Application of a Posttreatment to Improve the Viability and Antifungal Activity of Trichoderma asperellum Biomass Obtained in a Bioreactor during Submerged Cultivation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Microorganisms
2.2. Posttreatment of T. asperellum Biomass
- (1)
- HCl (Stanchem, Warszawa, Poland), adding 200 µL of 1 M HCl to achieve a pH of 4;
- (2)
- CuSO4×5H2O solution (Sigma-Aldrich, St. Louis, MO, USA) at 2 mg/mL, adding 50 µL to 20 µg/mL (or 100 µg/5 g of biomass) treatment or 150 µL to 60 µg/mL (or 300 µg/5 g of biomass) treatment; and
- (3)
- organic potato starch (Aloja Starkelsen Ltd., Ungurpils, Alojas pagasts, Latvia), 500 mg, thoroughly mixed with biomass.
2.3. Determination of Viability, Antifungal Activity and Micromorphology of T. asperellum
2.4. Statistical Analysis
3. Results
3.1. Viability of T. asperellum
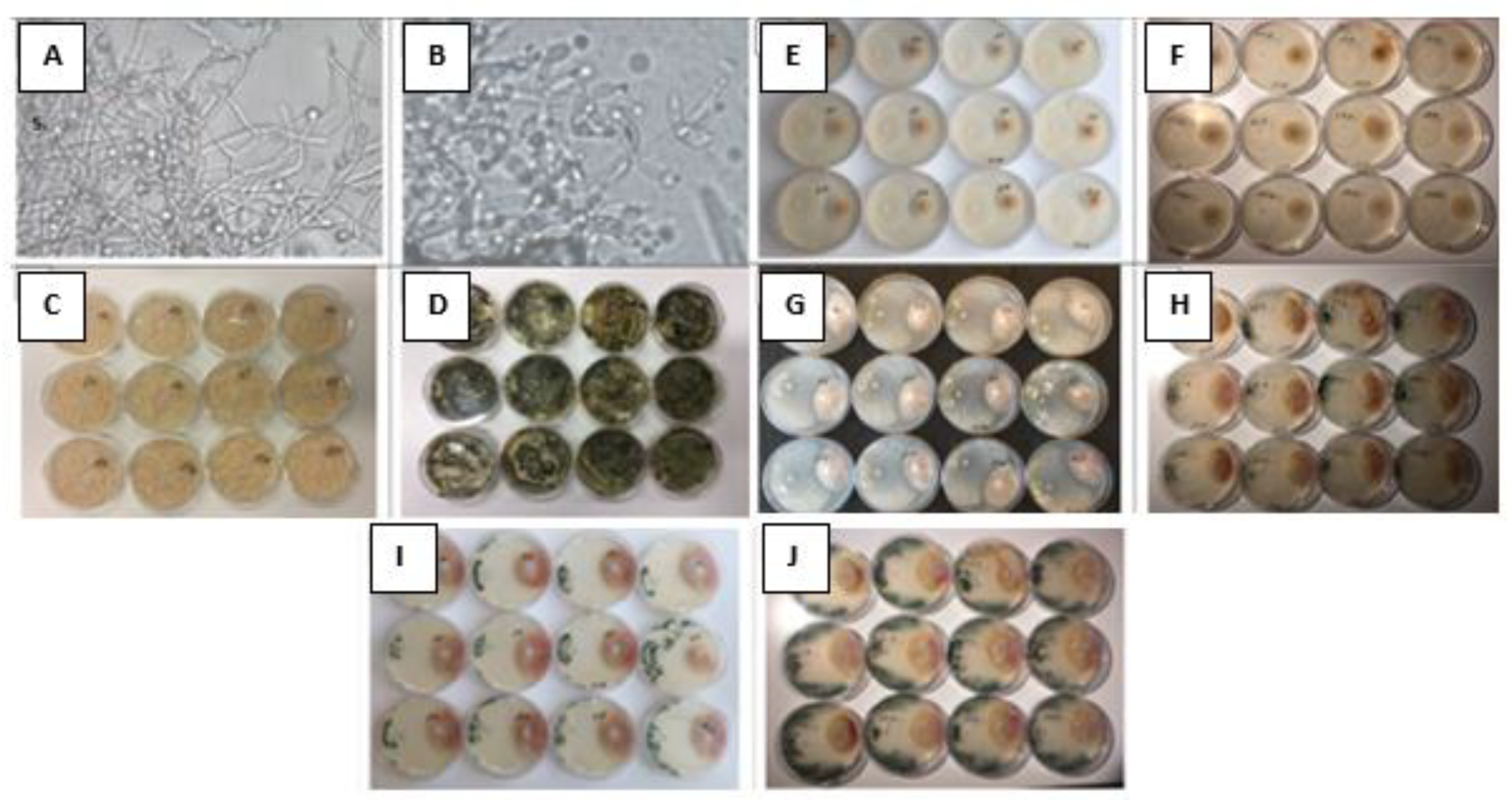
3.2. Morphological Features and Antifungal Activity of T. asperellum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Druzhinina, I.; Chenthamara, K.; Zhang, J.; Atanasova, L.; Yang, D.; Miao, Y.; Rahimi, M.; Grujic, M.; Cai, F.; Pourmehdi, S.; et al. Massive lateral transfer of genes encoding plant cell wall-degrading enzymes to the mycoparasitic fungus Trichoderma from its plant-associated hosts. PLoS Genet. 2018, 14, e1007322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 96, 109–123. [Google Scholar] [CrossRef]
- Kacprzak, M.J.; Rosikon, K.; Fijalkowski, K.; Grobelak, A. The effect of Trichoderma on heavy metal mobility and uptake by Miscanthus giganteus, Salix sp., Phalaris arundinacea, and Panicum virgatum. Appl. Environ. Soil Sci. 2014, 2014, 506142. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, P.; Singh, P.C.; Mishra, A.; Chauhan, P.S.; Dwivedi, S.; Bais, R.T.; Tripathi, R.D. Trichoderma: A potential bioremediator for environmental clean-up. Clean Technol. Environ. Policy 2013, 15, 541–550. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Kolombet, L.V.; Zhigletsova, S.K.; Kosareva, N.I.; Bystrova, E.V.; Derbyshev, V.V.; Krasnova, P.; Schisler, D. Development of fan extended shelf-life, liquid formulation of the biofungicide Trichoderma asperellum. World J. Microbiol. Biotechnol. 2008, 24, 123–131. [Google Scholar] [CrossRef]
- Díaz-Gutiérrez, C.; Arroyave, C.; Llugany, M.; Poschenrieder, C.; Martos, S.; Pelaez, C. Trichoderma asperellum as a preventive and curative agent to control Fusarium wilt in Stevia rebaudiana. Biol. Control 2020, 155, e104537. [Google Scholar] [CrossRef]
- Schuster, A.; Schmoll, M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799. [Google Scholar] [CrossRef] [Green Version]
- Prakash, V.; Basu, K. Mass multiplication of Trichoderma in bioreactor. In Trichoderma: Agricultural Applications and Beyond; Manoharachary, C., Singh, H.B., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 113–126. [Google Scholar]
- Onilude, A.A.; Adebayo-Tayo, B.C.; Odeniyi, A.O.; Banjo, D. Comparative mycelial and spore yield by Trichoderma viride in batch and fed-batch cultures. Ann. Microbiol. 2013, 63, 547–553. [Google Scholar] [CrossRef]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valero, J.R. Dissolved oxygen as principal parameter for conidia production of biocontrol fungi Trichoderma viride in non-Newtonian wastewater. J. Ind. Microbiol. Biotechnol. 2006, 33, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004, 22, 189–259. [Google Scholar] [CrossRef] [PubMed]
- Schisler, D.A.; Slininger, P.J. Effects of antagonist cell concentration and two strain mixtures on biological control of Fusarium dry rot of potatoes. Phytopathology 1997, 87, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Gams, W.; Bissett, J. Morphology and identification of Trichoderma. In Trichoderma and Gliocladium: Basic Biology, Taxonomy and Genetics; Harman, G.E., Kubicek, C.P., Eds.; Taylor and Francis: London, UK, 1998; pp. 3–34. [Google Scholar]
- Prasad, R.D.; Rangeshwaran, R.; Anuroop, C.P.; Phanikumar, P.R. Bioefficacy and shelf life of conidial and chlamydospore formulations of Trichoderma harzianum Rifai. J. Biol. Control 2002, 16, 145–148. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, S.; Zeng, X.; Bai, L.; Li, L.; Duan, R.; Wang, Y.; Wu, C. Inoculation with chlamydospores of Trichoderma asperellum SM-12F1 accelerated arsenic volatilization and influenced arsenic availability in soils. J. Integr. Agric. 2015, 14, 389–397. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Sivasithamparam, K.; Ghisalberti, E.L. Secondary metabolism in Trichoderma and Gliocladium. In Trichoderma and Gliocladium: Basic Biology, Taxonomy and Genetics; Harman, G.E., Kubicek, C.P., Eds.; Taylor and Francis: London, UK, 1998; pp. 139–191. [Google Scholar]
- Kobori, N.N.; Mascarin, G.M.; Jackson, M.A.; Schisler, D.A. Liquid culture production of microsclerotia and submerged conidia by Trichoderma harzianum active against damping-off disease caused by Rhizoctonia solani. Fungal Biol. 2015, 119, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.A.; Papavizas, G.C. Production of chlamydospores and conidia by Trichoderma spp. in liquid and solid growth media. Soil Biol. Biochem. 1983, 15, 351–357. [Google Scholar] [CrossRef]
- Muñoz, G.A.; Agosin, E.; Cotoras, M.; San Martin, R.; Volpe, D. Comparison of aerial and submerged spore properties for Trichoderma harzianum. FEMS Microbiol. Lett. 1995, 125, 63–70. [Google Scholar] [CrossRef]
- Lopes, R.B.; Faria, M.; Glare, T.R. A nonconventional two-stage fermentation system for the production of aerial conidia of entomopathogenic fungi utilizing surface tension. J. Appl. Microbiol. 2019, 126, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Nikolajeva, V.; Petrina, Z.; Vulfa, L.; Alksne, L.; Eze, D.; Grantina, L.; Gaitnieks, T.; Lielpetere, A. Growth and antagonism of Trichoderma spp. and conifer pathogen Heterobasidion annosum s.l. in vitro at different temperatures. Adv. Microbiol. 2012, 2, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Brito, J.P.; Ramada, M.H.; de Magalhães, M.T.; Luciano, P.S.; Ulhoa, C.J. Peptaibols from Trichoderma asperellum TR356 strain isolated from Brazilian soil. SpringerPlus 2014, 3, 600. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-García, B.M.; Espinosa-Huerta, E.; Villordo-Pineda, E.; Rodríguez-Guerra, R.; Mora-Avilés, M.A. Trichoderma spp. native strains molecular identification and in vitro antagonistic evaluation of root phitopathogenic fungus of the common bean (Phaseolus vulgaris L.) cv. Montcalm. Agrociencia 2017, 51, 63–79. [Google Scholar]
- Carreras-Villasenor, N.; Sanchez-Arreguin, J.; Herrera-Estrella, A. Trichoderma: Sensing the environment for survival and dispersal. Microbiology 2012, 158, 3–16. [Google Scholar] [CrossRef]
- Singh, A.; Shahid, M.; Srivastava, M.; Pandey, S.; Sharma, A.; Kumar, V. Optimal physical parameters for growth of Trichoderma species at varying pH, temperature and agitation. J. Virol. Mycol. 2014, 3, 1000127. [Google Scholar] [CrossRef] [Green Version]
- Steyaert, J.M.; Weld, R.J.; Stewart, A. Ambient pH intrinsically influences Trichoderma conidiation and colony morphology. Fungal Biol. 2010, 114, 198–208. [Google Scholar] [CrossRef]
- Petrovič, J.J.; Danilovič, G.; Čurčič, N.; Milinkovič, M.; Stošič, N.; Pankovič, D.; Raičevič, V. Copper tolerance of Trichoderma species. Arch. Biol. Sci. 2014, 66, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Velicogna, J.R.; Schwertfeger, D.; Jesmer, A.; Beer, C.; Kuo, J.; DeRosa, M.C.; Scroggins, R.; Smith, M.; Princz, J. Soil invertebrate toxicity and bioaccumulation of nano copper oxide and copper sulphate in soils, with and without biosolids amendment. Ecotoxicol. Environ. Saf. 2021, 217, 112222. [Google Scholar] [CrossRef]
- Carboué, Q.; Claeys-Bruno, M.; Bombarda, I.; Sergent, M.; Jolain, J.; Roussos, S. Experimental design and solid state fermentation: A holistic approach to improve cultural medium for the production of fungal secondary metabolites. Chemom. Intell. Lab. Syst. 2018, 176, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Kumar, R.; Om, H. Shelf-life of Trichoderma viride in talc and charcoal based formulations. Ind. J. Agric. Sci. 2013, 83, 566–569. [Google Scholar]
- Riquelme, M.; Aguirre, J.; Bartnicki-García, S.; Braus, G.H.; Feldbrügge, M.; Fleig, U.; Hansberg, W.; Herrera-Estrella, A.; Kämper, J.; Kück, U.; et al. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol. Mol. Biol. Rev. 2018, 82, e00068-17. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Oñate, M.A.; Esquivel-Naranjo, E.U.; Mendoza-Mendoza, A.; Stewart, A.; Herrera-Estrella, A.H. An injury-response mechanism conserved across kingdoms determines entry of the fungus Trichoderma atroviride into development. Proc. Natl. Acad. Sci. USA 2012, 109, 1491814923. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Kenerley, C.M. Regulation of morphogenesis and biocontrol properties in Trichoderma virens by a VELVET protein, Vel1. Appl. Environ. Microbiol. 2010, 76, 2345–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askun, T. Introductory chapter. Fusarium: Pathogenicity, infections, diseases, mycotoxins and management. In Fusarium: Plant Diseases, Pathogen Diversity, Genetic Diversity, Resistance and Molecular Markers; Askun, T., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bluhm, B.H.; Zhao, X.; Flaherty, J.E.; Xu, J.R.; Dunkle, L.D. RAS2 regulates growth and pathogenesis in Fusarium graminearum. Mol. Plant Microbe Interact. 2007, 20, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Wegulo, S.N. Factors influencing deoxynivalenol accumulation in small grain cereals. Toxins 2012, 4, 1157–1180. [Google Scholar] [CrossRef]
| Treatment | 1 M HCL | CuSO4×5H2O 2 mg/mL (Final Concentration, µg/mL) | Starch |
|---|---|---|---|
| 1 | - | - | - |
| 2 | X | - | - |
| 3 | - | X (20) | - |
| 4 | - | X (60) | - |
| 5 | X | X (20) | - |
| 6 | X | X (60) | - |
| 7 | - | - | X |
| 8 | X | - | X |
| 9 | - | X (20) | X |
| 10 | - | X (60) | X |
| 11 | X | X (20) | X |
| 12 | X | X (60) | X |
| Treatment | Week | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 6 | 8 | |
| 1 | 27.28 ± 0.74 c | 21.77 ± 0.00 b | 30.86 ± 0.00 b | 64.43 ± 6.78 b,c,d | 69.44 ± 1.18 b,c | 52.16 ± 1.02 e |
| 2 | 32.12 ± 0.80 a | 19.80 ± 1.25 c | 34.68 ± 0.83 a,b | 65.79 ± 1.14 c | 83.04 ± 2.57 a | 65.89 ± 3.43 a,b |
| 3 | 28.49 ± 1.50 c | 22.93 ± 2.02 a,b | 33.42 ± 1.63 a | 63.98 ± 0.00 d | 73.29 ± 3.62 b,c | 64.03 ± 2.26 a,b |
| 4 | 30.86 ± 0.00 b | 21.97 ± 2.63 b,c | 34.78 ± 2.49 a,b | 75.54 ± 7.34 a | 80.99 ± 1.27 a | 62.23 ± 1.11 c |
| 5 | 30.86 ± 1.50 b | 19.95 ± 2.51 b,c | 33.42 ± 1.63 a,b | 77.14 ± 3.72 a | 75.54 ± 7.34 a,b,c | 63.98 ± 0.00 b |
| 6 | 30.86 ± 0.00 b | 18.79 ± 0.61 c | 30.86 ± 0.00 b | 68.03 ± 6.97 a,b | 73.49 ± 6.04 a,b,c | 63.22 ± 10.03 a,b,c |
| 7 | 30.86 ± 0.00 b | 21.97 ± 2.63 b,c | 33.57 ± 3.26 a,b | 66.77 ± 10.31 a,b | 67.64 ± 2.32 c | 58.78 ± 1.00 d |
| 8 | 30.86 ± 0.00 b | 17.87 ± 1.19 b,c | 28.49 ± 1.50 c | 62.53 ± 5.57 c | 71.34 ± 2.38 b,c | 63.98 ± 0.00 b |
| 9 | 30.86 ± 0.00 b | 26.31 ± 2.88 a,b | 35.99 ± 0.00 a,b | 75.29 ± 4.89 a | 71.34 ± 2.38 b,c | 54.21 ± 6.21 d,e |
| 10 | 30.86 ± 0.00 b | 26.17 ± 1.44 a | 41.10 ± 8.05 a,b | 60.53 ± 2.19 d | 71.34 ± 2.38 b,c | 67.64 ± 2.32 a |
| 11 | 30.86 ± 0.00 b | 25.01 ± 0.70 a | 39.94 ± 8.79 a,b | 69.44 ± 1.18 b | 73.29 ± 3.62 b,c | 58.88 ± 3.24 b,c |
| 12 | 30.80 ± 1.50 b,c | 17.87 ± 1.19 b,c | 40.21 ± 2.68 a,b | 67.64 ± 2.32 c | 71.29 ± 0.00 b | 71.49 ± 4.77 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senkovs, M.; Dzierkale, M.T.; Rimkus, A.; Grigs, O.; Nikolajeva, V. Application of a Posttreatment to Improve the Viability and Antifungal Activity of Trichoderma asperellum Biomass Obtained in a Bioreactor during Submerged Cultivation. Biology 2022, 11, 1610. https://doi.org/10.3390/biology11111610
Senkovs M, Dzierkale MT, Rimkus A, Grigs O, Nikolajeva V. Application of a Posttreatment to Improve the Viability and Antifungal Activity of Trichoderma asperellum Biomass Obtained in a Bioreactor during Submerged Cultivation. Biology. 2022; 11(11):1610. https://doi.org/10.3390/biology11111610
Chicago/Turabian StyleSenkovs, Maris, Marija Tereze Dzierkale, Alina Rimkus, Oskars Grigs, and Vizma Nikolajeva. 2022. "Application of a Posttreatment to Improve the Viability and Antifungal Activity of Trichoderma asperellum Biomass Obtained in a Bioreactor during Submerged Cultivation" Biology 11, no. 11: 1610. https://doi.org/10.3390/biology11111610
APA StyleSenkovs, M., Dzierkale, M. T., Rimkus, A., Grigs, O., & Nikolajeva, V. (2022). Application of a Posttreatment to Improve the Viability and Antifungal Activity of Trichoderma asperellum Biomass Obtained in a Bioreactor during Submerged Cultivation. Biology, 11(11), 1610. https://doi.org/10.3390/biology11111610








