Climate Change Drives the Transmission and Spread of Vector-Borne Diseases: An Ecological Perspective
Abstract
:Simple Summary
Abstract
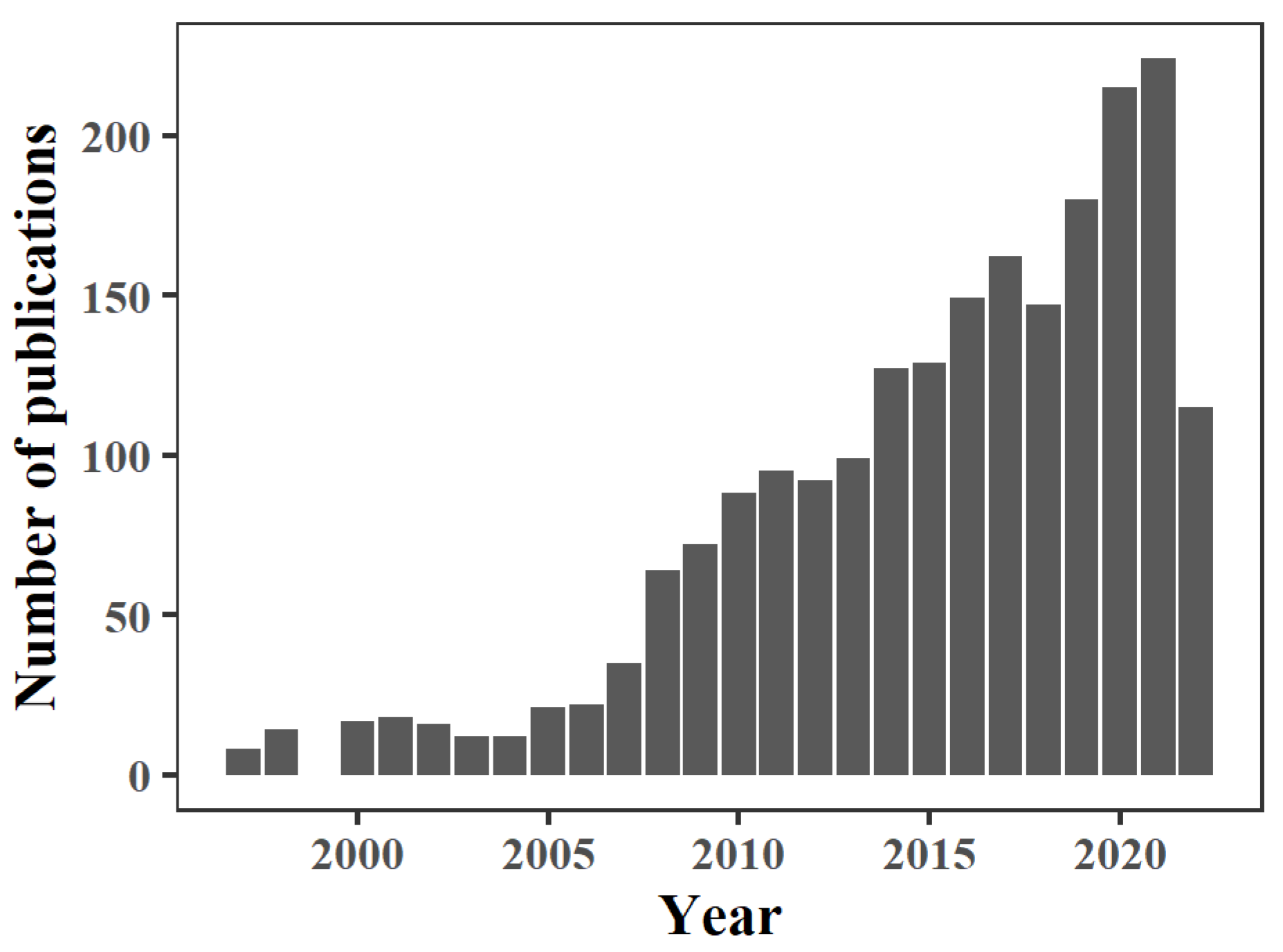
1. Introduction
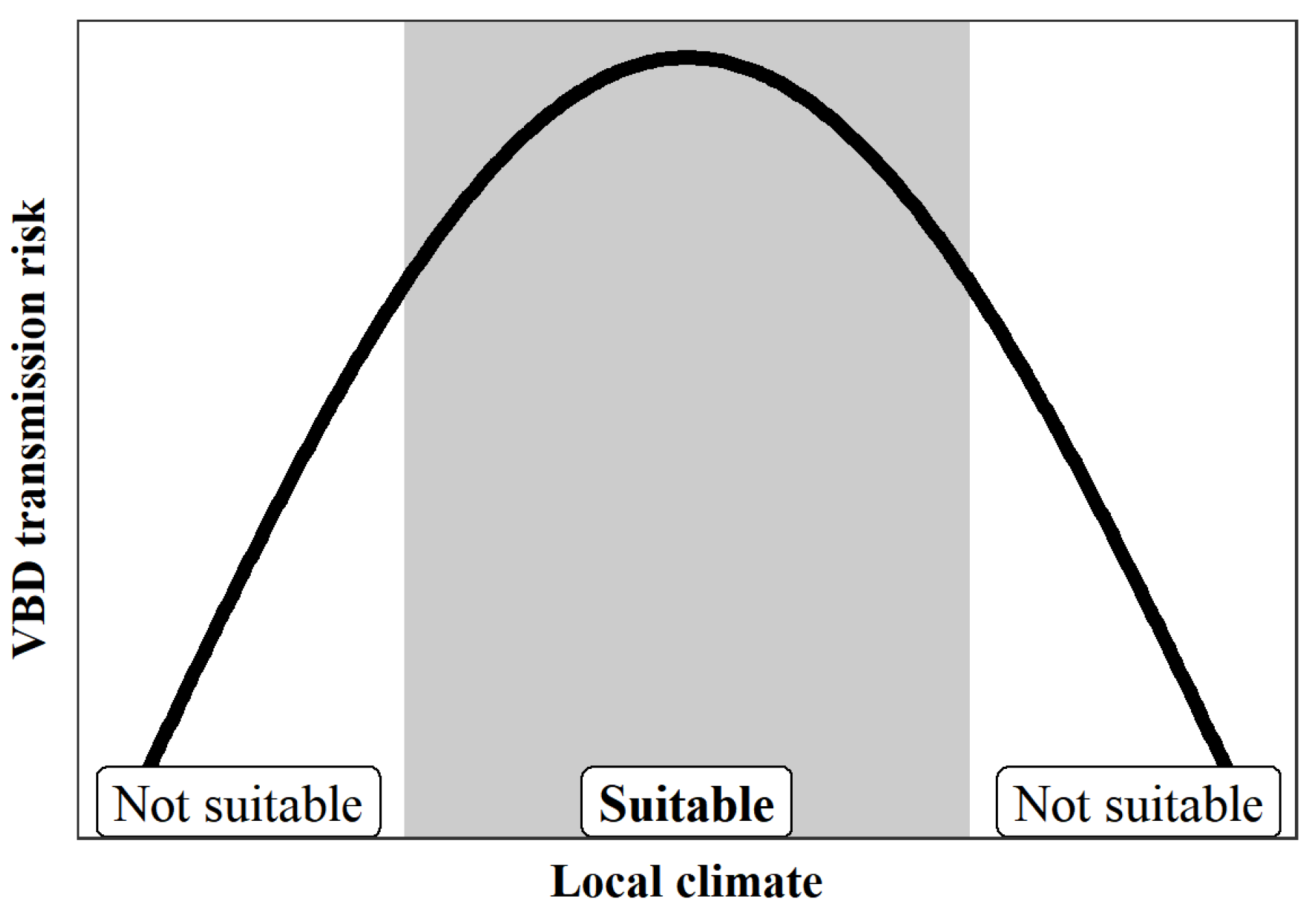
2. Non-Linear Effects of Local Climate on VBD Transmission
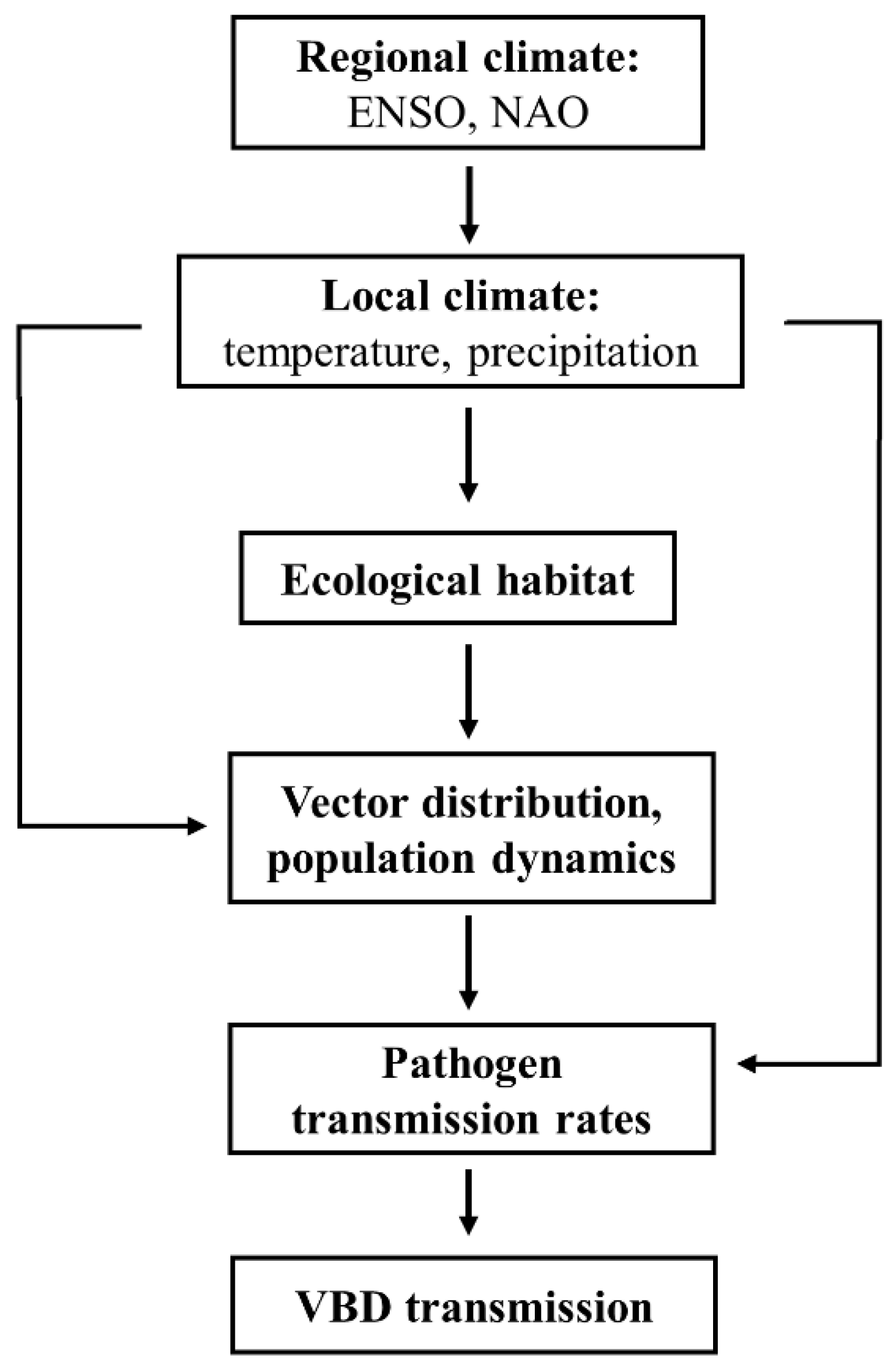
3. Time-Lag Effect of Local and Regional Climate Impacts on VBD Transmission
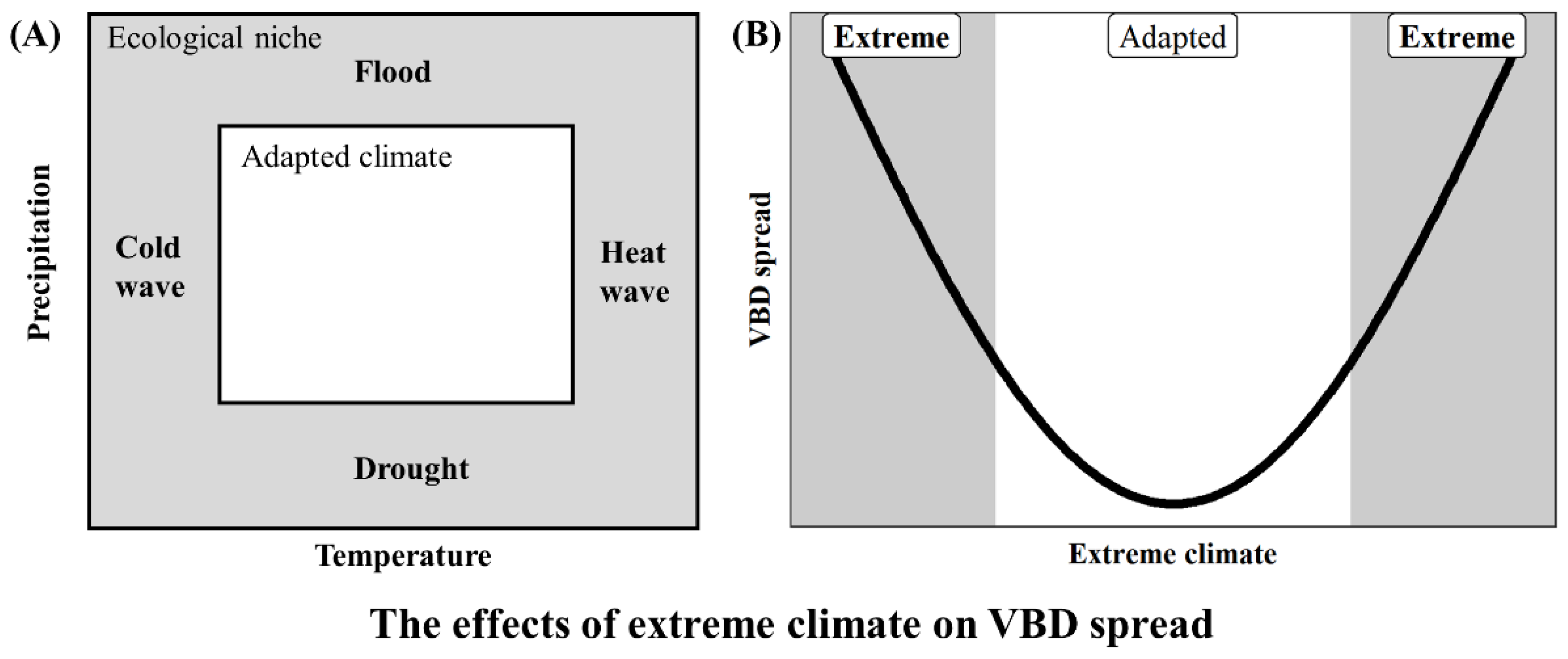
4. Impact of Extreme Climate Distribution Expansion on VBD Spread
5. Interaction between Non-Climate and Climate Factors Alters VBD Spread
6. The COVID-19 Pandemic Introduces a New Situation for VBD Epidemics
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 33–144. [Google Scholar] [CrossRef]
- Mora, C.; Frazier, A.G.; Longman, R.J.; Dacks, R.S.; Walton, M.M.; Tong, E.J.; Sanchez, J.J.; Kaiser, L.R.; Stender, Y.O.; Anderson, J.M.; et al. The Projected Timing of Climate Departure from Recent Variability. Nature 2013, 502, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Dousset, B.; Caldwell, I.; Powell, F.; Geronimo, R.; Bielecki, C.; Counsell, C.; Dietrich, B.; Johnston, E.; Louis, L.; et al. Global Risk of Deadly Heat. Nat. Clim. Chang. 2017, 7, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Patz, J.A.; Campbell-Lendrum, D.; Holloway, T.; Foley, J.A. Impact of Regional Climate Change on Human Health. Nature 2005, 438, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yang, L.; Li, H.; Wang, L. Spatiotemporal Variations of Plague Risk in the Tibetan Plateau from 1954-2016. Biology 2022, 11, 304. [Google Scholar] [CrossRef]
- Yin, Y.; He, Q.; Pan, X.; Liu, Q.; Wu, Y.; Li, X. Predicting Current Potential Distribution and the Range Dynamics of Pomacea Canaliculata in China under Global Climate Change. Biology 2022, 11, 110. [Google Scholar] [CrossRef]
- Kumari, P.; Wani, I.A.; Khan, S.; Verma, S.; Mushtaq, S.; Gulnaz, A.; Paray, B.A. Modeling of Valeriana Wallichii Habitat Suitability and Niche Dynamics in the Himalayan Region under Anticipated Climate Change. Biology 2022, 11, 498. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, D.; Li, C.; Zhou, R.; Wang, J.; Liu, Q. Projecting the Potential Distribution Areas of Ixodes Scapularis (Acari: Ixodidae) Driven by Climate Change. Biology 2022, 11, 107. [Google Scholar] [CrossRef]
- Zhou, R.; Gao, Y.; Chang, N.; Gao, T.; Ma, D.; Li, C.; Liu, Q. Projecting the Potential Distribution of Glossina Morsitans (Diptera: Glossinidae) under Climate Change Using the MaxEnt Model. Biology 2021, 10, 1150. [Google Scholar] [CrossRef]
- Ma, D.; Lun, X.; Li, C.; Zhou, R.; Zhao, Z.; Wang, J.; Zhang, Q.; Liu, Q. Predicting the Potential Global Distribution of Amblyomma Americanum (Acari: Ixodidae) under Near Current and Future Climatic Conditions, Using the Maximum Entropy Model. Biology 2021, 10, 1057. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; Chang, N.; Ma, D.; Zhou, R.; Zhao, Z.; Wang, J.; Zhang, Q.; Liu, Q. Risk Assessment of Anopheles Philippinensis and Anopheles Nivipes (Diptera: Culicidae) Invading China under Climate Change. Biology 2021, 10, 998. [Google Scholar] [CrossRef]
- Abubakr, M.; Sami, H.; Mahdi, I.; Altahir, O.; Abdelbagi, H.; Mohamed, N.S.; Ahmed, A. The Phylodynamic and Spread of the Invasive Asian Malaria Vectors, Anopheles Stephensi, in Sudan. Biology 2022, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gurgel, H.; Xu, L.; Yang, L.; Dong, J. Improving Dengue Forecasts by Using Geospatial Big Data Analysis in Google Earth Engine and the Historical Dengue Information-Aided Long Short Term Memory Modeling. Biology 2022, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Githeko, A.K.; Lindsay, S.W.; Confalonieri, U.E.; Patz, J.A. Climate Change and Vector-Borne Diseases: A Regional Analysis. Bull. World Health Organ. 2000, 78, 1136–1147. [Google Scholar]
- Kilpatrick, A.M.; Randolph, S.E. Drivers, Dynamics, and Control of Emerging Vector-Borne Zoonotic Diseases. Lancet 2012, 380, 1946–1955. [Google Scholar] [CrossRef] [Green Version]
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic Arboviral Diseases: Priorities for Research and Public Health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization & UNICEF/UNDP/World Bank. WHO Special Programme for Research and Training in Tropical Diseases. In Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151297-8. [Google Scholar]
- Leta, S.; Beyene, T.J.; Clercq, E.M.D.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global Risk Mapping for Major Diseases Transmitted by Aedes Aegypti and Aedes Albopictus. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Fouque, F.; Reeder, J.C. Impact of Past and On-Going Changes on Climate and Weather on Vector-Borne Diseases Transmission: A Look at the Evidence. Infect. Dis. Poverty 2019, 8, 51. [Google Scholar] [CrossRef]
- Kovats, R.S. El Niño and human health. Bull. World Health Organ. 2000, 78, 1127–1135. [Google Scholar]
- Morand, S.; Owers, K.A.; Waret-Szkuta, A.; McIntyre, K.M.; Baylis, M. Climate Variability and Outbreaks of Infectious Diseases in Europe. Sci. Rep. 2013, 3, 1774. [Google Scholar] [CrossRef] [Green Version]
- Ben Ari, T.; Gershunov, A.; Gage, K.L.; Snäll, T.; Ettestad, P.; Kausrud, K.L.; Stenseth, N.C. Human Plague in the USA: The Importance of Regional and Local Climate. Biol. Lett. 2008, 4, 737–740. [Google Scholar] [CrossRef] [Green Version]
- Kreppel, K.S.; Caminade, C.; Telfer, S.; Rajerison, M.; Rahalison, L.; Morse, A.; Baylis, M. A Non-Stationary Relationship between Global Climate Phenomena and Human Plague Incidence in Madagascar. PLoS Negl. Trop. Dis. 2014, 8, e3155. [Google Scholar] [CrossRef] [PubMed]
- Fisman, D.N.; Tuite, A.R.; Brown, K.A. Impact of El Niño Southern Oscillation on Infectious Disease Hospitalization Risk in the United States. Proc. Natl. Acad. Sci. USA 2016, 113, 14589–14594. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.; Kamel, M. Climatic Changes and Their Role in Emergence and Re-Emergence of Diseases. Environ. Sci. Pollut. Res. 2020, 27, 22336–22352. [Google Scholar] [CrossRef]
- Rosenthal, J. Climate Change and the Geographic Distribution of Infectious Diseases. EcoHealth 2009, 6, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Franklinos, L.H.V.; Jones, K.E.; Redding, D.W.; Abubakar, I. The Effect of Global Change on Mosquito-Borne Disease. Lancet Infect. Dis. 2019, 19, e302–e312. [Google Scholar] [CrossRef]
- Medlock, J.M.; Leach, S.A. Effect of Climate Change on Vector-Borne Disease Risk in the UK. Lancet Infect. Dis. 2015, 15, 721–730. [Google Scholar] [CrossRef]
- Singh, N.S.; Singh, D.P. Impact of Climate Change on Human Health Related Vector-Borne Diseases in the Present Scenario of COVID-19 in India. Entomol. Res. 2020, 44, 631–638. [Google Scholar] [CrossRef]
- Sternberg, E.D.; Thomas, M.B. Local Adaptation to Temperature and the Implications for Vector-Borne Diseases. Trends Parasitol. 2014, 30, 115–122. [Google Scholar] [CrossRef]
- Christiansen-Jucht, C.; Parham, P.E.; Saddler, A.; Koella, J.C.; Basáñez, M.-G. Temperature during Larval Development and Adult Maintenance Influences the Survival of Anopheles Gambiae s.s. Parasites Vectors 2014, 7, 489. [Google Scholar] [CrossRef]
- Westbrook, C.J.; Reiskind, M.H.; Pesko, K.N.; Greene, K.E.; Lounibos, L.P. Larval Environmental Temperature and the Susceptibility of Aedes Albopictus Skuse (Diptera: Culicidae) to Chikungunya Virus. Vector Borne Zoonotic Dis. 2010, 10, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Perkins, T.A.; Metcalf, C.J.E.; Grenfell, B.T.; Tatem, A.J. Estimating Drivers of Autochthonous Transmission of Chikungunya Virus in Its Invasion of the Americas. PLoS Curr. 2015, 7, ecurrents.outbreaks.a4c7b6ac10e0420b1788c9767946d1fc. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q. Impact of Climate Change on Vector-Borne Diseases and Related Response Strategies in China: Major Research Findings and Recommendations for Future Research. Chin. J. Vector Biol Control 2021, 32, 1–11. [Google Scholar] [CrossRef]
- Krauer, F.; Viljugrein, H.; Dean, K.R. The Influence of Temperature on the Seasonality of Historical Plague Outbreaks. Proc. R. Soc. B-Biol. Sci. 2021, 288, 20202725. [Google Scholar] [CrossRef]
- Reinhold, J.M.; Lazzari, C.R.; Lahondère, C. Effects of the Environmental Temperature on Aedes Aegypti and Aedes Albopictus Mosquitoes: A Review. Insects 2018, 9, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; et al. Thermal Biology of Mosquito-Borne Disease. Ecol. Lett. 2019, 22, 1690–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopp, M.J.; Foley, J.A. Global-Scale Relationships between Climate and the Dengue Fever Vector, Aedes Aegypti. Clim. Chang. 2001, 48, 441–463. [Google Scholar] [CrossRef]
- Orland, M.; Kelt, D. Responses of a Heteromyid Rodent Community to Large and Small-Scale Resource Pulses: Diversity, Abundance, and Home-Range Dynamics. J. Mammal 2007, 88, 1280–1287. [Google Scholar] [CrossRef]
- Matsushita, N.; Kim, Y.; Ng, C.F.S.; Moriyama, M.; Igarashi, T.; Yamamoto, K.; Otieno, W.; Minakawa, N.; Hashizume, M. Differences of Rainfall-Malaria Associations in Lowland and Highland in Western Kenya. Int. J. Environ. Res. Public Health 2019, 16, 3693. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Zhou, S.; Zhan, Y.; Wu, S.; Xue, J. Research Progress on the Correlations of Tick-Borne Diseases with Meteorological Factors and Their Prevention Measures in China. Chin. J. Parasitol. Parasit. Dis. 2020, 38, 123–127. [Google Scholar] [CrossRef]
- Ogden, N.H.; Lindsay, L.R. Effects of Climate and Climate Change on Vectors and Vector-Borne Diseases: Ticks Are Different. Trends Parasitol. 2016, 32, 646–656. [Google Scholar] [CrossRef]
- Stilianakis, N.I.; Syrris, V.; Petroliagkis, T.; Pärt, P.; Gewehr, S.; Kalaitzopoulou, S.; Mourelatos, S.; Baka, A.; Pervanidou, D.; Vontas, J.; et al. Identification of Climatic Factors Affecting the Epidemiology of Human West Nile Virus Infections in Northern Greece. PLoS ONE 2016, 11, e0161510. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Li, L.; Guo, X.; Wu, R.; Shi, B.; Wang, Y.; Liu, Y.; Wu, S.; Pan, Y.; Wang, Q.; et al. A Field-Based Modeling Study on Ecological Characterization of Hourly Host-Seeking Behavior and Its Associated Climatic Variables in Aedes Albopictus. Parasites Vectors 2019, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Cunze, S.; Glock, G.; Kochmann, J.; Klimpel, S. Ticks on the Move—Climate Change-Induced Range Shifts of Three Tick Species in Europe: Current and Future Habitat Suitability for Ixodes Ricinus in Comparison with Dermacentor Reticulatus and Dermacentor Marginatus. Parasitol. Res. 2022, 121, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A. Climate Change Decreases Habitat Suitability for Some Tick Species (Acari: Ixodidae) in South Africa. Onderstepoort J. Vet. Res. 2003, 70, 79–93. [Google Scholar] [PubMed]
- Fischer, D.; Thomas, S.M.; Suk, J.E.; Sudre, B.; Hess, A.; Tjaden, N.B.; Beierkuhnlein, C.; Semenza, J.C. Climate Change Effects on Chikungunya Transmission in Europe: Geospatial Analysis of Vector’s Climatic Suitability and Virus’ Temperature Requirements. Int. J. Health Geograph. 2013, 12, 51. [Google Scholar] [CrossRef] [Green Version]
- Carlson, C.J.; Bevins, S.N.; Schmid, B.V. Plague Risk in the Western United States over Seven Decades of Environmental Change. Glob. Chang. Biol. 2022, 28, 753–769. [Google Scholar] [CrossRef]
- Snäll, T.; Benestad, R.E.; Stenseth, N.C. Expected Future Plague Levels in a Wildlife Host under Different Scenarios of Climate Change. Glob. Chang. Biol. 2009, 15, 500–507. [Google Scholar] [CrossRef]
- Wan, X.; Holyoak, M.; Yan, C.; Le Maho, Y.; Dirzo, R.; Krebs, C.J.; Stenseth, N.C.; Zhang, Z. Broad-Scale Climate Variation Drives the Dynamics of Animal Populations: A Global Multi-Taxa Analysis. Biol. Rev. 2022; Early View. [Google Scholar] [CrossRef]
- Caminade, C.; Turner, J.; Metelmann, S.; Hesson, J.C.; Blagrove, M.S.C.; Solomon, T.; Morse, A.P.; Baylis, M. Global Risk Model for Vector-Borne Transmission of Zika Virus Reveals the Role of El Niño 2015. Proc. Natl. Acad. Sci. USA 2017, 114, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Hiko, A.; Malicha, G. Climate Change and Animal Health Risk. In Climate Change and the 2030 Corporate Agenda for Sustainable Development; Emerald group Publishing Limited Howard House: Bingley, UK, 2017; Volume 19, pp. 77–111. ISBN 978-1-78635-818-9. [Google Scholar]
- Ciota, A.T.; Keyel, A.C. The Role of Temperature in Transmission of Zoonotic Arboviruses. Viruses 2019, 11, 1013. [Google Scholar] [CrossRef] [Green Version]
- Rocklöv, J.; Dubrow, R. Climate Change: An Enduring Challenge for Vector-Borne Disease Prevention and Control. Nat. Immunol. 2020, 21, 479–483. [Google Scholar] [CrossRef]
- Tian, H.-Y.; Yu, P.-B.; Luis, A.D.; Bi, P.; Cazelles, B.; Laine, M.; Huang, S.-Q.; Ma, C.-F.; Zhou, S.; Wei, J.; et al. Changes in Rodent Abundance and Weather Conditions Potentially Drive Hemorrhagic Fever with Renal Syndrome Outbreaks in Xi’an, China, 2005–2012. PLoS Neglect. Trop. Dis. 2015, 9, e0003530. [Google Scholar] [CrossRef] [PubMed]
- Joshi, Y.P.; Kim, E.-H.; Cheong, H.-K. The Influence of Climatic Factors on the Development of Hemorrhagic Fever with Renal Syndrome and Leptospirosis during the Peak Season in Korea: An Ecologic Study. BMC Infect. Dis. 2017, 17, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubalek, Z. North Atlantic Weather Oscillation and Human Infectious Diseases in the Czech Republic, 1951–2003. Eur. J. Epidemiol. 2005, 20, 263–270. [Google Scholar] [CrossRef]
- Fernando Chaves, L.; Calzada, J.E.; Valderrama, A.; Saldana, A. Cutaneous Leishmaniasis and Sand Fly Fluctuations Are Associated with El Niño in Panamá. PLoS Neglect. Trop. Dis. 2014, 8, e3210. [Google Scholar] [CrossRef] [Green Version]
- Enscore, R.E.; Biggerstaff, B.J.; Brown, T.L.; Fulgham, R.E.; Reynolds, P.J.; Engelthaler, D.M.; Levy, C.E.; Parmenter, R.R.; Montenieri, J.A.; Cheek, J.E.; et al. Modeling Relationships between Climate and the Frequency of Human Plague Cases in the Southwestern United States, 1960-1997. Am. J. Trop. Med. Hyg. 2002, 66, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Hii, Y.L.; Rocklov, J.; Wall, S.; Ng, L.C.; Tang, C.S.; Ng, N. Optimal Lead Time for Dengue Forecast. PLoS Neglect. Trop. Dis. 2012, 6, e1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakarla, S.G.; Caminade, C.; Mutheneni, S.R.; Morse, A.P.; Upadhyayula, S.M.; Kadiri, M.R.; Kumaraswamy, S. Lag Effect of Climatic Variables on Dengue Burden in India. Epidemiol. Infect. 2019, 147, e170. [Google Scholar] [CrossRef] [Green Version]
- Bi, P.; Tong, S.L.; Donald, K.; Parton, K.A.; Ni, J.F. Climatic Variables and Transmission of Malaria: A 12-Year Data Analysis in Shuchen County, China. Public Health Rep. 2003, 118, 65–71. [Google Scholar] [CrossRef]
- Wangdi, K.; Singhasivanon, P.; Silawan, T.; Lawpoolsri, S.; White, N.J.; Kaewkungwal, J. Development of Temporal Modelling for Forecasting and Prediction of Malaria Infections Using Time-Series and ARIMAX Analyses: A Case Study in Endemic Districts of Bhutan. Malar. J. 2010, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Haddawy, P.; Hasan, A.H.M.I.; Kasantikul, R.; Lawpoolsri, S.; Sa-angchai, P.; Kaewkungwal, J.; Singhasivanon, P. Spatiotemporal Bayesian Networks for Malaria Prediction. Artif. Intell. Med. 2018, 84, 127–138. [Google Scholar] [CrossRef]
- Anyamba, A.; Linthicum, K.J.; Small, J.L.; Collins, K.M.; Tucker, C.J.; Pak, E.W.; Britch, S.C.; Eastman, J.R.; Pinzon, J.E.; Russell, K.L. Climate Teleconnections and Recent Patterns of Human and Animal Disease Outbreaks. PLoS Neglect. Trop. Dis. 2012, 6, e1465. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Tong, S.; Mengersen, K.; Oldenburg, B. Rainfall, Mosquito Density and the Transmission of Ross River Virus: A Time-Series Forecasting Model. Ecol. Model. 2006, 196, 505–514. [Google Scholar] [CrossRef]
- Jacups, S.P.; Whelan, P.I.; Markey, P.G.; Cleland, S.J.; Williamson, G.J.; Currie, B.J. Predictive Indicators for Ross River Virus Infection in the Darwin Area of Tropical Northern Australia, Using Long-Term Mosquito Trapping Data. Trop. Med. Int. Health 2008, 13, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Poh, K.C.; Chaves, L.F.; Reyna-Nava, M.; Roberts, C.M.; Fredregill, C.; Bueno, R.; Debboun, M.; Hamer, G.L. The Influence of Weather and Weather Variability on Mosquito Abundance and Infection with West Nile Virus in Harris County, Texas, USA. Sci. Total Environ. 2019, 675, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.-Y.; Bi, P.; Cazelles, B.; Zhou, S.; Huang, S.-Q.; Yang, J.; Pei, Y.; Wu, X.-X.; Fu, S.-H.; Tong, S.-L.; et al. How Environmental Conditions Impact Mosquito Ecology and Japanese Encephalitis: An Eco-Epidemiological Approach. Environ. Int. 2015, 79, 17–24. [Google Scholar] [CrossRef]
- Xu, L.; Stige, L.C.; Chan, K.-S.; Zhou, J.; Yang, J.; Sang, S.; Wang, M.; Yang, Z.; Yan, Z.; Jiang, T.; et al. Climate Variation Drives Dengue Dynamics. Proc. Natl. Acad. Sci. USA 2017, 114, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Bezirtzoglou, C.; Dekas, K.; Charvalos, E. Climate Changes, Environment and Infection: Facts, Scenarios and Growing Awareness from the Public Health Community within Europe. Anaerobe 2011, 17, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Kreppel, K.; Caminade, C.; Govella, N.; Morse, A.P.; Ferguson, H.M.; Baylis, M. Impact of ENSO 2016-17 on Regional Climate and Malaria Vector Dynamics in Tanzania. Environ. Res. Lett. 2019, 14, 075009. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Tao, Y.; Chen, M.; Wen, X.; Xu, L.; Tian, H.; Stenseth, N.C. Relationship between Increase Rate of Human Plague in China and Global Climate Index as Revealed by Cross-Spectral and Cross-Wavelet Analyses. Integr. Zool. 2007, 2, 144–153. [Google Scholar] [CrossRef]
- Yue, R.P.H.; Lee, H.F. The Delayed Effect of Cooling Reinforced the NAO-Plague Connection in Pre-Industrial Europe. Sci. Total Environ. 2021, 762, 143122. [Google Scholar] [CrossRef]
- Flahault, A.; de Castaneda, R.R.; Bolon, I. Climate Change and Infectious Diseases. Public Health Rev. 2016, 37, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, G.J.; Coronel, G.; Pasten, M.; Baez, J.; Monte-Domecq, R.; Galeano-Rojas, A.; Flores, L.; Ciganda, C.; Bidegain, M.; Aparicio-Effen, M.; et al. Impacts on Well-Being and Health by Excessive Rainfall and Floods in Paraguay, Uruguay and Bolivia. In Climate Change and Health: Improving Resilience and Reducing Risks; Springer: Berlin/Heidelberg, Germany, 2016; pp. 475–514. ISBN 978-3-319-24660-4. [Google Scholar]
- Thomson, M.C.; Munoz, A.G.; Cousin, R.; Shumake-Guillemot, J. Climate Drivers of Vector-Borne Diseases in Africa and Their Relevance to Control Programmes. Infect. Dis. Poverty 2018, 7, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, P.; Liang, L.; Tan, X.; Chen, J.; Chen, X. Potential Effects of Heat Waves on the Population Dynamics of the Dengue Mosquito Aedes Albopictus. PLoS Neglect. Trop. Dis. 2019, 13, e0007528. [Google Scholar] [CrossRef]
- Nosrat, C.; Altamirano, J.; Anyamba, A.; Caldwell, J.M.; Damoah, R.; Mutuku, F.; Ndenga, B.; LaBeaud, A.D. Impact of Recent Climate Extremes on Mosquito-Borne Disease Transmission in Kenya. PLoS Neglect. Trop. Dis. 2021, 15, e0009182. [Google Scholar] [CrossRef]
- Duchet, C.; Moraru, G.M.; Segev, O.; Spencer, M.; Hayoon, A.G.; Blaustein, L. Effects of Flash Flooding on Mosquito and Community Dynamics in Experimental Pools. J. Vector Ecol. 2017, 42, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Shaman, J.; Stieglitz, M.; Stark, C.; Le Blancq, S.; Cane, M. Using a Dynamic Hydrology Model to Predict Mosquito Abundances in Flood and Swamp Water. Emerg. Infect. Dis 2002, 8, 6–13. [Google Scholar] [CrossRef]
- Benedum, C.M.; Seidahmed, O.M.E.; Eltahir, E.A.B.; Markuzon, N. Statistical Modeling of the Effect of Rainfall Flushing on Dengue Transmission in Singapore. PLoS Neglect. Trop. Dis. 2018, 12, e0006935. [Google Scholar] [CrossRef] [Green Version]
- Adekunle, A.; Adegboye, O.A.; Rahman, K.M. Flooding in Townsville, North Queensland, Australia, in February 2019 and Its Effects on Mosquito-Borne Diseases. Int. J. Environ. Res. Public Health 2019, 16, 1393. [Google Scholar] [CrossRef] [Green Version]
- Chase, J.M.; Knight, T.M. Drought-Induced Mosquito Outbreaks in Wetlands. Ecol. Lett. 2003, 6, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Liu, Q.; Stige, L.C.; Ben Ari, T.; Fang, X.; Chan, K.-S.; Wang, S.; Stenseth, N.C.; Zhang, Z. Nonlinear Effect of Climate on Plague during the Third Pandemic in China. Proc. Natl. Acad. Sci. USA 2011, 108, 10214–10219. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, L.; Schmid, B.V.; Dean, K.R.; Zhang, Z.; Xie, Y.; Fang, X.; Wang, S.; Liu, Q.; Lyu, B.; et al. Human Plague System Associated with Rodent Diversity and Other Environmental Factors. Royal Soc. Open Sci. 2019, 6, 190216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, W.; Zhang, C.; Zhang, S.; Ai, S.; Bai, Y.; Bao, J.; Chen, B.; Chang, N.; Chen, H.; Cheng, L.; et al. The 2021 China Report of the Lancet Countdown on Health and Climate Change: Seizing the Window of Opportunity. Lancet Public Health 2021, 6, e932–e947. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, C.; Suen, H.P.; Ai, S.; Bai, Y.; Bao, J.; Chen, B.; Cheng, L.; Cui, X.; Dai, H.; et al. The 2020 China Report of the Lancet Countdown on Health and Climate Change. Lancet Public Health 2021, 6, e64–e81. [Google Scholar] [CrossRef]
- Evander, M.; Ahlm, C. Milder Winters in Northern Scandinavia May Contribute to Larger Outbreaks of Haemorrhagic Fever Virus. Glob. Health Action 2009, 2, 2020. [Google Scholar] [CrossRef]
- Karmakar, M.; Pradhan, M.M. Climate Change and Public Health: A Study of Vector-Borne Diseases in Odisha, India. Nat. Hazards 2020, 102, 659–671. [Google Scholar] [CrossRef]
- Lin, S.; Shrestha, S.; Prusinski, M.A.; White, J.L.; Lukacik, G.; Smith, M.; Lu, J.; Backenson, B. The Effects of Multiyear and Seasonal Weather Factors on Incidence of Lyme Disease and Its Vector in New York State. Sci. Total Environ. 2019, 665, 1182–1188. [Google Scholar] [CrossRef]
- Boeckmann, M.; Joyner, T.A. Old Health Risks in New Places? An Ecological Niche Model for I. Ricinus Tick Distribution in Europe under a Changing Climate. Health Place 2014, 30, 70–77. [Google Scholar] [CrossRef]
- Ali, S.; Gugliemini, O.; Harber, S.; Harrison, A.; Houle, L.; Ivory, J.; Kersten, S.; Khan, R.; Kim, J.; LeBoa, C.; et al. Environmental and Social Change Drive the Explosive Emergence of Zika Virus in the Americas. PLoS Negl. Trop. Dis. 2017, 11, e0005135. [Google Scholar] [CrossRef]
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Bansal, S. Climate Change Increases Cross-Species Viral Transmission Risk. Nature 2022, 607, 555–562. [Google Scholar] [CrossRef]
- Wilson, M. Infectious Diseases: An Ecological Perspective. BMJ 1995, 311, 1681–1684. [Google Scholar] [CrossRef]
- Ratnam, I.; Leder, K.; Black, J.; Torresi, J. Dengue Fever and International Travel. J. Travel Med. 2013, 20, 384–393. [Google Scholar] [CrossRef]
- Muhammad, S.; Long, X.; Salman, M. COVID-19 Pandemic and Environmental Pollution: A Blessing in Disguise? Sci. Total Environ. 2020, 728, 138820. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, D.; Markard, J. A COVID-19 Recovery for Climate. Science 2020, 368, 447. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ren, L.; Li, H.; Wang, H.; Wang, P.; Chen, L.; Yue, X.; Liao, H. Fast Climate Responses to Aerosol Emission Reductions During the COVID-19 Pandemic. Geophys. Res. Lett. 2020, 47, e2020GL089788. [Google Scholar] [CrossRef]
- Dutheil, F.; Baker, J.; Navel, V. COVID-19 as a Factor Influencing Air Pollution? Environ. Pollut. 2020, 263, 114466. [Google Scholar] [CrossRef] [PubMed]
- Forster, P.M.; Forster, H.I.; Evans, M.J.; Gidden, M.J.; Jones, C.D.; Keller, C.A.; Lamboll, R.D.; Quere, C.L.; Rogelj, J.; Rosen, D.; et al. Current and Future Global Climate Impacts Resulting from COVID-19. Nat. Clim. Chang. 2020, 10, 913–919. [Google Scholar] [CrossRef]
- Castaneda-Gomez, J.; Gonzalez-Acosta, C.; Jaime-Rodriguez, J.L.; Villegas-Trejo, A.; Moreno-Garcia, M. COVID-19 and its impact on the control of Aedes (Stegomyia) aegypti mosquito and the epidemiological surveillance of arbovirus infections. Gac. Med. Mex. 2021, 157, 194–200. [Google Scholar] [CrossRef]
- Olive, M.-M.; Baldet, T.; Devillers, J.; Fite, J.; Paty, M.-C.; Paupy, C.; Quenel, P.; Quillery, E.; Raude, J.; Stahl, J.-P.; et al. The COVID-19 Pandemic Should Not Jeopardize Dengue Control. PLoS Neglect. Trop. Dis. 2020, 14, e0008716. [Google Scholar] [CrossRef]
- Seelig, F.; Bezerra, H.; Cameron, M.; Hii, J.; Hiscox, A.; Irish, S.; Jones, R.T.; Lang, T.; Lindsay, S.W.; Lowe, R.; et al. The COVID-19 Pandemic Should Not Derail Global Vector Control Efforts. PLoS Neglect. Trop. Dis. 2020, 14, e0008606. [Google Scholar] [CrossRef]
- Webb, C.E. Reflections on a Highly Unusual Summer: Bushfires, COVID-19 and Mosquito-Borne Disease in NSW, Australia. Public Health Res. Pract. 2020, 30, e3042027. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Tissera, H.; Ooi, E.E.; Coloma, J.; Scott, T.W.; Gubler, D.J. Preventing Dengue Epidemics during the COVID-19 Pandemic. Am. J. Trop. Med. Hyg. 2020, 103, 570–571. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Webb, C.E.; Abu Kassim, N.F. Prioritizing Mosquito-Borne Diseases during and after the COVID-19 Pandemic. West. Pac. Surveill. Response J. 2021, 12, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Reegan, A.D.; Gandhi, M.R.; Asharaja, A.C.; Devi, C.; Shanthakumar, S.P. COVID-19 Lockdown: Impact Assessment on Aedes Larval Indices, Breeding Habitats, Effects on Vector Control Programme and Prevention of Dengue Outbreaks. Heliyon 2020, 6, e05181. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Roncon, L. Reduction of West Nile Virus Infections in Italy during 2020 Early Summer: A Secondary “COVID-19” Effect? Pathog. Glob. Health 2020, 114, 345–346. [Google Scholar] [CrossRef]
- Lim, J.T.; Dickens, B.S.L.; Chew, L.Z.X.; Choo, E.L.W.; Koo, J.R.; Aik, J.; Ng, L.C.; Cook, A.R. Impact of Sars-Cov-2 Interventions on Dengue Transmission. PLoS Neglect. Trop. Dis. 2020, 14, e0008719. [Google Scholar] [CrossRef]
- McCormick, D.W.; Kugeler, K.J.; Marx, G.E.; Jayanthi, P.; Dietz, S.; Mead, P.; Hinckley, A.F. Effects of COVID-19 Pandemic on Reported Lyme Disease, United States, 2020. Emerg. Infect. Dis 2021, 27, 2715–2717. [Google Scholar] [CrossRef]
- Ullrich, A.; Schranz, M.; Rexroth, U.; Hamouda, O.; Schaade, L.; Diercke, M.; Boender, T.S. Impact of the COVID-19 Pandemic and Associated Non-Pharmaceutical Interventions on Other Notifiable Infectious Diseases in Germany: An Analysis of National Surveillance Data during Week 1-2016—Week 32-2020. Lancet Reg. Health-Eur. 2021, 6, 100103. [Google Scholar] [CrossRef]
- Lai, C.-C.; Chen, S.-Y.; Yen, M.-Y.; Lee, P.-I.; Ko, W.-C.; Hsueh, P.-R. The Impact of the Coronavirus Disease 2019 Epidemic on Notifiable Infectious Diseases in Taiwan: A Database Analysis. Travel Med. Infect. Dis. 2021, 40, 101997. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Guo, Y.; Gao, J.; Tang, H.; Xu, K.; Liu, Q.; Xu, L. Climate Change Drives the Transmission and Spread of Vector-Borne Diseases: An Ecological Perspective. Biology 2022, 11, 1628. https://doi.org/10.3390/biology11111628
Ma J, Guo Y, Gao J, Tang H, Xu K, Liu Q, Xu L. Climate Change Drives the Transmission and Spread of Vector-Borne Diseases: An Ecological Perspective. Biology. 2022; 11(11):1628. https://doi.org/10.3390/biology11111628
Chicago/Turabian StyleMa, Jian, Yongman Guo, Jing Gao, Hanxing Tang, Keqiang Xu, Qiyong Liu, and Lei Xu. 2022. "Climate Change Drives the Transmission and Spread of Vector-Borne Diseases: An Ecological Perspective" Biology 11, no. 11: 1628. https://doi.org/10.3390/biology11111628





