Plasticity in Compensatory Growth to Artificial Defoliation and Light Availability in Four Neotropical Understory and Forest Edge Herb Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Species
2.2. Experimental Methods
2.3. Physiological and Morphological Measurements
2.4. Statistical Analysis
3. Results
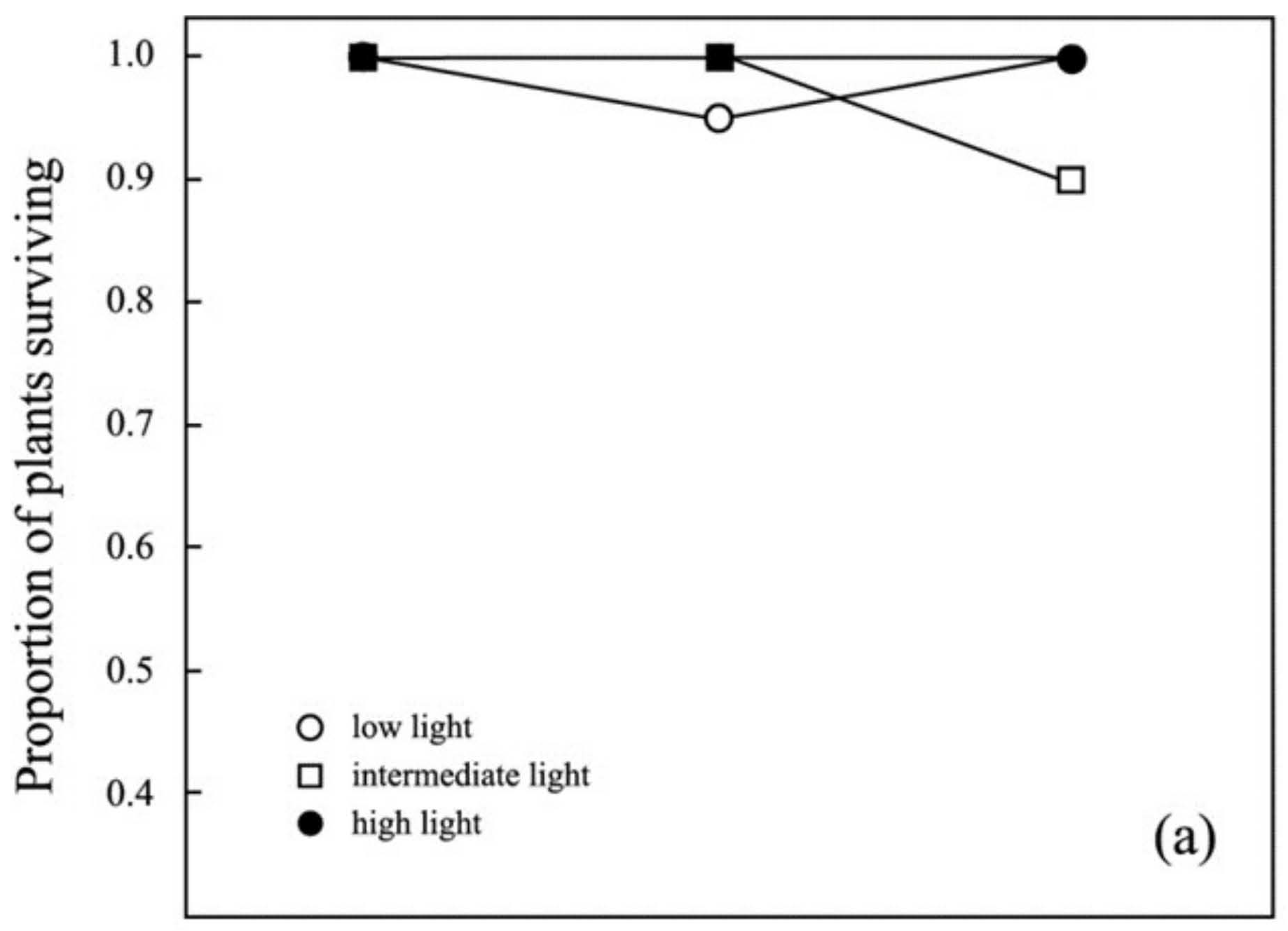
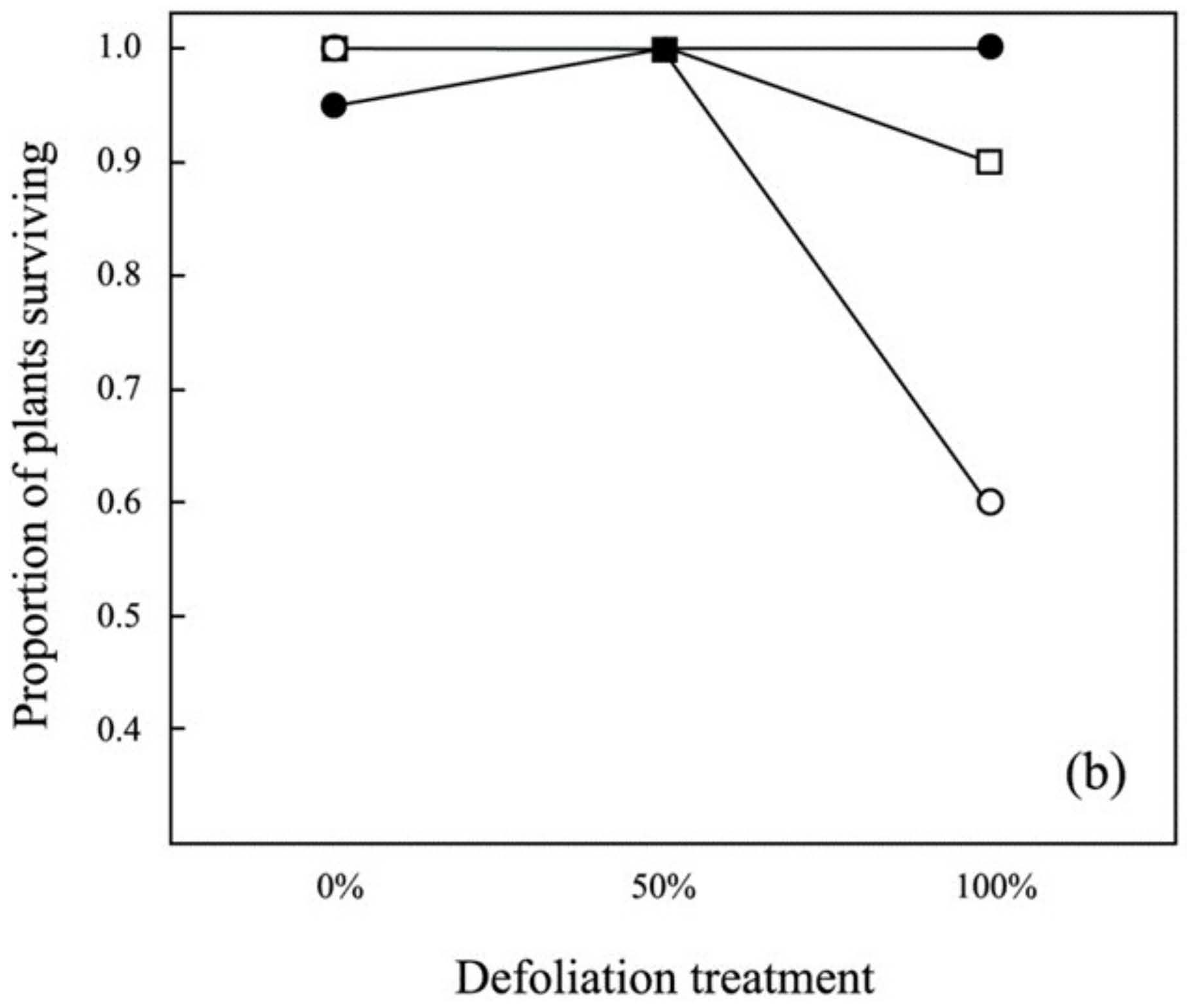
3.1. Survival
3.2. General Light and Defoliation Effects
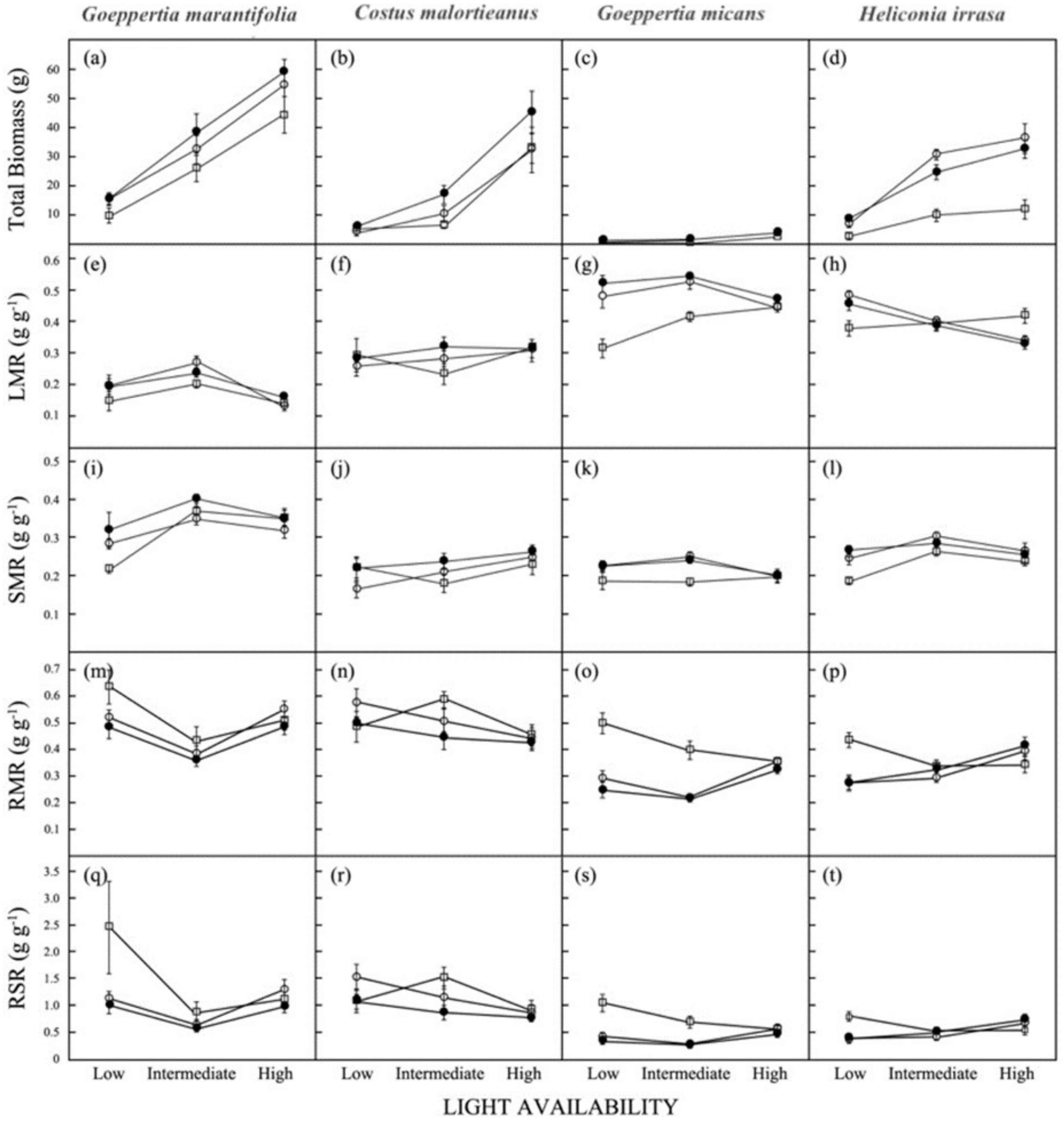
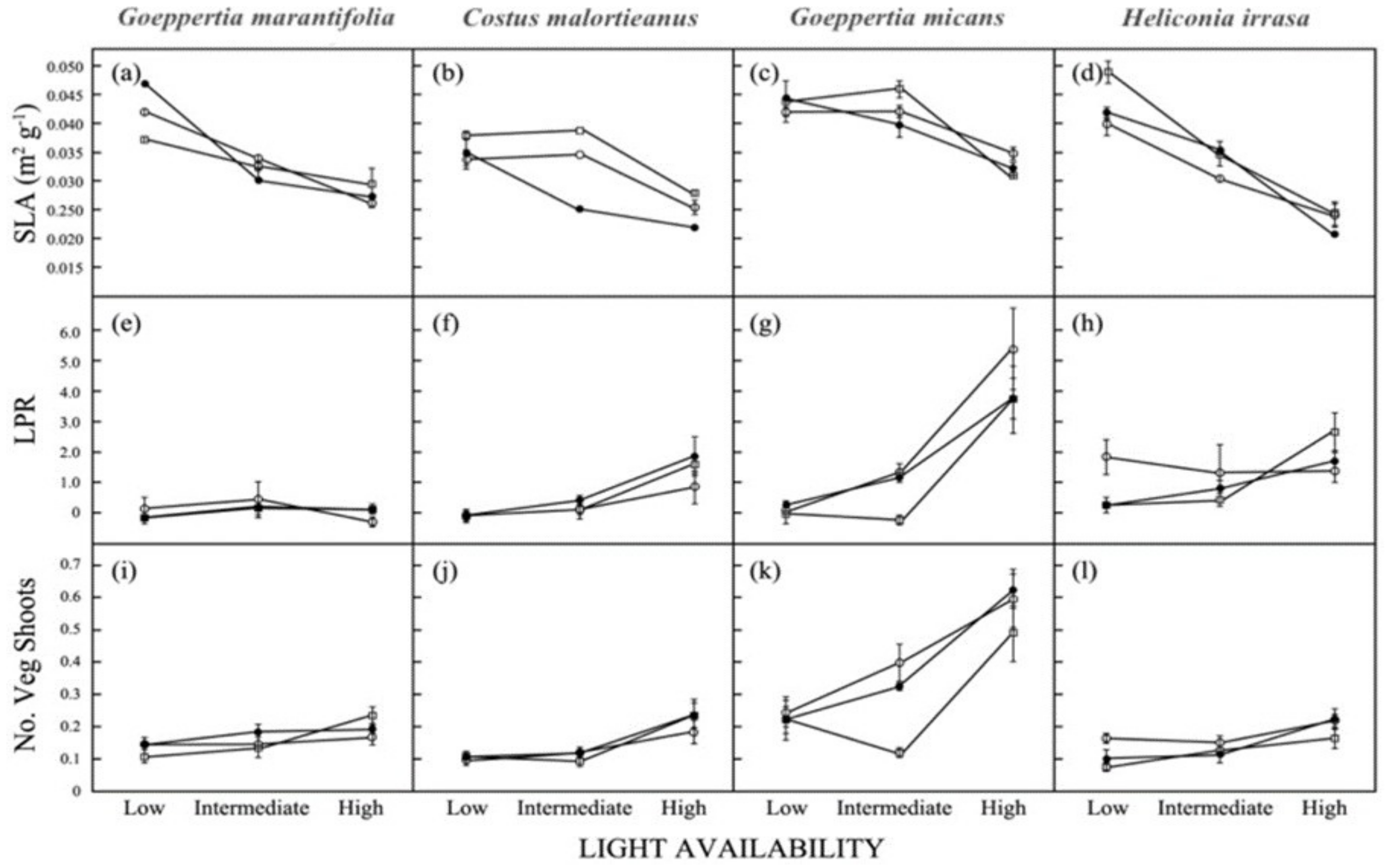
3.3. Biomass Allocation
3.4. Shoot Production
3.5. Photosynthetic Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aide, T.M. Limbfalls: A major cause of sapling mortality for tropical forest plants. Biotropica 1987, 19, 284–285. [Google Scholar] [CrossRef]
- Clark, D.B.; Clark, D.A. The role of physical damage in the seedling mortality regime of a tropical rainforest. Oikos 1989, 55, 225–230. [Google Scholar] [CrossRef]
- Clark, D.B.; Clark, D.A. The impact of physical damage on canopy tree regeneration in tropical rainforest. J. Ecol. 1991, 79, 447–457. [Google Scholar] [CrossRef]
- Scariot, A. Seedling mortality by litterfall in Amazonian forest fragments. Biotropica 2000, 32, 662–669. [Google Scholar] [CrossRef]
- Álvarez-Clare, S.; Kitajima, K. Susceptibility of tree seedlings to biotic and abiotic hazards in the understory of a moist tropical forest in Panamá. Biotropica 2009, 31, 47–56. [Google Scholar] [CrossRef]
- Dirzo, R.; Horvid, C.C.; Quevedo, H.; López, M.A. The effect of gap size and age on the understory herb community of a tropical Mexican rain forest. J. Ecol. 1992, 80, 809–822. [Google Scholar] [CrossRef]
- Marquis, R.J. Leave herbivores decrease fitness of a tropical plant. Science 1984, 226, 537–539. [Google Scholar] [CrossRef]
- Coley, P.D.; Barone, J.A. Herbivory and plant defenses in tropical forests. Ann. Rev. Ecol. Syst. 1996, 27, 305–335. [Google Scholar] [CrossRef]
- García-Guzmán, G.; Dirzo, R. Patterns of leaf-pathogen infection in the understory of a Mexican rain forest: Incidence, spatiotemporal variation and mechanisms of infection. Am. J. Bot. 2001, 88, 634–645. [Google Scholar] [CrossRef]
- McNaughton, S.J. Grazing as an optimization process: Grass-ungulate relationships in the Serengeti. Am. Nat. 1979, 113, 691–703. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Ackerly, D.D. Canopy-level photosynthetic compensation after defoliation in a tropical understory palm. Funct. Ecol. 2001, 15, 252–262. [Google Scholar]
- Crawley, M.J. Insect herbivores and plant population dynamics. Ann. Rev. Entom. 1989, 34, 531–564. [Google Scholar] [CrossRef]
- Ehrlén, J. Fitness components versus total demographic effects: Evaluating herbivore impacts on a perennial herb. Am. Nat. 2003, 162, 796–810. [Google Scholar] [CrossRef]
- Stowe, K.A.; Marquis, J.R.; Hochwender, C.G.; Simms, E.L. The evolutionary ecology of tolerance to consumer damage. Ann. Rev. Ecol. Syst. 2000, 31, 565–595. [Google Scholar] [CrossRef]
- Núñez-Farfán, J.; Fornoni, J.; Valverde, P.L. The evolution of resistance and tolerance to herbivores. Ann. Rev. Ecol. Evol. Syst. 2007, 2007, 541–566. [Google Scholar] [CrossRef]
- Fornoni, J. Ecological and evolutionary implications of plant tolerance to herbivory. Funct. Ecol. 2011, 25, 399–407. [Google Scholar] [CrossRef]
- Belsky, A.J. Does herbivory benefit plants? A review of the evidence. Am. Nat. 1986, 127, 870–892. [Google Scholar] [CrossRef]
- Paige, K.N.; Whitham, T.G. Overcompensation in response to mammalian herbivory: The advantage of being eaten. Am. Nat. 1987, 129, 407–416. [Google Scholar] [CrossRef]
- Chazdon, R.L. Light variation and carbon gain in rain forest understory palms. J. Ecol. 1991, 74, 995–1012. [Google Scholar] [CrossRef]
- Clark, D.B.; Clark, D.A.; Rich, P.M.; Weiss, S.; Oberbauer, S.F. Landscape-scale evaluation of understory light and canopy structure: Methods and application in a neotropical lowland rainforest. Can. J. For. Res. 1996, 26, 747–757. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Fetcher, N. Photosynthetic light environments in a lowland tropical rainforest in Costa Rica. J. Ecol. 1984, 72, 553–564. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Chazdon, R.L. Forest structure, canopy architecture, and light transmittance in old-growth and second-growth tropical rain forests. Ecology 2001, 82, 2707–2718. [Google Scholar] [CrossRef]
- Watling, J.R.; Robinson, J.E.; Woodrow, L.E.; Osmond, C.B. Responses of rainforest understory plants to excess light during sunflecks. Austr. J. Plant Physiol. 1996, 24, 17–25. [Google Scholar]
- Lovelock, C.E.; Kursar, T.A.; Skillman, J.B.; Winter, K. Photo inhibition in tropical forest understory plants with short- and long-lived leaves. Funct. Ecol. 1998, 12, 553–560. [Google Scholar] [CrossRef]
- Gold, W.G.; Caldwell, M.M. The effects of the spatial pattern of defoliation on regrowth of a tussock grass. III. Photosynthesis, canopy structure and light interception. Oecologia 1990, 82, 12–17. [Google Scholar] [CrossRef]
- Senock, R.S.; Sisson, W.B.; Donart, G.B. Compensatory photosynthesis of Sporobolus flexuosus (Thurb.) Rydb. following simulated herbivory in the Chihuahuan desert. Bot. Gaz. 1991, 152, 275–2881. [Google Scholar] [CrossRef]
- Hikosaka, K.; Sudoh, S.; Hirose, T. Light acquisition and use by individuals competing in a dense stand of an annual herb Xanthium canadense. Oecologia 1999, 118, 388–396. [Google Scholar] [CrossRef]
- Ashmun, J.W.; Thomas, R.J.; Pitelka, L.F. Translocation of photoassimilates between sister ramets in two rhizomatous forest herbs. Ann. Bot. 1982, 49, 403–415. [Google Scholar] [CrossRef]
- Nobel, J.C.; Marshall, C. The population biology of plants with clonal growth. I. The nutrient strategy and modular physiology of Carex arenaria. J. Ecol. 1983, 71, 865–877. [Google Scholar] [CrossRef]
- Chazdon, R.L. Effects of leaf and ramet removal on growth and reproduction of Geonoma congesta, a clonal understory palm. J. Ecol. 1986, 79, 1137–1146. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Martínez-Ramos, M.; Ackerly, D.D. Defoliation and growth in an understory palm: Quantifying the contributions of compensatory responses. Ecology 2003, 84, 2905–2918. [Google Scholar] [CrossRef]
- Martínez-Ramos, M.; Anten, N.P.R.; Ackerly, D.D. Defoliation and ENSO effects on vital rates of an understorey tropical rain forest palm. J. Ecol. 2009, 97, 1050–1061. [Google Scholar] [CrossRef]
- Canto, A.; Parra-Tabla, V.; García-Franco, J.G. Variations in leaf production and floral display of Anthurium schlechtendalii. (Araceae) in response to herbivory and environment. Funct. Ecol. 2004, 18, 692–699. [Google Scholar] [CrossRef]
- Bruna, E.M.; Ribeiro, M.B.N. The compensatory response of an understory herb to experimental damage is habitat-dependent. Am. J. Bot. 2005, 92, 2101–2106. [Google Scholar] [CrossRef]
- Benítez-Malvido, J. Effect of low vegetation on the recruitment of plants in successional habitat types. Biotropica 2006, 38, 171–182. [Google Scholar] [CrossRef]
- Gómez-Díaz, J.A.; Krömer, T.; Kreft, H.; Gerold, G.; Carvajal-Hernández, C.I.; Heitkamp, F. Diversity and composition of herbaceous angiosperms along gradients of elevation and forest-use intensity. PLoS ONE 2017, 12, e0182893. [Google Scholar] [CrossRef] [Green Version]
- Rundel, P.W.; Sharifi, M.R.; Gibson, A.C.; Esler, K.J. Structural and physiological adaptation to light environments in neotropical Heliconia (Heliconiaceae). J. Trop. Ecol. 1998, 14, 789–801. [Google Scholar] [CrossRef]
- Cooley, A.M.; Reich, J.A.; Rundel, P.W. Petiole biomechanics of neotropical understory monocots. Am. J. Bot. 2004, 91, 573–581. [Google Scholar] [CrossRef]
- Swenson, N.G. Herbaceous monocot plant form and function along a tropical rain–forest light gradient: A reversal of dicot strategy. J. Trop. Ecol. 2009, 25, 103–106. [Google Scholar] [CrossRef] [Green Version]
- García-Robledo, C.; Horvid, C.C.; Staines, C.L. Adult and larval morphology, host plants, adult longevity and notes on natural history in Cephaloleia rolled-leaf beetles (Coleoptera: Chrysomelidae: Cassidinae). Zootaxa 2010, 2610, 50–68. [Google Scholar] [CrossRef]
- Holdridge, L.R.; Grenk, W.G.; Hatheway, W.H.; Liang, T.; Tosi, J.A. Forest Environments in Tropical Life Zones; Pergamon Press: New York, NY, USA, 1975. [Google Scholar]
- Strauss-Debenedetti, S.; Bazzaz, F.A. Plasticity and acclimation to light in tropical Moraceae of different successional positions. Oecologia 1991, 87, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Matlaga, D.P.; Horvid, C. Growth and survival across a gap-understory gradient: Contrast in performance of sexually vs. clonally produced offspring. Am. J. Bot. 2009, 96, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Matlaga, D.P.; Horvid, C.C. Large size and high light do not lower the cost of reproduction for the Neotropical herb Goeppertia marantifolia. Am. J. Bot. 2015, 102, 350–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matlaga, D.P.; Snyder, R.K.; Horvid, C.C. Dispersal of Goeppertia marantifolia clonal offspring increases with greater canopy openness and larger plant size. J. Trop. Ecol. 2017, 33, 107–113. [Google Scholar] [CrossRef]
- Matlaga, D.; Sternberg, L. Ephemeral clonal integration in Calathea marantifolia (Marantaceae): Evidence of diminished integration over time. Am. J. Bot. 2009, 96, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Levesque, R. SPSS Programming and Data Management: A Guide for SPSS and SAS Users, 4th ed.; SPSS Inc.: Chicago, IL, USA, 2007. [Google Scholar]
- Jansen, M.; Zuidema, P.A.; van Ast, A.; Bongers, F.; Malosetti, M.; Martínez-Ramos, M.; Núñez-Farfán, J.; Anten, N.P. Heritability of growth and leaf loss compensation in a long-lived tropical understorey palm. PLoS ONE 2019, 14, e0209631. [Google Scholar] [CrossRef]
- Camargo, I.D.; Tapia-López, R.; Nűñez-Farfán, J. Ecotypic variation in growth responses to simulated herbivory: Trade-off between maximum relative growth rate and tolerance to defoliation in an annual plant. AoB Plants 2015, 7, plv015. [Google Scholar] [CrossRef] [Green Version]
- Blundell, A.G.; Peart, D.R. Growth strategies of a shade-tolerant tropical tree: The interactive effects of canopy gaps and simulated herbivory. J. Ecol. 2001, 89, 608–615. [Google Scholar] [CrossRef]
- Parra-Tabla, V.; Rico-Gray, V.V.; Carbajal, M. Effect of defoliation on leaf growth, sexual expression and reproductive success of Cnidoscolus aconitifolius (Euphorbiaceae). Plant Ecol. 2004, 173, 153–160. [Google Scholar] [CrossRef]
- Ballina-Gómez, H.S.; Iriarte-Vivar, S.; Orellana, R.; Santiago, L.S. Compensatory growth response to defoliation and light availability in two native Mexican woody plant species. J. Trop. Ecol. 2010, 26, 163–171. [Google Scholar] [CrossRef]
- Bazzaz, F.A.; Carlson, R.W. Photosynthetic acclimation to variability in the light environment of early and late successional plants. Oecologia 1982, 54, 313–316. [Google Scholar] [CrossRef]
- Sims, D.A.; Pearcy, R.W. Photosynthetic characteristics of a tropical forest understory herb, Alocasia macrorrhiza, and a related crop species, Colocasia esculenta grown in contrasting light environments. Oecologia 1989, 79, 53–59. [Google Scholar] [CrossRef]
- Crawley, M.J. Herbivory: The Dynamics of Animal-Plant Interactions; University of California Press: Berkeley, CA, USA, 1983. [Google Scholar]
- LaBarbera, M. Analyzing body size as a factor in ecology and evolution. Ann. Rev. Ecol. System. 1989, 20, 97–117. [Google Scholar] [CrossRef]
- Mendoza, A.; Piñero, D.; Sarukhán, J. Effects of experimental defoliation on growth, reproduction, and survival of Astrocaryum mexicanum. J. Ecol. 1987, 75, 545–554. [Google Scholar] [CrossRef]
- Oyama, K.; Mendoza, A. Effects of defoliation on growth, reproduction and survival of a Neotropical dioecious Palm, Chamaedorea tepejilote. Biotropica 1990, 22, 119–123. [Google Scholar] [CrossRef]
- Boebe, K. Influence of plant ontogeny on compensation to leaf damage. Am. J. Bot. 2005, 92, 1632–1640. [Google Scholar]
- Hernández-Barrios, J.C.; Anten, N.P.R.; Ackerly, D.D.; Martínez-Ramos, M. Defoliation and gender effects on fitness components in three congeneric and sympatric understorey palms. J. Ecol. 2012, 100, 1544–1556. [Google Scholar] [CrossRef] [Green Version]
- Massey, F.P.; Pross, M.C.; Hartley, S.E. Long- and short-term induction of defences in seedlings of Shorea leprosula (Dipterocarpaceae): Support for the carbon:nutrient balance hypothesis. J. Trop. Ecol. 2005, 21, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Zuidema, P.A.; de Koon, H.; Werger. Testing sustainability by prospective and retrospective demographic analyses: Evaluation for palm harvesting. Ecol. Appl. 2007, 17, 118–128. [Google Scholar] [CrossRef] [Green Version]
- McNaughton, S.J. Compensatory plant growth as a response to herbivory. Oikos 1983, 40, 329–336. [Google Scholar] [CrossRef]
- Coughenour, M.B.; Detling, J.K.; Bamberg, I.E.; Mugambi, M.M. Production and nitrogen response of the African dwarf shrub. Indigofera spinosa to defoliation and water limitation. Oecologia 1990, 83, 546–552. [Google Scholar] [CrossRef]
- Silvertown, S.; Franco, M.; Menan der Meijden, E.; Wijn, M.; Verkaar, H.J. Defense and regrowth, alternative plant strategies in the struggle against herbivores. Oikos 1988, 51, 355–363. [Google Scholar]
- Batista, W.B.; Platt, W.J.; Machiavelli, R.E. Demography of a shade-tolerant tree (Fagus grandifolia) in a hurricane-disturbed forest. Ecology 1998, 79, 39–53. [Google Scholar] [CrossRef] [Green Version]
- De Kroon, H.; van Groenendael, J. The Ecology and Evolution of Clonal Plants; Backhuys: Leiden, The Netherlands, 1997. [Google Scholar]
- Rosenthal, J.P.; Kotanen, P.M. Terrestrial plant tolerance to herbivory. Trends Ecol. Evol. 1994, 9, 145–148. [Google Scholar] [CrossRef]
- Kobe, R.K. Carbohydrate allocation to storage as a basis of interspecific variation in sapling survivorship and growth. Oikos 1997, 80, 226–233. [Google Scholar] [CrossRef]
- Canham, C.D.; Kobe, R.K.; Latty, E.F.; Chazdon, R.L. Interspecific and intraspecific variation in tree seedling survival: Effects of allocation to roots versus carbohydrate reserves. Oecologia 1999, 121, 1–11. [Google Scholar] [CrossRef]
- Van Staalduinen, M.; Anten, N.P.R. Difference in the capacity for compensatory growth of two co-occurring grass species in relation to water availability. Oecologia 2005, 146, 190–199. [Google Scholar] [CrossRef]
- Wise, M.J.; Abrahamson, W.G. Effects of resource availability on tolerance of herbivory: A review and assessment of three opposing models. Am. Nat. 2007, 169, 443–454. [Google Scholar] [CrossRef]
- Lend, K.A.; Cipollini, D.F. Effect of light and simulated herbivory on growth of endangered northeastern bulrush, Scirpus ancistrochaetus Schulyer. Plant Ecol. 1998, 139, 125–131. [Google Scholar]
- McPherson, K.; Williams, K. The role of carbohydrate reserves the growth, resilience, and persistence of cabbage palm seedlings (Sabal palmetto). Oecologia 1998, 117, 460–468. [Google Scholar] [CrossRef]
- Hicks, R.; Turkington, R. Compensatory growth of three herbaceous perennial species: The effects of clipping and nutrient availability. Can. J. Bot. 2000, 78, 759–767. [Google Scholar]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Pud, F.E.; Brokaw, N.V.L. Sprouting of broken trees on Barro Colorado Island, Panamá. Ecology 1989, 70, 508–512. [Google Scholar]
- Gartner, B.L. Breakage and regrowth of Piper species in rainforest understory. Biotropica 1989, 21, 303–307. [Google Scholar] [CrossRef]
- Fong, F.W. Perspectives for sustainable resource utilization and management of Nipa vegetation. Econ. Bot. 1995, 46, 45–54. [Google Scholar] [CrossRef]
- Joyal, E. The palm has its time: An ethnoecology of Sabal uresana in Sonora, Mexico. Econ. Bot. 1996, 50, 446–462. [Google Scholar] [CrossRef]
- Ratsirarsort, J.; Silander, J.A.; Richard, A.F. Conservation and management of a threatened Madagascar palm species, Neodypsis decaryi. Conserv. Biol. 1996, 10, 40–52. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Westerband, A.C.; Horvid, C.C. Interactions between plant size and canopy openness influence vital rates and life-history tradeoffs in two neotropical understory herbs. Am. J. Bot. 2015, 102, 1290–1299. [Google Scholar] [CrossRef] [Green Version]
- Westerband, A.C.; Horvid, C.C. Early life conditions and precipitation influence the performance of widespread understory herbs in variable light environments. J. Ecol. 2017, 105, 1298–1308. [Google Scholar] [CrossRef]
- Westerband, A.C.; Horvid, C.C. Photosynthetic rates influence the population dynamics of understory herbs in stochastic light environments. Ecology 2017, 98, 370–381. [Google Scholar] [CrossRef]

| Dependent Variable | Light | Defoliation | Light × Defoliation |
|---|---|---|---|
| Goeppertia marantifolia | |||
| Total Biomass | 66.44 *** | 6.15 * | 0.30 |
| LMR | 11.88 *** | 2.40 | 0.74 |
| SMR | 7.92 ** | 2.32 | 1.68 |
| RMR | 14.49 *** | 2.44 | 0.78 |
| RSR | 11.83 *** | 3.03 | 1.20 |
| SLA | 41.72 *** | 1.54 | 0.28 |
| LPR | 1.64 | 0.12 | 1.08 |
| No. Vegetative Shoots | 8.53 *** | 0.45 | 2.68 * |
| Costus malortieanus | |||
| LMR | 1.56 | 1.06 | 0.99 |
| SMR | 2.98 | 1.31 | 1.05 |
| RMR | 2.51 | 1.06 | 1.26 |
| RSR | 3.05 | 1.43 | 1.35 |
| SLA | 24.38 *** | 8.41 | 2.19 |
| LPR | 20.38 *** | 1.71 | 0.90 |
| No. Vegetative Shoots | 20.754 *** | 0.64 | 1.05 |
| Goeppertia micans | |||
| Total Biomass | 100.98 *** | 34.40 *** | 2.28 |
| LMR | 8.36 *** | 36.39 *** | 9.42 *** |
| SMR | 2.07 | 7.95 *** | 1.51 |
| RMR | 10.11 *** | 33.73 *** | 6.78 *** |
| RSR | 8.58 *** | 34.01 *** | 6.98 *** |
| SLA | 33.92 *** | 0.24 | 0.15 |
| LPR | 76.58 *** | 7.52 ** | 2.41 |
| No. Vegetative Shoots | 17.15 *** | 12.19 *** | 2.33 |
| Heliconia irrasa | |||
| Total Biomass | 70.66 *** | 46.47 *** | 0.572 |
| LMR | 11.66 *** | 0.62 | 6.93 *** |
| SMR | 11.65 *** | 8.92 ** | 2.24 |
| RMR | 4.81 * | 3.16 * | 5.30 *** |
| RSR | 4.89 ** | 3.11 | 5.53 *** |
| SLA | 58.10 *** | 1.92 | 1.38 |
| LPR | 7.96 *** | 1.24 | 3.02 * |
| No. Vegetative Shoots | 7.69 ** | 8.37 *** | 1.06 |
| Dependent Variable | Low | Intermediate | High |
|---|---|---|---|
| Goeppertia marantifolia | |||
| Amax (μmol m−2 s−1) | 4.49 ± 0.38 | 6.91 ± 0.15 | 9.80 ± 0.30 |
| Respiration rate (μmol m−2 s−1) | −0.31 ± 0.05 | −0.33 ± 0.02 | −0.50 ± 0.08 |
| Light compensation point (μmol photons m−2 s−1) | 2 | 4 | 7 |
| Saturating PFD (mmol photons m−2 s−1) | 300 | 600 | 800 |
| Apparent quantum yield (mole CO2 mole photon−1) | 2.83 ± 0.39 | 4.86 ± 0.51 | 0.04 ± 0.46 |
| Costus malortieanus | |||
| Amax (μmol m−2 s−1) | 3.25 ± 0.08 | 5.20 ± 0.23 | 11.70 ± 0.22 |
| Respiration rate (μmol m−2 s−1) | −0.35 ± 0.02 | −0.35 ± 0.01 | −0.59 ± 0.02 |
| Light compensation point (μmol photons m−2 s−1) | 2 | 5 | 7 |
| Saturating PFD (mmol photons m−2 s−1) | 150 | 600 | 800 |
| Apparent quantum yield (mole CO2 mole photon−1) | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.02 ± 0.04 |
| Goeppertia micans | |||
| Amax (μmol m−2 s−1) | 2.55 ± 0.19 | 2.90 ± 0.21 | 4.56 ± 0.19 |
| Respiration rate (μmol m-−2 s−1) | −0.23 ± 0.02 | −0.26 ± 0.05 | −0.32 ± 0.03 |
| Light compensation point (μmol photons m−2 s−1) | 4 | 4 | 5 |
| Saturating PFD (mmol photons m−2 s−1) | 250 | 300 | 400 |
| Apparent quantum yield (mole CO2 mole photon-1) | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.00 |
| Heliconia irrasa | |||
| Amax (μmol m−2 s−1) | 4.35 ± 0.30 | 7.88 ± 0.06 | 10.20 ± 0.58 |
| Respiration rate (μmol m−2 s−1) | −0.18 ± 0.13 | −0.23 ± 0.02 | −0.34 ± 0.08 |
| Light compensation point (μmol photons m−2 s−1) | 4 | 5 | 7 |
| Saturating PFD (mmol photons m−2 s−1) | 250 | 500 | 800 |
| Apparent quantum yield (mole CO2 mole photon−1) | 0.07 ± 0.00 | 0.01 ± 0.01 | 0.06 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.W.C.; Sharifi, M.R.; Rundel, P.W. Plasticity in Compensatory Growth to Artificial Defoliation and Light Availability in Four Neotropical Understory and Forest Edge Herb Species. Biology 2022, 11, 1532. https://doi.org/10.3390/biology11101532
Sun JWC, Sharifi MR, Rundel PW. Plasticity in Compensatory Growth to Artificial Defoliation and Light Availability in Four Neotropical Understory and Forest Edge Herb Species. Biology. 2022; 11(10):1532. https://doi.org/10.3390/biology11101532
Chicago/Turabian StyleSun, Jennifer W. C., M. Rasoul Sharifi, and Philip W. Rundel. 2022. "Plasticity in Compensatory Growth to Artificial Defoliation and Light Availability in Four Neotropical Understory and Forest Edge Herb Species" Biology 11, no. 10: 1532. https://doi.org/10.3390/biology11101532
APA StyleSun, J. W. C., Sharifi, M. R., & Rundel, P. W. (2022). Plasticity in Compensatory Growth to Artificial Defoliation and Light Availability in Four Neotropical Understory and Forest Edge Herb Species. Biology, 11(10), 1532. https://doi.org/10.3390/biology11101532





