Co-Expression of Pig IL-2 and Fusion Bovine Cathelicidin Gene by Recombinant Plasmids in Yeast and Their Promotion of Mouse Antibacterial Defense
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of Recombinant Plasmid
2.2. Yeast Transformation
2.3. Fusion PIL2/FBC Protein Expressed by Pichia Pastoris
2.4. Digestion Analysis In Vitro
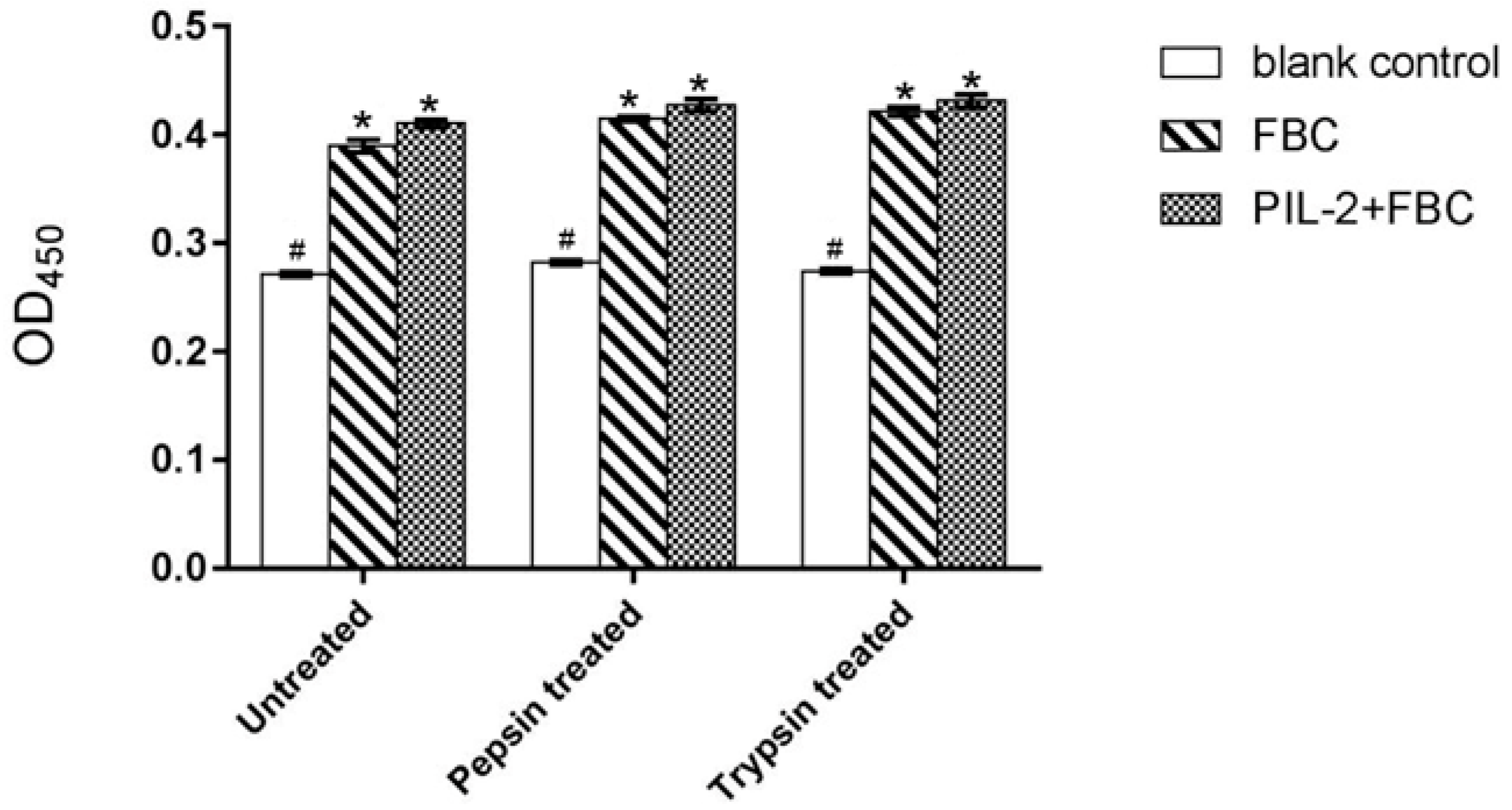
2.5. Pig Lymphocyte Proliferation Assay
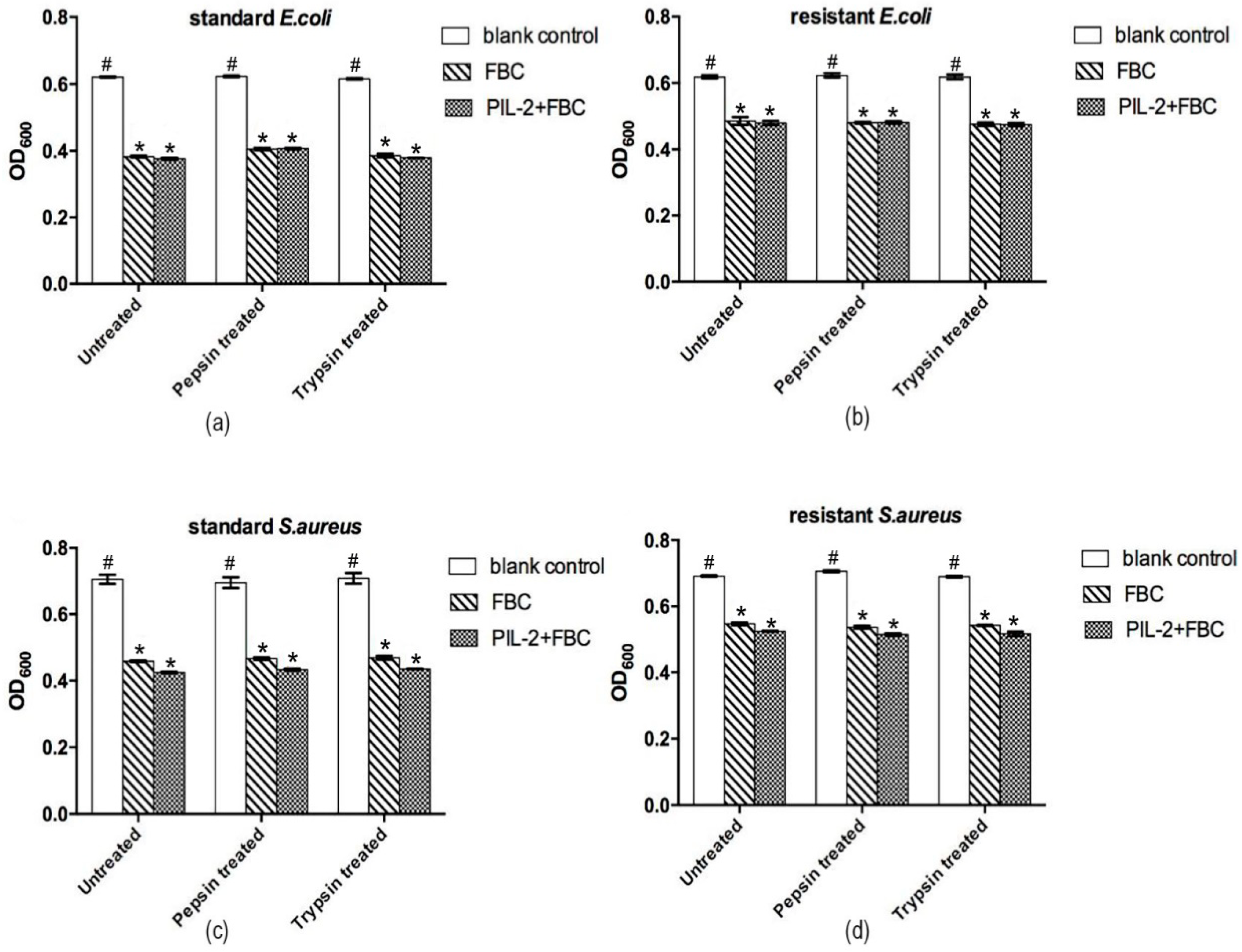
2.6. Antimicrobial Assay
2.7. Animal Experiment
2.8. Immunological Assays
2.8.1. Assay of Immune Cells Quantity in Peripheral Blood
2.8.2. Measurement of Th and Tc Cells by Flow Cytometry
2.8.3. IgG Determination by Sandwich ELISA
2.8.4. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Identification of Recombinant Plasmid
3.2. Identification of Pichia Pastoris Recombinants
3.3. Bioactivity of Pichia Pastoris Recombinants In Vitro
3.4. In Vitro Antimicrobial Activity of Pichia Pastoris Recombinants
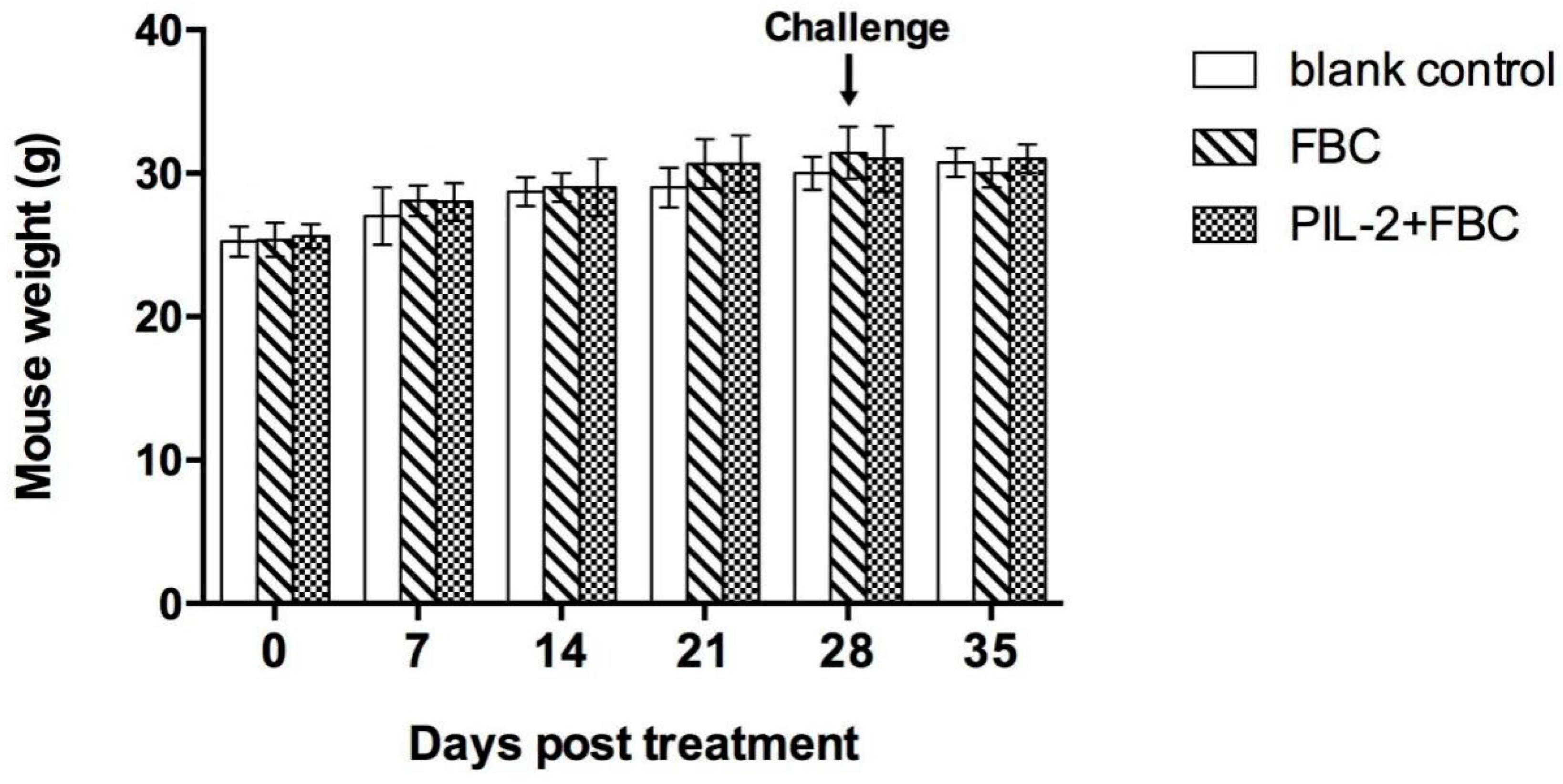
3.5. Body Weight
3.6. Leukocytes in Peripheral Blood
3.7. CD4+ and CD8+ T Cells Numbers
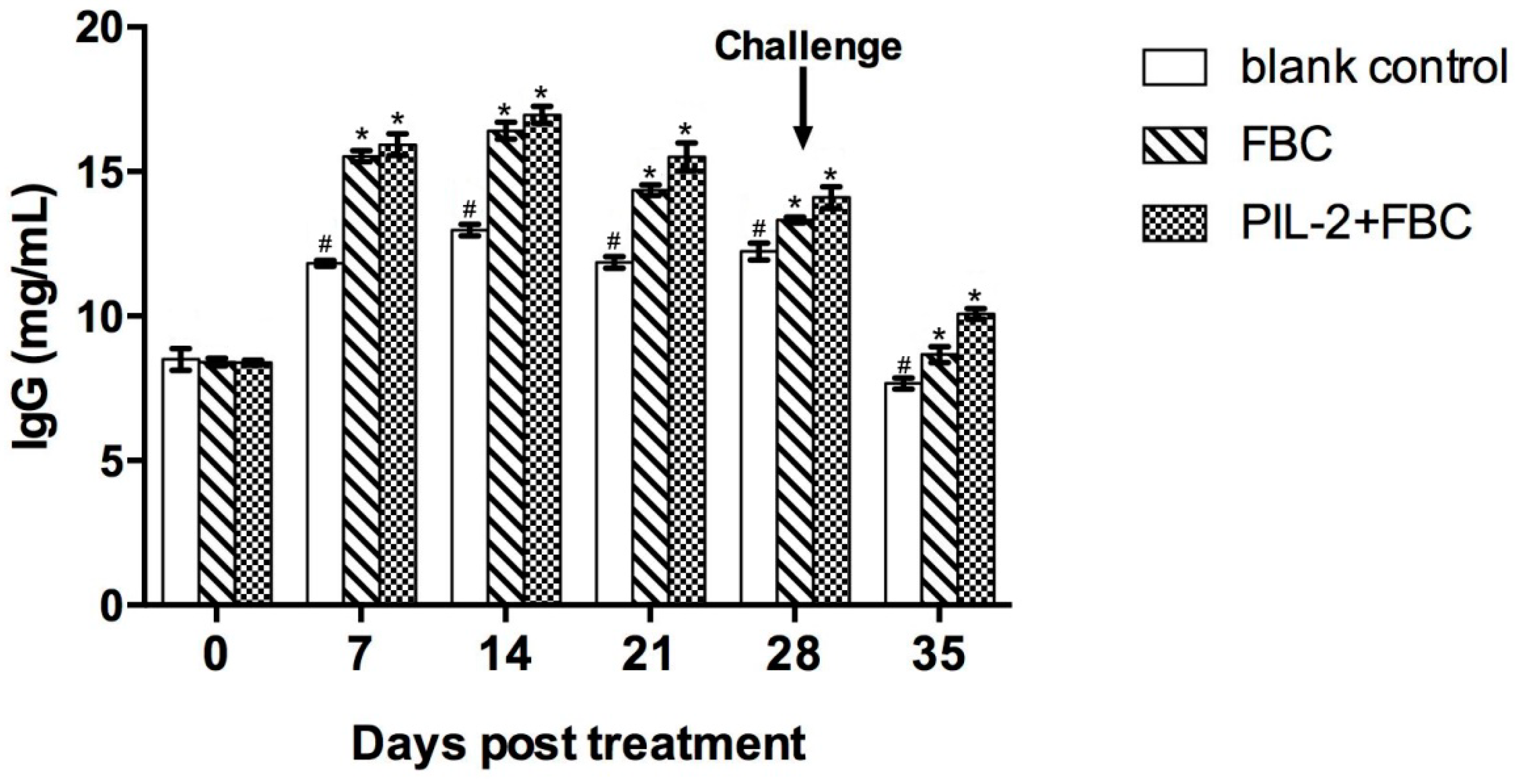
3.8. Levels of IgG
3.9. Immune Gene Expression
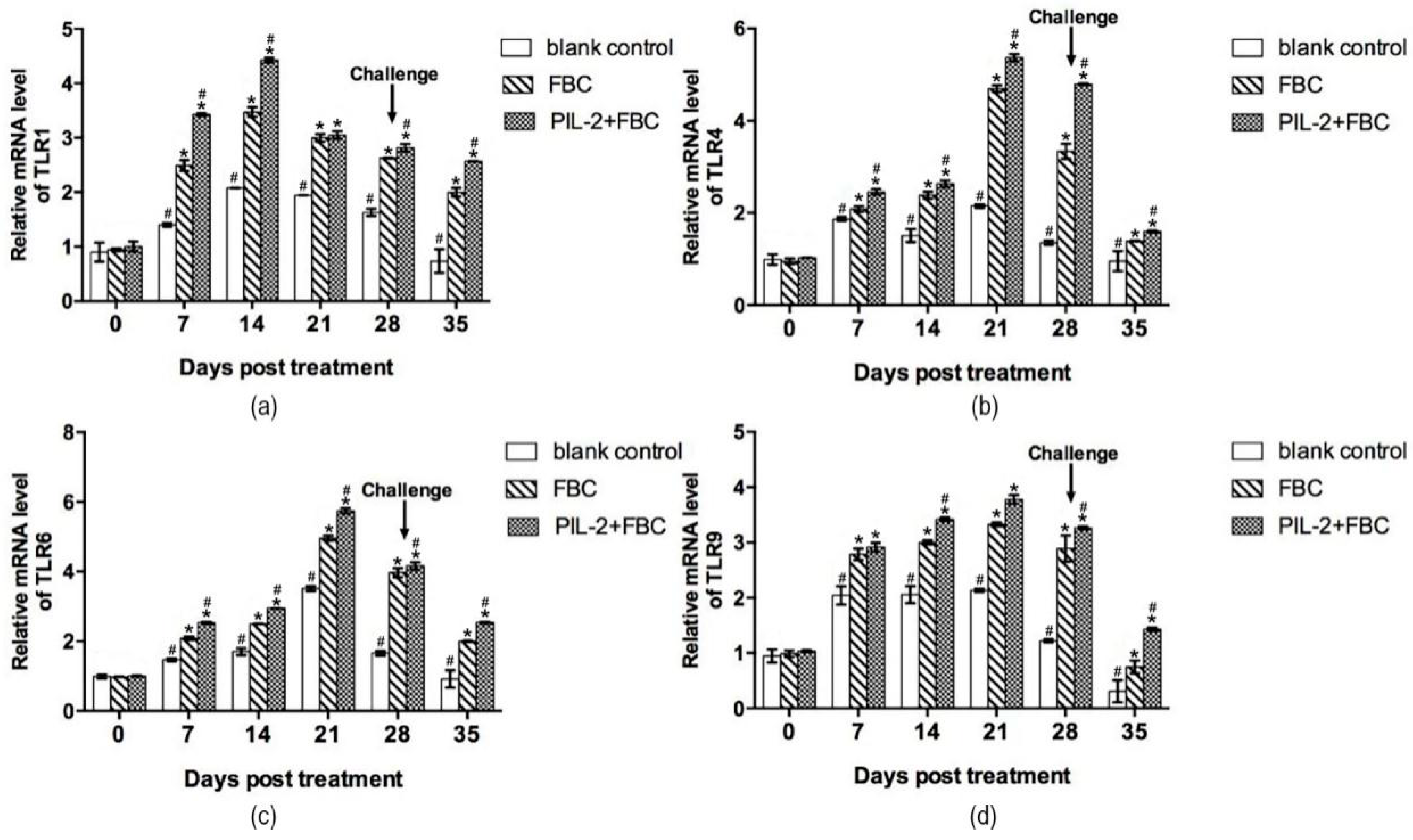
3.9.1. Toll-Like Receptor Gene
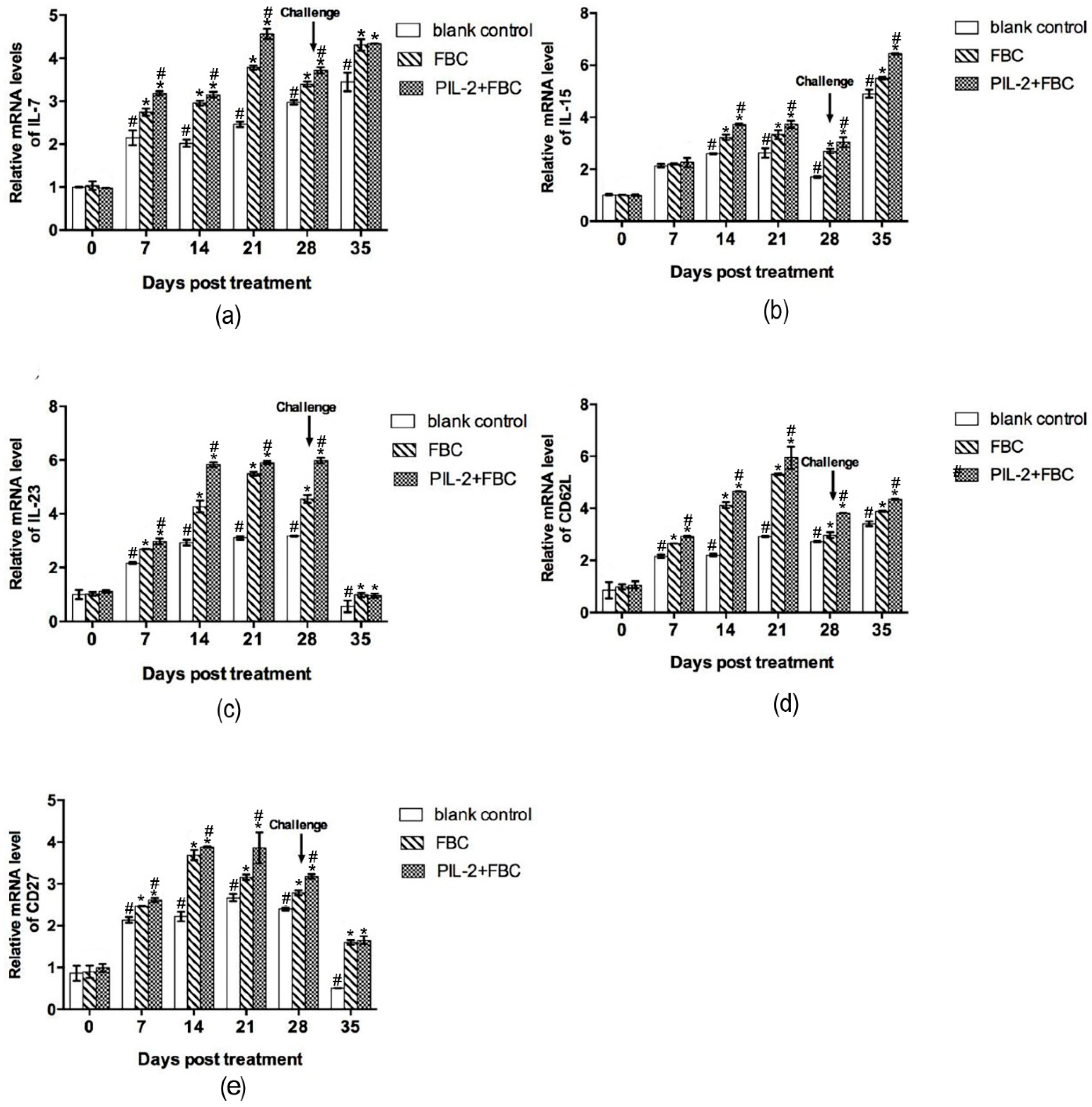
3.9.2. Immune Memory-Related Genes
3.9.3. Cytokine Genes
3.9.4. Antimicrobial Peptides
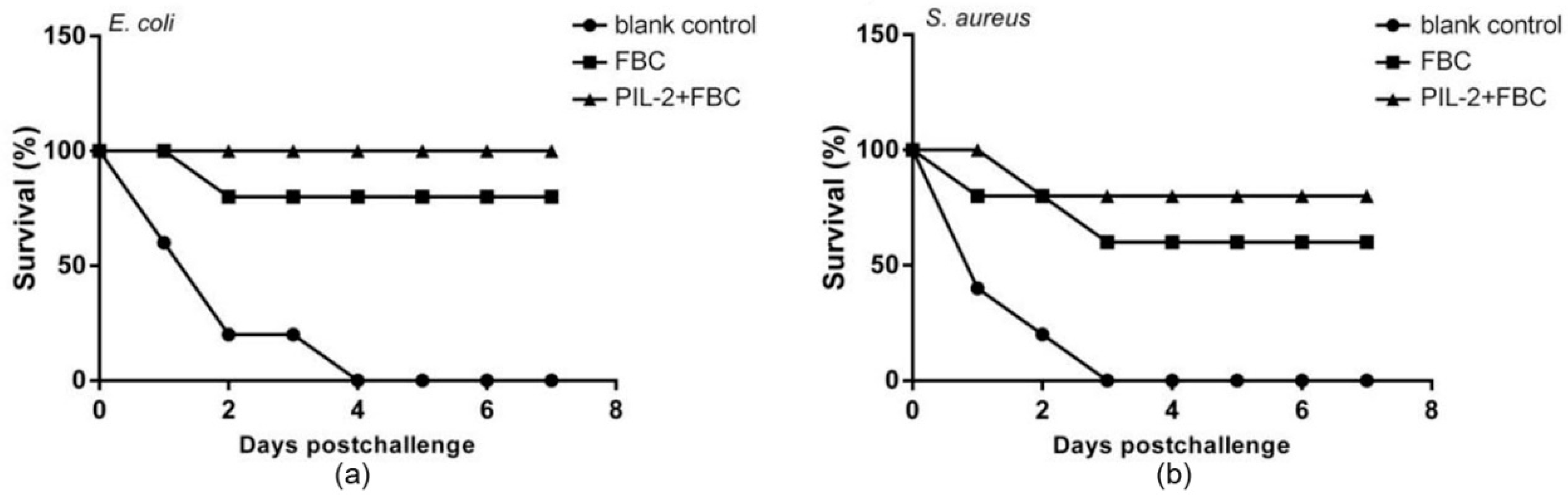
3.10. Virulent Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial stewardship: Fighting antimicrobial resistance and protecting global public health. Infect. Drug Resist. 2020, 13, 4713. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.C.; Moutinho, C.G.; Pinto, F.C.; Del Fiol, F.S.; Jozala, A.; Chaud, M.V.; Vila, M.M.; Teixeira, J.A.; Balcão, V.M. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol. Res. 2016, 191, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide design principles for antimicrobial applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Cheng, L.; Zhang, S.; Wang, L.; Hu, J. The role of natural antimicrobial peptides during infection and chronic inflammation. Antonie Van Leeuwenhoek 2018, 111, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Van Harten, R.M.; van Woudenbergh, E.; van Dijk, A.; Haagsman, H.P. Cathelicidins: Immunomodulatory Antimicrobials. Vaccines 2018, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro da Silva, F.; Machado, M.C. The dual role of cathelicidins in systemic inflammation. Immunol. Lett. 2017, 182, 57–60. [Google Scholar] [CrossRef]
- Piyadasa, H.; Hemshekhar, M.; Altieri, A.; Basu, S.; van der Does, A.M.; Halayko, A.J.; Hiemstra, P.S.; Mookherjee, N. Immunomodulatory innate defence regulator (IDR) peptide alleviates airway inflammation and hyper-responsiveness. Thorax 2018, 73, 908–917. [Google Scholar] [CrossRef]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef]
- Young-Speirs, M.; Drouin, D.; Cavalcante, P.A.; Barkema, H.W.; Cobo, E.R. Host defense cathelicidins in cattle: Types, production, bioactive functions and potential therapeutic and diagnostic applications. Int. J. Antimicrob. Agents 2018, 51, 813–821. [Google Scholar] [CrossRef]
- Le, C.F.; Fang, C.M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef] [Green Version]
- Boyman, O.; Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012, 12, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, J.; Li, H.; Wang, Y.; Xie, Z.; Wu, M.; Zhang, H.; Zhao, Z.; Chen, Q.; Fu, M.; et al. Porcine interleukin-2 gene encapsulated in chitosan nanoparticles enhances immune response of mice to piglet paratyphoid vaccine. Comp. Immunol. Microbiol. Infect. Dis. 2007, 30, 19–32. [Google Scholar] [CrossRef]
- Liu, S.P.; Zhou, L.; Lakshminarayanan, R.; Beuerman, R.W. Multivalent Antimicrobial Peptides as Therapeutics: Design Principles and Structural Diversities. Int. J. Pept. Res. Ther. 2010, 16, 199–213. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Kumar, P. Yeast-based vaccines: New perspective in vaccine development and application. FEMS Yeast Res. 2019, 19, foz007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, S.; Mansell, T.J. Yeasts as probiotics: Mechanisms, outcomes, and future potential. Fungal Genet. Biol. 2020, 137, 103333. [Google Scholar] [CrossRef] [PubMed]
- Garrait, G.; Jarrige, J.F.; Blanquet-Diot, S.; Alric, M. Genetically engineered yeasts as a new delivery vehicle of active compounds to the digestive tract: In vivo validation of the concept in the rat. Metab. Eng. 2009, 11, 148–154. [Google Scholar] [CrossRef]
- Horton, R.M.; Hunt, H.D.; Ho, S.N.; Pullen, J.K.; Pease, L.R. Engineering hybrid genes without the use of restriction enzymes: Gene splicing by overlap extension. Gene 1989, 77, 61–68. [Google Scholar] [CrossRef]
- Garrett, D.; Failla, M.; Sarama, R. Development of an in Vitro Digestion Method To Assess Carotenoid Bioavailability from Meals. J. Agric. Food Chem. 1999, 47, 4301–4309. [Google Scholar] [CrossRef]
- Hancock, R.; Haney, E.; Gill, E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Choudhry, H.; Helmi, N.; Abdulaal, W.H.; Zeyadi, M.; Zamzami, M.A.; Wu, W.; Mahmoud, M.M.; Warsi, M.K.; Rasool, M.; Jamal, M.S. Prospects of IL-2 in Cancer Immunotherapy. Biomed. Res. Int. 2018, 2018, 9056173. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.; Min, R.; Dai, G.-q.; Wang, Y.-L.; Nan, L.; Yang, Z.; Xia, J.; Pan, S.-Y.; Mao, H.; Xie, W.-P.; et al. Clinical and Immunological Effects of rhIL-2 Therapy in Eastern Chinese Patients with Multidrug-resistant Tuberculosis. Sci. Rep. 2017, 7, 17854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Li, H.; Chen, J.; Zhang, H.B.; Wang, Y.Y.; Chen, Q.; Zhao, Z.Z.; Cheng, C.; Zhang, H.; Yang, Y.; et al. Shuffling of pig interleukin-2 gene and its enhancing of immunity in mice to Pasteurella multocida vaccine. Vaccine 2007, 25, 8163–8171. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Madera, L.; Afacan, N.; Okumura, K.; Ogawa, H.; Hancock, R. The innate defense regulator peptides IDR-HH2, IDR-1002, and IDR-1018 modulate human neutrophil functions. J. Leukoc. Biol. 2013, 94, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efimova, V.S.; Isaeva, L.V.; Orekhov, P.S.; Bozdaganyan, M.E.; Rubtsov, M.A.; Novikova, L.A. Using a viral 2A peptide-based strategy to reconstruct the bovine P450scc steroidogenic system in S. cerevisiae: Bovine P450scc system expression using 2A peptides. J. Biotechnol. 2021, 325, 186–195. [Google Scholar] [CrossRef]
- Szymczak-Workman, A.L.; Vignali, K.M.; Vignali, D.A. Design and construction of 2A peptide-linked multicistronic vectors. Cold Spring Harb. Protoc. 2012, 2012, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spolski, R.; Li, P.; Leonard, W.J. Biology and regulation of IL-2: From molecular mechanisms to human therapy. Nat. Rev. Immunol. 2018, 18, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Kisuya, J.; Chemtai, A.; Raballah, E.; Keter, A.; Ouma, C. The diagnostic accuracy of Th1 (IFN-gamma, TNF-alpha, and IL-2) and Th2 (IL-4, IL-6 and IL-10) cytokines response in AFB microscopy smear negative PTB-HIV co-infected patients. Sci. Rep. 2019, 9, 2966. [Google Scholar] [CrossRef] [Green Version]
- Behr, F.M.; Chuwonpad, A.; Stark, R.; van Gisbergen, K. Armed and Ready: Transcriptional Regulation of Tissue-Resident Memory CD8 T Cells. Front. Immunol. 2018, 9, 1770. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Rojas, J.M.; Avia, M.; Martin, V.; Sevilla, N. IL-10: A Multifunctional Cytokine in Viral Infections. J. Immunol. Res. 2017, 2017, 6104054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maynard, C.L.; Weaver, C.T. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol. Rev. 2008, 226, 219–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippart, M.; Schmidt, J.; Bittner, B. Oral Delivery of Therapeutic Proteins and Peptides: An Overview of Current Technologies and Recommendations for Bridging from Approved Intravenous or Subcutaneous Administration to Novel Oral Regimens. Drug Res. 2016, 66, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Yoo, H.S. Animal vaccines based on orally presented yeast recombinants. Vaccine 2013, 31, 4287–4292. [Google Scholar] [CrossRef] [PubMed]
- Embregts, C.W.E.; Reyes-Lopez, F.; Pall, A.C.; Stratmann, A.; Tort, L.; Lorenzen, N.; Engell-Sorensen, K.; Wiegertjes, G.F.; Forlenza, M.; Sunyer, J.O.; et al. Pichia pastoris yeast as a vehicle for oral vaccination of larval and adult teleosts. Fish Shellfish Immunol. 2019, 85, 52–60. [Google Scholar] [CrossRef]




| Primer | Oligonucleotide Sequences (5′-3′) |
|---|---|
| IL-2-F | GAAGCTGAATTCATGTATAAGATGCAGCTCTTGT |
| IL-2-R | CGCATGTTAGAAGACTTCCCCTGCCCTCTCCGCTTCCAGTCAGTGTTGAGTAGATGCT |
| 2A-α-F | CTTCTAACATGCGGGGACGTGGAGGAAAATCCCGGGCCAATGAGATTTCCTTCAATTTTTAC |
| 2A-α-R | TGCCAATCGAGCTTCAGCCTCTCTTTTCT |
| FBC-F | GAGGCTGAAGCTCGATTGGCACGTATCGTCGT |
| FBC-R | CGGTGTTCTAGACTAATGGTGATGGTGATGAT |
| FBC-F’ | GAAGCTGAATTCCGATTGGCACGTATCGTCGT |
| FBC-R’ | AAACGGGCCCTCTAGACTAATGGTGATGGTGATGATGCT |
| Gene | Oligonucleotide Sequences (5′-3′) |
|---|---|
| β-actin-F | TACGCCAACACGGTGCTGTC |
| β-actin-R | GTACTCCTGCTTGCTGATCCACAT |
| TLR-1-F | GGACCTACCCTTGCAAACAA |
| TLR-1-R | GGTGGCACAAGATCACCTTT |
| TLR-4-F | ACCTGGCTGGTTTACACGTC |
| TLR-4-R | CTGCCAGAGACATTGCAGAA |
| TLR-6-F | CCAAGAACAAAAGCCCTGAG |
| TLR-6-R | TGTTTTGCAACCGATTGTGT |
| TLR-9-F | ACTGAGCACCCCTGCTTCTA |
| TLR-9-R | AGATTAGTCAGCGGCAGGAA |
| IL-2-F | AAGCACAGCAGCAGCAGCAG |
| IL-2-R | GCCGCAGAGGTCCAAGTTCATC |
| IL-4-F | GCCATATCCACGGATGCGACAA |
| IL-4-R | GGTGTTCTTCGTTGCTGTGAGGA |
| IL-6-F | TCTTGGGACTGATGCTGGTGACA |
| IL-6-R | AGCCTCCGACTTGTGAAGTGGTAT |
| IL-7-F | TTCCTCCACTGATCCTTGTTCT |
| IL-7-R | AGCAGCTTCCTTTGTATCATCAC |
| IL-10-F | GCTCTTACTGACTGGCATGAG |
| IL-10-R | CGCAGCTCTAGGAGCATGTG |
| IL-12-F | CAATCACGCTACCTCCTCTTTT |
| IL-12-R | CAGCAGTGCAGGAATAATGTTTC |
| IL-15-F | CATCCATCTCGTGCTACTTGTG |
| IL-15-R | GCCTCTGTTTTAGGGAGACCT |
| IL-23-F | TGCTGGATTGCAGAGCAGTAA |
| IL-23-R | GCATGCAGAGATTCCGAGAGA |
| IFN-γ-F | AGGCCATCAGCAACAACATA |
| IFN-γ-R | TGAGCTCATTGAATGCTTGG |
| TNF-α-F | CCTGTAGCCCACGTCGTAG |
| TNF-α-R | GGGAGTAGACAAGGTACAACCC |
| CRP4-F | AGAGGTTTGTTATGCTATTGTAGA |
| CRP4-R | TCAGCGGCGGGGGCAG |
| CAMP-F | CAGCAGTCCCTAGACACCAAT |
| CAMP-R | CACAGACTTGGGAGTATCTGGA |
| CD62L-F | TACATTGCCCAAAAGCCCTTAT |
| CD62L-R | CATCGTTCCATTTCCCAGAGTC |
| CD27-F | CAGCTTCCCAACTCGACTGTC |
| CD27-R | GCACCCAGGACGAAGATAAGAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Peng, J.; Ma, C.; Zhang, L.; Wu, X.; Wei, H.; Li, J.; Lü, X.; Gao, R. Co-Expression of Pig IL-2 and Fusion Bovine Cathelicidin Gene by Recombinant Plasmids in Yeast and Their Promotion of Mouse Antibacterial Defense. Biology 2022, 11, 1491. https://doi.org/10.3390/biology11101491
Chen J, Peng J, Ma C, Zhang L, Wu X, Wei H, Li J, Lü X, Gao R. Co-Expression of Pig IL-2 and Fusion Bovine Cathelicidin Gene by Recombinant Plasmids in Yeast and Their Promotion of Mouse Antibacterial Defense. Biology. 2022; 11(10):1491. https://doi.org/10.3390/biology11101491
Chicago/Turabian StyleChen, Jianlin, Junjie Peng, Changjun Ma, Linhan Zhang, Xueyin Wu, Hong Wei, Jianglin Li, Xuebin Lü, and Rong Gao. 2022. "Co-Expression of Pig IL-2 and Fusion Bovine Cathelicidin Gene by Recombinant Plasmids in Yeast and Their Promotion of Mouse Antibacterial Defense" Biology 11, no. 10: 1491. https://doi.org/10.3390/biology11101491
APA StyleChen, J., Peng, J., Ma, C., Zhang, L., Wu, X., Wei, H., Li, J., Lü, X., & Gao, R. (2022). Co-Expression of Pig IL-2 and Fusion Bovine Cathelicidin Gene by Recombinant Plasmids in Yeast and Their Promotion of Mouse Antibacterial Defense. Biology, 11(10), 1491. https://doi.org/10.3390/biology11101491









