Impact of Lidocaine on Pain-Related Grooming in Cuttlefish
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.1.1. Animals
2.1.2. Aquarium System
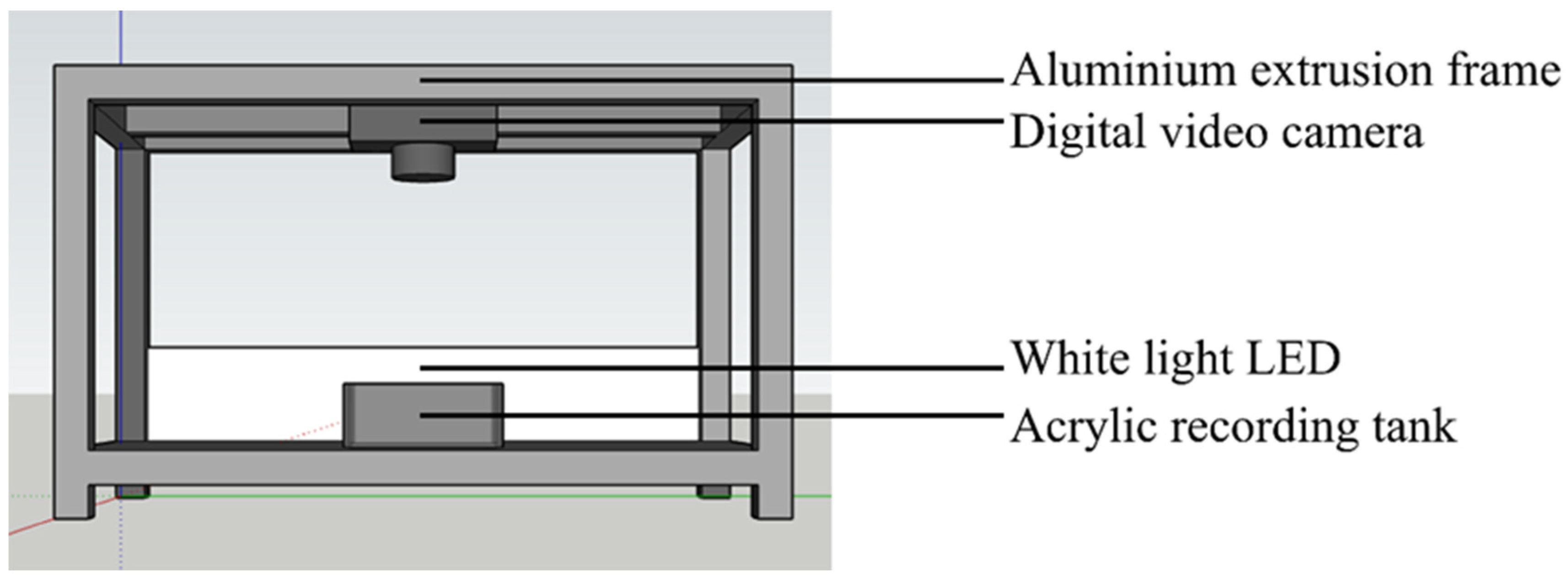
2.2. Experimental Apparatus and Procedure
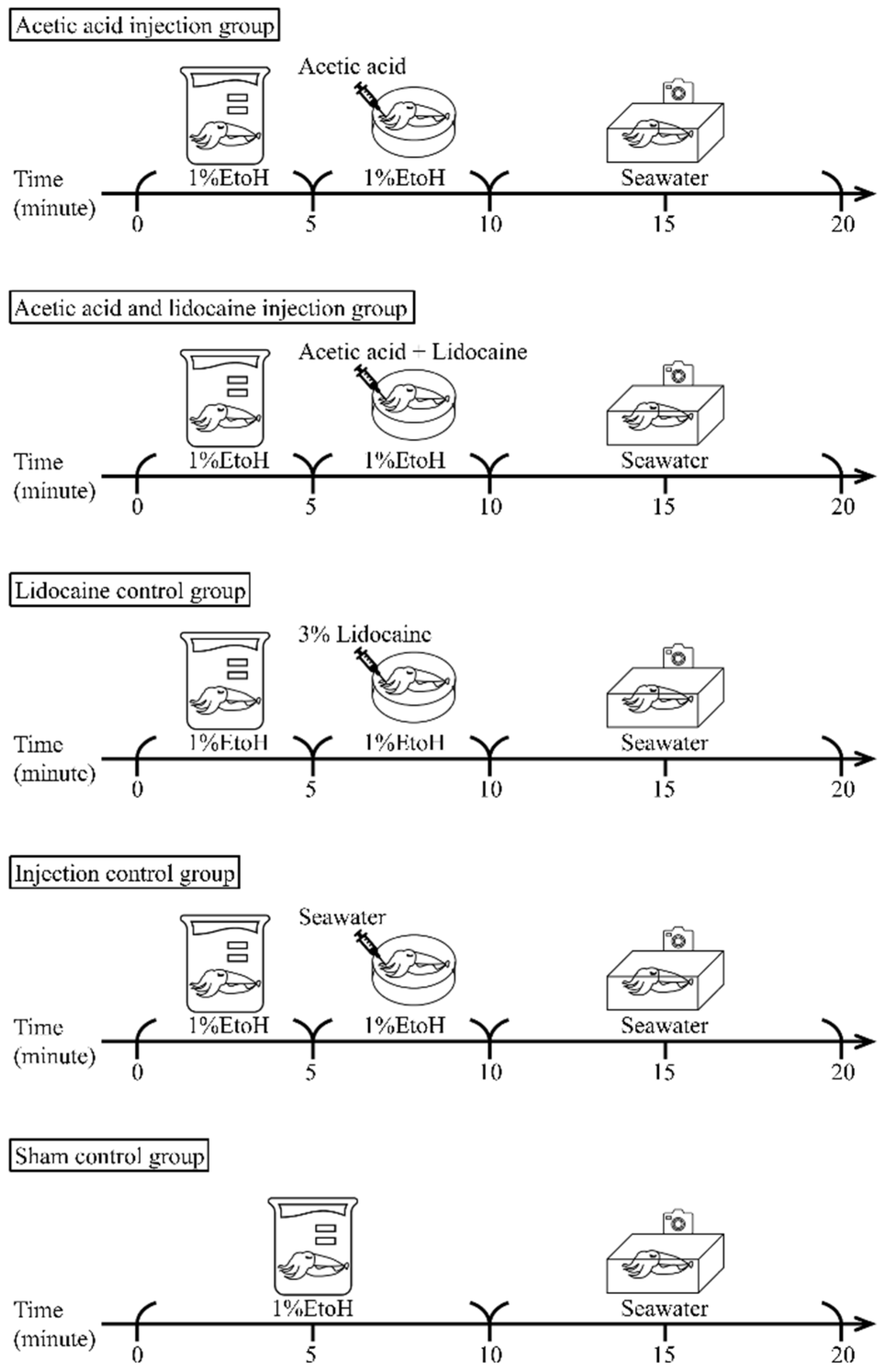
2.3. Experimental Design
2.3.1. Acetic Acid Injection Group
2.3.2. Acetic Acid and Lidocaine Injection Group
2.3.3. Lidocaine Control Group
2.3.4. Injection Control Group
2.3.5. Sham Control Group
2.4. Data Analysis
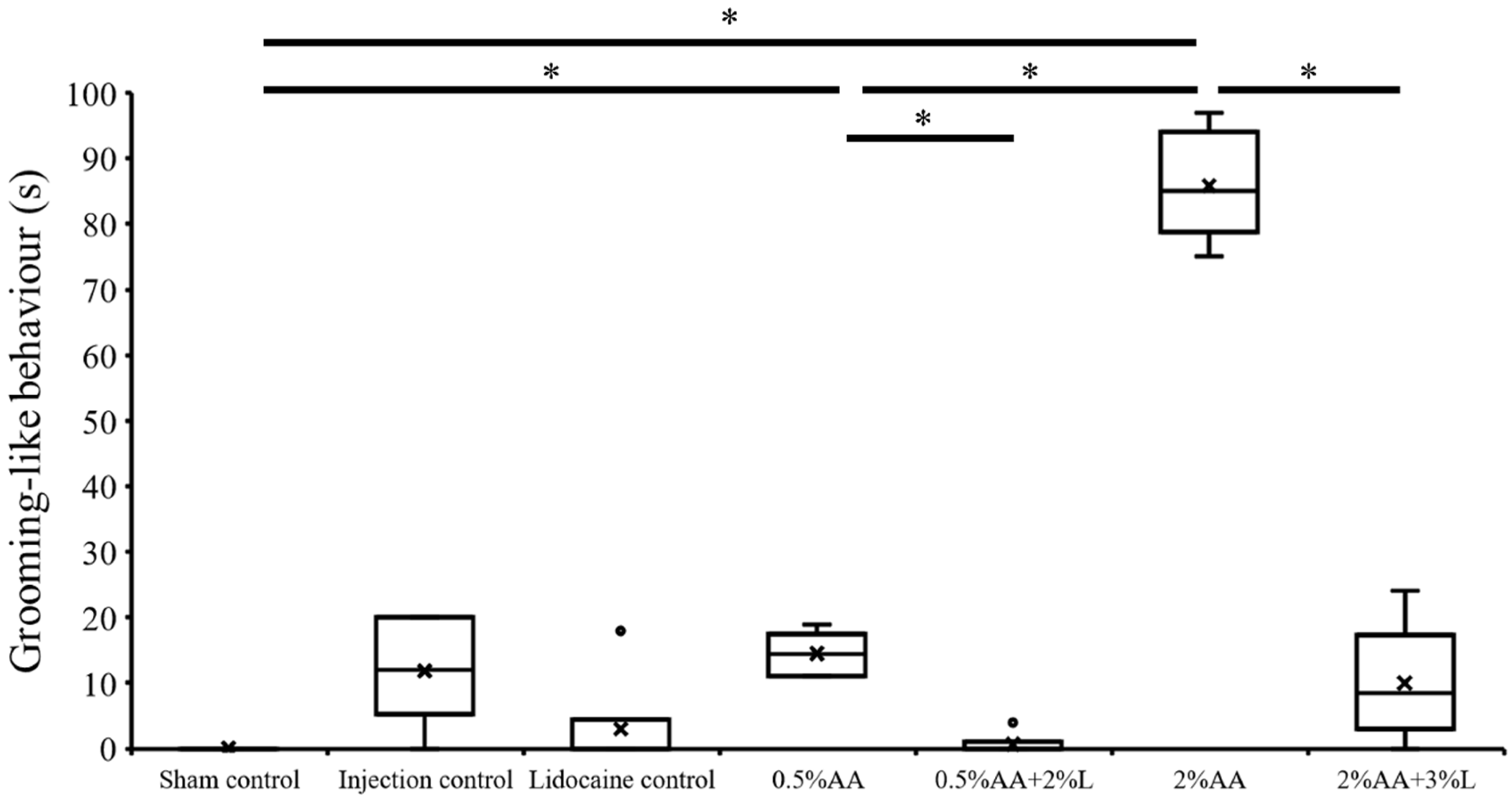
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sneddon, L.U.; Elwood, R.W.; Adamo, S.A.; Leach, M.C. Defining and assessing animal pain. Anim. Behav. 2014, 97, 201–212. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A. The revised IASP definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, L.U. Evolution of nociception and pain: Evidence from fish models. Philos. Trans. R. Soc. B 2019, 374, 20190290. [Google Scholar] [CrossRef] [PubMed]
- Bufalari, A.; Adami, C.; Angeli, G.; Short, C.E. Pain Assessment in Animals. Vet. Res. Commun. 2007, 31 (Suppl. 1), 55–58. [Google Scholar] [CrossRef]
- Mathews, K.; Kronen, P.W.; Lascelles, D.; Nolan, A.; Robertson, S.; Steagall, P.V.; Wright, B.; Yamashita, K. Guidelines for Recognition, Assessment and Treatment of Pain: WSAVA Global Pain Council members and co-authors of this document. J. Small Anim. Pract. 2014, 55, E10–E68. [Google Scholar] [CrossRef]
- Stasiak, K.L.; Maul, D.; French, E.; Hellyer, P.W.; Vandewoude, S. Species-specific assessment of pain in laboratory animals. J. Am. Assoc. Lab. Anim. Sci. 2003, 42, 13–20. [Google Scholar]
- Molony, V.; Kent, J.E. Assessment of acute pain in farm animals using behavioral and physiological measurements. J. Anim. Sci. 1997, 75, 266–272. [Google Scholar] [CrossRef]
- Darmaillacq, A.-S.; Dickel, L.; Mather, J. Cephalopod Cognition; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Hanlon, R.T.; Messenger, J.B. Cephalopod Behaviour; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Smith, J.A.; Andrews, P.L.; Hawkins, P.; Louhimies, S.; Ponte, G.; Dickel, L. Cephalopod research and EU Directive 2010/63/EU: Requirements, impacts and ethical review. J. Exp. Mar. Biol. Ecol. 2013, 447, 31–45. [Google Scholar] [CrossRef]
- Fiorito, G.; Affuso, A.; Anderson, D.B.; Basil, J.; Bonnaud, L.; Botta, G.; Cole, A.; D’Angelo, L.; De Girolamo, P.; Dennison, N. Cephalopods in neuroscience: Regulations, research and the 3Rs. Invertebr. Neurosci. 2014, 14, 13–36. [Google Scholar] [CrossRef]
- Howard, R.B.; Lopes, L.N.; Lardie, C.R.; Perez, P.P.; Crook, R.J. Early-life injury produces lifelong neural hyperexcitability, cognitive deficit and altered defensive behaviour in the squid Euprymna scolopes. Philos. Trans. R. Soc. B 2019, 374, 20190281. [Google Scholar] [CrossRef]
- Darmaillacq, A.-S.; Dickel, L.; Chichery, M.-P.; Agin, V.; Chichery, R. Rapid taste aversion learning in adult cuttlefish, Sepia officinalis. Anim. Behav. 2004, 68, 1291–1298. [Google Scholar] [CrossRef]
- Darmaillacq, A.-S.; Chichery, R.; Dickel, L. Food imprinting, new evidence from the cuttlefish Sepia officinalis. Biol. Lett. 2006, 2, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Darmaillacq, A.-S.; Lesimple, C.; Dickel, L. Embryonic visual learning in the cuttlefish, Sepia officinalis. Anim. Behav. 2008, 76, 131–134. [Google Scholar] [CrossRef]
- Yang, T.-I.; Chiao, C.-C. Number sense and state-dependent valuation in cuttlefish. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161379. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-H.; Chiao, C.-C. Learned valuation during forage decision-making in cuttlefish. R. Soc. Open Sci. 2020, 7, 201602. [Google Scholar] [CrossRef]
- Jozet-Alves, C.; Bertin, M.; Clayton, N.S. Evidence of episodic-like memory in cuttlefish. Curr. Biol. 2013, 23, R1033–R1035. [Google Scholar] [CrossRef]
- Alupay, J.S.; Hadjisolomou, S.P.; Crook, R.J. Arm injury produces long-term behavioral and neural hypersensitivity in octopus. Neurosci. Lett. 2014, 558, 137–142. [Google Scholar] [CrossRef]
- Crook, R.J.; Hanlon, R.T.; Walters, E.T. Squid Have Nociceptors That Display Widespread Long-Term Sensitization and Spontaneous Activity after Bodily Injury. J. Neurosci. 2013, 33, 10021–10026. [Google Scholar] [CrossRef]
- Crook, R.J.; Dickson, K.; Hanlon, R.T.; Walters, E.T. Nociceptive Sensitization Reduces Predation Risk. Curr. Biol. 2014, 24, 1121–1125. [Google Scholar] [CrossRef]
- Oshima, M.; von Treuheim, T.d.P.; Carroll, J.; Hanlon, R.T.; Walters, E.T.; Crook, R.J. Peripheral injury alters schooling behavior in squid, Doryteuthis pealeii. Behav. Process. 2016, 128, 89–95. [Google Scholar] [CrossRef]
- Crook, R.J. Behavioral and neurophysiological evidence suggests affective pain experience in octopus. iScience 2021, 24, 102229. [Google Scholar] [CrossRef] [PubMed]
- Crook, R.J.; Lewis, T.; Hanlon, R.T.; Walters, E.T. Peripheral injury induces long-term sensitization of defensive responses to visual and tactile stimuli in the squid Loligo pealeii, Lesueur 1821. J. Exp. Biol. 2011, 214, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- Butler-Struben, H.M.; Brophy, S.M.; Johnson, N.A.; Crook, R.J. In Vivo Recording of Neural and Behavioral Correlates of Anesthesia Induction, Reversal, and Euthanasia in Cephalopod Molluscs. Front. Physiol. 2018, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Collymore, C.; Banks, E.K.; Turner, P.V. Lidocaine hydrochloride compared with MS222 for the euthanasia of zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 816–820. [Google Scholar]
- Imperadore, P.; Shah, S.B.; Makarenkova, H.P.; Fiorito, G. Nerve degeneration and regeneration in the cephalopod mollusc Octopus vulgaris: The case of the pallial nerve. Sci. Rep. 2017, 7, 46564. [Google Scholar] [CrossRef]
- Deakin, A.G.; Spencer, J.W.; Cossins, A.R.; Young, I.S.; Sneddon, L.U. Welfare Challenges Influence the Complexity of Movement: Fractal Analysis of Behaviour in Zebrafish. Fishes 2019, 4, 8. [Google Scholar] [CrossRef]
- Deakin, A.G.; Buckley, J.; AlZu’bi, H.S.; Cossins, A.R.; Spencer, J.W.; Al’Nuaimy, W.; Young, I.S.; Thomson, J.S.; Sneddon, L.U. Automated monitoring of behaviour in zebrafish after invasive procedures. Sci. Rep. 2019, 9, 9042. [Google Scholar] [CrossRef]
- Mettam, J.J.; Oulton, L.J.; McCrohan, C.R.; Sneddon, L.U. The efficacy of three types of analgesic drugs in reducing pain in the rainbow trout, Oncorhynchus mykiss. Appl. Anim. Behav. Sci. 2011, 133, 265–274. [Google Scholar] [CrossRef]
- Thomson, J.S.; Al-Temeemy, A.A.; Isted, H.; Spencer, J.W.; Sneddon, L.U. Assessment of behaviour in groups of zebrafish (Danio rerio) using an intelligent software monitoring tool, the chromatic fish analyser. J. Neurosci. Methods 2019, 328, 108433. [Google Scholar] [CrossRef]
- Koster, R. Acetic acid for analgesic screening. In Proceedings of the Federation Proceedings, Godesberg, Germany, 14 September 1959; p. 412. [Google Scholar]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal Models of Nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar]
- Dai, G.; Li, B.; Xu, Y.; Li, Z.; Mo, F.; Wei, C. Synergistic interaction between matrine and paracetamol in the acetic acid writhing test in mice. Eur. J. Pharmacol. 2021, 895, 173869. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.W.; MacIver, D.N.; Newman, L.C. Testing and Comparison of Non-Opioid Analgesics in Amphibians. J. Am. Assoc. Lab. Anim. Sci. 2001, 40, 23–27. [Google Scholar]
- Sneddon, L.U.; Braithwaite, V.A.; Gentle, M.J. Do fishes have nociceptors? Evidence for the evolution of a vertebrate sensory system. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 1115–1121. [Google Scholar] [CrossRef]
- Dubuisson, D.; Dennis, S.G. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Roveroni, R.C.; Parada, C.A.; Cecılia, M.; Veiga, F.; Tambeli, C.H. Development of a behavioral model of TMJ pain in rats: The TMJ formalin test. Pain 2001, 94, 185–191. [Google Scholar] [CrossRef]
- Sneddon, L.U. The evidence for pain in fish: The use of morphine as an analgesic. Appl. Anim. Behav. Sci. 2003, 83, 153–162. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P.D. Pain Mechanisms: A New Theory: A gate control system modulates sensory input from the skin before it evokes pain perception and response. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Burrell, B.D. Endocannabinoid-Dependent LTD in a Nociceptive Synapse Requires Activation of a Presynaptic TRPV-like Receptor. J. Neurophysiol. 2010, 104, 2766–2777. [Google Scholar] [CrossRef]
- Yuan, S.; Burrell, B.D. Nonnociceptive afferent activity depresses nocifensive behavior and nociceptive synapses via an endocannabinoid-dependent mechanism. J. Neurophysiol. 2013, 110, 2607–2616. [Google Scholar] [CrossRef][Green Version]
- Lynagh, T.; Mikhaleva, Y.; Colding, J.M.; Glover, J.C.; Pless, S.A. Acid-sensing ion channels emerged over 600 Mya and are conserved throughout the deuterostomes. Proc. Natl. Acad. Sci. USA 2018, 115, 8430–8435. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, T.-H.; Sneddon, L.U.; Spencer, J.W.; Chiao, C.-C. Impact of Lidocaine on Pain-Related Grooming in Cuttlefish. Biology 2022, 11, 1560. https://doi.org/10.3390/biology11111560
Kuo T-H, Sneddon LU, Spencer JW, Chiao C-C. Impact of Lidocaine on Pain-Related Grooming in Cuttlefish. Biology. 2022; 11(11):1560. https://doi.org/10.3390/biology11111560
Chicago/Turabian StyleKuo, Tzu-Hsin, Lynne U. Sneddon, Joseph W. Spencer, and Chuan-Chin Chiao. 2022. "Impact of Lidocaine on Pain-Related Grooming in Cuttlefish" Biology 11, no. 11: 1560. https://doi.org/10.3390/biology11111560
APA StyleKuo, T.-H., Sneddon, L. U., Spencer, J. W., & Chiao, C.-C. (2022). Impact of Lidocaine on Pain-Related Grooming in Cuttlefish. Biology, 11(11), 1560. https://doi.org/10.3390/biology11111560






