Bone Molecular Modifications Induced by Diagenesis Followed-Up for 12 Months
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Samples
2.2. Raman Microspectrometry
2.3. Statistical Analysis
3. Results
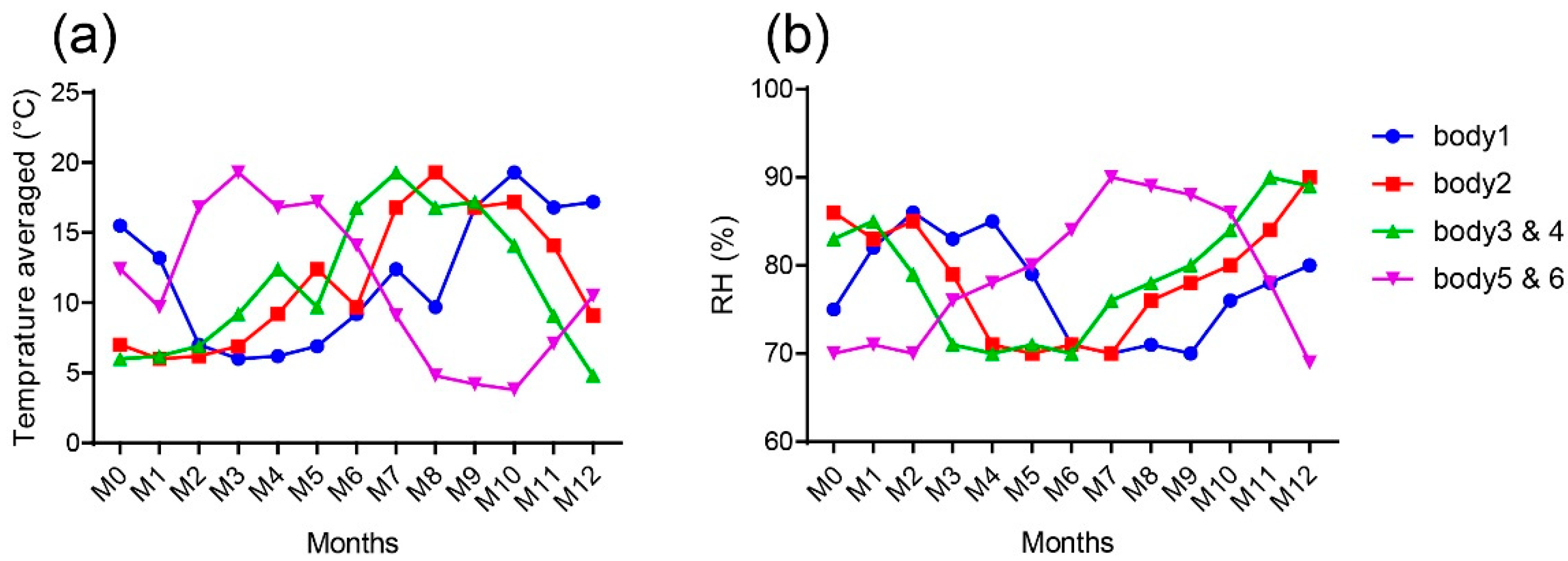
Significant Variations of the Molecular Composition Were Observed in the Mineral and Organic Matrices as Function of Burial Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hedges, R.E.M. Bone diagenesis: An overview of processes. Archaeometry 2002, 44, 319–328. [Google Scholar] [CrossRef]
- Nielsen-Marsh, C.M.; Hedges, R.E. Patterns of Diagenesis in Bone I: The Effects of Site Environments. J. Archaeol. Sci. 2000, 27, 1139–1150. [Google Scholar] [CrossRef]
- Fernández-Jalvo, Y.; Andrews, P.; Pesquero, D.; Smith, C.; Marín-Monfort, D.; Sánchez, B.; Geigl, E.-M.; Alonso, A. Early bone diagenesis in temperate environments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 288, 62–81. [Google Scholar] [CrossRef]
- De Sousa, D.V.; Eltink, E.; Oliveira, R.A.P.; Félix, J.F.; Guimarães, L.D.M. Diagenetic processes in Quaternary fossil bones from tropical limestone caves. Sci. Rep. 2020, 10, 21425. [Google Scholar] [CrossRef]
- Kendall, C.; Eriksen, A.M.H.; Kontopoulos, I.; Collins, M.J.; Turner-Walker, G. Diagenesis of archaeological bone and tooth. Palaeogeogr. Palaeoclim. Palaeoecol. 2018, 491, 21–37. [Google Scholar] [CrossRef] [Green Version]
- López-Costas, O.; Lantes-Suárez, O.; Cortizas, A.M. Chemical compositional changes in archaeological human bones due to diagenesis: Type of bone vs soil environment. J. Archaeol. Sci. 2016, 67, 43–51. [Google Scholar] [CrossRef]
- Procopio, N.; Mein, C.; Starace, S.; Bonicelli, A.; Williams, A. Bone Diagenesis in Short Timescales: Insights from an Exploratory Proteomic Analysis. Biology 2021, 10, 460. [Google Scholar] [CrossRef]
- Keenan, S.W.; Engel, A. Early diagenesis and recrystallization of bone. Geochim. Et Cosmochim. Acta 2017, 196, 209–223. [Google Scholar] [CrossRef]
- Delannoy, Y.; Colard, T.; Cannet, C.; Mesli, V.; Hédouin, V.; Penel, G.; Ludes, B. Characterization of bone diagenesis by histology in forensic contexts: A human taphonomic study. Int. J. Leg. Med. 2017, 132, 219–227. [Google Scholar] [CrossRef]
- Ermida, C.; Navega, D.; Cunha, E. Luminol chemiluminescence: Contribution to postmortem interval determination of skeletonized remains in Portuguese forensic context. Int. J. Leg. Med. 2017, 131, 1149–1153. [Google Scholar] [CrossRef]
- Falgayrac, G.; Vitale, R.; Delannoy, Y.; Behal, H.; Penel, G.; Duponchel, L.; Colard, T. Critical aspects of Raman spectroscopy as a tool for postmortem interval estimation. Talanta 2022, 249, 123589. [Google Scholar] [CrossRef] [PubMed]
- Mandair, G.S.; Morris, M.D. Contributions of Raman spectroscopy to the understanding of bone strength. BoneKEy Rep. 2015, 4, 620. [Google Scholar] [CrossRef] [Green Version]
- Paschalis, E.P.; Gamsjaeger, S.; Klaushofer, K. Vibrational spectroscopic techniques to assess bone quality. Osteoporos. Int. 2017, 28, 2275–2291. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, M.; Müller, R.; Schneider, P. Techniques to assess bone ultrastructure organization: Orientation and arrangement of mineralized collagen fibrils. J. R. Soc. Interface 2016, 13, 20160088. [Google Scholar] [CrossRef] [Green Version]
- Trueman, C.N.; Privat, K.; Field, J. Why do crystallinity values fail to predict the extent of diagenetic alteration of bone mineral? Palaeogeogr. Palaeoclim. Palaeoecol. 2008, 266, 160–167. [Google Scholar] [CrossRef]
- Woess, C.; Unterberger, S.H.; Roider, C.; Ritsch-Marte, M.; Pemberger, N.; Cemper-Kiesslich, J.; Hatzer-Grubwieser, P.; Parson, W.; Pallua, J.D. Assessing various Infrared (IR) microscopic imaging techniques for post-mortem interval evaluation of human skeletal remains. PLoS ONE 2017, 12, e0174552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creagh, D.; Cameron, A. Estimating the Post-Mortem Interval of skeletonized remains: The use of Infrared spectroscopy and Raman spectro-microscopy. Radiat. Phys. Chem. 2017, 137, 225–229. [Google Scholar] [CrossRef]
- McLaughlin, G.; Lednev, I.K. Potential application of Raman spectroscopy for determining burial duration of skeletal remains. Anal. Bioanal. Chem. 2011, 401, 2511–2518. [Google Scholar] [CrossRef]
- Baptista, A.; Pedrosa, M.; Curate, F.; Ferreira, M.T.; Marques, M.P.M. Estimation of the post-mortem interval in human bones by infrared spectroscopy. Int. J. Leg. Med. 2021, 136, 309–317. [Google Scholar] [CrossRef]
- Zhang, K.; Fan, F.; Tu, M.; Cui, J.-H.; Li, J.-S.; Peng, Z.; Deng, Z.-H. The role of multislice computed tomography of the costal cartilage in adult age estimation. Int. J. Leg. Med. 2017, 132, 791–798. [Google Scholar] [CrossRef]
- Navadijo, M.P.; Zapata, I.F.; Miranda, E.A.B.; Aguilera, I.A. Discriminant functions for sex estimation using the rib necks in a Spanish population. Int. J. Leg. Med. 2021, 135, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Pascart, T.; Cortet, B.; Olejnik, C.; Paccou, J.; Migaud, H.; Cotten, A.; Delannoy, Y.; During, A.; Hardouin, P.; Penel, G.; et al. Bone Samples Extracted from Embalmed Subjects Are Not Appropriate for the Assessment of Bone Quality at the Molecular Level Using Raman Spectroscopy. Anal. Chem. 2016, 88, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Adlam, R.E.; Simmons, T. The Effect of Repeated Physical Disturbance on Soft Tissue Decomposition—Are Taphonomic Studies an Accurate Reflection of Decomposition? J. Forensic Sci. 2007, 52, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Falgayrac, G.; Cortet, B.; Devos, O.; Barbillat, J.; Pansini, V.; Cotten, A.; Pasquier, G.; Migaud, H.; Penel, G. Comparison of Two-Dimensional Fast Raman Imaging versus Point-by-Point Acquisition Mode for Human Bone Characterization. Anal. Chem. 2012, 84, 9116–9123. [Google Scholar] [CrossRef] [PubMed]
- Gamsjaeger, S.; Robins, S.P.; Tatakis, D.N.; Klaushofer, K.; Paschalis, E.P. Identification of Pyridinoline Trivalent Collagen Cross-Links by Raman Microspectroscopy. Calcif. Tissue Int. 2017, 100, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Penel, G.; Delfosse, C.; Descamps, M.; Leroy, G. Composition of bone and apatitic biomaterials as revealed by intravital Raman microspectroscopy. Bone 2005, 36, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Patonai, Z.; Maasz, G.; Avar, P.; Schmidt, J.; Lorand, T.; Bajnoczky, I.; Mark, L. Novel dating method to distinguish between forensic and archeological human skeletal remains by bone mineralization indexes. Int. J. Leg. Med. 2012, 127, 529–533. [Google Scholar] [CrossRef]
- Sasso, G.D.; Angelini, I.; Maritan, L.; Artioli, G. Raman hyperspectral imaging as an effective and highly informative tool to study the diagenetic alteration of fossil bones. Talanta 2018, 179, 167–176. [Google Scholar] [CrossRef]
- Unal, M.; Yang, S.; Akkus, O. Molecular spectroscopic identification of the water compartments in bone. Bone 2014, 67, 228–236. [Google Scholar] [CrossRef]
- Turner-Walker, G. The Chemical and Microbial Degradation of Bones and Teeth. Adv. Hum. Palaeopathology 2008, 592, 3–29. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, J.C. Chapter 4—Mineral, Synthetic and Biological Carbonate Apatites. In Studies in Inorganic Chemistry; Elliott, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 1994; pp. 191–304. [Google Scholar]
- Berna, F.; Matthews, A.; Weiner, S. Solubilities of bone mineral from archaeological sites: The recrystallization window. J. Archaeol. Sci. 2004, 31, 867–882. [Google Scholar] [CrossRef]
- Collins, M.; Riley, M.S.; Child, A.M.; Turner-Walker, G. A Basic Mathematical Simulation of the Chemical Degradation of Ancient Collagen. J. Archaeol. Sci. 1995, 22, 175–183. [Google Scholar] [CrossRef]
- Delannoy, Y.; Colard, T.; Le Garff, E.; Mesli, V.; Aubernon, C.; Penel, G.; Hedouin, V.; Gosset, D. Effects of the environment on bone mass: A human taphonomic study. Leg. Med. 2016, 20, 61–67. [Google Scholar] [CrossRef]
- Steadman, D.W. Hard Evidence: Case Studies in Forensic Anthropology; Routledge: New York, NY, USA, 2015. [Google Scholar]
- Pfretzschner, H.-U. Collagen gelatinization: The key to understand early bone-diagenesis. Palaeontogr. Abt. A 2006, 278, 135–148. [Google Scholar] [CrossRef]
- Lebon, M.; Müller, K.; Bahain, J.-J.; Fröhlich, F.; Falguères, C.; Bertrand, L.; Sandt, C.; Reiche, I. Imaging fossil bone alterations at the microscale by SR-FTIR microspectroscopy. J. Anal. At. Spectrom. 2011, 26, 922–929. [Google Scholar] [CrossRef]
- Leskovar, T.; Pajnič, I.Z.; Jerman, I.; Črešnar, M. Separating forensic, WWII, and archaeological human skeletal remains using ATR-FTIR spectra. Int. J. Leg. Med. 2019, 134, 811–821. [Google Scholar] [CrossRef]
- France, C.A.; Thomas, D.B.; Doney, C.R.; Madden, O. FT-Raman spectroscopy as a method for screening collagen diagenesis in bone. J. Archaeol. Sci. 2014, 42, 346–355. [Google Scholar] [CrossRef]
- Amadasi, A.; Cappella, A.; Cattaneo, C.; Cofrancesco, P.; Cucca, L.; Merli, D.; Milanese, C.; Pinto, A.; Profumo, A.; Scarpulla, V.; et al. Determination of the post mortem interval in skeletal remains by the comparative use of different physico-chemical methods: Are they reliable as an alternative to 14C? HOMO 2017, 68, 213–221. [Google Scholar] [CrossRef]
- Burr, D.B.; Akkus, O. Chapter 1—Bone Morphology and Organization. In Basic and Applied Bone Biology; Allen, D.B.B.R., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 3–25. [Google Scholar]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2005, 17, 319–336. [Google Scholar] [CrossRef]
- Rappu, P.; Salo, A.M.; Myllyharju, J.; Heino, J. Role of prolyl hydroxylation in the molecular interactions of collagens. Essays Biochem. 2019, 63, 325–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arseni, L.; Lombardi, A.; Orioli, D. From Structure to Phenotype: Impact of Collagen Alterations on Human Health. Int. J. Mol. Sci. 2018, 19, 1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yerramshetty, J.S.; Lind, C.; Akkus, O. The compositional and physicochemical homogeneity of male femoral cortex increases after the sixth decade. Bone 2006, 39, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Gourion-Arsiquaud, S.; Burket, J.C.; Havill, L.M.; Dicarlo, E.; Doty, S.B.; Mendelsohn, R.; Van Der Meulen, M.C.H.; Boskey, A.L. Spatial Variation in Osteonal Bone Properties Relative to Tissue and Animal Age. J. Bone Miner. Res. 2009, 24, 1271–1281. [Google Scholar] [CrossRef]
- Allen, M.R.; Burr, D.B. Chapter 4—Bone Modeling and Remodeling. In Basic and Applied Bone Biology; Allen, D.B.B.R., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 75–90. [Google Scholar]
- Andronowski, J.M.; Crowder, C.; Martinez, M.S. Recent advancements in the analysis of bone microstructure: New dimensions in forensic anthropology. Forensic Sci. Res. 2018, 3, 278–293. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, D.; Vassalo, A.R.; Mamede, A.; Makhoul, C.I.; Piga, G.; Cunha, E.; Marques, M.P.M.; de Carvalho, L.B. Crystal clear: Vibrational spectroscopy reveals intrabone, intraskeleton, and interskeleton variation in human bones. Am. J. Phys. Anthr. 2018, 166, 296–312. [Google Scholar] [CrossRef]

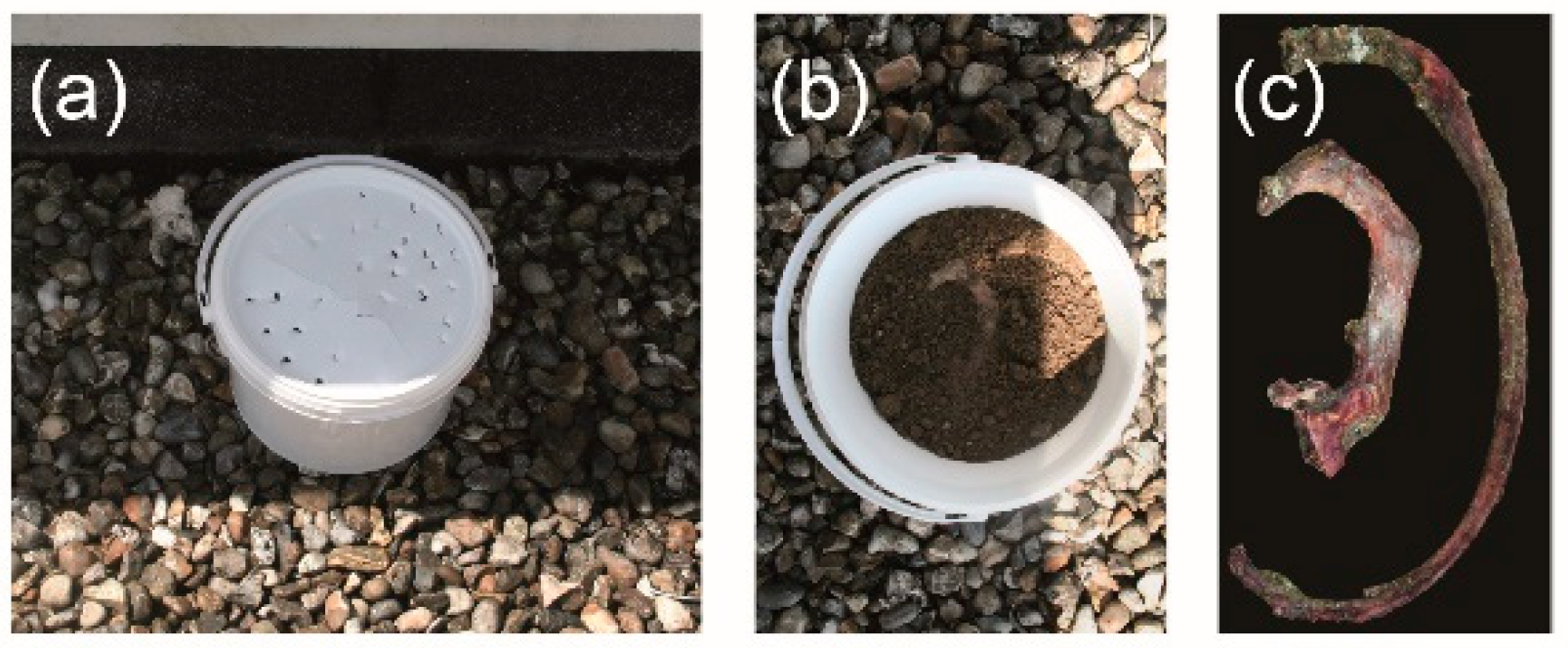


| ID | Sex | Age | Observations |
|---|---|---|---|
| #1 | male | 72 | no records |
| #2 | female | 92 | coxarthrosis, knee surgery, osteonecrosis of the femoral head |
| #3 | female | 80 | heart prosthetic stent, vascular issues, Alzheimer’s |
| #4 | male | 88 | hypertension |
| #5 | male | 82 | acute myeloid leukaemia, alcoholism, smoker |
| #6 | male | 87 | no records |
| Variables | ΔPPV (95%CI) | p Value |
|---|---|---|
| Mineral/organic | −0.020 (−0.036 to −0.004) | 0.015 |
| Carbonation type-B | −0.35 × 10−3 (−0.48 × 10−3 to −0.23 × 10−3) | <0.001 |
| Crystallinity | +0.04 × 10−3 (+0.02 × 10−3 to +0.06 × 10−3) | <0.001 |
| Hydroxyproline/proline | +0.28 × 10−3 (−0.49 × 10−3 to 1.05 × 10−3) | 0.47 |
| Collagen cross-links | −0.012 (−0.014 to −0.009) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falgayrac, G.; Vitale, R.; Delannoy, Y.; Behal, H.; Penel, G.; Olejnik, C.; Duponchel, L.; Colard, T. Bone Molecular Modifications Induced by Diagenesis Followed-Up for 12 Months. Biology 2022, 11, 1542. https://doi.org/10.3390/biology11101542
Falgayrac G, Vitale R, Delannoy Y, Behal H, Penel G, Olejnik C, Duponchel L, Colard T. Bone Molecular Modifications Induced by Diagenesis Followed-Up for 12 Months. Biology. 2022; 11(10):1542. https://doi.org/10.3390/biology11101542
Chicago/Turabian StyleFalgayrac, Guillaume, Raffaele Vitale, Yann Delannoy, Hélène Behal, Guillaume Penel, Cécile Olejnik, Ludovic Duponchel, and Thomas Colard. 2022. "Bone Molecular Modifications Induced by Diagenesis Followed-Up for 12 Months" Biology 11, no. 10: 1542. https://doi.org/10.3390/biology11101542





