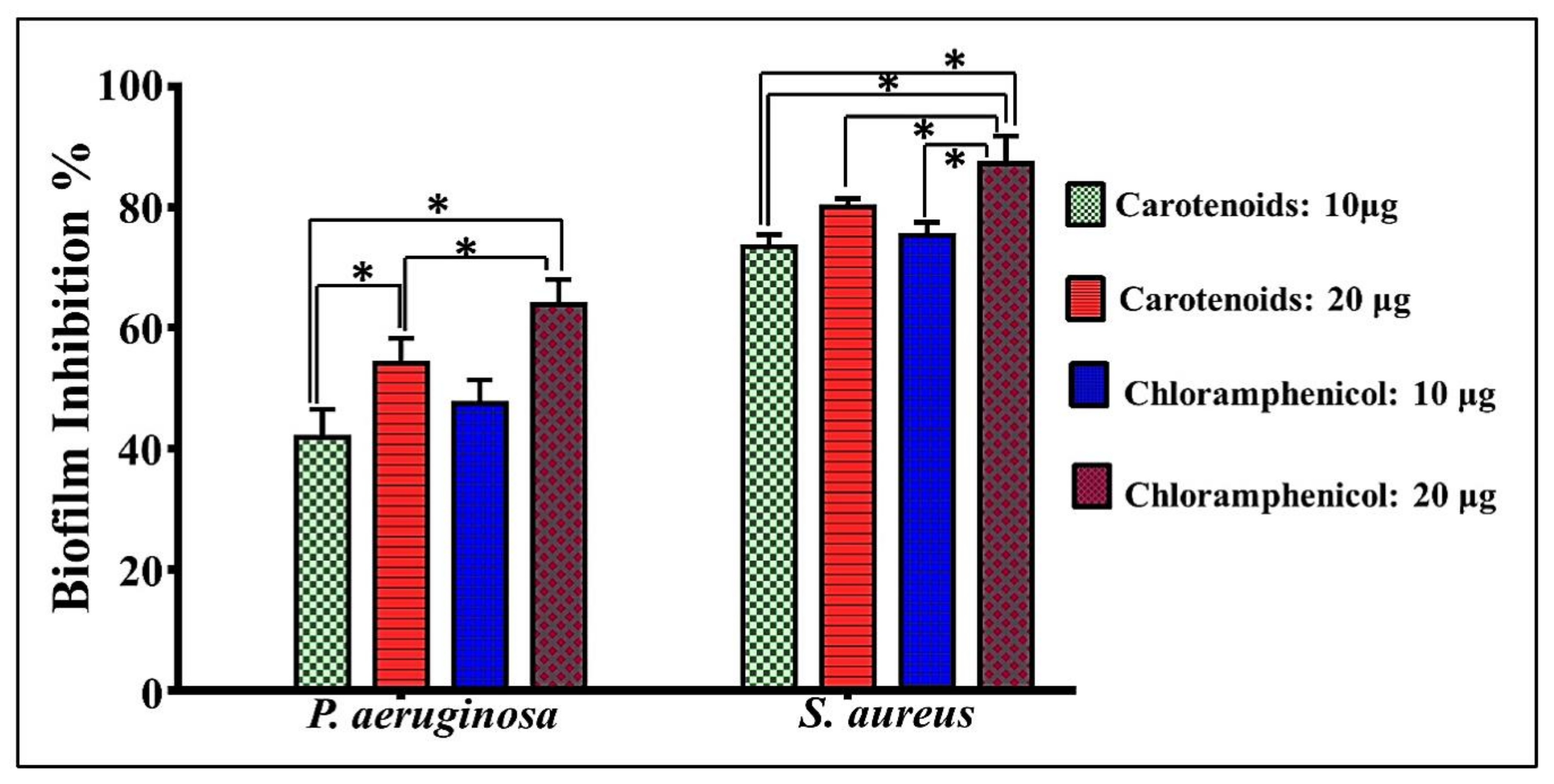
3.7.2. Antibiofilm Potency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kusmita, L.; Nuryadi, H.; Abi Widyananto, P.; Muchlissin, S.; Sabdono, A.; Trianto, A.; Radjasa, O.K. Bioactivity of carotenoid produced by soft coral symbiotic microorganisms from Panjang and Karimunjawa Island, Central Java, Indonesia. J. Biol. Divers. 2021, 2, 19–22. [Google Scholar]
- Ming-Hua, L.; Jianhua, Z.; Jian-Guo, J. Carotenoids biosynthesis and cleavage related genes from bacteria to plants. Crit. Rev. Food Sci. Nutr. 2018, 14, 2314–2333. [Google Scholar]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Neagu, S.; Cojoc, R.; Enache, M.; Mocioiu, O.C.; Precupas, A.; Popa, V.T.; Enache, M. Biotransformation of Waste Glycerol from Biodiesel Industry in Carotenoids Compounds by Halophilic Microorganisms. Waste Biomass Valorization 2019, 10, 45–52. [Google Scholar] [CrossRef]
- Kusmita, L.; Mutiara, E.V.; Nuryadi, H.; Pratama, P.A.; Wiguna, A.S.; Radjasa, O.K. Characterization of carotenoid pigments from bacterial symbionts of soft-coral Sarcophyton sp. from North Java Sea. Int. Aquat. Res. 2017, 9, 61–69. [Google Scholar] [CrossRef]
- Hegazy, G.E.; Abu-Serie, M.M.; Abo-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Abdel-Fattah, Y.R. In vitro dual (anticancer and antiviral) activity of the carotenoids produced by haloalkaliphilic archaeon Natrialba sp. M6. Sci. Rep. 2020, 10, 5986. [Google Scholar] [CrossRef]
- Waditee-Sirisattha, R.; Kageyama, H.; Takabe, T. Halophilic microorganism resources and their applications in industrial and environmental biotechnology. AIMS Microbiol. 2016, 2, 42–54. [Google Scholar] [CrossRef]
- Galaviz-Silva, L.; Iracheta-Villarreal, J.M.; Molina-Garza, Z.J. Bacillus and Virgibacillus strains isolated from three Mexican coasts antagonize Staphylococcus aureus and Vibrio parahaemolyticus. FEMS Microbiol. Lett. 2018, 365, fny202. [Google Scholar] [CrossRef] [PubMed]
- Kusmita, E. Antibacterial Activity of Carotenoid from Bacterial Symbiont Virgibacillus salarius Strain 19. PP. Sc. 1.6 against MDR E. coli and MRSA. Egypt. J. Aquat. Biol. Fish. 2021, 25, 147–157. [Google Scholar] [CrossRef]
- Salem, S.M.; Gammal, E.S. Salt minerals at Wadi El Natrun Saline Lakes, Egypt. New implications from remote sensing data. Eur. Chem. Bull. 2018, 7, 72–80. [Google Scholar] [CrossRef]
- Salgaonkar, B.B.; Mani, K.; Braganca, J.M. Characterization of polyhydroxyalkanoates accumulated by a moderately halophilic salt pan isolate Bacillus megaterium strain H16. J. Appl. Microbiol. 2013, 114, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Agunbiade, M.; Pohl, C.; Ashafa, O. Bioflocculant production from Streptomyces platensis and its potential for river and wastewater treatment. Braz. J. Microbiol. 2018, 49, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.; Ponce, A.; del Valle, C.; Roura, S. Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT 2005, 38, 565–570. [Google Scholar] [CrossRef]
- Eltarahony, M.; Zaki, S.; Abd-El-Haleem, D. Aerobic and anaerobic removal of lead and mercury via calcium carbonate precipitation mediated by statistically optimized nitrate reductases. Sci. Rep. 2020, 10, 4029. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elreesh, G.; El-Shall, H.; Eltarahony, M.; Abdelhaleem, D. Conversion of Cost-Effective agricultural wastes into valued oil using the fungus Curvularia Sp: Isolation, Optimization and Statistical analysis. Biosci. Res. 2019, 16, 3006–3024. [Google Scholar]
- Liu, J.; Miao, S.; Wen, X.; Sun, Y. Optimization of polysaccharides (ABP) extraction from the fruiting bodies of Agaricus blazei Murill using response surface methodology (RSM). Carbohydr. Polym. 2009, 78, 704–709. [Google Scholar] [CrossRef]
- Williams, R.P.; Green, J.A.; Rappoport, D.A. Studies on pigmentation of Serratia marcescens. I. Spectral and paper chromatographic properties of prodigiosin. J. Bacteriol. 1955, 71, 115. [Google Scholar] [CrossRef]
- El-Ziney, M.G.; Zaid, E.A.A.; El-Naggar, M.Y. Characterization of Carotenogenic Rhodotorula Strains Isolated from Delta Region, Egypt and their Potential for Carotenoids Production. J. Pure Appl. Microbiol. 2018, 12, 587–599. [Google Scholar] [CrossRef]
- Caitlin, S.B.; Julian, D.; Jochenl, W.; Eric, A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 6, 515–532. [Google Scholar]
- Eltarahony, M.; Abu-Serie, M.; Hamad, H.; Zaki, S.; Abd-El-Haleem, D. Unveiling the role of novel biogenic functionalized CuFe hybrid nanocomposites in boosting anticancer, antimicrobial and biosorption activities. Sci. Rep. 2021, 11, 7790. [Google Scholar] [CrossRef]
- Veerapagu, M.; Narayanan, A.S.; Ponmurugan, K.; Jeya, K.R. Screening, selection, identification, production and optimization of bacterial lipase from oil spilled soil. Asian J. Pharm. Clin. Res. 2013, 6, 62–67. [Google Scholar]
- Shukla, R.J.; Singh, S.P. Production optimization, purification and characterization of α-amylase from thermophilic Bacillus licheniformis TSI-14. Starch-Stärke 2015, 67, 629–639. [Google Scholar] [CrossRef]
- Elbanna, K.; Ibrahim, I.M.; Revol-Junelles, A.-M. Purification and characterization of halo-alkali-thermophilic protease from Halobacterium sp. strain HP25 isolated from raw salt, Lake Qarun, Fayoum, Egypt. Extremophiles 2015, 19, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Pal, T.K.; Dan, S.; Dan, N. Application of Response Surface Methodology (RSM) in statistical optimization and pharmaceutical characterization of a matrix tablet formulation using metformin HCl as a model drug. Innoriginal Int. J. Sci. 2014, 1, 1–6. [Google Scholar]
- Ibrahim, A.; El-Fakharany, E.M.; Abu-Serie, M.M.; ElKady, M.F.; Eltarahony, M. Methyl Orange Biodegradation by Immobilized Consortium Microspheres: Experimental Design Approach, Toxicity Study and Bioaugmentation Potential. Biology 2022, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Noordin, M.Y.; Venkatesh, V.C.; Sharif, S.; Elting, S.; Abdullah, A. Application of response surface methodology in describing the performance of coated carbide tools when turning AISI 1045 steel. J. Mater. Process. Technol. 2004, 145, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Taavitsainen, V.M.T. Experimental optimization and response surfaces. In Chemometrics in Practical Applications; IntechOpen: London, UK, 2012; pp. 91–138. [Google Scholar]
- Wang, Y.; Ren, W.; Gao, D.; Wang, L.; Yang, Y.; Bai, Q. One-step refolding and purification of recombinant human tumor necrosis factor-α (rhTNF-α) using ion-exchange chromatography. Biomed. Chromatogr. 2015, 29, 305–311. [Google Scholar] [CrossRef]
- Dutka, M.; Ditaranto, M.; Løvås, T. Application of a Central Composite Design for the Study of NOx Emission Performance of a Low NOx Burner. Energies 2015, 8, 3606–3627. [Google Scholar] [CrossRef]
- Zainal, S.; Nadzirah, K.; Noriham, A.; Normah, I. Optimisation of beef tenderisation treated with bromelain using response surface methodology (RSM). Agric. Sci. 2013, 4, 65–72. [Google Scholar] [CrossRef]
- El-Hadi, A.; El-Refai, H.; Shafei, M.; Zaki, R.; Mostafa, H. Statistical optimization of L-asparaginase production by using Fusarium solani. Egypt. Pharm. J. 2017, 16, 16–23. [Google Scholar] [CrossRef]
- Bose, G.K.; Mahapatra, K.K. Parametric study of Die Sinking EDM process on AISI H13 tool steel using statistical techniques. J. Adv. Prod. Eng. Manag. 2014, 9, 168–180. [Google Scholar] [CrossRef]
- Vila, E.; Hornero-Méndez, D.; Azziz, G.; Lareo, C.; Saravia, V. Carotenoids from heterotrophic bacteria isolated from Fildes Peninsula, King George Island, Antarctica. Biotechnol. Rep. 2019, 21, e00306. [Google Scholar] [CrossRef] [PubMed]
- Horiue, H.; Sasaki, M.; Yoshikawa, Y. Raman spectroscopic signatures of carotenoids and polyenes enable label-freevisuilzation of microbial distributionwithin pink biofilms. Sci. Rep. 2020, 10, 7704. [Google Scholar] [CrossRef]
- Lorand, T.; Deli, J.; Molnar, P.; Toth, G. FT-IR study of some carotenoids. Helv. Chim. Acta 2002, 85, 1691–1697. [Google Scholar] [CrossRef]
- Prasad, K.; Jha, A.K.; Kulkarni, A.R. Synthesis of nickel nanoparticles: Bioreduction method. Nanosci. Nanotechnol. 2008, 2, 83–86. [Google Scholar]
- Sorokin, D.Y.; Banciu, H.L.; Muyzer, G. Functional microbiology of soda lakes. Curr. Microbiol. 2015, 25, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, O.E.; Sudagidan, M.; Yurt, M.N.; Tasbasi, B.B.; Acar, E.E.; Ozalp, V.C. Microbial community of soda Lake Van as obtained from direct and enriched water, sediment and fish samples. Sci. Rep. 2021, 11, 18364. [Google Scholar] [CrossRef]
- Heer, K.; Sharma, S. Microbial pigments as a natural color. Int. J. Pharm. Sci. Res. 2017, 8, 1913. [Google Scholar]
- Usman, H.M.; Farouq, A.A.; Baki, A.S.; Abdulkadir, N.; Gani, M. Production and characterization of orange pigment produced by Halophilic bacterium Salinococcus roseus isolated from Abattoir soil. J. Microbiol. Exp. 2018, 6, 238–243. [Google Scholar] [CrossRef]
- Seel, W.; Baust, D.; Sons, D.; Albers, M.; Etzbach, L.; Fuss, J.; Lipski, A. Carotenoids are used as regulators for membrane fluidity by Staphylococcus xylosus. Sci. Rep. 2020, 10, 330. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Gleichenhagen, M.; Heller, A.; Esquivel, P.; Schulze-Kaysers, N.; Schieber, A. Carotenoid profile, antioxidant capacity, and chromoplasts of pink guava (Psidium guajava L. Cv. ‘Criolla’) during fruit ripening. J. Agric. Food Chem. 2017, 65, 3737–3747. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, S.J.; Srivastava, P.; Ramachandran, S.; Sivashanmugam, K.; Gothandam, K.M. Lutein: A potential antibiofilm and antiquorum sensing molecule from green microalga Chlorella pyrenoidosa. Microb. Pathog. 2019, 135, 103658. [Google Scholar] [CrossRef] [PubMed]
- Guardini, Z.; Dall’Osto, L.; Barera, S.; Jaberi, M.; Cazzaniga, S.; Vitulo, N.; Bassi, R. High carotenoid mutants of Chlorella vulgaris show enhanced biomass yield under high irradiance. Plants 2021, 5, 911. [Google Scholar] [CrossRef]
- Asker, D.; Awad, T.S.; Beppu, T.; Ueda, K. Isolation, characterization, and diversity of novel radiotolerant carotenoid-producing bacteria. J. Mol. Biol. 2012, 892, 21–60. [Google Scholar]
- Xueqin, L.; Yanli, F.; Xiaohua, L.; Tian, D.; Xin, L.; Mingsheng, L.; Shujun, W. Virgibacillus halodenitrificans ST-1 for fermentation of shrimp paste and hydrolysates of its protease. Food Sci. Nutr. 2020, 8, 5352–5361. [Google Scholar]
- Hardijito, L.; Huq, A.; Colwell, R.R. The Influence of environmental conditions on the production of pigment by Serratia marcescens. Biotechnol. Bioprocess Engineer. 2002, 7, 100–104. [Google Scholar] [CrossRef]
- Ibrahim, A.S. Production of carotenoids by a newly isolated marine Micrococcus sp. Biotechnology 2008, 7, 469–474. [Google Scholar] [CrossRef]
- Arora, P.K.; Sharma, A.; Mehta, R.; Shenoy, B.D.; Srivastava, A.; Singh, V.P. Metabolism of 4-chloro-2-nitrophenol in a Gram-positive bacterium, Exiguo bacterium sp. PMA. Microb. Cell Factories 2012, 11, 150. [Google Scholar] [CrossRef]
- Mondal, S.K.; Samantaray, D.P.; Mishra, B.B. Optimization of pigment production by a novel Bacillus sp. BBMRH isolated from cow dung. J. Pure Appl. Microbiol. 2015, 9, 2321–2326. [Google Scholar]
- Hamidi, M.; Abdin, Z.M.; Nazemyieh, H.; Hejazi, A.M.; Hejazi, S.M. Optimization of total carotenoid production by Halorubrum sp. TBZ126 using response surface methodology. J. Microb. Biochem. Technol. 2014, 6, 286–294. [Google Scholar] [CrossRef]
- Rodriguez-Valera, F.; Ventosa, A.; Juez, G.; Imhoff, J.F. Variation of environmental features and microbial populations with salt concentrations in a multi-pond saltern. Microb. Ecol. 1985, 11, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, C.; Pancha, I.; Ghosh, T.; Maurya, R.; Chokshi, K.; Bharadwaj, S.V.; Ram, S.; Mishra, S. Selective carotenoid accumulation by varying nutrient media and salinity in Synechocystis sp. CCNM 2501. Bioresour. Technol. 2015, 197, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Al Disi, Z.A.; Bontognali, T.R.; Jaoua, S.; Attia, E.; Al-Kuwari, H.A.; Zouari, N. Influence of temperature, salinity and Mg2+: Ca2+ ratio on microbially-mediated formation of Mg-rich carbonates by Virgibacillus strains isolated from a sabkha environment. Sci. Rep. 2019, 9, 19633. [Google Scholar] [CrossRef] [PubMed]
- Allahkarami, S.; Sepahi, A.A.; Hosseini, H.; Razavi, M.R. Isolation and identification of carotenoid-producing Rhodotorula sp. from Pinaceae forest ecosystems and optimization of in vitro carotenoid production. Biotechnol. Rep. 2021, 32, e00687. [Google Scholar] [CrossRef]
- El-banna, A.; Abd El-Razek, A.; Elmahdy, A. Isolation, identification and screening of carotenoid-producing strains of Rhodotorula glutinis. Food Nutr. Sci. 2012, 3, 627–633. [Google Scholar]
- Zhao, Y.; Guo, L.; Xia, Y.; Zhuang, X.; Chu, W. Isolation, identification of carotenoid-producing Rhodotorula sp. from marine environment and optimization for carotenoid production. Mar. Drugs 2019, 17, 161. [Google Scholar] [CrossRef]
- Silva, C.M.; de Borba, T.D.; Kalil, S.J.; de Medeiros, J.F. Raw glycerol and parboiled rice effluent for carotenoid production: Effect of the composition of culture medium and initial pH. Food Technol. Biotechnol. 2016, 54, 489–496. [Google Scholar]
- Sumathi, C.; MohanaPriya, D.; Swarnalatha, S.; Dinesh, M.G.; Sekaran, G. Production of prodigiosin using tannery fleshing and evaluating its pharmacological effects. Sci. World J. 2014, 2014, 290327. [Google Scholar] [CrossRef]
- Abdel-Raheam, H.; Hassan, S.; Ali, M. Production and application of natural food pigments by Monascus ruber using potato chips manufacturing wastes. Bull. Pharm. Sci. 2021, 44, 521–533. [Google Scholar] [CrossRef]
- Gutiérrez, F.J.; Albillos, S.M.; Casas-Sanz, E.; Cruz, Z.; GarcíaEstrada, C.; García-Guerra, A.; García-Reverter, J.; García-Suárez, M.; Gatón, P.; González-Ferrero, C.; et al. Methods for the nanoencapsulation of β-carotene in the food sector. Trends Food Sci. Technol. 2013, 32, 73–83. [Google Scholar] [CrossRef]
- Liang, R.; Huang, Q.; Ma, J.; Shoemaker, C.F.; Zhong, F. Effect of relative humidity on the store stability of spray-dried beta carotene nanoemulsions. Food Hydrocoll. 2013, 33, 225–233. [Google Scholar] [CrossRef]
- Mao, L.K.; Xu, D.X.; Yang, J.; Yuan, F.; Gao, X.Y.; Zhao, J. Effect of small and large molecule emulsifiers on the characteristics of beta-carotene nanoemulsions prepared by high pressure homogenization. Food Technol. Biotechnol. 2009, 47, 336–342. [Google Scholar]
- Shatila, F.; Yusef, H.; Holail, H. Pigment production by Exiguobacterium aurantiacum FH, a novel Lebanese strain. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 176–191. [Google Scholar]
- Anuradha, M.; Puradha, M.; Priyanka, K.; Rama, B. Production and partial characterization of pigments produced by Kocuria sp Bri36: Influence of heavy metals. Int. J. Pharm. Pharm. Sci. 2017, 10, 137–145. [Google Scholar]
- Rajan, S.; Gargi, G. Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: A statistical approach. Biotechnol. Rep. 2020, 25, e00407. [Google Scholar]
- Asker, D.; Awad, T.S.; Beppu, T.; Ueda, K. Screening, Isolation, and Identification of Zeaxanthin-Producing Bacteria. Methods Mol. Biol. 2018, 1852, 193–209. [Google Scholar]
- Etzbach, L.; Pfeiffer, A.; Weber, F.; Schieber, A. Characterization of carotenoid profiles in goldenberry (Physalis peruviana L.) fruits at various ripening stages and in different plant tissues by (HPLC-DADAPCI-MS). Food Chem. 2018, 245, 508–517. [Google Scholar] [CrossRef]
- Manimala, M.R.A.; Murugesan, R. In vitro antioxidant and antimicrobial activity of carotenoid pigment extracted from Sporobolomyces sp. isolated from natural source. J. Appl. Nat. Sci. 2014, 6, 649–653. [Google Scholar] [CrossRef]
- Haddad, M.; Aghaei, S.; Zargar, M. Antimicrobial and Antioxidant Activity of Carotenoid Pigment Produced by Native Rhodococcus spp. Isolated from Soil. Int. J. Mol. Clin. Microbiol. 2017, 7, 809–815. [Google Scholar]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An antibacterial carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Alam, R.; Łochyńska, M.; Stasiewicz, M. What Do We Know about Antimicrobial Activity of Astaxanthin and Fucoxanthin? Mar. Drugs 2021, 29, 36. [Google Scholar] [CrossRef] [PubMed]
- Jemil, N.; Ayed, B.A.; Manresa, A.; Nasri, M.; Hamidet, N. Antioxidant properties, antimicrobial and anti-adhesive activities of DCS1 lipopeptides from Bacillus methylotrophicus DCS1. BMC Microbiol. J. 2017, 17, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Source | DF | Seq SS | Adj SS | Adj MS | F | p |
|---|---|---|---|---|---|---|
| Main Effects | 8 | 0.33246 | 0.33246 | 0.04156 | 19.1 | 0.017 |
| Residual Error | 3 | 0.00653 | 0.00653 | 0.00218 | ||
| Total | 11 | 0.33899 |
| Run Order | Yeast Extract (g) | Peptone (g) | NaCl (g) | Inoculum Size (%) | Experimental Pigment Weight (mg/mL) | Predicted Pigment Weight (mg/mL) | St. Residual |
|---|---|---|---|---|---|---|---|
| 1 | −1 | 1 | −1 | 1 | 7.89 | 9.84 | −1.24 |
| 2 | 0 | 0 | 0 | 2 | 18.57 | 20.78 | −1.41 |
| 3 | 1 | −1 | −1 | −1 | 12.31 | 13.21 | −0.58 |
| 4 | −1 | 1 | 1 | −1 | 6.68 | 8.05 | −0.88 |
| 5 | 2 | 0 | 0 | 0 | 8.36 | 10.21 | −1.18 |
| 6 | 1 | −1 | 1 | −1 | 8.68 | 9.31 | −0.39 |
| 7 | 1 | 1 | 1 | −1 | 13.36 | 12.73 | 0.4 |
| 8 | −1 | −1 | −1 | 1 | 15.73 | 14.57 | 0.75 |
| 9 | 0 | 0 | 0 | 0 | 22.36 | 21.1 | 0.57 |
| 10 | 1 | −1 | 1 | 1 | 12.89 | 10.36 | 1.62 |
| 11 | 1 | 1 | −1 | −1 | 6.26 | 6.47 | −0.15 |
| 12 | −1 | −1 | 1 | 1 | 7.0 | 9.31 | −1.49 |
| 13 | −1 | −1 | 1 | −1 | 0.263 | 1.47 | −0.76 |
| 14 | 1 | 1 | 1 | 1 | 12.36 | 12.68 | −0.19 |
| 15 | 0 | 0 | 0 | 0 | 21.1 | 21.1 | 0 |
| 16 | 0 | 0 | 0 | 0 | 21.68 | 21.1 | 0.26 |
| 17 | −1 | −1 | −1 | −1 | 3.05 | 5.31 | −1.45 |
| 18 | 0 | 0 | 2 | 0 | 0.21 | 0.631 | −0.26 |
| 19 | 1 | 1 | −1 | 1 | 10.78 | 7.84 | 1.91 |
| 20 | 0 | −2 | 0 | 0 | 17.36 | 15.36 | 1.28 |
| 21 | 1 | −1 | −1 | 1 | 14.52 | 15.68 | −0.76 |
| 22 | −1 | 1 | −1 | −1 | 1.0 | 1.73 | −0.47 |
| 23 | 0 | 0 | 0 | 0 | 20.78 | 21.1 | −0.14 |
| 24 | 0 | 0 | −2 | 0 | 0.789 | 0.368 | 0.75 |
| 25 | −2 | 0 | 0 | 0 | 6.94 | 4.36 | 1.66 |
| 26 | 0 | 0 | 0 | 0 | 20.94 | 21.1 | −0.07 |
| 27 | 0 | 2 | 0 | 0 | 12.84 | 14.1 | −0.8 |
| 28 | 0 | 0 | 0 | 0 | 17.73 | 21.1 | −1.51 |
| 29 | 0 | 0 | 0 | 0 | 23.1 | 21.1 | 0.9 |
| 30 | 0 | 0 | 0 | −2 | 14.52 | 11.57 | 1.89 |
| 31 | −1 | 1 | 1 | 1 | 17.42 | 14.73 | 1.73 |
| Variable | Coded Levels/Experimental Values | ||||||
| −2 | −1 | 0 | 1 | 2 | |||
| Yeast Extract (g) | 0.5 | 1 | 2 | 4 | 7 | ||
| Peptone (g) | 3 | 5 | 10 | 15 | 20 | ||
| NaCl (g) | 58.4 | 116.8 | 233.6 | 350.4 | 467.2 | ||
| Inoculum Size (%) | 0.1% | 0.3% | 0.5% | 2% | 4% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayez, D.; Youssif, A.; Sabry, S.; Ghozlan, H.; Eltarahony, M. Carotegenic Virgibacillus halodenitrificans from Wadi El-Natrun Salt Lakes: Isolation, Optimization, Characterization and Biological Activities of Carotenoids. Biology 2022, 11, 1407. https://doi.org/10.3390/biology11101407
Fayez D, Youssif A, Sabry S, Ghozlan H, Eltarahony M. Carotegenic Virgibacillus halodenitrificans from Wadi El-Natrun Salt Lakes: Isolation, Optimization, Characterization and Biological Activities of Carotenoids. Biology. 2022; 11(10):1407. https://doi.org/10.3390/biology11101407
Chicago/Turabian StyleFayez, Doaa, Asmaa Youssif, Soraya Sabry, Hanan Ghozlan, and Marwa Eltarahony. 2022. "Carotegenic Virgibacillus halodenitrificans from Wadi El-Natrun Salt Lakes: Isolation, Optimization, Characterization and Biological Activities of Carotenoids" Biology 11, no. 10: 1407. https://doi.org/10.3390/biology11101407






