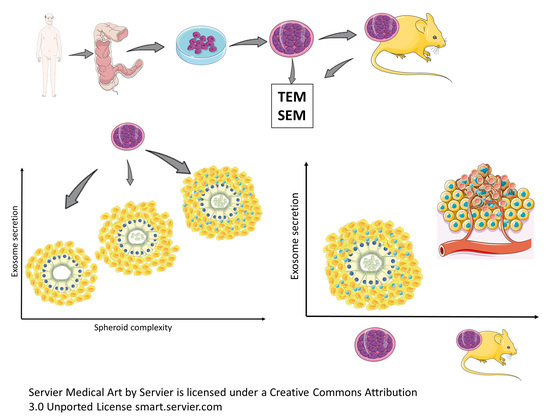
A Different Exosome Secretion Pattern Characterizes Patient-Derived Colorectal Cancer Multicellular Spheroids and Their Mouse Xenografts
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. CSC MTSs Isolation and Culture
- Patient
- Cancer biopsies management (immediately after recovery)
- Biopsies dissociation procedure
- Cell culture
2.2. Animal Procedures
- Animals
- Xenograft extraction and treatment
2.3. Scanning Electron Microscopy (SEM) Protocol for MTSs
- Primary fixation (immediately upon recovery)
- Washing: samples were rinsed overnight in Phosphate buffer solution 0.1 M, pH 7.4 at 4 °C.
- Post-fixation
- Dehydration procedure
- Critical point drying procedure (Emitech K850, Emitech, Corato, Italy).
- Samples were mounted on aluminum stubs using carbon tape.
- Sputter coating procedure.
- Observation
2.4. Transmission Electron Microscopy (TEM) Protocol for MTSs and Xenograft
- Primary fixation (immediately upon recovery)
- Washing: samples were rinsed overnight in Phosphate buffer solution 0.1 M, pH 7.4 at 4 °C.
- Post-fixation
- Washing: phosphate buffer solution 0.1 M, pH 7.4 for 20 min (10 min + 10 min) was used to remove (OsO4) residuals.
- Dehydration procedure
- Substitution procedure
- Embedding procedure
- Semithin sections (1 m thick) were collected on glass slides, stained blue by methylene blue, to perform light microscopy observations by a Zeiss Axioskop-40 (Carl Zeiss, Oberkochen, Germany) equipped with Axiovision image acquisition software.
- Ultrathin sections for TEM observations were cut using an ultramicrotome (Leica EM UC6, Vienna, Austria). Ultrathin sections were collected on 100-mesh copper grids (Assing, Rome, Italy). Staining was performed using Uranyless© solution and lead citrate 3% solution (Electron Microscopy Science, 1560 Industry Road, Hatfield, PA, USA).
- Observation under a transmission electron microscope (Carl Zeiss EM10, Thornwood, NY, USA) set with an accelerating voltage of 60 kV.
- Digital image acquisition system: CCD digital camera (AMT CCD, Deben UK Ltd., Suffolk, UK).
2.5. Exosome and Multivesicular Bodies (MVBs) Size Measurement and Statistical Analysis
3. Results
3.1. SEM Analysis of MTSs Morphology and Exosomes Secretion
3.2. LM (Light Microscopy) and TEM Analysis of MTSs Cells Morphology and Exosomes Secretion
3.3. LM and TEM Analysis of Xenograft Morphology and Exosomes Secretion
3.4. Analysis of Exosomes and MVBs Morphology and Size by Transmission Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer Stat Facts: Colorectal Cancer. National Cancer Institute. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 15 July 2022).
- Naxerova, J.G.; Reiter, E.; Brachtel, J.K.; Lennerz, M.; van de Wetering, A.; Rowan, T.; Cai, H.; Clevers, C.; Swanton, M.A.; Nowak, M.A.; et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science 2017, 357, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Linnekamp, J.F.; Wang, X.; Medema, J.P.; Vermeulen, L. Colorectal cancer heterogeneity and targeted therapy: A case for molecular disease subtypes. Cancer Res. 2015, 75, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Wasmuth, H.H.; Rekstad, L.C.; Trano, G. The outcome and the frequency of pathological complete response after neoadjuvant radiotherapy in curative resections for advanced rectal cancer: A population-based study. Color. Dis. 2016, 18, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Moradi Marjaneh, R.; Hassanian, S.M.; Ghobadi, N.; Ferns, G.A.; Karimi, A.; Jazayeri, M.H.; Nasiri, M.; Avan, A.; Khazaei, M. Targeting the death receptor signaling pathway as a potential therapeutic target in the treatment of colorectal cancer. J. Cell. Physiol. 2018, 233, 6538–6549. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Sholl, L.M.; Aisner, D.L.; Varella-Garcia, M.; Berry, L.D.; Dias-Santagata, D.; Wistuba, I.I.; Chen, H.; Fujimoto, J.; Kugler, K.; Franklin, W.A.; et al. Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: The Lung Cancer Mutation Consortium experience. J. Thorac. Oncol. 2015, 10, 768–777. [Google Scholar] [CrossRef]
- De Angelis, M.L.; Francescangeli, F.; Zeuner, A.; Baiocchi, M. Colorectal Cancer Stem Cells: An Overview of Evolving Methods and Concepts. Cancers 2021, 13, 5910. [Google Scholar] [CrossRef]
- De Angelis, M.L.; Zeuner, A.; Policicchio, E.; Russo, G.; Bruselles, A.; Signore, M.; Vitale, S.; De Luca, G.; Pilozzi, E.; Boe, A.; et al. Cancer Stem Cell-Based Models of Colorectal Cancer Reveal Molecular Determinants of Therapy Resistance. Stem Cells Transl. Med. 2016, 5, 511–523. [Google Scholar] [CrossRef]
- Nikolouzakis, T.K.; Vassilopoulou, L.; Fragkiadaki, P.; Mariolis Sapsakos, T.; Papadakis, G.Z.; Spandidos, D.A.; Tsatsakis, A.M.; Tsiaoussis, J. Improving diagnosis, prognosis and prediction by using biomarkers in CRC patients. Oncol. Rep. 2018, 39, 2455–2472. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Zhou, S.; Yuan, T. The “sugar-coated bullets” of cancer: Tumor-derived exosome surface glycosylation from basic knowledge to applications. Clin. Transl. Med. 2020, 10, e204. [Google Scholar] [CrossRef]
- Bracci, L.; Lozupone, F.; Parolini, I. The role of exosomes in colorectal cancer disease progression and response to therapy. Cytokine Growth Factor Rev. 2020, 51, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, X.; Tang, W.; Familiari, G.; Relucenti, M.; Aschner, M.; Li, X.; Chen, R. Chemotherapeutic Risk lncRNA-PVT1 SNP Sensitizes Metastatic Colorectal Cancer to FOLFOX Regimen. Front. Oncol. 2022, 12, 808889. [Google Scholar] [CrossRef]
- Hashemipour, M.; Boroumand, H.; Mollazadeh, S.; Tajiknia, V.; Nourollahzadeh, Z.; Borj, M.R.; Pourghadamyari, H.; Rahimian, N.; Hamblin, M.R.; Mirzaei, H. Exosomal microRNAs and exosomal long non-coding RNAs in gynecologic cancers. Gynecol. Oncol. 2021, 161, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Meng, Q.; Shi, C.; Yang, H.; Li, X.; Wu, S.; Familiari, G.; Relucenti, M.; Aschner, M.; Wang, X.; et al. Hypoxia-Inducible Exosomes Facilitate Liver-Tropic Premetastatic Niche in Colorectal Cancer. Hepatology 2021, 74, 2633–2651. [Google Scholar] [CrossRef]
- Xu, J.; Meng, Q.; Li, X.; Yang, H.; Xu, J.; Gao, N.; Sun, H.; Wu, S.; Familiari, G.; Relucenti, M.; et al. Long Noncoding RNA MIR17HG Promotes Colorectal Cancer Progression via miR-17-5p. Cancer Res. 2019, 79, 4882–4895. [Google Scholar] [CrossRef]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012, 151, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Scavo, M.P.; Rizzi, F.; Depalo, N.; Fanizza, E.; Ingrosso, C.; Curri, M.L.; Giannelli, G. A possible role of FZD10 delivering exosomes derived from colon cancers cell lines in inducing activation of epithelial–mesenchymal transition in normal colon epithelial cell line. Int. J. Mol. Sci. 2020, 21, 6705. [Google Scholar] [CrossRef]
- Deng, X.; Ruan, H.; Zhang, X.; Xu, X.; Zhu, Y.; Peng, H.; Zhang, X.; Kong, F.; Guan, M. Long noncoding RNA CCAL transferred from fibroblasts by exosomes promotes chemoresistance of colorectal cancer cells. Int. J. Cancer 2020, 146, 1700–1716. [Google Scholar] [CrossRef]
- Glass, S.E.; Coffey, R.J. Recent Advances in the Study of Extracellular Vesicles in Colorectal Cancer. Gastroenterology 2022, 18. [Google Scholar] [CrossRef]
- Nabariya, D.K.; Pallu, R.; Yenuganti, V.R. Exosomes: The protagonists in the tale of colorectal cancer? Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188426. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.L.; Chen, K.C.; Hsieh, J.T.; Shen, T.L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Relucenti, M.; Francescangeli, F.; De Angelis, M.L.; D’Andrea, V.; Miglietta, S.; Pilozzi, E.; Li, X.; Boe, A.; Chen, R.; Zeuner, A.; et al. The Ultrastructural Analysis of Human Colorectal Cancer Stem Cell-Derived MTSs and Their Mouse Xenograft Shows That the Same Cells Types Have Different Ratios. Biology 2021, 10, 929. [Google Scholar] [CrossRef]
- Correr, S.; Makabe, S.; Heyn, R.; Relucenti, M.; Naguro, T.; Familiari, G. Microplicae-like structures of the fallopian tube in postmenopausal women as shown by electron microscopy. Histol. Histopathol. 2006, 21, 219–226. [Google Scholar] [PubMed]
- Cottignoli, V.; Relucenti, M.; Agrosì, G.; Cavarretta, E.; Familiari, G.; Salvador, L.; Maras, A. Biological Niches within Human Calcified Aortic Valves: Towards Understanding of the Pathological Biomineralization Process. BioMed Res. Int. 2015, 2015, 542687. [Google Scholar] [CrossRef] [PubMed]
- Lo Torto, F.; Relucenti, M.; Familiari, G.; Vaia, N.; Casella, D.; Matassa, R.; Miglietta, S.; Marinozzi, F.; Bini, F.; Fratoddi, I.; et al. The Effect of Postmastectomy Radiation Therapy on Breast Implants: Material Analysis on Silicone and Polyurethane Prosthesis. Ann. Plast. Surg. 2018, 81, 228–234. [Google Scholar] [CrossRef]
- Forte, M.; Bianchi, F.; Cotugno, M.; Marchitti, S.; De Falco, E.; Raffa, S.; Stanzione, R.; Di Nonno, F.; Chimenti, I.; Palmerio, S.; et al. Pharmacological restoration of autophagy reduces hypertension-related stroke occurrence. Autophagy 2020, 16, 1468–1481. [Google Scholar] [CrossRef]
- Relucenti, M.; Heyn, R.; Petruzziello, L.; Pugliese, G.; Taurino, M.; Familiari, G. Detecting microcalcifications in atherosclerotic plaques by a simple trichromic staining method for epoxy embedded carotid endarterectomies. Eur. J. Histochem. 2010, 54, e33. [Google Scholar] [CrossRef]
- Nardoni, M.; Della Valle, E.; Liberti, M.; Relucenti, M.; Casadei, M.A.; Paolicelli, P.; Apollonio, F.; Petralito, S. Can Pulsed Electromagnetic Fields Trigger On-Demand Drug Release from High-Tm Magnetoliposomes? Nanomaterials 2018, 8, 196. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Relucenti, M.; Miglietta, S.; Bove, G.; Donfrancesco, O.; Battaglione, E.; Familiari, P.; Barbaranelli, C.; Covelli, E.; Barbara, M.; Familiari, G. SEM BSE 3D Image Analysis of Human Incus Bone Affected by Cholesteatoma Ascribes to Osteoclasts the Bone Erosion and VpSEM dEDX Analysis Reveals New Bone Formation. Scanning 2020, 2020, 9371516. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; Raposo, G.; Candalh, C.; Boussac, M.; Hershberg, R.; Cerf-Bensussan, N.; Heyman, M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 2001, 121, 337–349. [Google Scholar] [CrossRef]
- Mallegol, J.; van Niel, G.; Heyman, M. Phenotypic and functional characterization of intestinal epithelial exosomes. Blood Cells Mol. Dis. 2005, 35, 11–16. [Google Scholar] [CrossRef]
- Zeuner, A.; Todaro, M.; Stassi, G.; De Maria, R. Colorectal cancer stem cells: From the crypt to the clinic. Cell Stem Cell 2014, 15, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Francescangeli, F.; Contavalli, P.; De Angelis, M.L.; Baiocchi, M.; Gambara, G.; Pagliuca, A.; Fiorenzano, A.; Prezioso, C.; Boe, A.; Todaro, M.; et al. Dynamic regulation of the cancer stem cell compartment by Cripto-1 in colorectal cancer. Cell Death Differ. 2015, 22, 1700–1713. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Gao, X.; Kang, B.; Liu, Y.; Wang, D.; Wang, Y. The Role of Tumor Stem Cell Exosomes in Cancer Invasion and Metastasis. Front. Oncol. 2022, 12, 836548. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Mathivanan, S.; Ji, H.; Simpson, R.J. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol. Cell. Proteom. 2013, 12, 587–598. [Google Scholar] [CrossRef]
- Dang, Y.; Zhang, S.; Wang, Y.; Zhao, G.; Chen, C.; Jiang, W. State-of-the-Art: Exosomes in Colorectal Cancer. Curr. Cancer Drug Targets 2022, 22, 2–17. [Google Scholar] [CrossRef]
- Li, Q. Role of exosomes in cellular communication between tumor cells and the tumor microenvironment. Oncol. Lett. 2022, 24, 240. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed]














| MBVs (200 Analyzed Cells for Each Group) | Arithmetic Mean | Std. Error | 95% CI |
|---|---|---|---|
| MVBs Sph Apical | 3.07 | 0.22 | 2.6272 to 3.5128 |
| MVBs Sph Lateral | 0.65 | 0.56 | 0.5504 to 0.7496 |
| MVBs Xeno Apical | 41.52 | 0.05 | 40.4102 to 42.6298 |
| MVBs Xeno Lateral | 3.94 | 0.09 | 3.7659 to 4.1241 |
| Sample Diameter (N = 200 Each Group) | Lower Value (nm) | Higher Value (nm) | Mean | Std. Error | 95% CI |
|---|---|---|---|---|---|
| Sph Apical | 50 | 71 | 61.2637 | 0.4633 | 60.3502 to 62.1772 |
| Sph Lateral | 50 | 72 | 60.7761 | 0.4829 | 59.8240 to 61.7283 |
| Xeno Apical | 51 | 73 | 60.8756 | 0.4776 | 59. 9338 to 61.8174 |
| Xeno Lateral | 50 | 71 | 61.1841 | 0.4791 | 60.2394 to 62.1288 |
| (N = 200 Each) | Lower Value (nm) | Higher Value (nm) | Mean | 95% CI | Variance | Standard Deviation |
|---|---|---|---|---|---|---|
| MVB Sph | 134 | 240 | 196.8550 | 192.0112–201.6988 | 1206.7377 | 34.7381 |
| MVB Xeno | 131 | 243 | 197.4200 | 192.5860–202.2540 | 1201.8529 | 34.6677 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Relucenti, M.; Francescangeli, F.; De Angelis, M.L.; D’Andrea, V.; Miglietta, S.; Donfrancesco, O.; Li, X.; Chen, R.; Zeuner, A.; Familiari, G. A Different Exosome Secretion Pattern Characterizes Patient-Derived Colorectal Cancer Multicellular Spheroids and Their Mouse Xenografts. Biology 2022, 11, 1427. https://doi.org/10.3390/biology11101427
Relucenti M, Francescangeli F, De Angelis ML, D’Andrea V, Miglietta S, Donfrancesco O, Li X, Chen R, Zeuner A, Familiari G. A Different Exosome Secretion Pattern Characterizes Patient-Derived Colorectal Cancer Multicellular Spheroids and Their Mouse Xenografts. Biology. 2022; 11(10):1427. https://doi.org/10.3390/biology11101427
Chicago/Turabian StyleRelucenti, Michela, Federica Francescangeli, Maria Laura De Angelis, Vito D’Andrea, Selenia Miglietta, Orlando Donfrancesco, Xiaobo Li, Rui Chen, Ann Zeuner, and Giuseppe Familiari. 2022. "A Different Exosome Secretion Pattern Characterizes Patient-Derived Colorectal Cancer Multicellular Spheroids and Their Mouse Xenografts" Biology 11, no. 10: 1427. https://doi.org/10.3390/biology11101427
APA StyleRelucenti, M., Francescangeli, F., De Angelis, M. L., D’Andrea, V., Miglietta, S., Donfrancesco, O., Li, X., Chen, R., Zeuner, A., & Familiari, G. (2022). A Different Exosome Secretion Pattern Characterizes Patient-Derived Colorectal Cancer Multicellular Spheroids and Their Mouse Xenografts. Biology, 11(10), 1427. https://doi.org/10.3390/biology11101427