Genetic Relationship, SPAD Reading, and Soluble Sugar Content as Indices for Evaluating the Graft Compatibility of Citrus Interstocks
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Statistics on Genetic Relationship Map Construction, Grafting Survival Ratio, and Preservation Ratio
2.3. Determination of Vegetative Growth and Chlorophyll Content in Leaves
2.4. Determination of Enzyme Activities and Osmotic Regulatory Substances in Leaves
2.5. Evaluation of ‘Translocated’ Incompatibility and ‘Localized’ Incompatibility
2.6. Statistical Analysis
3. Results
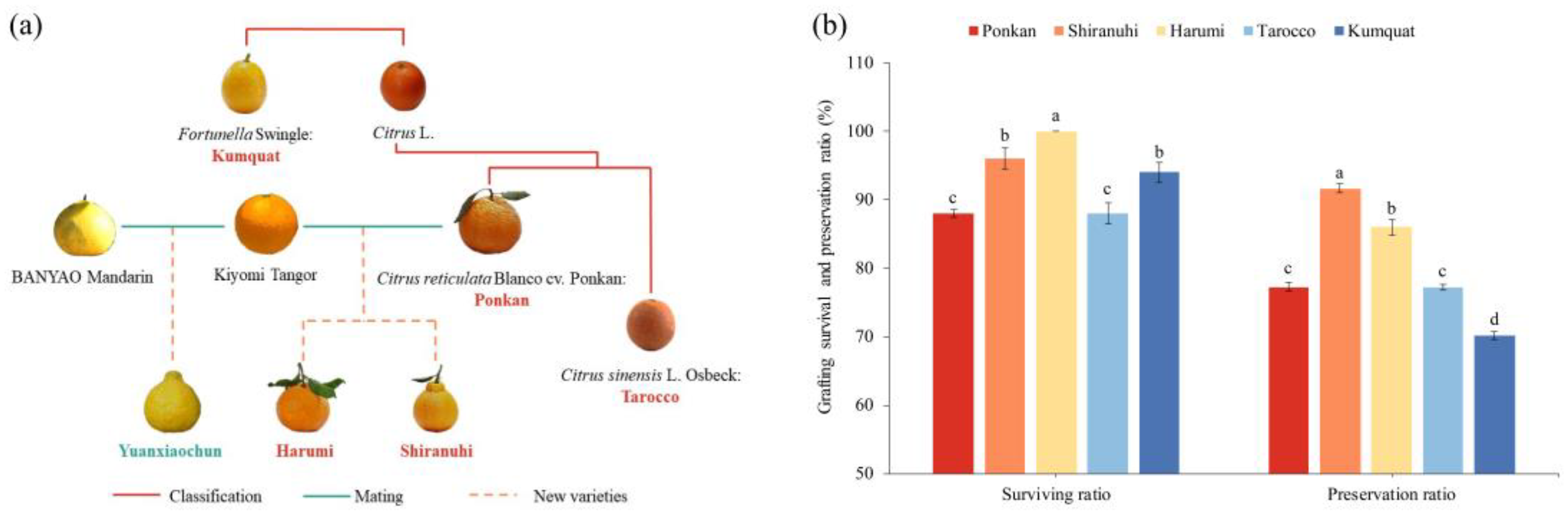
3.1. Genetic Relationship, Survival Ratio, and Preservation Ratio of Grafting
3.2. Vegetative Growth
3.3. Correlation Analysis of SPAD Reading and Vegetative Growth
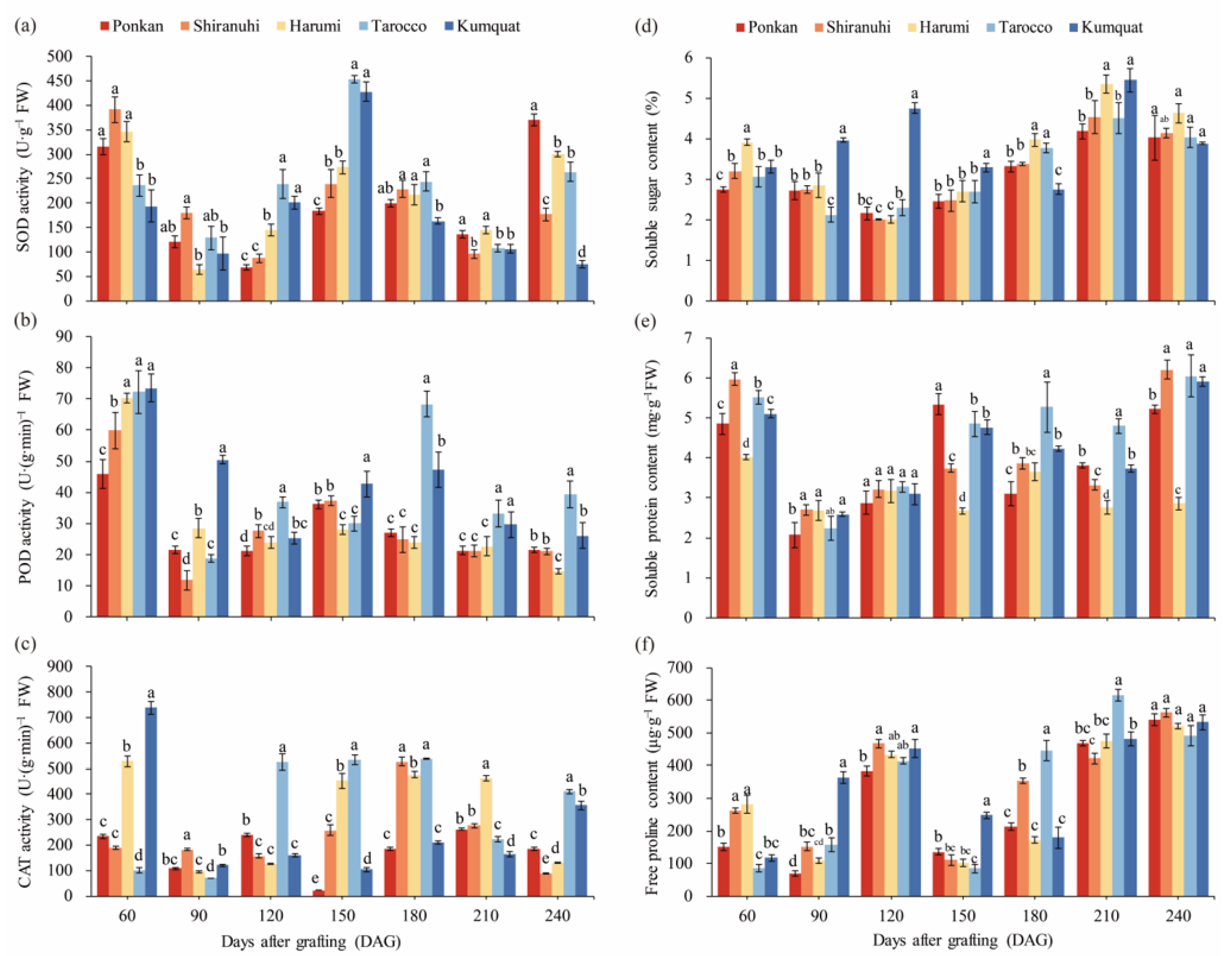
3.4. Antioxidant Enzyme Activities
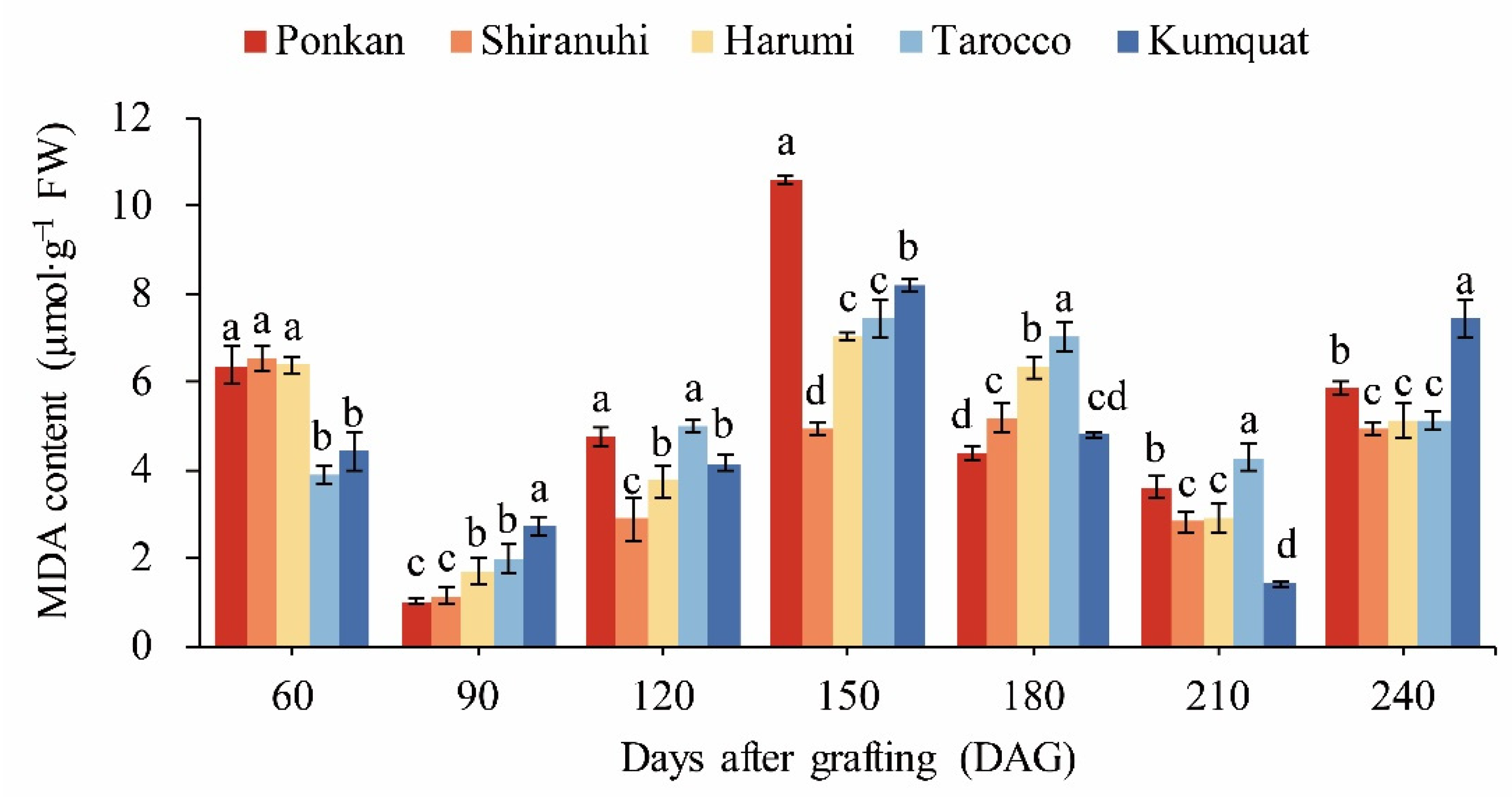
3.5. Osmotic Regulatory Substances and MDA Content
3.6. Translocated’ Incompatibility and ‘Localized’ Incompatibility
4. Discussion
4.1. Response of Grafting Survival Ratio and Preservation Ratio to Interstocks
4.2. Interstocks Affect the Accumulation of Scion Biomass
4.3. Response of Antioxidant Enzymes and Osmoregulatory Substances to Graft Compatibility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Xu, Y.T.; Jiang, X.L.; Yu, H.W.; Jia, H.H.; Tan, C.M.; Hu, G.; Hu, Y.B.; Junaid, R.M.; Deng, X.X.; et al. Genome of a citrus rootstock and global DNA demethylation caused by heterografting. Hortic. Res. 2021, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rodriguez, M.M.; Estan, M.T.; Moyano, E.; Garcia-Abellan, J.O.; Flores, F.B.; Campos, J.F.; Al-Azzawi, M.J.; Flowers, T.J.; Bolarin, M.C. The effectiveness of grafting to improve salt tolerance in tomato when an ‘excluder’ genotype is used as scion. Environ. Exp. Bot. 2008, 63, 392–401. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Meyerowitz, E.M. Plant grafting. Curr. Biol. 2015, 25, R183–R188. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Yang, C.; Zhang, L.; Feng, J.; Xi, W. Effect of light-emitting diodes and ultraviolet irradiation on the soluble sugar, organic acid, and carotenoid content of postharvest sweet oranges (Citrus sinensis (L.) Osbeck). Molecules 2019, 24, 3440. [Google Scholar] [CrossRef] [Green Version]
- Morales, J.; Bermejo, A.; Navarro, P.; Forner-Giner, M.N.; Salvador, A. Rootstock effect on fruit quality, anthocyanins, sugars, hydroxycinnamic acids and flavanones content during the harvest of blood oranges ‘Moro’ and ‘Tarocco Rosso’ grown in Spain. Food Chem. 2020, 342, 128305. [Google Scholar] [CrossRef]
- Wang, T.; Xiong, B.; Tan, L.P.; Yang, Y.T.; Zhang, Y.; Ma, M.M.; Xu, Y.H.; Liao, L.; Sun, G.C.; Liang, D.; et al. Effects of interstocks on growth and photosynthetic characteristics in ‘Yuanxiaochun’ Citrus seedlings. Funct. Plant Biol. 2020, 47, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Chen, X.Y.; Zhao, S.A.; Zhan, Q.L.; Chen, S.M.; Jiang, J.F.; Fang, W.M.; Chen, F.D.; Guan, Z.Y. Effect of grafting on the growth and flowering of sprays chrysanthemums. Sci. Hortic. 2022, 291, 110607. [Google Scholar] [CrossRef]
- Askari-Khorasgani, O.; Jafarpour, M.; Hadad, M.M.; Pessarakli, M. Fruit yield and quality characteristics of ‘Shahmiveh’ pear cultivar grafted on six rootstocks. J. Plant Nutr. 2019, 42, 323–332. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, C.B.; Li, Y.F.; Jang, C.L.; Chun, C.P.; Peng, L.Z. Effects and cost comparison of different interstock sprout inhibition and removal treatments in top grafted citrus trees. J. Fruit Sci. 2018, 35, 711–717. [Google Scholar] [CrossRef]
- He, W.; Xie, R.; Wang, Y.; Chen, Q.; Wang, H.; Yang, S.F.; Luo, Y.; Zhang, Y.; Tang, H.R.; Gmitter, F.G., Jr.; et al. Comparative transcriptomic analysis on compatible/incompatible grafts in citrus. Hortic. Res. 2022, 9, uhab072. [Google Scholar] [CrossRef] [PubMed]
- Lockard, R.G. Stock and scion growth relationships and the dwarfing mechanism in apple. Hortic. Rev. 1981, 3, 315–375. [Google Scholar] [CrossRef]
- Mosse, B. Graft-Incompatibility in Fruit Trees: With Particular Reference to Its Underlying Causes; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1962. [Google Scholar]
- Darikova, J.A.; Savva, Y.V.; Vaganov, E.A.; Grachev, A.M.; Kuznetsova, G.V. Grafts of woody plants and the problem of incompatibility between scion and rootstock (a review). J. Sib. Fed. Univ. Biol. 2011, 4, 54–63. [Google Scholar]
- Moing, A.; Carbonne, F.; Gaudillère, J.P. Growth and carbon partitioning in compatible and incompatible peach/plum grafts. Physiol. Plant. 1990, 79, 540–546. [Google Scholar] [CrossRef]
- Reig, G.; Salazar, A.; Zarrouk, O.; Font I Forcada, C.; Val, J.; Moreno, M.Á. Long-term graft compatibility study of peach-almond hybrid and plum based rootstocks budded with European and Japanese plums. Sci. Hortic. 2019, 243, 392–400. [Google Scholar] [CrossRef]
- Yan, Y.; Gao, Z.; He, C.Z.; Li, X.Z. Advances in Activity of Related Enzymes during Graft Healing Process of Citrus Paradisi Macf. Agric. Sci. Technol. 2011, 12, 1472–1476. [Google Scholar] [CrossRef]
- Zhao, Y.; Ruan, C.J.; Ding, G.J.; Mopper, S. Genetic relationships in a germplasm collection of Camellia japonica and Camellia oleifera using SSR analysis. Genet. Mol. Res. GMR 2017, 16, 16019526. [Google Scholar] [CrossRef]
- Miao, L.; Li, Q.; Sun, T.; Chai, S.; Wang, C.L.; Bai, L.Q.; Sun, M.T.; Li, Y.S.; Qin, X.; Zhang, Z.H.; et al. Sugars promote graft union development in the heterograft of cucumber onto pumpkin. Hortic. Res. 2021, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, M.M.; Kilpatrick, D.C.; Miedzybrodzka, M.B.; Gould, A.R. Cellular interactions during graft formation in plants, a recognition phenomenon? Symp. Soc. Exp. Biol. 1978, 32, 139–160. [Google Scholar]
- Aloni, B.; Karni, L.; Deventurero, G.; Levin, Z.; Cohen, R.; Katzir, N.; Lotan-Pompan, M.; Edelstein, M.; Aktas, H.; Turhan, E.; et al. Physiological and biochemical changes at the rootstock-scion interface in graft combinations between Cucurbita rootstocks and a melon scion. Horticult. Sci. Biotechnol. 2008, 83, 777–783. [Google Scholar] [CrossRef]
- Zhang, H.M.; Huang, D.F.; Ding, M.; Xue, W.X. Changes in three enzyme activities in the process of watermelon seedlings grafted with different ages of scion. Plant Physiol. J. 2005, 41, 302–304. [Google Scholar] [CrossRef]
- Chen, S.Y. Injury of membrane lipid peroxidation to plant cell. Plant Physiol. J. 1991, 27, 84–90. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Su, Y.; Han, F.Y.; Su, M.L.; Cui, S.M. The research of anatomical structures and three activity of ant oxidases’ change of grafted cucumber seeding. J. Inn. Mong. Agric. Univ. (Nat. Sci. Ed.) 2007, 28, 224–230. [Google Scholar]
- Chen, Z.; Zhao, J.T.; Qin, Y.H.; Hu, G.B. Study on the graft compatibility between ‘Jingganghongnuo’ and other litchi cultivars. Sci. Hortic. 2016, 199, 56–62. [Google Scholar] [CrossRef]
- Milien, M.; Renault-Spilmont, A.S.; Cookson, S.J.; Sarrazin, A.; Verdeil, J.L. Visualization of the 3D structure of the graft union of grapevine using X-ray tomography. Sci. Hortic. 2012, 144, 130–140. [Google Scholar] [CrossRef]
- Thomas, H.; Van den Broeck, L.; Spurney, R.; Sozzani, R.; Frank, M. Gene regulatory networks for compatible versus incompatible grafts identify a role for SlWOX4 during junction formation. Plant Cell 2021, 34, 535–556. [Google Scholar] [CrossRef]
- Wakiyama, Y. The Relationship between SPAD Values and Leaf Blade Chlorophyll Content throughout the Rice Development Cycle. Jpn. Agric. Res. Q. JARQ 2016, 50, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Zarrouk, O.; Gogorcena, Y.; Moreno, M.A.; Pinochet, J. Graft compatibility between peach cultivars and prunus rootstocks. HortScience 2006, 41, 1389–1394. [Google Scholar] [CrossRef]
- Ulas, F.; Fricke, A.; Stutzel, H. Leaf physiological and root morphological parameters of grafted tomato plants under drought stress conditions. Fresenius Environ. Bull. 2019, 28, 3423–3434. [Google Scholar]
- Habibi, F.; Liu, T.; Folta, K.; Sarkhosh, A. Physiological, biochemical, and molecular aspects of grafting in fruit trees. Hortic. Res. 2022, 9, uhac032. [Google Scholar] [CrossRef]
- Sharma, A.; Zheng, B. Molecular Responses during Plant Grafting and Its Regulation by Auxins, Cytokinins, and Gibberellins. Biomolecules 2019, 9, 397. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.A.; Terol, J.; Ibanez, V.; Lopez-Garcia, A.; Perez-Roman, E.; Borreda, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safaa, N.; Rabab, S.; Ramzi, M. Morphological and biochemical changes in two parsley varieties upon water stress. Physiol. Mol. Biol. Plants. 2012, 18, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.L.; Liu, X.B.; Liu, R.F.; Shan, L.N. Characterization and effects of two algicidal isolates on antioxidase activities of Chlorella pyrenoidosa. Environ. Prog. Sustain. Energy 2015, 34, 1647–1651. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Bian, W.J.; Bao, G.Z.; Qian, H.M.; Song, Z.W.; Qi, Z.M.; Zhang, M.Y.; Chen, W.W.; Dong, W.Y. Physiological response characteristics in medicago sativa under freeze-thaw and deicing salt stress. Water Air Soil Pollut. 2018, 229, 8. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Moreno, M.A.; Moing, A.; Lansac, M.; Gaudillere, J.P.; Salesses, G. Peach/myrobalan plum graft incompatibility in the nursery. J. Hortic. Sci. 2015, 68, 705–714. [Google Scholar] [CrossRef]
- Mosse, B.; Herrero, J. Studies on incompatibility between some pear and quince grafts. J. Hortic. Sci. 2015, 26, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, A. The Effects of Different Rootstocks on the Graft Success and Stion Development of Some Pear Cultivars. Int. J. Fruit Sci. 2021, 21, 932–944. [Google Scholar] [CrossRef]
- Almqvist, C. Interstock effects on topgraft vitality and strobili production after topgrafting in Pinus sylvestris. Can. J. For. Res. 2013, 43, 584–588. [Google Scholar] [CrossRef]
- Goldschmidt, E.E. Plant grafting: New mechanisms, evolutionary implications. Front. Plant Sci. 2014, 5, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Zhang, S.; Ren, H.; Zheng, X.; Qi, X. The Interspecific Grafting and Phylogenetic Analysis of Myrica cerifera and Myrica rubra. Linye Kexue/Sci. Silvae Sin. 2019, 55, 171–177. [Google Scholar] [CrossRef]
- Li, X.L.; Li, M.J.; Zhou, B.B.; Yang, Y.Z.; Zhang, J.; Wei, Q.P.; Zhang, J.K. Na+ efflux from apple dwarfing rootstocks is associated with high-salt resistance of their scions. J. Plant Growth Regul. 2020, 40, 2139–2147. [Google Scholar] [CrossRef]
- Mondragón-Valero, A.; Velázquez-Martí, B.; Salazar, D.M.; López-Cortés, I. Influence of fertilization and rootstocks in the biomass energy characterization of prunus dulcis (Miller). Energies 2018, 11, 1189. [Google Scholar] [CrossRef]
- Soumelidou, K.; Battey, N.H.; John, P.; Barnett, J.R. The Anatomy of the Developing Bud Union and its Relationship to Dwarfing in Apple. Ann. Bot. 1994, 74, 605–611. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Li, M.K.; Yao, J.J.; Zhou, Y.M.; Wang, Y.; Zhang, X.Z.; Li, W.; Wu, T.; Han, Z.H.; Xu, X.F.; et al. Root architecture characteristics of differing size-controlling rootstocks and the influence on the growth of ‘Red Fuji’ apple trees. Sci. Hortic. 2021, 281, 109959. [Google Scholar] [CrossRef]
- Camara Zapata, J.M.; Cerda, A.; Nieves, M. Interstock-induced mechanism of increased growth and salt resistance of orange (Citrus sinensis) trees. Tree Physiol. 2004, 24, 1109–1117. [Google Scholar] [CrossRef] [Green Version]
- Reig, G.; Carolina, F.; Mestre, L.; Jiménez, S.; Betrán, J.; Moreno, M. Horticultural, leaf mineral and fruit quality traits of two ‘Greengage’ plum cultivars budded on plum based rootstocks in Mediterranean conditions. Sci. Hortic. 2018, 232, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Moreno, M.A.; Gaudillere, J.P.; Moing, A. Protein and amino acid content in compatible and incompatible peach/plum grafts. J. Pomol. Hortic. Sci. 1994, 69, 955–962. [Google Scholar] [CrossRef]
- Dogra, K.; Kour, K.; Kumar, R.; Bakshi, P.; Kumar, V. Graft-incompatibility in horticultural crops. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1805–1820. [Google Scholar] [CrossRef]
- Assunção, M.; Pinheiro, J.; Cruz, S.; Brazão, J.; Queiroz, J.; Eiras Dias, J.E.; Canas, S. Gallic acid, sinapic acid and catechin as potential chemical markers of vitis graft success. Sci. Hortic. 2019, 246, 129–135. [Google Scholar] [CrossRef]
- Dan, T.; Jiang, X.; Gong, B.; Liu, T.; Yan, X.; Wu, K. Early selection of interstocks for improving grafting compatibility in diospyros kaki ‘Fuyu’. Sci. Silvae Sin. 2017, 53, 54–62. [Google Scholar] [CrossRef]
- Lidon, F.C.; Teixeira, M.G. Oxy radicals production and control in the chloroplast of Mn-treated rice. Plant Sci. 2000, 152, 7–15. [Google Scholar] [CrossRef]
- Schmid, P.; Feucht, W. Carbohydrates in the phloem of prunus-avium prunus-cerasus graftings and of homospecific controls. Angew. Bot. 1986, 60, 201–208. [Google Scholar]
- Trinchera, A.; Pandozy, G.; Rinaldi, S.; Crino, P.; Temperini, O.; Rea, E. Graft union formation in artichoke grafting onto wild and cultivated cardoon: An anatomical study. J. Plant Physiol. 2013, 170, 1569–1578. [Google Scholar] [CrossRef]
- Liu, C.J. Deciphering the Enigma of Lignification: Precursor Transport, Oxidation, and the Topochemistry of Lignin Assembly. Mol. Plant. 2012, 5, 304–317. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Guo, S.R.; Li, L.; An, Y.H.; Shu, S.; Sun, J. Proteomics analysis of compatibility and incompatibility in grafted cucumber seedlings. Plant Physiol. Biochem. 2016, 105, 21–28. [Google Scholar] [CrossRef]
- Markhart, A.H., III. Chilling injury: A review of possible causes. HortScience 1986, 21, 1329–1333. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Lv, Z.; Khanzada, A.; Huang, M.; Cai, J.; Zhou, Q.; Huo, Z.; Jiang, D. Effects of Cold and Salicylic Acid Priming on Free Proline and Sucrose Accumulation in Winter Wheat under Freezing Stress. J. Plant Growth Regul. 2022, 41, 2171–2184. [Google Scholar] [CrossRef]
| ‘Translocated’ Incompatibility Symptoms | ‘Localized’ Incompatibility Category |
|---|---|
| Leaf and wood yellowing and reddening, defoliation, tree vigor reduction, and death. Moreover, the tree body may have low SPAD values. | Category A: Perfect unions (the line of the union between bark and wood is hardly visible); Category B: Good unions (the bark and wood are continuous although the line of a union in the wood is often clearly distinguished by excessive ray formation); Category C: Unions with discontinuities in the bark (the bark tissues of rootstock and scion are separated by a dark brown layer of corky appearance); Category D: Unions showing vascular and wood discontinuities (the woody tissues of rootstock and scion are separated in many places by clusters of living, non-lignified parenchyma, whereas bark tissues are generally as in Category C); Category E: Observed breakage of the tree at the graft union in the nursery or orchard. Categories D and E were considered ‘incompatible’ unions because breakage might occur caused by mechanical damage or wind. |
| Treatment | Rootstock Diameter /mm | Interstock Diameter /mm | Scion Diameter /mm | Lower Graft Union Diameter /mm | Upper Graft Union Diameter /mm | Interstock/Rootstock Diameter | Scion/Interstock Diameter | Upper/Lower Graft Union Diameter |
|---|---|---|---|---|---|---|---|---|
| Ponkan | 17.08 ± 0.61 a | 8.73 ± 0.16 bc | 9.83 ± 0.44 a | 13.24 ± 1.13 bc | 11.32 ± 0.63 bc | 0.51 ± 0.05 c | 1.13 ± 0.06 a | 0.86 ± 0.04 ab |
| Shiranuhi | 11.97 ± 0.70 b | 7.60 ± 0.10 c | 5.91 ± 0.18 c | 12.58 ± 0.19 c | 11.07 ± 0.17 c | 0.64 ± 0.03 ab | 0.78 ± 0.01 b | 0.88 ± 0.02 ab |
| Harumi | 16.77 ± 1.01 a | 8.43 ± 0.63 bc | 7.51 ± 0.27 b | 15.67 ± 0.82 b | 10.22 ± 0.85 c | 0.50 ± 0.01 c | 0.90 ± 0.08 b | 0.66 ± 0.08 b |
| Tarocco | 18.00 ± 1.56 a | 10.18 ± 1.04 ab | 8.94 ± 0.18 a | 15.67 ± 1.26 b | 14.49 ± 0.48 ab | 0.56 ± 0.02 bc | 0.90 ± 0.11 b | 0.93 ± 0.07 a |
| Kumquat | 17.26 ± 1.15 a | 11.42 ± 0.52 a | 10.05 ± 0.66 a | 18.94 ± 0.70 a | 15.37 ± 1.95 a | 0.66 ± 0.02 a | 0.88 ± 0.03 b | 0.82 ± 0.14 ab |
| Treatment | Leaf Length /cm | Leaf Width /cm | Leaf Shape Index | Fresh Mass of 100 Leaves /g | Dry Mass of 100 Leaves /g | Dry/Fresh Quality | Water Content /% | SPAD Reading |
|---|---|---|---|---|---|---|---|---|
| Ponkan | 8.72 ± 1.04 bc | 3.33 ± 0.35 ab | 2.66 ± 0.24 b | 26.84 ± 0.48 c | 10.86 ± 0.09 c | 0.40 ± 0.00 bc | 59.52 ± 0.40 ab | 61.87 ± 1.85 c |
| Shiranuhi | 9.55 ± 0.28 ab | 3.07 ± 0.26 ab | 3.16 ± 0.29 ab | 28.96 ± 0.48 b | 11.21 ± 0.05 c | 0.39 ± 0.00 c | 61.26 ± 0.82 a | 70.02 ± 2.07 b |
| Harumi | 8.00 ± 0.46 c | 2.70 ± 0.24 b | 2.98 ± 0.12 ab | 29.86 ± 0.40 b | 13.29 ± 0.10 b | 0.45 ± 0.00 a | 55.49 ± 0.28 c | 66.42 ± 2.14 bc |
| Tarocco | 10.53 ± 0.30 a | 3.02 ± 0.11 b | 3.50 ± 0.07 a | 26.80 ± 0.51 c | 10.43 ± 0.02 d | 0.39 ± 0.00 bc | 61.04 ± 0.67 ab | 80.03 ± 0.03 a |
| Kumquat | 9.80 ± 0.56 ab | 3.80 ± 0.10 a | 2.58 ± 0.09 b | 35.68 ± 0.39 a | 14.50 ± 0.27 a | 0.41 ± 0.00 b | 59.36 ± 0.31 b | 76.12 ± 1.33 a |
| Rootstock Diameter | Interstock Diameter | Scion Diameter | Lower Graft Union Diameter | Upper Graft Union Diameter | SPAD Reading | |
|---|---|---|---|---|---|---|
| Rootstock diameter | 1 | |||||
| Interstock diameter | 0.709 ** | 1 | ||||
| Scion diameter | 0.663 ** | 0.649 ** | 1 | |||
| Lower graft union diameter | 0.402 | 0.651 ** | 0.426 | 1 | ||
| Upper graft union diameter | 0.552 * | 0.836 ** | 0.577 * | 0.455 | 1 | |
| SPAD reading | 0.289 | 0.554 * | 0.133 | 0.379 | 0.733 ** | 1 |
| Treatment | ‘Translocated’ Incompatibility Symptoms | ‘Localized’ Incompatibility Category |
|---|---|---|
| Ponkan | N | 1A, 5B |
| Shiranuhi | N | 4B, 2D |
| Harumi | Ab | 6B |
| Tarocco | N | 2A, 4B |
| Kumquat | N | 1A, 5B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Deng, L.; Huang, S.; Xiong, B.; Ihtisham, M.; Zheng, Z.; Zheng, W.; Qin, Z.; Zhang, M.; Sun, G.; et al. Genetic Relationship, SPAD Reading, and Soluble Sugar Content as Indices for Evaluating the Graft Compatibility of Citrus Interstocks. Biology 2022, 11, 1639. https://doi.org/10.3390/biology11111639
Wang T, Deng L, Huang S, Xiong B, Ihtisham M, Zheng Z, Zheng W, Qin Z, Zhang M, Sun G, et al. Genetic Relationship, SPAD Reading, and Soluble Sugar Content as Indices for Evaluating the Graft Compatibility of Citrus Interstocks. Biology. 2022; 11(11):1639. https://doi.org/10.3390/biology11111639
Chicago/Turabian StyleWang, Tie, Lijun Deng, Shengjia Huang, Bo Xiong, Muhammad Ihtisham, Zhendong Zheng, Wei Zheng, Zeyu Qin, Mingfei Zhang, Guochao Sun, and et al. 2022. "Genetic Relationship, SPAD Reading, and Soluble Sugar Content as Indices for Evaluating the Graft Compatibility of Citrus Interstocks" Biology 11, no. 11: 1639. https://doi.org/10.3390/biology11111639






