A Non-Stressful Temperature Rise and Greater Food Availability Could Increase Tolerance to Calcium Limitation of Daphnia cf. pulex (Sensu Hebert, 1995) Populations in Cold Soft-Water Lakes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
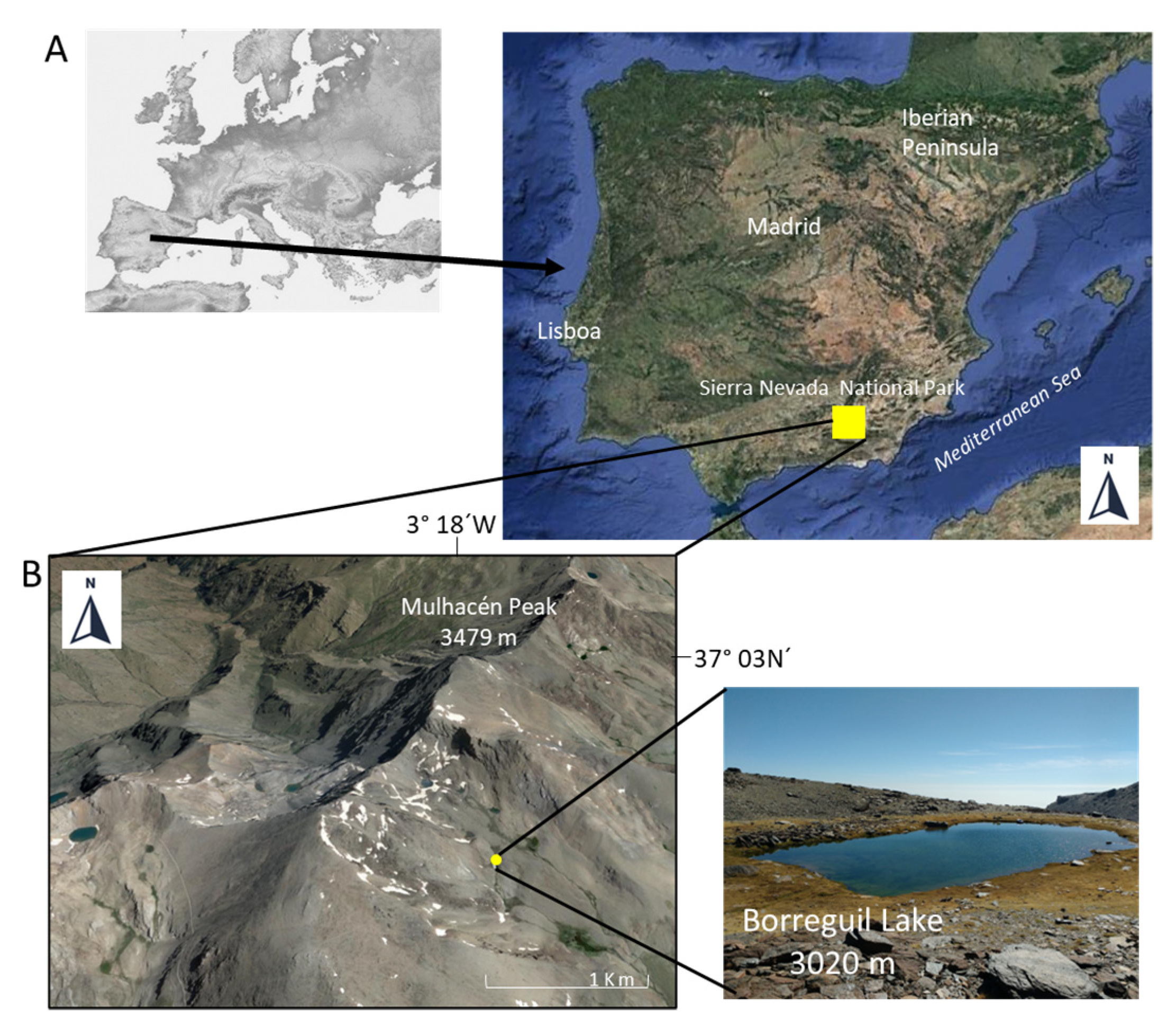
2.1. Collection Site
2.2. Culturing Daphnia and Algae
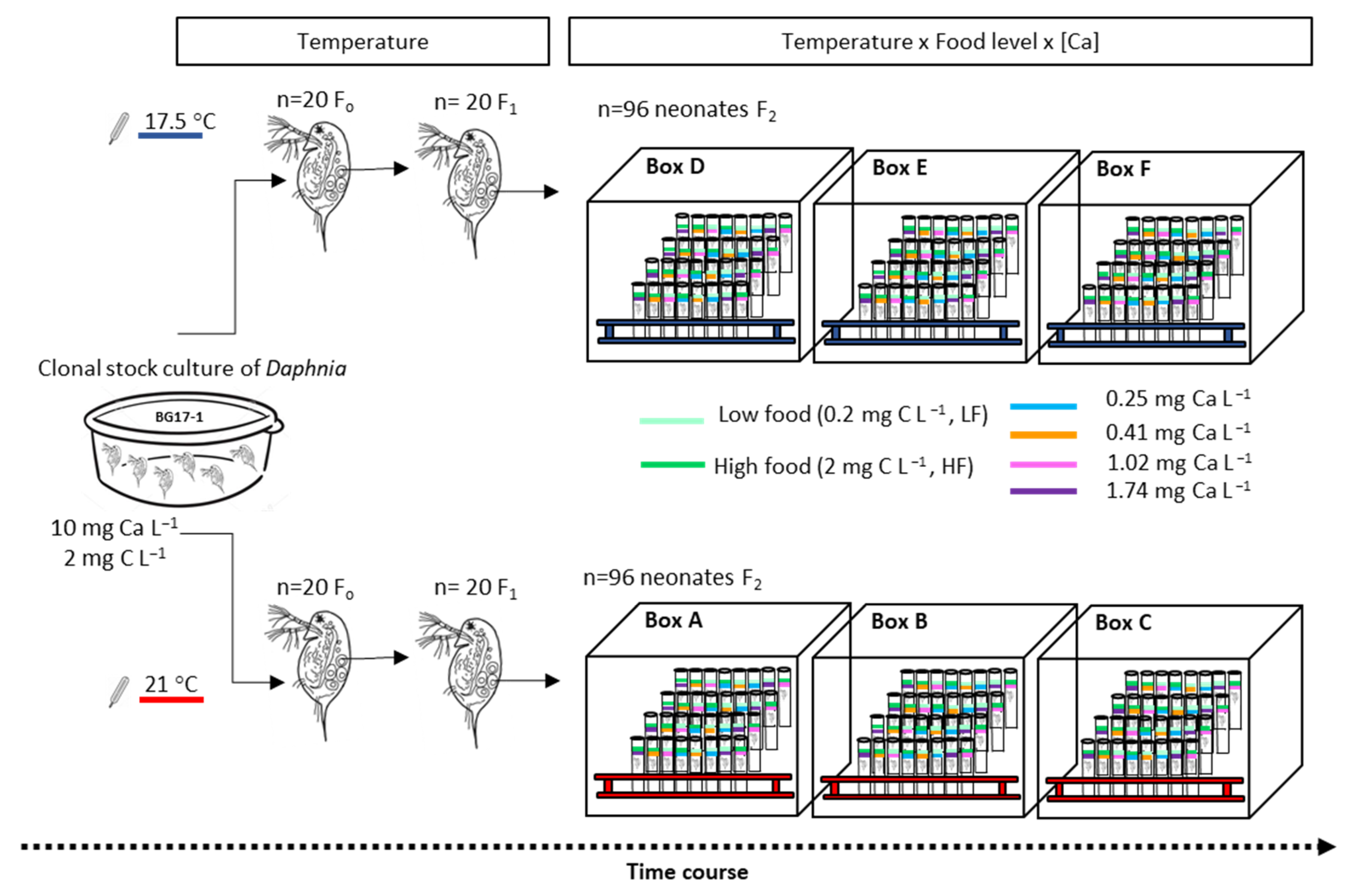
2.3. Experimental Design
Quantified Life History Parameters
2.4. Statistical Analyses
3. Results
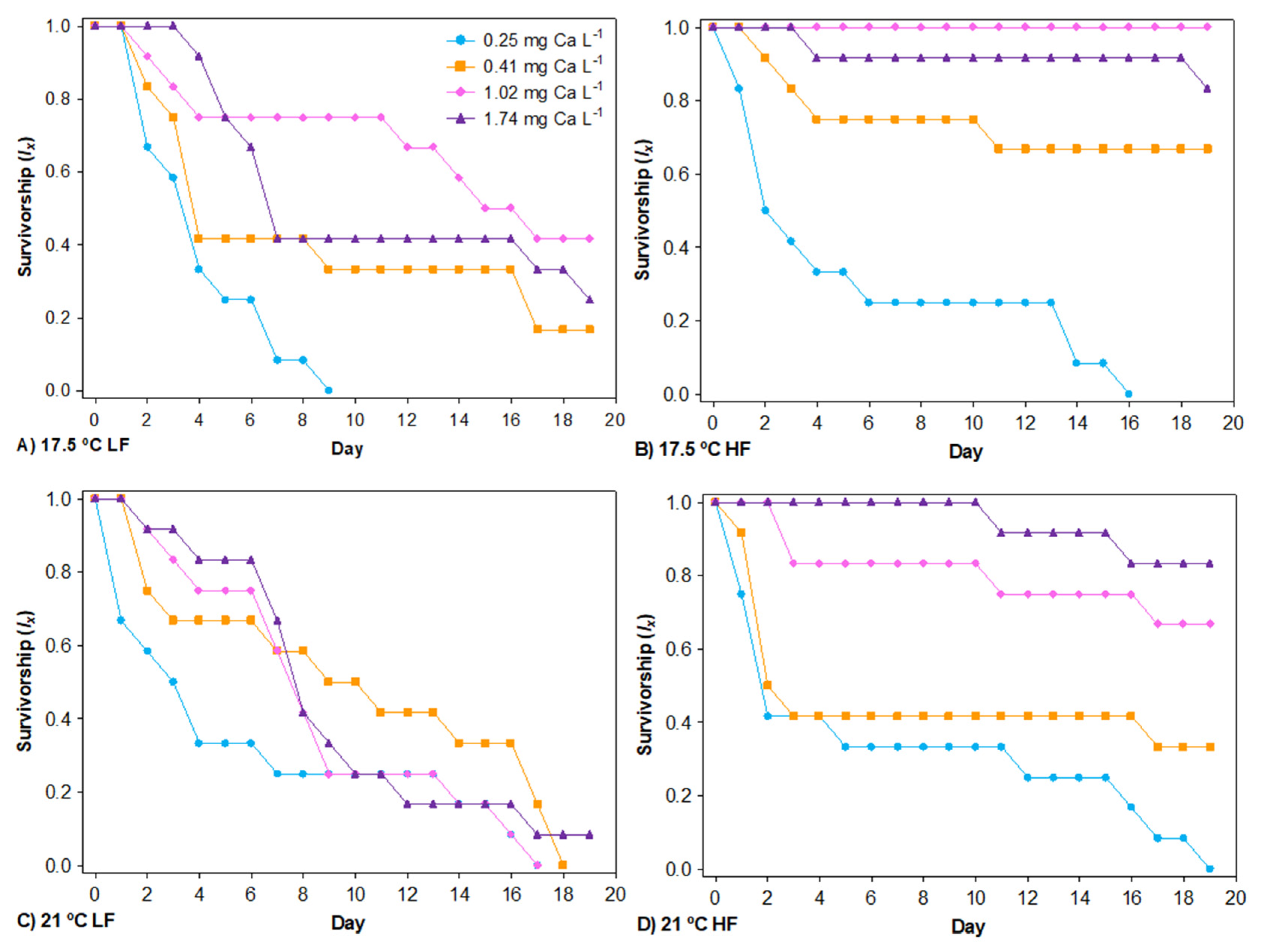
3.1. Survival
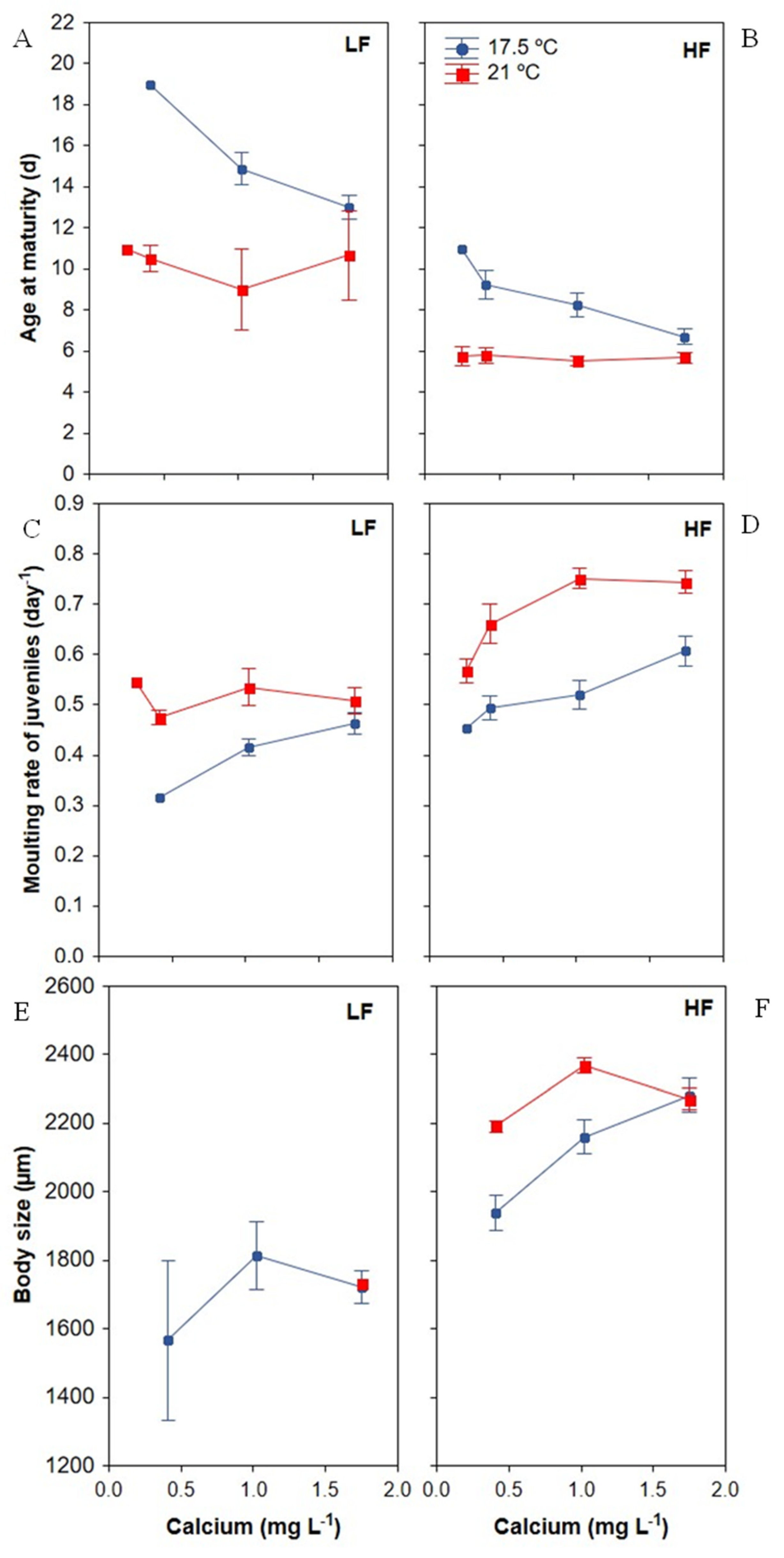
3.2. Development Traits
3.2.1. Age of Maturity
3.2.2. Moulting Frequency of Juveniles
3.2.3. Body Size
3.3. Reproductive Traits
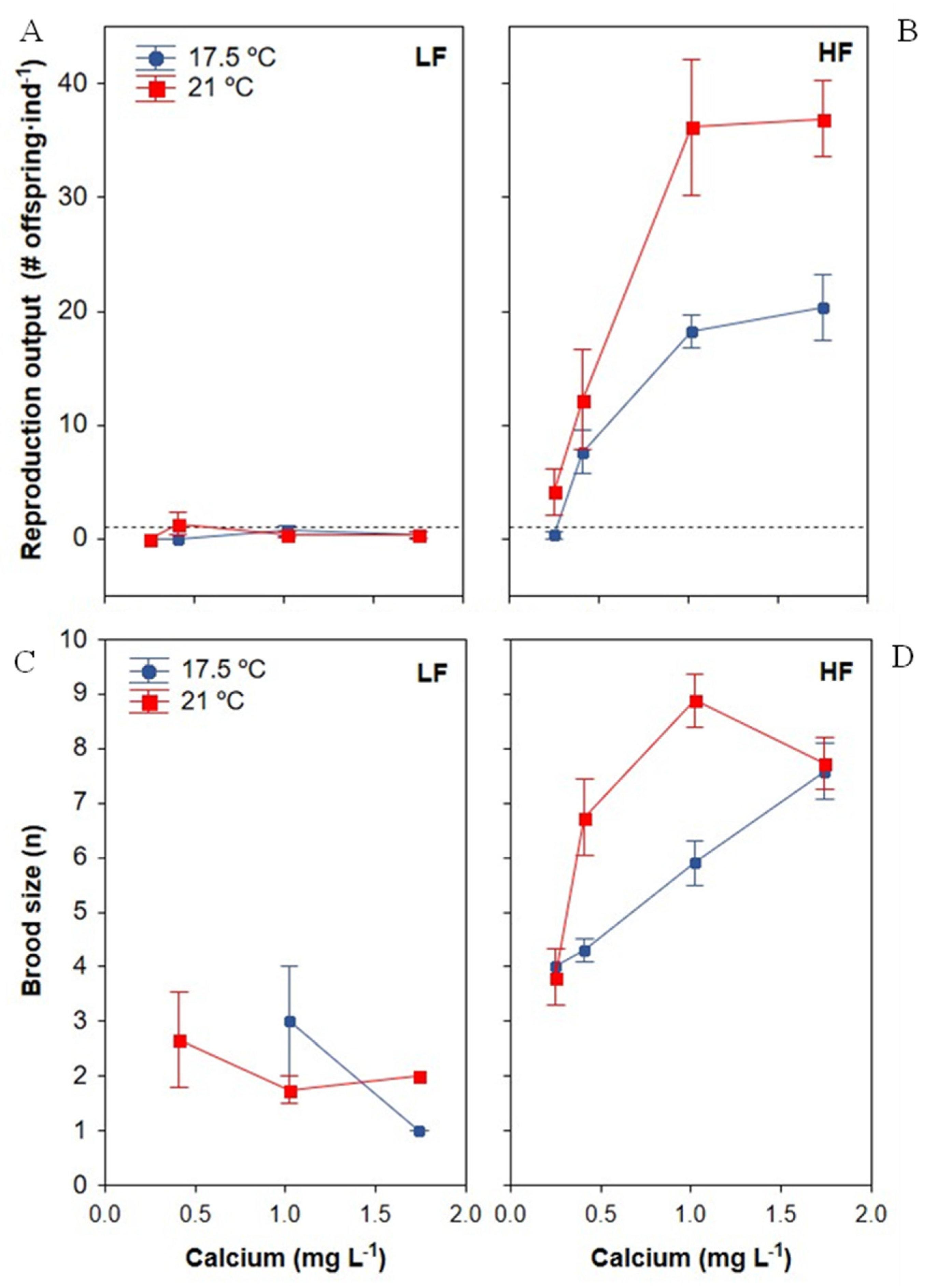
3.3.1. Reproduction Output
3.3.2. Brood Size
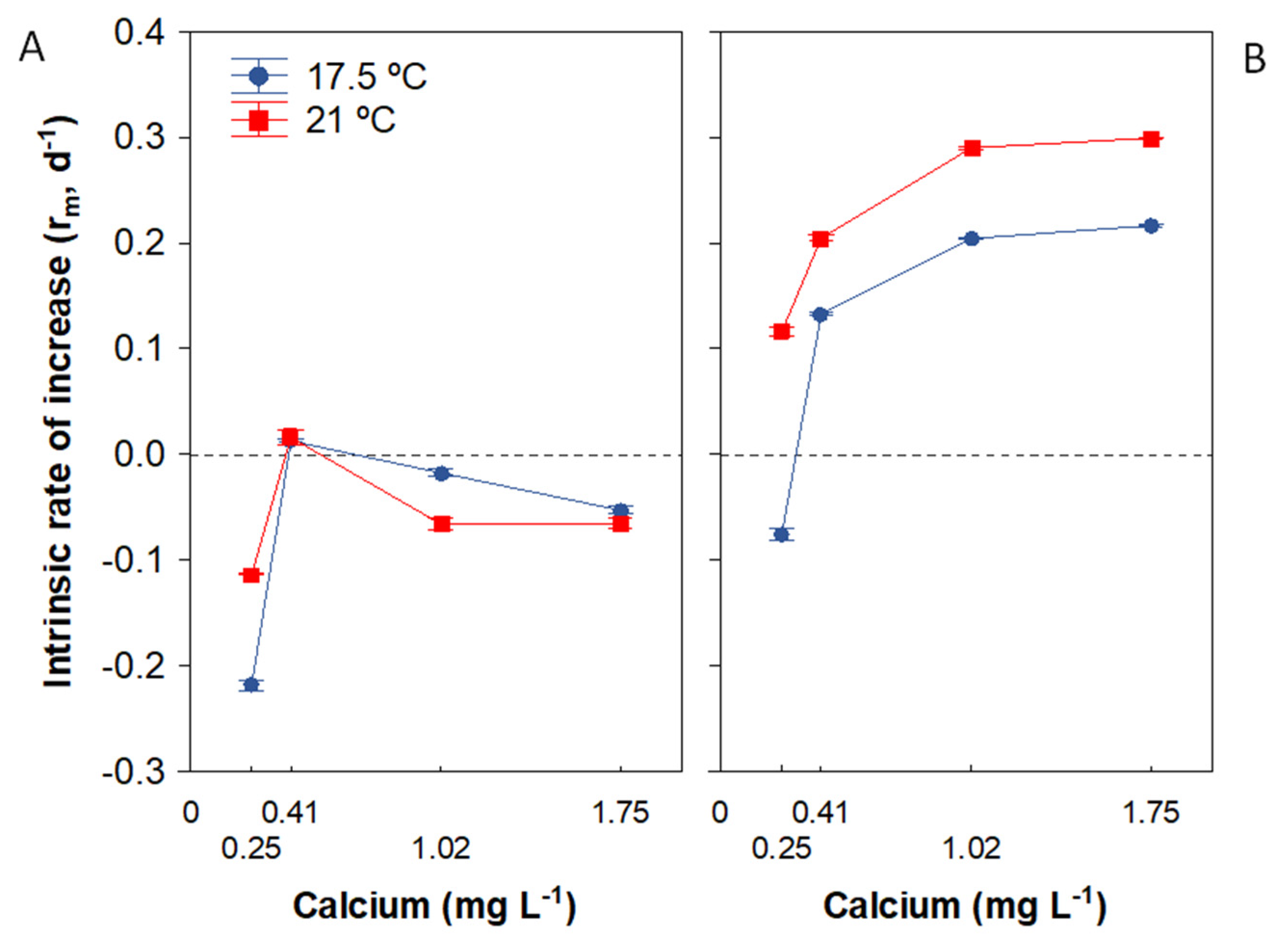
3.4. Population Growth Rate
4. Discussion
4.1. Food and Temperature Influence on the Calcium Threshold for Survival
4.2. Food and Temperature Influence on the Calcium Threshold for Reproduction
4.3. Food and Temperature Influence on the Calcium Threshold for Population Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ormerod, S.J.; Dobson, M.; Hildrew, A.G.; Townsend, C.R. Multiple stressors in freshwater ecosystems. Freshw. Biol. 2010, 55, 1–4. [Google Scholar] [CrossRef]
- Cuenca Cambronero, M.; Marshall, H.; De Meester, L.; Davidson, T.A.; Beckerman, A.P.; Orsini, L. Predictability of the impact of multiple stressors on the keystone species Daphnia. Sci. Rep. 2018, 8, 17572. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rodríguez, E.; Moreno, E.; Conde-Porcuna, C.P. Intraspecific variation in sensitivity to food availability and temperature-induced phenotypic plasticity in the rotifer Keratella Cochlearis. J. Exp. Biol. 2020, 223, jeb209676. [Google Scholar] [CrossRef]
- Crain, C.M.; Kroeker, K.; Halpern, B.S. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 2008, 11, 1304–1315. [Google Scholar] [CrossRef]
- Eads, B.; Andrews, J.; Colbourne, J. Ecological genomics in Daphnia: Stress responses and environmental sex determination. Heredity 2008, 100, 184–190. [Google Scholar] [CrossRef]
- Dodson, S.I.; Hanazato, T. Commentary on Effects of Anthropogenic and Natural Organic-Chemicals on Development, Swimming Behavior, and Reproduction of Daphnia, a Key Member of Aquatic Ecosystems. Environ. Health Persp. 1995, 103, 7–11. [Google Scholar] [CrossRef][Green Version]
- Ashforth, D.; Yan, N.D. The interactive effects of calcium concentration and temperature on the survival and reproduction of Daphnia pulex at high and low food concentrations. Limnol. Oceanogr. 2008, 53, 420–432. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, L.; Li, Q.; Zhang, H.; Chen, Y. Combined effects of calcium deficiency, cyanobacteria, and warming on the population fitness of three cladocerans. Fundam. Appl. Limnol. 2014, 185, 321–328. [Google Scholar] [CrossRef]
- Pérez-Fuentetaja, A.; Goodberry, F. Daphnia’s challenge: Survival and reproduction when calcium and food are limiting. J. Plankton Res. 2016, 38, 1379–1388. [Google Scholar] [CrossRef]
- Cairns, A.; Yan, N. A review of the influence of low ambient calcium concentrations on freshwater daphniids, gammarids, and crayfish. Environ. Rev. 2009, 17, 67–79. [Google Scholar] [CrossRef]
- Hessen, D.O.; Alstad, N.E.W.; Skardal, L. Ca limitation in Daphnia magna. J. Plankton Res. 2000, 22, 553–568. [Google Scholar] [CrossRef]
- Rukke, N.A. Effects of low calcium concentrations on two common freshwater crustaceans, Gammarus lacustris and Astacus astacus. Funct. Ecol. 2002, 16, 357–366. [Google Scholar] [CrossRef]
- Jeziorski, A.; Smol, J.P. The ecological impacts of lakewater calcium decline on softwater boreal ecosystems. Environ. Rev. 2017, 25, 245–253. [Google Scholar] [CrossRef]
- Hessen, D.O.; Rukke, N.A. UV radiation and low calcium as mutual stressors for Daphnia. Limnol. Oceanogr. 2000, 45, 1834–1838. [Google Scholar] [CrossRef]
- Celis-Salgado, M.P.; Keller, W.; Yan, N.D. Calcium and sodium as regulators of the recovery of four Daphnia species along a gradient of metals and base cations in metal contaminated lakes in Sudbury, Ontario, Canada. J. Limnol. 2016, 75, 36–49. [Google Scholar] [CrossRef]
- Liorti, A.; Crease, T.; Heyland, A. Interactive effects of copper and calcium in Daphnia pulex. J. Limnol. 2017, 76, 281–291. [Google Scholar] [CrossRef]
- Steinkey, D.; Lari, E.; Woodman, S.G.; Steinkey, R.; Luong, K.H.; Wong, C.S.; Pyle, G.G. The effects of diltiazem on growth, reproduction, energy reserves, and calcium-dependent physiology in Daphnia magna. Chemosphere 2019, 232, 424–429. [Google Scholar] [CrossRef]
- Prater, C.; Wagner, N.D.; Frost, P.C. Effects of calcium and phosphorus limitation on the nutritional ecophysiology of Daphnia. Limnol. Oceanogr. 2016, 61, 268–278. [Google Scholar] [CrossRef]
- Akbar, S.; Du, J.; Jia, Y.; Tian, X. The importance of calcium in improving resistance of Daphnia to Microcystis. PLoS ONE 2017, 12, e0175881. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Zhou, Q.; Sun, Y.; Zhang, L.; Gu, L.; Lyu, K.; Huang, Y.; Chen, Y.; Yang, Z. Non-toxic and toxic Microcystis aeruginosa reduce the tolerance of Daphnia pulex to low calcium in different degrees: Based on the changes in the key life-history traits. Chemosphere 2020, 248, 126101. [Google Scholar] [CrossRef]
- Rice, M.J.; Jones, C.L.C.; Starke, C.W.E.; Frost, P.C. Calcium stress in Daphnia pulicaria and exposure to predator-derived cues: Making a bad situation worse? Freshw. Sci. 2021, 40, 449–462. [Google Scholar] [CrossRef]
- Betini, G.S.; Roszell, J.; Heyland, A.; Fryxell, J.M. Calcium interacts with temperature to influence Daphnia movement rates. R Soc. Open. Sci. 2016, 3, 16053. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Z.; Yu, B.; Sun, Y.; Gu, L.; Zhang, L.; Huang, Y.; Yang, Z. Population changes of Daphnia caused by declined calcium concentration: Evidences from population dynamics and sexual reproduction. Ecotoxicol. Environ. Saf. 2022, 233, 113352. [Google Scholar] [CrossRef] [PubMed]
- Weyhenmeyer, G.A.; Hartmann, J.; Hessen, D.O.; Kopáček, J.; Hejzlar, J.; Jacquet, S.; Zechmeister, T. Widespread diminishing anthropogenic effects on calcium in freshwaters. Sci. Rep. 2019, 9, 10450. [Google Scholar] [CrossRef]
- Jeziorski, A.; Yan, N.D.; Paterson, A.M.; DeSellas, A.M.; Turner, M.A.; Jeffries, D.S.; Smol, J.P. The widespread threat of calcium decline in fresh waters. Science 2008, 322, 1374–1377. [Google Scholar] [CrossRef]
- Hessen, D.O.; Andersen, T.; Tominaga, K.; Finstad, A.G. When soft waters becomes softer; drivers of critically low levels of Ca in Norwegian lakes. Limnol. Oceanogr. 2017, 62, 289–298. [Google Scholar] [CrossRef]
- Rogora, M.; Mosello, R.; Marchetto, A. Long-term trends in the chemistry of atmospheric deposition in northwestern Italy: The role of increasing Saharan dust deposition. Tellus B 2004, 56, 426–434. [Google Scholar] [CrossRef]
- Preunkert, S.; Legrand, M. Towards a quasi-complete reconstruction of past atmospheric aerosol load and composition (organic and inorganic) over Europe since 1920 inferred from Alpine ice cores. Clim. Past. 2013, 9, 1403–1416. [Google Scholar] [CrossRef]
- Pérez-Martínez, C.; Conde-Porcuna, J.M.; Ramos-Rodríguez, E.; Moreno, E.; Rühland, K.M.; Jeziorski, A.; Smol, J.P.; García-Alix, A.; Heiri, O.; Corral-Arredondo, E.; et al. Paleolimnological Indicators of Global Change. In The Landscape of the Sierra Nevada; Zamora, R., Oliva, M., Eds.; Springer Nature Switzerland AG: Gewerbestrasse, Switzerland, 2022; pp. 283–295. [Google Scholar]
- Rostási, Á.; Topa, B.A.; Gresina, F.; Weiszburg, T.G.; Gelencsér, A.; Varga, G. Saharan Dust Deposition in Central Europe in 2016—A Representative Year of the Increased North African Dust Removal Over the Last Decade. Front. Earth Sci. 2022, 10, 869902. [Google Scholar] [CrossRef]
- Goss, L.B.; Bunting, L.D. Daphnia development and reproduction: Responses to temperature. J. Therm. Biol. 1983, 4, 375–380. [Google Scholar] [CrossRef]
- Hoefnagel, K.N.; De Vries, E.; Jongejans, E.; Verberk, W.C. The temperature-size rule in Daphnia magna across different genetic lines and ontogenetic stages: Multiple patterns and mechanisms. Ecol. Evol. 2018, 8, 3828–3841. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.F.; Colomer, J.; Serra, T. Temperature-driven response reversibility and short-term quasi-acclimation of Daphnia magna. PLoS ONE 2018, 13, e0209705. [Google Scholar] [CrossRef] [PubMed]
- Goss, L.B.; Bunting, L.D. Thermal tolerance of zooplankton. Wat. Res. 1976, 10, 387–398. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Pörtner, H.O. Climate variations and the physiological basis of temperature dependent biogeography: Systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. Part A 2002, 132, 739–761. [Google Scholar] [CrossRef]
- Walczyńska, A.; Kiełbasa, A.; Sobczyk, M. ‘Optimal thermal range’ in ectotherms: Defining criteria for tests of the temperature- size-rule. J. Therm. Biol. 2016, 60, 41–48. [Google Scholar] [CrossRef]
- MacIsaac, H.; Hugh, P.; Schwartz, S.S. Inter- and intraspecific variation in acute thermal tolerance of Daphnia. Physiol. Zool. 1985, 58, 350–355. [Google Scholar] [CrossRef]
- Burns, C.W. Predictors of invasion success by Daphnia species: Influence of food, temperature and species identity. Biol. Invasions 2013, 15, 859–869. [Google Scholar] [CrossRef]
- Benzie, J.A.H. Cladocera: The genus Daphnia (including Daphniopsis). In Guide to the Identification of the Microinvertebrates of the Continental Waters of the World; Dumont, H.J.F., Ed.; Backhuys Publishers: Leiden, The Netherlands, 2005; Volume 21, pp. 1–376. [Google Scholar]
- Duggan, I.C.; Robinson, K.V.; Burns, C.W.; Banks, J.C.; Hogg, I.D. Identifying invertebrate invasions using morphological and molecular analyses: North American Daphnia ‘pulex’ in New Zealand fresh waters. Aquat. Invasions 2012, 7, 585–590. [Google Scholar] [CrossRef]
- Williams, P.J.; Dick, K.B.; Yampolsky, L.Y. Heat tolerance, temperature acclimation, acute oxidative damage and canalization of haemoglobin expression in Daphnia. Evol. Ecol. 2012, 26, 591–609. [Google Scholar] [CrossRef]
- Paul, R.J.; Lamkemeyer, T.; Maurer, J.; Pinkhaus, O.; Pirow, R.; Seidl, M.; Zeis, B. Thermal acclimation in the microcrustacean Daphnia: A survey of behavioural, physiological and biochemical mechanisms. J. Therm. Biol. 2004, 29, 655–662. [Google Scholar] [CrossRef]
- Steffen, W.; Persson, Ǻ.; Deutsch, L.; Zalasiewicz, J.; Williams, M.; Richardson, K.; Crumley, C.; Crutzen, P.; Folke, C.; Gordon, L.; et al. The Anthropocene: From global change to planetary steward-ship. AMBIO 2011, 40, 739–761. [Google Scholar] [CrossRef] [PubMed]
- Nogués-Bravo, D.; López-Moreno, J.I.; Vicente-Serrano, S.M. Climate change and its impact. In Mediterranean Mountain Environments; Vogiatzakis, I.N., Ed.; Wiley-Blackwell: Chichester, UK, 2012; pp. 185–201. [Google Scholar]
- Preston, D.L.; Caine, N.; McKnight, D.M.; Williams, M.W.; Hell, K.; Miller, M.P.; Hart, S.J.; Johnson, P.T.J. Climate regulates alpine lake ice cover phenology and aquatic ecosystem structure. Geophys. Res. Lett. 2016, 43, 5353–5360. [Google Scholar] [CrossRef]
- Sadro, S.; Melack, J.M.; Sickman, J.O.; Skeen, K. Climate warming response of mountain lakes affected by variations in snow. LO-Lett. 2019, 4, 9–17. [Google Scholar] [CrossRef]
- Schindler, D.W. Lakes as sentinels and integrators for the effects of climate change on watersheds, airsheds, and landscapes. Limnol. Oceanogr. 2009, 54, 2349–2358. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Karlsson, J. Nonlinear response of dissolved organic carbon concentrations in boreal lakes to increasing temperatures. Limnol. Oceanogr. 2009, 54, 2513–2519. [Google Scholar] [CrossRef]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.Z.; Liu, X.D. and others. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Chang. 2015, 5, 424–430. [Google Scholar] [CrossRef]
- Dokulil, M.T.; de Eyto, E.; Maberly, S.C.; May, L.; Weyhenmeyer, G.A.; Woolway, R.I. Increasing maximum lake surface temperature under climate change. Clim. Chang. 2021, 165, 56. [Google Scholar] [CrossRef]
- Jiménez, L.; Rühland, K.M.; Jeziorski, A.; Smol, J.P.; Pérez-Martínez, C. Climate change and Saharan dust drive recent cladoceran and primary production changes in remote alpine lakes of Sierra Nevada, Spain. Glob. Chang. Biol. 2018, 24, e139–e158. [Google Scholar] [CrossRef]
- Pérez-Martínez, C.; Conde-Porcuna, J.M.; Moreno, E.; Ramos- Rodríguez, E.; Jiménez, L. Cladoceran assemblage distribution in shallow alpine lakes of Sierra Nevada (Spain) and its relationship with environmental variables. Aquat. Sci. 2020, 82, 4. [Google Scholar] [CrossRef]
- Conde-Porcuna, J.M.; Veiga, J.; Moreno, E.; Jiménez, L.; Ramos-Rodríguez, E.; Pérez-Martínez, C. Spatiotemporal genetic structure in the Daphnia pulex complex from Sierra Nevada lakes (Spain): Reproductive mode and first record of North American D. cf. pulex in European alpine lakes. J. Plankton Res. 2021, 43, 380–395. [Google Scholar] [CrossRef]
- Crease, T.J.; Omilian, A.R.; Costanzo, K.S.; Taylor, D.J. Transcontinental phylogeography of the Daphnia pulex species complex. PLoS ONE 2012, 7, e46620. [Google Scholar] [CrossRef]
- Conde-Porcuna, J.M.; Ramos-Rodríguez, E.; Pérez-Martínez, C. In situ production of empty ephippia and resting eggs by an obligate parthenogenetic Daphnia population. J. Plankton Res. 2014, 36, 157–169. [Google Scholar] [CrossRef][Green Version]
- Gutiérrez-Parejo, P. Biodiversidad del Zooplancton de lagunas con Distintas Características Ambientales: Patrones y Consecuencias ante el Cambio Global. Master’s Thesis, Universidad de Granada, Granada, Spain, 2019; pp. 1–50. [Google Scholar]
- Kilham, S.S.; Kreeger, D.A.; Lynn, S.G.; Goulden, C.E.; Herrera, L. COMBO: A defined freshwater culture medium for algae and zooplankton. Hydrobiologia 1998, 377, 147–159. [Google Scholar] [CrossRef]
- Lampert, W. A directly coupled, artificial two-step food chain for long-term experiments with filter-feeders at constant food concentrations. Mar. Biol. 1976, 37, 349–355. [Google Scholar] [CrossRef]
- Wood, A.M.; Everroad, R.C.; Wingard, L.M. Measuring growth rates in microalgal cultures. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 269–285. [Google Scholar]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dynoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Klausmeier, C.A.; Litchman, E.; Levin, S.A. Phytoplankton growth and stoichiometry under multiple nutrient limitation. Limnol. Oceanogr. 2004, 49, 1463–1470. [Google Scholar] [CrossRef]
- Hurlbert, S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Pérez-Martínez, C.; Jimenez, L.; Moreno, E.; Conde-Porcuna, J.M. Emergence pattern and hatching cues of Daphnia pulicaria (Crustacea, Cladocera) in an alpine lake. Hydrobiologia 2013, 707, 47–57. [Google Scholar] [CrossRef]
- Sakwińska, O. Persistent maternal identity effects on life history traits in Daphnia. Oecologia 2004, 138, 379–386. [Google Scholar] [CrossRef]
- Guinnee, M.A.; West, S.A.; Little, T.J. Testing small clutch size models with Daphnia. Am. Nat. 2004, 163, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Montero-Pau, J.; Gabaldón, C.; Carmona, M.J.; Serra, M. Measuring the potential for growth in populations investing in diapause. Ecol. Modell. 2014, 272, 76–83. [Google Scholar] [CrossRef]
- Ramos-Rodríguez, E.; Conde-Porcuna, J.M. Nutrient limitation on a planktonic rotifer: Life history consequences and starvation resistance. Limnol. Oceanogr. 2003, 48, 933–938. [Google Scholar] [CrossRef]
- Rellstab, C.; Spaak, P. Lake origin determines Daphnia population growth under winter conditions. J. Plankton Res. 2009, 31, 261–271. [Google Scholar] [CrossRef]
- Meyer, J.S.; Ingersoll, C.G.; McDonald, L.L.; Boyce, M.S. Estimating uncertainty in population growth rates: Jackknife vs. bootstrap techniques. Ecology 1986, 67, 1156–1166. [Google Scholar] [CrossRef]
- Austin, P.C. A Tutorial on Multilevel Survival Analysis: Methods, Models and Applications. Int. Stat. Rev. 2017, 85, 185–203. [Google Scholar] [CrossRef]
- Therneau, T. A Package for Survival Analysis in R. 2022. R Package Version 3.3-1. Available online: https://CRAN.R-project.org/package=survival (accessed on 13 June 2022).
- Fournier, D.A.; Skaug, H.J.; Ancheta, J.; Ianelli, J.; Magnusson, A.; Maunder, M.; Nielsen, A.; Sibert, J. AD Model Builder: Using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Methods Softw. 2012, 27, 233–249. [Google Scholar] [CrossRef]
- Skaug, H.; Fournier, D.; Nielsen, A.; Magnusson, A.; Bolker, B. Generalized Linear Mixed Models Using AD Model Builder. 2016. R Package Version 0.8.3.3. Available online: https://rdrr.io/rforge/glmmADMB/ (accessed on 13 June 2022).
- Rabe-Hesketh, S.; Skrondal, A. Multilevel and Longitudinal Modeling Using Stata, Volume 2: Categorical Responses, Counts, and Survival, 3rd ed.; Stata Press: College Station, TX, USA, 2012; Volume 2. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and SPLUS; Springer: New York, NY, USA, 2000; p. 527. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Luo, D.; Ganesh, S.; Koolaard, J. Predictmeans: Calculate Predicted Means for Linear Models. 2021. Available online: https://CRAN.R-project.org/package=predictmeans (accessed on 13 June 2022).
- Rukke, N.A. Tolerance to low ambient calcium shows inter- population differences in Daphnia galeata. J. Plankton Res. 2002, 24, 527–531. [Google Scholar] [CrossRef]
- Porcella, D.B.; Rixford, C.E.; Slater, J.V. Molting and calcification in Daphnia magna. Physiol. Zool. 1969, 42, 148–159. [Google Scholar] [CrossRef]
- Cowgill, U.M.; Emmel, H.W.; Hopkins, D.L.; Applegath, S.L.; Takahashi, I.T. Influence of water on reproductive success and chemical composition of laboratory reared populations of Daphnia magna. Water Res. 1986, 20, 317–323. [Google Scholar] [CrossRef]
- He, X.J.; Wang, W.X. Calcium balance in Daphnia grown on diets differing in food quantity, phosphorus and calcium. Freshw. Biol. 2009, 54, 2200–2211. [Google Scholar] [CrossRef]
- Muyssen, B.T.A.; De Schamphelaere, K.A.C.; Janssen, C.R. Calcium accumulation and regulation in Daphnia magna: Links with feeding, growth and reproduction. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 152, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.W. Relation between filtering rate, temperature, and body size in four species of Daphnia. Limnol. Oceanogr. 1969, 14, 693–700. [Google Scholar] [CrossRef]
- Oleksy, I.A.; Baron, J.S.; Leavitt, P.R.; Spaulding, S.A. Nutrients and warming interact to force mountain lakes into unprecedented ecological states. Proc. R. Soc. B 2020, 287, 20200304. [Google Scholar] [CrossRef]
| Effects | Estimate | SE | Wald z |
|---|---|---|---|
| Intercept | −5.422 | 1.750 | −3.098 ** |
| Calcium (Ca) | −0.237 | 0.228 | −1.038 ns |
| Food | 0.144 | 0.183 | 0.784 ns |
| Temperature | 0.191 | 0.089 | 2.140 * |
| Interval | −0.017 | 0.017 | −1.018 ns |
| Ca × food | −1.058 | 0.267 | −3.969 *** |
| Age of Maturity | Molting Rate | Body Size | Brood Size | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effects | Estimate | SE | Wald z | Estimate | SE | Wald z | Estimate | SE | Wald z | Estimate | SE | Wald z |
| Intercept | 5.47 | 0.40 | 13.73 *** | −0.46 | 0.33 | −1.40 ns | 999.5 | 215.2 | 4.64 *** | −10.39 | 3.50 | −2.97 ** |
| Calcium (Ca) | −2.07 | 0.54 | −3.84 *** | 0.04 | 0.01 | 3.56 *** | 1172.4 | 395.0 | 2.97 ** | −0.50 | 0.92 | −0.59 ns |
| Food | −0.36 | 0.02 | −14.62 *** | −0.33 | 0.19 | −1.72 ns | 239.7 | 24.1 | 8.86 *** | 2.65 | 0.27 | 9.85 *** |
| Temperature | −0.15 | 0.02 | −7.24 *** | 0.05 | 0.02 | 2.77 ** | 38.6 | 11.9 | 3.24 ** | 0.63 | 0.18 | 3.45 *** |
| Ca × food | - | - | - | - | - | - | - | - | - | 1.19 | 0.49 | 2.43 * |
| Ca × temperature | 0.11 | 0.03 | 3.73 *** | - | - | - | −54.9 | 21.1 | −2.60 ** | - | - | - |
| Food × temperature | - | - | - | 0.02 | 0.01 | 2.23 * | - | - | - | - | - | - |
| Reproduction Output | Population Growth Rate | |||||
|---|---|---|---|---|---|---|
| Effects | Chi-Square | df | p-Value from Permutation | Chi-Square | df | p-Value from Permutation |
| Calcium (Ca) | 1.4974 | 1 | 0.22426 | 25.99 | 1 | 0.00033 |
| Food | 19.9 | 1 | 0.00033 | 18.458 | 1 | 0.00033 |
| Temperature | 0.2692 | 1 | 0.56548 | 2.0402 | 1 | 0.17727 |
| Ca × food | 8.3336 | 1 | 0.00533 | 54.956 | 1 | 0.00033 |
| Ca × temperature | - | - | - | 23.914 | 1 | 0.00033 |
| Food × temperature | 28.78 | 1 | 0.00033 | 35.359 | 1 | 0.00033 |
| Ca × food × temperature | 13.37 | 1 | 0.00100 | - | - | - |
| Life-History Traits | Relevant Outcomes from This Study |
|---|---|
| Survival | Ca survival threshold ≥0.25 mg Ca L−1 (19 d endpoint); at HF, higher Ca survival threshold when temperature increases from 17.5 to 21 °C Survival greatly reduced by temperature rising from 17.5 to 21 °C, regardless of the availability of food and water Ca Ca × food: Higher hazard of mortality by Ca stress at HF |
| Development traits | |
| Age of maturity | Ca × temperature: Earlier with increasing Ca at lower temperature (17.5 °C); no effect of Ca gradient at higher temperature (21 °C) |
| Moulting frequency of juveniles | Greater when Ca increased Food × temperature: increased at higher temperature and HF |
| Body size | Ca × temperature: Greater increase in body size with highest Ca at lowest temperature; at Ca < 1.5 mg Ca L−1, interestingly, Daphnia was larger in warmer conditions |
| Reproduction traits | |
| Reproduction output | Ca reproduction threshold < 0.25 mg Ca L−1 (at 21 °C) At HF, Ca saturation point for reproduction at 1 mg Ca L−1 Ca × food: At HF, increased when Ca increased; at LF, higher Ca had no effect Food × temperature: Greater effect of temperature increases at HF than at LF Ca × food × temperature: Positive synergistic effect of Ca and temperature at HF; At HF, a higher temperature improved Ca tolerance with a lower minimum Ca required for reproduction output > 1 offspring·ind−1 |
| Fertility (brood size) | Ca × food: Increased at higher Ca at HF, but not at LF |
| Population growth rate | Ca × food: Ca effect stronger at HF Ca × temperature: Ca threshold for population growth (CaZPG) varied between temperatures under HF; CaZPG markedly lower for 21 ° versus 17.5 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Rodríguez, E.; Pérez-Martínez, C.; Conde-Porcuna, J.M. A Non-Stressful Temperature Rise and Greater Food Availability Could Increase Tolerance to Calcium Limitation of Daphnia cf. pulex (Sensu Hebert, 1995) Populations in Cold Soft-Water Lakes. Biology 2022, 11, 1539. https://doi.org/10.3390/biology11101539
Ramos-Rodríguez E, Pérez-Martínez C, Conde-Porcuna JM. A Non-Stressful Temperature Rise and Greater Food Availability Could Increase Tolerance to Calcium Limitation of Daphnia cf. pulex (Sensu Hebert, 1995) Populations in Cold Soft-Water Lakes. Biology. 2022; 11(10):1539. https://doi.org/10.3390/biology11101539
Chicago/Turabian StyleRamos-Rodríguez, Eloísa, Carmen Pérez-Martínez, and José María Conde-Porcuna. 2022. "A Non-Stressful Temperature Rise and Greater Food Availability Could Increase Tolerance to Calcium Limitation of Daphnia cf. pulex (Sensu Hebert, 1995) Populations in Cold Soft-Water Lakes" Biology 11, no. 10: 1539. https://doi.org/10.3390/biology11101539
APA StyleRamos-Rodríguez, E., Pérez-Martínez, C., & Conde-Porcuna, J. M. (2022). A Non-Stressful Temperature Rise and Greater Food Availability Could Increase Tolerance to Calcium Limitation of Daphnia cf. pulex (Sensu Hebert, 1995) Populations in Cold Soft-Water Lakes. Biology, 11(10), 1539. https://doi.org/10.3390/biology11101539





