Dunaliella salina Microalga Restores the Metabolic Equilibrium and Ameliorates the Hepatic Inflammatory Response Induced by Zinc Oxide Nanoparticles (ZnO-NPs) in Male Zebrafish
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Zinc Oxide Nanoparticles Preparation
2.2. Zebrafish Maintenance
2.3. Diets Formulation
2.4. Acute Toxicity Study (Determining the Median Lethal Concentration; 96-h LC50)
2.5. Antidotal Study
2.6. Determination of Fishes’ Whole Body Chemical Composition
2.7. ZnO-NPs Residues Assessments in the Whole Fish Body
2.8. Determination of Total Intestinal Bacteria and Aeromonas Counts
2.9. Assessments of Hepatic Glycogen, Lipids, and Histopathological Analysis
2.10. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
2.11. Immunohistochemistry and Image Analysis
2.12. Statistical Analysis
3. Results
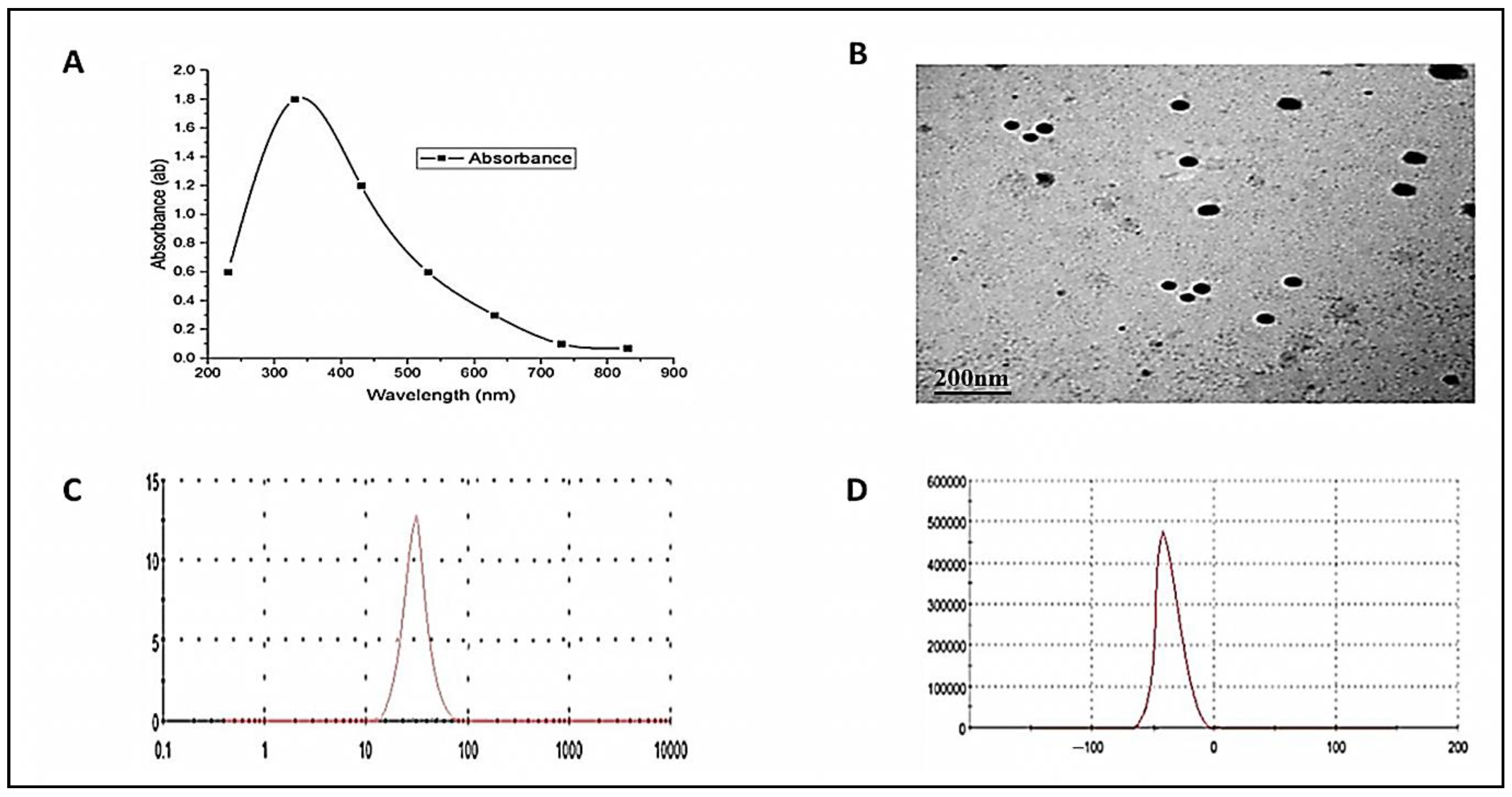
3.1. ZnO-NPs Characterization
3.2. The Value of Estimated 96-h LC50 and Behavioral Responses in ZnO-NPs Exposed Male Zebrafish
3.3. D. salina Restores the Appetite Loss of ZnO-NPs Exposed Males
3.4. Determination of ZnO-NPs Residues and their Effects on the Whole-Body Composition
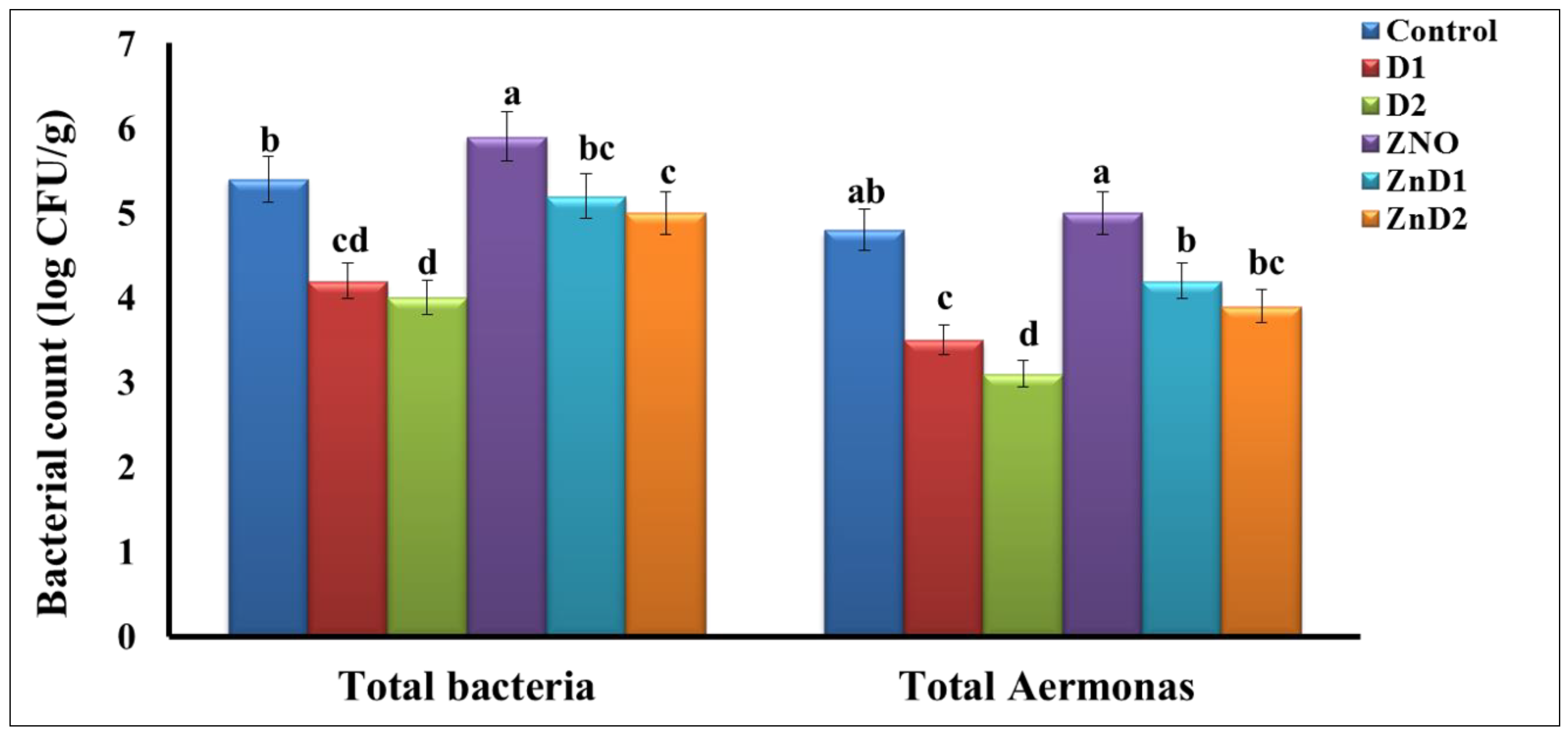
3.5. D. salina Reduced Intestinal Total Bacterial and Aeromonas Counts
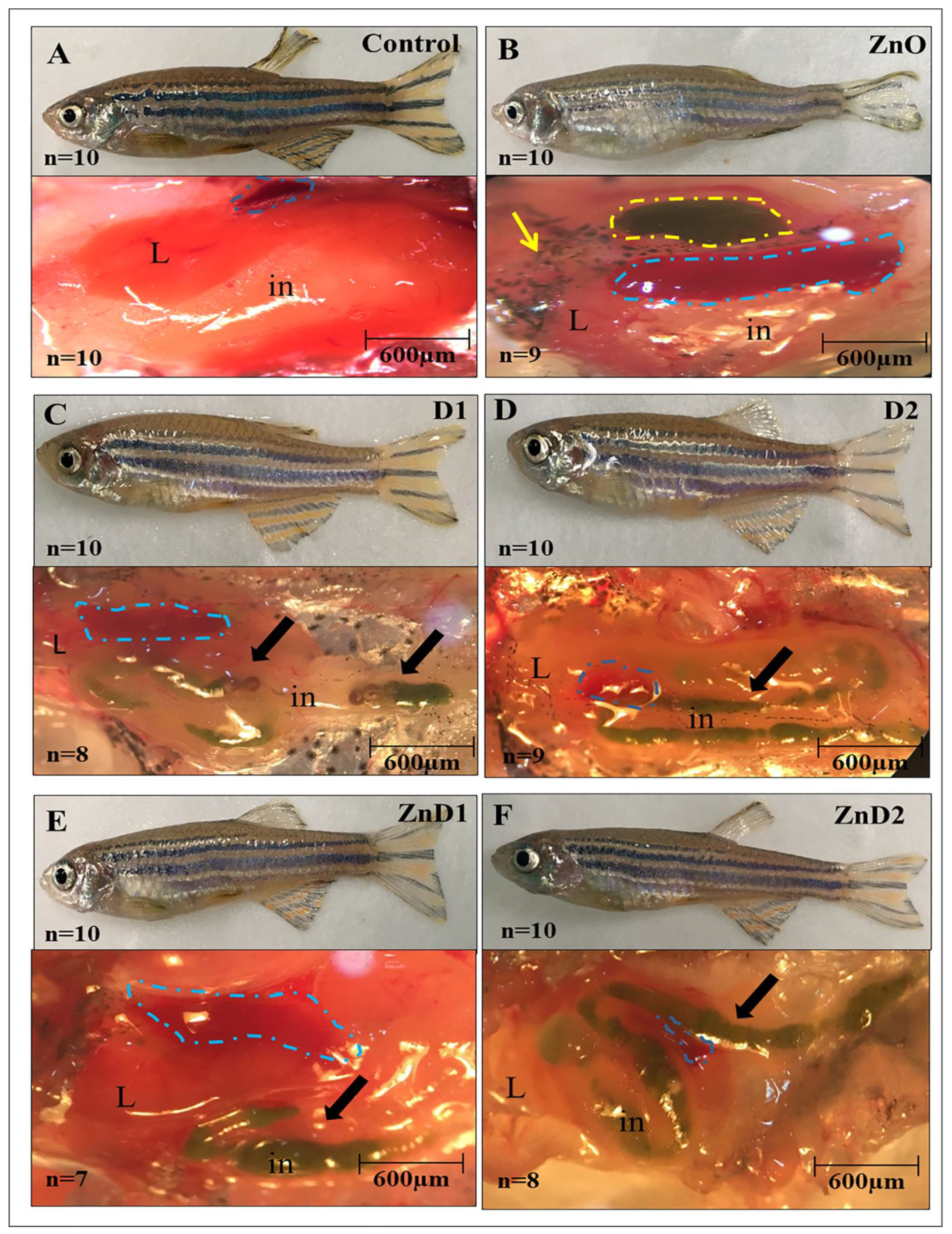
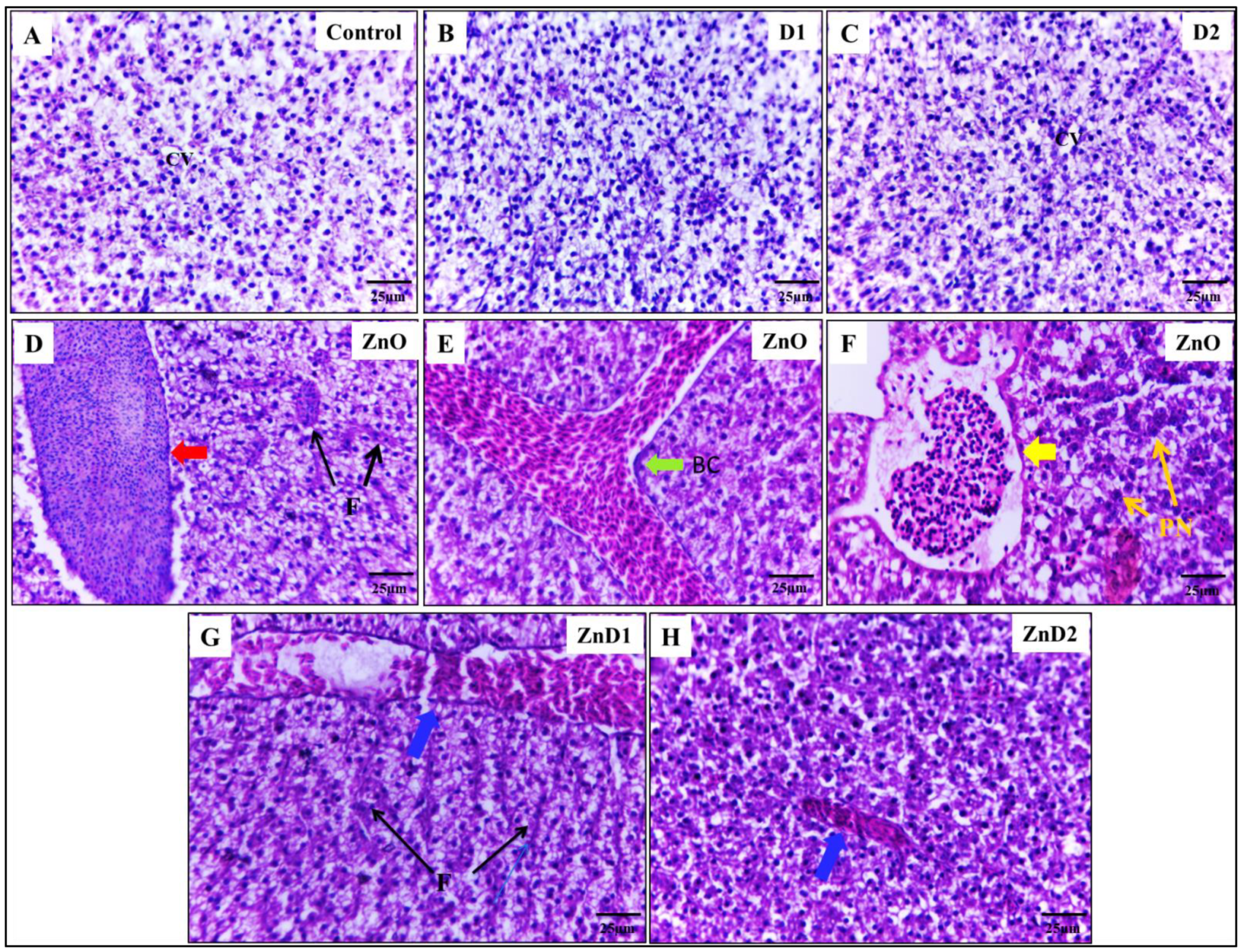
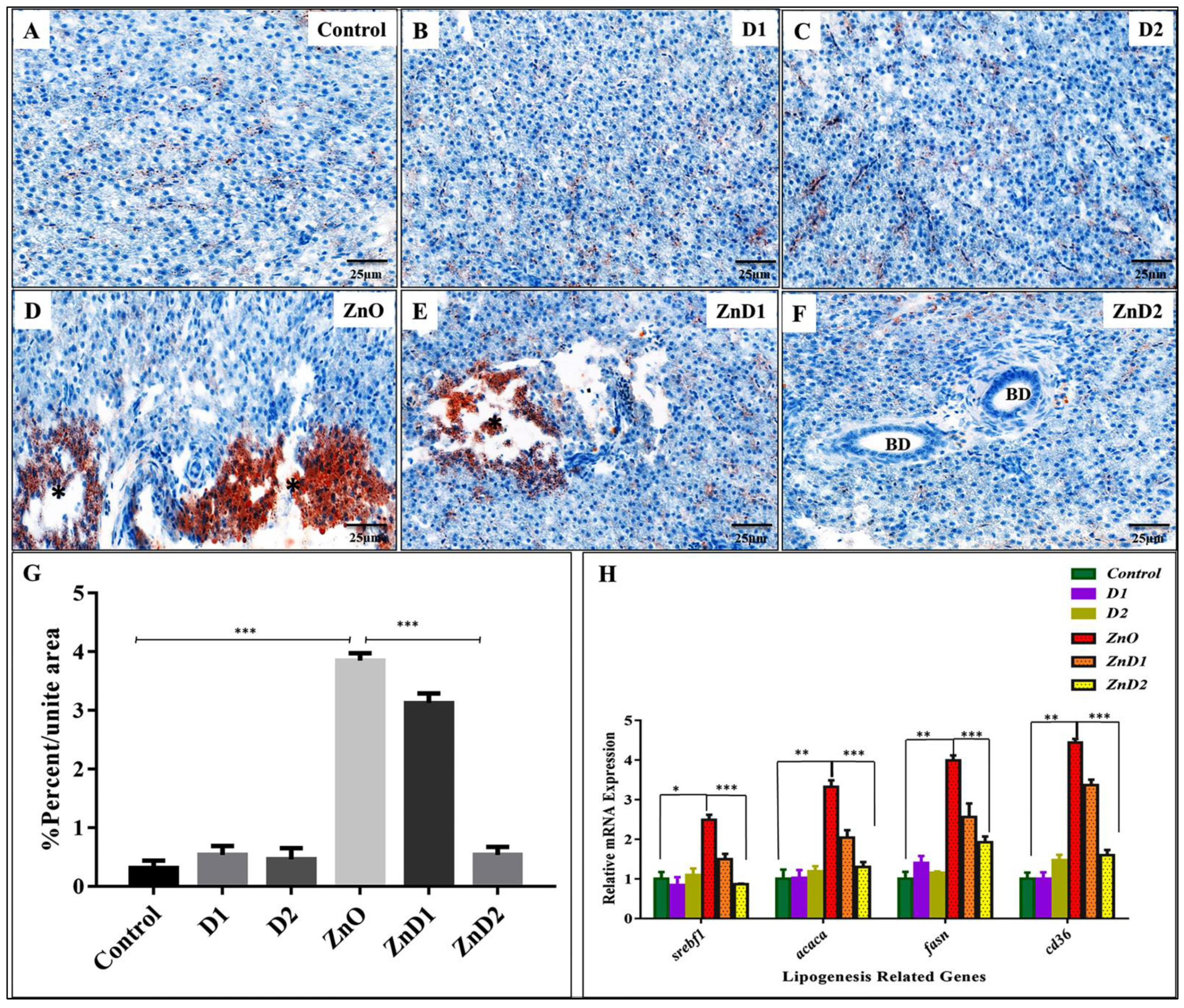
3.6. D. salina Ameliorates the Hepatic Histopathological Changes Induced by ZnO-NPs
3.7. ZnO-NPs Altered Hepatic Glyco-Lipid Equilibrium
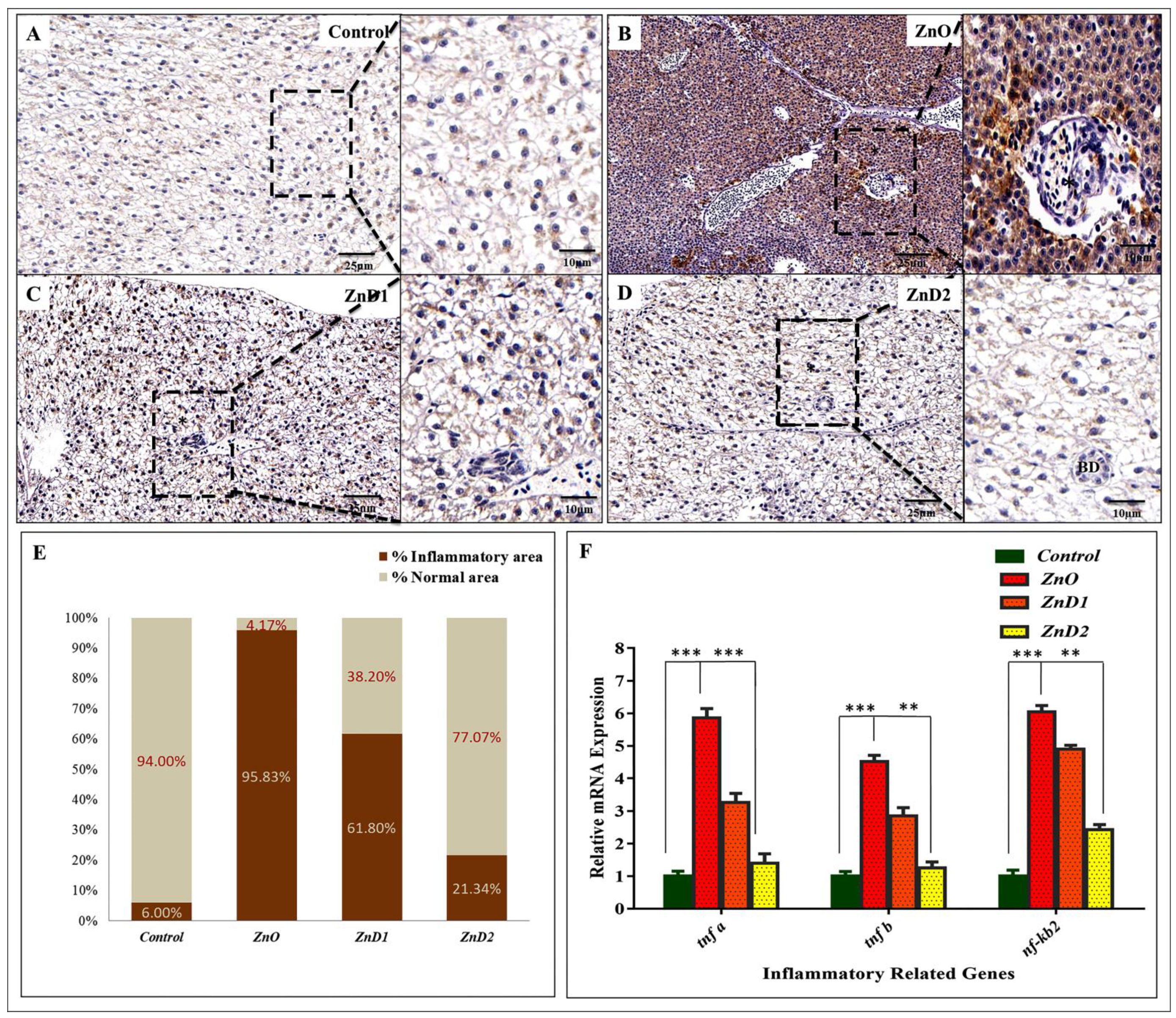
3.8. Hepatic Inflammation Could Be Effectively Prevented by D. salina after ZnO-NPs Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, A.; Kumar, N.; Kumar, R.; Salar, R.K. Antimicrobial activity of zinc oxide nanoparticles synthesized from Aloe vera peel extract. SN Appl. Sci. 2018, 1, 136. [Google Scholar] [CrossRef] [Green Version]
- Raj, N.B.; PavithraGowda, N.T.; Pooja, O.S.; Purushotham, B.; Kumar, M.R.A.; Sukrutha, S.K.; Ravikumar, C.R.; Nagaswarupa, H.P.; Murthy, H.C.A.; Boppana, S.B. Harnessing ZnO nanoparticles for antimicrobial and photocatalytic activities. J. Photochem. Photobiol. 2021, 6, 100021. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Pezzuto, F.; Scarano, A. Cytotoxic and Bacteriostatic Activity of Nanostructured TiO2 Coatings. Pol. J. Microbiol. 2016, 65, 225–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guildford, A.L.; Poletti, T.; Osbourne, L.H.; Di Cerbo, A.; Gatti, A.M.; Santin, M. Nanoparticles of a different source induce different patterns of activation in key biochemical and cellular components of the host response. J. R. Soc. Interf. 2009, 6, 1213–1221. [Google Scholar] [CrossRef]
- Pandurangan, M.; Kim, D.H. In vitro toxicity of zinc oxide nanoparticles: A review. J. Nanopart. Res. 2015, 17, 158. [Google Scholar] [CrossRef]
- Saad, S.R.; Mahmed, N.; Abdullah, M.M.A.B.; Sandu, A.V. Self-Cleaning Technology in Fabric: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2016, 133, 012028. [Google Scholar] [CrossRef]
- Batsmanova, L.; Taran, N.; Konotop, Y.; Kalenska, S.; Novytska, N. Use of a colloidal solution of metal and metal oxide-containing nanoparticles as fertilizer for increasing soybean productivity. J. Cent. Eur. Agric. 2020, 21, 311–319. [Google Scholar] [CrossRef]
- Shaw, B.J.; Handy, R.D. Physiological effects of nanoparticles on fish: A comparison of nanometals versus metal ions. Environ. Int. 2011, 37, 1083–1097. [Google Scholar] [CrossRef]
- Mawed, S.A.; Marini, C.; Alagawany, M.; Farag, M.R.; Reda, R.M.; El-Saadony, M.T.; Elhady, W.M.; Magi, G.E.; Di Cerbo, A.; El-Nagar, W.G. Zinc Oxide Nanoparticles (ZnO-NPs) Suppress Fertility by Activating Autophagy, Apoptosis, and Oxidative Stress in the Developing Oocytes of Female Zebrafish. Antioxidants 2022, 11, 1567. [Google Scholar] [CrossRef]
- He, M.; Li, X.; Yu, L.; Deng, S.; Gu, N.; Li, L.; Jia, J.; Li, B. Double-Sided Nano-ZnO: Superior Antibacterial Properties and Induced Hepatotoxicity in Zebrafish Embryos. Toxics 2022, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Li, N.; Sheng, W.; Ji, X.; Liang, X.; Kong, B.; Yin, P.; Li, Y.F.; Zhang, X.; Liu, K. Toxicity of different zinc oxide nanomaterials and dose-dependent onset and development of Parkinson’s disease-like symptoms induced by zinc oxide nanorods. Environ. Int. 2021, 146, 106179. [Google Scholar] [CrossRef]
- Rajkumar, K.S.; Sivagaami, P.; Ramkumar, A.; Murugadas, A.; Srinivasan, V.; Arun, S.; Senthil Kumar, P.; Thirumurugan, R. Bio-functionalized zinc oxide nanoparticles: Potential toxicity impact on freshwater fish Cyprinus carpio. Chemosphere 2022, 290, 133220. [Google Scholar] [CrossRef] [PubMed]
- Alkaladi, A.; El-Deen, N.A.M.N.; Afifi, M.; Zinadah, O.A.A. Hematological and biochemical investigations on the effect of vitamin E and C on Oreochromis niloticus exposed to zinc oxide nanoparticles. Saudi J. Biol. Sci. 2015, 22, 556–563. [Google Scholar] [CrossRef] [Green Version]
- Paul, V.; Krishnakumar, S.; Gowd, G.S.; Nair, S.V.; Koyakutty, M.; Paul-Prasanth, B. Sex-Dependent Bioaccumulation of Nano Zinc Oxide and Its Adverse Effects on Sexual Behavior and Reproduction in Japanese Medaka. ACS Appl. Bio Mater. 2021, 4, 7408–7421. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, R.O.; Yoon, S.; Kim, W.K. Developmental Toxicity of Zinc Oxide Nanoparticles to Zebrafish (Danio rerio): A Transcriptomic Analysis. PLoS ONE 2016, 11, e0160763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Crupi, R.; Marino, Y.; Franco, G.A.; Cuzzocrea, S.; Spanò, N.; Gugliandolo, E.; Peritore, A.F. Environmental Toxicity Assessment of Sodium Fluoride and Platinum-Derived Drugs Co-Exposure on Aquatic Organisms. Toxics 2022, 10, 272. [Google Scholar] [CrossRef]
- Abou-Zeid, S.M.; Aljuaydi, S.H.; AbuBakr, H.O.; Tahoun, E.A.; Di Cerbo, A.; Alagawany, M.; Khalil, S.R.; Farag, M.R. Astaxanthin Mitigates Thiacloprid-Induced Liver Injury and Immunotoxicity in Male Rats. Mar. Drugs 2021, 19, 525. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Canello, S.; Guidetti, G.; Fiore, F.; Corsi, L.; Rubattu, N.; Testa, C.; Cocco, R. Adverse food reactions in dogs due to antibiotic residues in pet food: A preliminary study. Vet. Ital. 2018, 54, 137–146. [Google Scholar] [CrossRef]
- Ghafari Farsani, H.; Binde Doria, H.; Jamali, H.; Hasanpour, S.; Mehdipour, N.; Rashidiyan, G. The protective role of vitamin E on Oreochromis niloticus exposed to ZnONP. Ecotoxicol. Environ. Saf. 2017, 145, 1–7. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Rashidian, G.; Hoseinifar, S.H.; Kamel, S.; Zare, M.; Ghafarifarsani, H.; Algharib, S.A.; Moonmanee, T.; Van Doan, H. Protective effects of Allium hirtifolium extract against foodborne toxicity of Zinc oxide nanoparticles in Common carp (Cyprinus carpio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 257, 109345. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Mohammed, A.T.; Farag, M.R.; Hassan, M.A.; Mawed, S.A.; Alagawany, M.; Zizzadoro, C.; Di Cerbo, A.; Abdel-Latif, H.M.R. Dietary Supplementation of Nile Tilapia (Oreochromis niloticus) With Panax ginseng Essential Oil: Positive Impact on Animal Health and Productive Performance, and Mitigating Effects on Atrazine- Induced Toxicity. Front. Mar. Sci. 2022, 9, 920057. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Khalil, S.R.; Abd El-Aziz, R.M.; Zaglool, A.W.; Moselhy, A.A.A.; Abou-Zeid, S.M. Effect of parsley essential oil on digestive enzymes, intestinal morphometry, blood chemistry and stress-related genes in liver of Nile tilapia fish exposed to Bifenthrin. Aquaculture 2022, 546, 737322. [Google Scholar] [CrossRef]
- Rahman, H.S.; Othman, H.H.; Abdullah, R.; Edin, H.; Al-Haj, N.A. Beneficial and toxicological aspects of zinc oxide nanoparticles in animals. Vet. Med. Sci. 2022, 8, 1769–1779. [Google Scholar] [CrossRef]
- Sun, L.; Gao, M.; Qian, Q.; Guo, Z.; Zhu, P.; Wang, X.; Wang, H. Triclosan-induced abnormal expression of miR-30b regulates fto-mediated m(6)A methylation level to cause lipid metabolism disorder in zebrafish. Sci. Total Environ. 2021, 770, 145285. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, F.; Bahadar, H.; Hassani, S.; Niaz, K.; Baeeri, M.; Rahimifard, M.; Ghasemi-Niri, S.F.; Abdollahi, M. Biochemical evidence on the potential role of methyl mercury in hepatic glucose metabolism through inflammatory signaling and free radical pathways. J. Cell. Biochem. 2019, 120, 16195–16205. [Google Scholar] [CrossRef]
- Marqueno, A.; Flores, C.; Casado, M.; Porte, C. Dysregulation of lipid metabolism in PLHC-1 and ZFL cells exposed to tributyltin an all-trans retinoic acid. Aquat. Toxicol. 2021, 231, 105733. [Google Scholar] [CrossRef]
- Filippi, C.; Pryde, A.; Cowan, P.; Lee, T.; Hayes, P.; Donaldson, K.; Plevris, J.; Stone, V. Toxicology of ZnO and TiO2 nanoparticles on hepatocytes: Impact on metabolism and bioenergetics. Nanotoxicology 2015, 9, 126–134. [Google Scholar] [CrossRef]
- Chen, S.W.; Lv, W.H.; Wu, K.; Chen, G.H.; Chen, F.; Song, C.C.; Luo, Z. Dietary Nano-ZnO Is Absorbed via Endocytosis and ZIP Pathways, Upregulates Lipogenesis, and Induces Lipotoxicity in the Intestine of Yellow Catfish. Int. J. Mol. Sci. 2021, 22, 12047. [Google Scholar] [CrossRef]
- Chen, G.-H.; Song, C.-C.; Zhao, T.; Hogstrand, C.; Wei, X.-L.; Lv, W.-H.; Song, Y.-F.; Luo, Z. Mitochondria-Dependent Oxidative Stress Mediates ZnO Nanoparticle (ZnO NP)-Induced Mitophagy and Lipotoxicity in Freshwater Teleost Fish. Environ. Sci. Technol. 2022, 56, 2407–2420. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced Antibacterial Activity of Nanocrystalline ZnO Due to Increased ROS-Mediated Cell Injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 2012, 17, 852–870. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, Q.F.; Jin, K.X.; Zhu, S.Q.; Wang, X.F.; Lu, J.Y. [Toxic Effect of Nano-ZnO in Liver of Zebrafish]. Huan Jing Ke Xue 2015, 36, 3884–3891. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Lee, Y.-H.; Wang, B., Jr.; Chen, R.-J.; Wang, Y.-J. Skin damage induced by zinc oxide nanoparticles combined with UVB is mediated by activating cell pyroptosis via the NLRP3 inflammasome–autophagy–exosomal pathway. Part. Fibre Toxicol. 2022, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Landi, R.; Rubino, V.; Di Cerbo, A.; Giovazzino, A.; Palatucci, A.T.; Centenaro, S.; Guidetti, G.; Canello, S.; Cortese, L.; et al. Oxytetracycline induces DNA damage and epigenetic changes: A possible risk for human and animal health? PeerJ 2017, 5, e3236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacelli, C.; Di Cerbo, A.; Lecce, L.; Piccoli, C.; Canello, S.; Guidetti, G.; Capitanio, N. Effect of Chicken Bone Extracts on Metabolic and Mitochondrial Functions of K562 Cell Line. Pharmaceuticals 2020, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Brun, N.R.; Lenz, M.; Wehrli, B.; Fent, K. Comparative effects of zinc oxide nanoparticles and dissolved zinc on zebrafish embryos and eleuthero-embryos: Importance of zinc ions. Sci. Total Environ. 2014, 476-477, 657–666. [Google Scholar] [CrossRef]
- Rashidian, G.; Mahboub, H.H.; Hoseinifar, S.H.; Ghafarifarsani, H.; Zare, M.; Punyatong, M.; Doan, H.V. Allium hirtifolium protects Cyprinus carpio against the detrimental responses mediated by foodborne zinc oxide nanoparticle. Aquaculture 2022, 555, 738252. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Kabir Chowdhury, M.A.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Pourkarimi, S.; Hallajisani, A.; Alizadehdakhel, A.; Nouralishahi, A.; Golzary, A. Factors affecting production of beta-carotene from Dunaliella salina microalgae. Biocatal. Agric. Biotechnol. 2020, 29, 101771. [Google Scholar] [CrossRef]
- Pratiwi, D.Y. A mini review-effect of Dunaliella salina on growth and health of shrimps. Int. J. Fish. Aquat. Stud. 2020, 8, 317–319. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Salama, A.A.A.; Hussein, R.A. Dunaliella salina microalgae oppose thioacetamide-induced hepatic fibrosis in rats. Toxicol. Rep. 2020, 7, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Chitranjali, T.; Anoop Chandran, P.; Muraleedhara Kurup, G. Omega-3 fatty acid concentrate from Dunaliella salina possesses anti-inflammatory properties including blockade of NF-kappaB nuclear translocation. Immunopharmacol. Immunotoxicol. 2015, 37, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Kumudha, A.; Sarada, R. Characterization of vitamin B12 in Dunaliella salina. J. Food Sci. Technol. 2016, 53, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Alishahi, M.; Karamifar, M.; Mesbah, M.; Zarei, M. Hemato-immunological responses of Heros severus fed diets supplemented with different levels of Dunaliella salina. Fish Physiol. Biochem. 2014, 40, 57–65. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Salama, A.A.A.; Salama, R.A.A. Dunaliella salina Attenuates Diabetic Neuropathy Induced by STZ in Rats: Involvement of Thioredoxin. BioMed Res. Int. 2020, 2020, 1295492. [Google Scholar] [CrossRef] [Green Version]
- Gallego, R.; Valdes, A.; Sanchez-Martinez, J.D.; Suarez-Montenegro, Z.J.; Ibanez, E.; Cifuentes, A.; Herrero, M. Study of the potential neuroprotective effect of Dunaliella salina extract in SH-SY5Y cell model. Anal. Bioanal. Chem. 2022, 414, 5357–5371. [Google Scholar] [CrossRef]
- Bassem, S.; Abd El Tawab, M.I.; Temraz, T.; Bassaly, W.K.; El-baz, F.K.; Ali, G.; Abdel Gawad, F.K. Dunaliella salina Extract Alleviates The Toxic Impact of Dioxin Induced Endocrine Disruption in Nile Tilapia. Egypt. J. Chem. 2020, 63, 1787–1798. [Google Scholar] [CrossRef]
- Srinivasan, R.; Chaitanyakumar, A.; Mageswari, A.; Gomathi, A.; Pavan Kumar, J.G.S.; Jayasindu, M.; Bharath, G.; Shravan, J.S.; Gothandam, K.M. Oral administration of lyophilized Dunaliella salina, a carotenoid-rich marine alga, reduces tumor progression in mammary cancer induced rats. Food Funct. 2017, 8, 4517–4527. [Google Scholar] [CrossRef]
- Chidambara Murthy, K.N.; Rajesha, J.; Vanitha, A.; Swamy, M.M.; Ravishankar, G.A. Protective effect of Dunaliella salina-A marine micro alga, against carbon tetrachloride-induced hepatotoxicity in rats. Hepatol. Res. 2005, 33, 313–319. [Google Scholar] [CrossRef]
- Madkour, F.F.; Abdel-Daim, M.M. Hepatoprotective and Antioxidant Activity of Dunaliella salina in Paracetamol-induced Acute Toxicity in Rats. Indian J. Pharm. Sci. 2013, 75, 642–648. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, F.K.; Elgohary, R.; Salama, A. Amelioration of Hepatic Encephalopathy Using Dunaliella salina Microalgae in Rats: Modulation of Hyperammonemia/TLR4. BioMed Res. Int. 2021, 2021, 8843218. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.P.; Gantar, M.; Gibbs, P.D.; Schmale, M.C. The zebrafish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Tocher, D.R. Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Dong, T.; Kang, X.; Liu, Z.; Zhao, S.; Ma, W.; Xuan, Q.; Liu, H.; Wang, Z.; Zhang, Q. Altered glycometabolism affects both clinical features and prognosis of triple-negative and neoadjuvant chemotherapy-treated breast cancer. Tumour Biol. 2016, 37, 8159–8168. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Alkhatib, F.M.; Alzahrani, S.O.; Shafi, M.E.; El Abdel-Hamid, S.; Taha, T.F.; Aboelenin, S.M.; Soliman, M.M.; Ahmed, N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021, 28, 4592–4604. [Google Scholar] [CrossRef]
- AOAC. Official Method 989.05, Fat in Milk, Modified Mojonnier, Ether Extrac tion Method. 2006. Available online: https://d163axztg8am2h.cloudfront.net/static/doc/33/39/67e2a818ad56f4785aa08ee21f10.pdf (accessed on 20 July 2022).
- Di Cerbo, A.; Miraglia, D.; Marino, L.; Stocchi, R.; Loschi, A.R.; Fisichella, S.; Cammertoni, N.; Menchetti, L.; Farneti, S.; Ranucci, D.; et al. “Burrata di Andria” PGI Cheese: Physicochemical and Microbiological Features. Foods 2020, 9, 1694. [Google Scholar] [CrossRef]
- Mahaffey, K.R.; Capar, S.G.; Gladen, B.C.; Fowler, B.A. Concurrent exposure to lead, cadmium, and arsenic. Effects on toxicity and tissue metal concentrations in the rat. J. Lab. Clin. Med. 1981, 98, 463–481. [Google Scholar]
- Julshamn, K.; Andersen, K.J. Subcellular distribution of major and minor elements in unexposed molluscs in Western Norway—III. The distribution and binding of cadmium, zinc, copper, magnesium, manganese, iron and lead in the kidney and the digestive system of the horse mussel Modiolus modiolus. Comp. Biochem. Physiol. Part A Physiol. 1983, 75, 17–20. [Google Scholar] [CrossRef]
- Saad, A.M.; Sitohy, M.Z.; Ahmed, A.I.; Rabie, N.A.; Amin, S.A.; Aboelenin, S.M.; Soliman, M.M.; El-Saadony, M.T. Biochemical and Functional Characterization of Kidney Bean Protein Alcalase-Hydrolysates and Their Preservative Action on Stored Chicken Meat. Molecules 2021, 26, 4690. [Google Scholar] [CrossRef]
- Praveen, P.K.; Debnath, C.; Shekhar, S.; Dalai, N.; Ganguly, S. Incidence of Aeromonas spp. infection in fish and chicken meat and its related public health hazards: A review. Vet. World 2016, 9, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- MacAulay, C.; Keyes, M.; Hayes, M.; Lo, A.; Wang, G.; Guillaud, M.; Gleave, M.; Fazli, L.; Korbelik, J.; Collins, C.; et al. Quantification of large scale DNA organization for predicting prostate cancer recurrence. Cytom. A 2017, 91, 1164–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iseppi, R.; Di Cerbo, A.; Messi, P.; Sabia, C. Antibiotic Resistance and Virulence Traits in Vancomycin-Resistant Enterococci (VRE) and Extended-Spectrum beta-Lactamase/AmpC-producing (ESBL/AmpC) Enterobacteriaceae from Humans and Pets. Antibiotics 2020, 9, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, T.; Narumiya, S. Roles of hepatic stellate cells in liver inflammation: A new perspective. Inflamm. Regen. 2016, 36, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.W.; Wang, J.; Guo, H.; Zhao, Y.Y.; Sun, H.H.; Li, Y.F.; Lai, X.Y.; Zhao, N.; Wang, X.; Xie, C.; et al. CD36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis. Nat. Commun. 2020, 11, 4765. [Google Scholar] [CrossRef] [PubMed]
- Tiegs, G.; Horst, A.K. TNF in the liver: Targeting a central player in inflammation. Semin. Immunopathol. 2022, 44, 445–459. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, C.; Yang, L.; Lu, R.; Zhao, X.; Nie, G. A comparative genomics study of carbohydrate/glucose metabolic genes: From fish to mammals. BMC Genom. 2018, 19, 246. [Google Scholar] [CrossRef] [Green Version]
- Benchoula, K.; Khatib, A.; Jaffar, A.; Ahmed, Q.U.; Sulaiman, W.; Wahab, R.A.; El-Seedi, H.R. The promise of zebrafish as a model of metabolic syndrome. Exp. Anim. 2019, 68, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Miller, Y.I. Emerging applications for zebrafish as a model organism to study oxidative mechanisms and their roles in inflammation and vascular accumulation of oxidized lipids. Free Radic. Biol. Med. 2012, 53, 1411–1420. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.; Raghuraman, B.; Gopish, A.R. Aspergillus niger mediated synthesis of ZnO nanoparticles and their antimicrobial and invitro anticancerous activity. World J. Pharm. Res. 2013, 3, 3044–3054. [Google Scholar]
- Fakhari, S.; Jamzad, M.; Kabiri Fard, H. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Sandiya, K.; Santhiya, S.; Pradeep, R.S.; Kumar, N.M.; Suriyanarayanan, N.; Thirumarimurugan, M. Biosynthesis, characterization, and antibacterial activity of zinc oxide nanoparticles derived from Bauhinia tomentosa leaf extract. J. Nanostruct. Chem. 2018, 8, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Khalek, A.A.; Badran, S.R.; Marie, M.A. Toxicity evaluation of copper oxide bulk and nanoparticles in Nile tilapia, Oreochromis niloticus, using hematological, bioaccumulation and histological biomarkers. Fish Physiol. Biochem. 2016, 42, 1225–1236. [Google Scholar] [CrossRef]
- Yu, L.-p.; Fang, T.; Xiong, D.-w.; Zhu, W.-t.; Sima, X.-f. Comparative toxicity of nano-ZnO and bulk ZnO suspensions to zebrafish and the effects of sedimentation, ˙OH production and particle dissolution in distilled water. J. Environ. Monit. 2011, 13, 1975–1982. [Google Scholar] [CrossRef]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef]
- Chen, T.H.; Lin, C.C.; Meng, P.J. Zinc oxide nanoparticles alter hatching and larval locomotor activity in zebrafish (Danio rerio). J. Hazard. Mater. 2014, 277, 134–140. [Google Scholar] [CrossRef]
- Bencsik, A.; Lestaevel, P.; Guseva Canu, I. Nano- and neurotoxicology: An emerging discipline. Prog. Neurobiol. 2018, 160, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Liu, H.; Zhang, S.; Liu, X.; Hayat, T.; Alsaedi, A.; Wang, X. Mechanism of toxic effects of Nano-ZnO on cell cycle of zebrafish (Danio rerio). Chemosphere 2019, 229, 206–213. [Google Scholar] [CrossRef]
- Yoo, A.; Lin, M.; Mustapha, A. Zinc Oxide and Silver Nanoparticle Effects on Intestinal Bacteria. Materials 2021, 14, 2489. [Google Scholar] [CrossRef]
- Ma, K.; Chen, S.; Wu, Y.; Ma, Y.; Qiao, H.; Fan, J.; Wu, H. Dietary supplementation with microalgae enhances the zebrafish growth performance by modulating immune status and gut microbiota. Appl. Microbiol. Biotechnol. 2022, 106, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Fadl, S.E.; El-Habashi, N.; Gad, D.M.; Elkassas, W.M.; Elbialy, Z.I.; Abdelhady, D.H.; Hegazi, S.M. Effect of adding Dunaliella algae to fish diet on lead acetate toxicity and gene expression in the liver of Nile tilapia. Toxin Rev. 2021, 40, 1155–1171. [Google Scholar] [CrossRef]
- Colgan, S.M.; Tang, D.; Werstuck, G.H.; Austin, R.C. Endoplasmic reticulum stress causes the activation of sterol regulatory element binding protein-2. Int. J. Biochem. Cell. Biol. 2007, 39, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Thematic review series: Adipocyte Biology. Adipocyte stress: The endoplasmic reticulum and metabolic disease. J. Lipid Res. 2007, 48, 1905–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colgan, S.M.; Al-Hashimi, A.A.; Austin, R.C. Endoplasmic reticulum stress and lipid dysregulation. Expert Rev. Mol. Med. 2011, 13, e4. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Capelle, M.; Fent, K. Silver nanoparticles induce endoplasmatic reticulum stress response in zebrafish. Toxicol. Appl. Pharmacol. 2013, 272, 519–528. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Saleh, D.O.; Abdel Jaleel, G.A.; Hussein, R.A. Attenuation of Age-Related Hepatic Steatosis by Dunaliella salina Microalgae in Senescence Rats through the Regulation of Redox Status, Inflammatory Indices, and Apoptotic Biomarkers. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 3797218. [Google Scholar] [CrossRef]
- Sarni, R.O.; Suano de Souza, F.I.; Ramalho, R.A.; Schoeps Dde, O.; Kochi, C.; Catherino, P.; Dias, M.C.; Pessotti, C.F.; Mattoso, L.C.; Colugnat, F.A. Serum retinol and total carotene concentrations in obese pre-school children. Med. Sci. Monit. 2005, 11, CR510-514. [Google Scholar]
- Seif El-Din, S.H.; El-Lakkany, N.M.; El-Naggar, A.A.; Hammam, O.A.; Abd El-Latif, H.A.; Ain-Shoka, A.A.; Ebeid, F.A. Effects of rosuvastatin and/or beta-carotene on non-alcoholic fatty liver in rats. Res. Pharm. Sci. 2015, 10, 275–287. [Google Scholar]
- Duvnjak, M.; Tomasic, V.; Gomercic, M.; Smircic Duvnjak, L.; Barsic, N.; Lerotic, I. Therapy of nonalcoholic fatty liver disease: Current status. J. Physiol. Pharmacol. 2009, 60 (Suppl. S7), 57–66. [Google Scholar]
- Tan, H.H.; Fiel, M.I.; Sun, Q.; Guo, J.; Gordon, R.E.; Chen, L.C.; Friedman, S.L.; Odin, J.A.; Allina, J. Kupffer cell activation by ambient air particulate matter exposure may exacerbate non-alcoholic fatty liver disease. J. Immunotoxicol. 2009, 6, 266–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Daim, M.M.; Farouk, S.M.; Madkour, F.F.; Azab, S.S. Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol. Immunotoxicol. 2015, 37, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.W.; Liu, C.W.; Yang, D.J.; Chen, C.C.; Chen, S.Y.; Tseng, J.K.; Chang, T.J.; Chang, Y.Y. Dunaliella salina alga extract inhibits the production of interleukin-6, nitric oxide, and reactive oxygen species by regulating nuclear factor-kappaB/Janus kinase/signal transducer and activator of transcription in virus-infected RAW264.7 cells. J. Food Drug Anal. 2017, 25, 908–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khayyal, M.T.; El-Baz, F.K.; Meselhy, M.R.; Ali, G.H.; El-Hazek, R.M. Intestinal injury can be effectively prevented by Dunaliella salina in gamma irradiated rats. Heliyon 2019, 5, e01814. [Google Scholar] [CrossRef] [Green Version]
- El-Baz, F.K.; Abdel Jaleel, G.A.; Hussein, R.A.; Saleh, D.O. Dunalialla salina microalgae and its isolated zeaxanthin mitigate age-related dementia in rats: Modulation of neurotransmission and amyloid-β protein. Toxicol. Rep. 2021, 8, 1899–1908. [Google Scholar] [CrossRef]
- Morales-Medina, J.C.; Aguilar-Alonso, P.; Di Cerbo, A.; Iannitti, T.; Flores, G. New insights on nitric oxide: Focus on animal models of schizophrenia. Behav. Brain Res. 2021, 409, 113304. [Google Scholar] [CrossRef]

| Gene | Accession (Gene ID) | Sequences (5′–3′) | Gene Name |
|---|---|---|---|
| Gluconeogenesis | |||
| pck1 | NM_214751 | F: 5′ ATCACGCATCGCTAAAGAGG 3′ | Phosphoenolpyruvate carboxykinase 1 |
| R: 5′ CCGCTGCGAAATACTTCTTC 3′ | |||
| gys1 | NM_201180 | F: 5′ GCAGCTCAGTGTGACGAACC 3′ | glycogen synthase 1 |
| R: 5′ GGTCCCCTGCTTCCTTATCC 3′ | |||
| g6pca.1 | NM_001003512 | F: 5′ TCACAGCGTTGCTTTCAATC 3′ | glucose-6-phosphatase a, catalytic subunit, tandem duplicate 1 |
| R: 5′ AACCCAGAAACATCCACAGC 3′ | |||
| Lipogenesis | |||
| srepf1 | NM_001105129 | F: 5′CATCCACATGGCTCTGAGTG 3′ | sterol regulatory element binding transcription factor 1 |
| R: 5′CTCATCCACAAAGAAGCGGT 3′ | |||
| acaca | NM_001271308 | F: 5′GGACGGACCCTTGCACAATA 3′ | acetyl-CoA carboxylase 1 |
| R: 5′CCTCTGCAGGTCGATACGTC 3′ | |||
| fasn | XM_009306807 | F: 5′GAGAAAGCTTGCCAAACAGG 3′ | Fatty acid synthase |
| R: 5′GAGGGTCTTGCAGGAGACAG-3′ | |||
| cd36 | NM_001002363 | F: 5′AGGCCACTGTGAACCTGAAG 3′ | Thrombospondin receptor |
| R: 5′AAGTTGGGGTTCATTCCGAC 3′ | |||
| Inflammation | |||
| tnf-α | NM_212859 | F: 5′AGACCTTAGACTGGAGAGATGAC 3′ | Tumor necrosis factor α |
| R: 5′ CAAAGACACCTGGCTGTAGAC 3′ | |||
| tnf-β | NM_001024447 | F: 5′TCAGAAACCCAACAGAGAACATC 3′ | tumor necrosis factor β |
| R: 5′ ACCCATTTCAGCGATTGTCC 3′ | |||
| nf-κb2 | NM_001001840 | F: 5′ATGAGAACGGAGACACG 3′ | kappaB kinase/NF-kappaB cascade |
| R: 5′CAGCAATCGCAAACAA 3′ | |||
| β-actin | |||
| β-actin | NM_131031 | F: 5′ATGGATGAGGAAATCGCTGC 3′ | actin, beta 1 (actb1) |
| R: 5′CTTTCTGTCCCATGCCAACC 3′ | |||
| Behavior | ZnO-NPs Concentration (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 0.05 | 0.1 | 0.2 | 0.4 | 0.8 | 1.6 | 3.2 | 6.4 | |
| Air gulping | − | − | − | − | − | + | + | ++ | ++++ |
| Respiratory distress | − | − | − | − | − | + | + | ++ | ++++ |
| Sluggish movement | − | − | − | − | − | + | + | ++ | ++++ |
| Uncoordinated swimming | − | − | − | − | + | + | ++ | +++ | ++++ |
| Hyperventilation | − | − | − | − | − | + | ++ | ++++ | ++++ |
| Groups | Zn Residues (µg/g Wet Weight) |
|---|---|
| Control | 18.47 ± 0.25 d |
| D1 | 13.21 ± 0.01 e |
| D2 | 10.02 ± 0.01 f |
| ZnO | 60.85 ± 0.02 a |
| ZnD1 | 41.23 ± 0.01 b |
| ZnD2 | 30.09 ± 0.53 c |
| SEM | 4.30 |
| * p value | < 0.001 |
| Composition | Control | D1 | D2 | ZnO | ZnD1 | ZnD2 | * p Value |
|---|---|---|---|---|---|---|---|
| Moisture (%) | 75.13 ± 0.01 | 76.17 ± 0.02 | 75.83 ± 0.01 | 76.03 ± 0.01 | 76.18 ± 0.01 | 76.61 ± 0.01 | 0.610 |
| Ash (%) | 4.86 ± 0.02 | 4.61 ± 0.01 | 4.62 ± 0.01 | 5.67 ± 0.02 | 5.01 ± 0.01 | 4.98 ± 0.08 | 0.104 |
| Crude lipids (%) | 6.21 ± 0.02 a | 5.40 ± 0.01 b | 5.02 ± 0.01 c | 3.07 ± 0.03 f | 4.05 ± 0.03 e | 4.73 ± 0.01 d | <0.001 |
| Crude protein (%) | 13.91 ± 0.01 | 13.52 ± 0.02 | 13.40 ± 0.03 | 13.20 ± 0.01 | 13.44 ± 0.05 | 13.47 ± 0.56 | 0.100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mawed, S.A.; Centoducati, G.; Farag, M.R.; Alagawany, M.; Abou-Zeid, S.M.; Elhady, W.M.; El-Saadony, M.T.; Di Cerbo, A.; Al-Zahaby, S.A. Dunaliella salina Microalga Restores the Metabolic Equilibrium and Ameliorates the Hepatic Inflammatory Response Induced by Zinc Oxide Nanoparticles (ZnO-NPs) in Male Zebrafish. Biology 2022, 11, 1447. https://doi.org/10.3390/biology11101447
Mawed SA, Centoducati G, Farag MR, Alagawany M, Abou-Zeid SM, Elhady WM, El-Saadony MT, Di Cerbo A, Al-Zahaby SA. Dunaliella salina Microalga Restores the Metabolic Equilibrium and Ameliorates the Hepatic Inflammatory Response Induced by Zinc Oxide Nanoparticles (ZnO-NPs) in Male Zebrafish. Biology. 2022; 11(10):1447. https://doi.org/10.3390/biology11101447
Chicago/Turabian StyleMawed, Suzan Attia, Gerardo Centoducati, Mayada R. Farag, Mahmoud Alagawany, Shimaa M. Abou-Zeid, Walaa M. Elhady, Mohamed T. El-Saadony, Alessandro Di Cerbo, and Sheren A. Al-Zahaby. 2022. "Dunaliella salina Microalga Restores the Metabolic Equilibrium and Ameliorates the Hepatic Inflammatory Response Induced by Zinc Oxide Nanoparticles (ZnO-NPs) in Male Zebrafish" Biology 11, no. 10: 1447. https://doi.org/10.3390/biology11101447
APA StyleMawed, S. A., Centoducati, G., Farag, M. R., Alagawany, M., Abou-Zeid, S. M., Elhady, W. M., El-Saadony, M. T., Di Cerbo, A., & Al-Zahaby, S. A. (2022). Dunaliella salina Microalga Restores the Metabolic Equilibrium and Ameliorates the Hepatic Inflammatory Response Induced by Zinc Oxide Nanoparticles (ZnO-NPs) in Male Zebrafish. Biology, 11(10), 1447. https://doi.org/10.3390/biology11101447










