Feeding Ecology of Odontaster validus under Different Environmental Conditions in the West Antarctic Peninsula
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
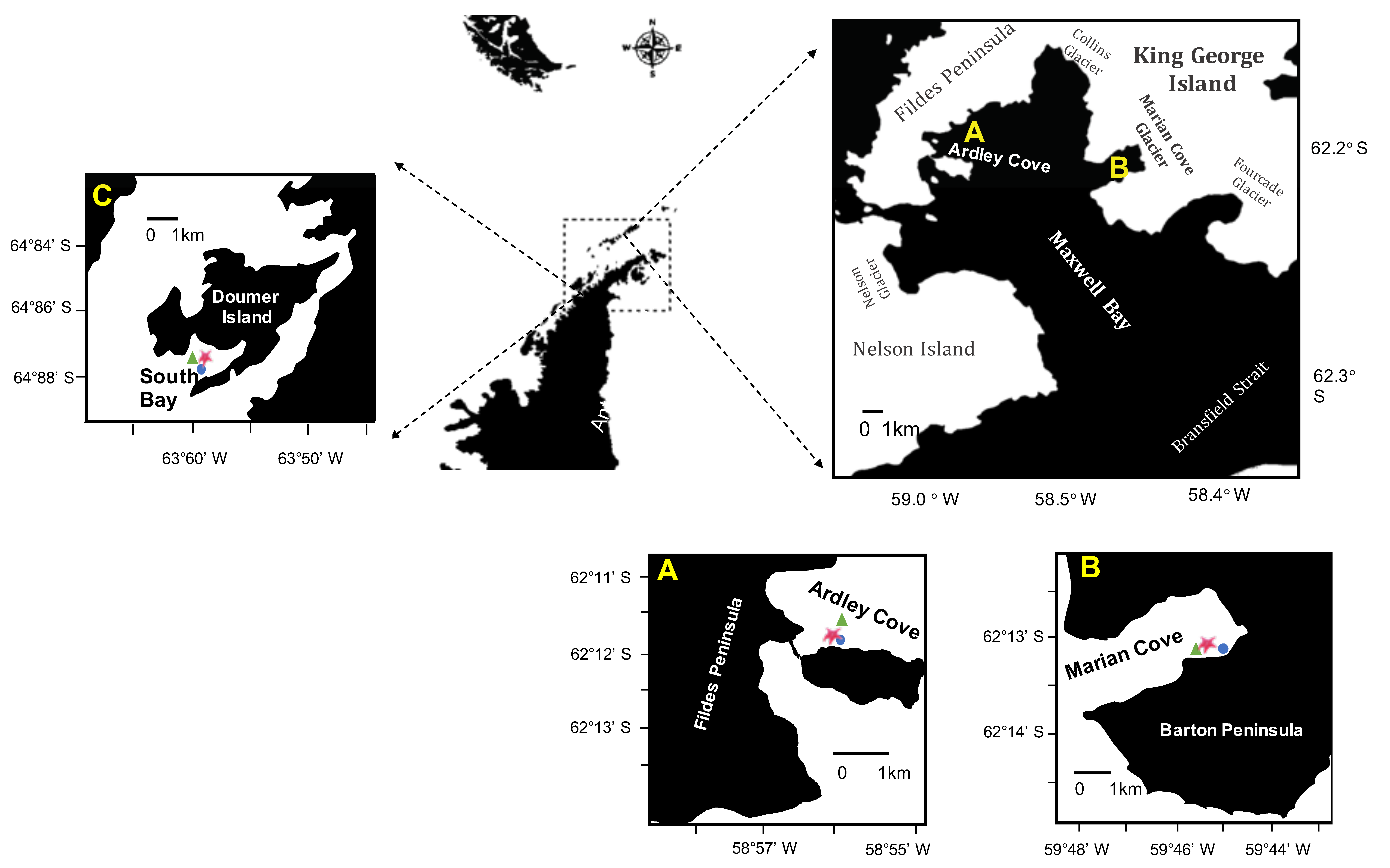
2.1. Study Sites
2.1.1. Marian Cove—High Activity of Glacier Meltwater and Ice Disturbance
2.1.2. Ardley Cove—Low Seasonal Sea Ice
2.1.3. South Bay—High Sea Ice Cover
2.2. Field Sampling
2.2.1. O. validus
2.2.2. Potential Prey
2.2.3. Suspended Particulate Organic Matter (POM)
2.3. Stable Isotope Analysis
2.4. Stomach Contents Analysis
2.5. Statistical Analysis
3. Results
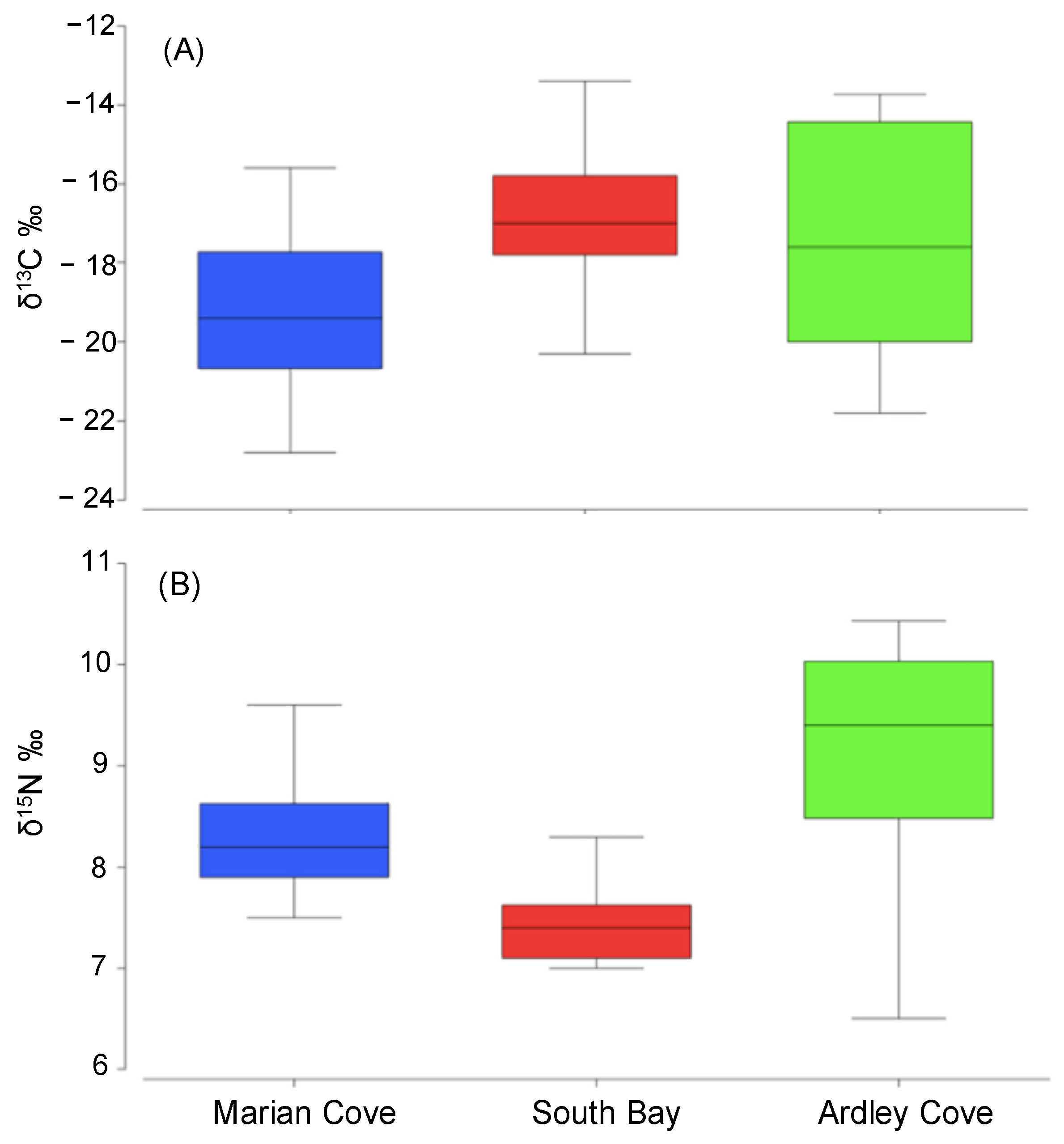
3.1. Divergence of O. validus δ15N–δ13C Values among Regions
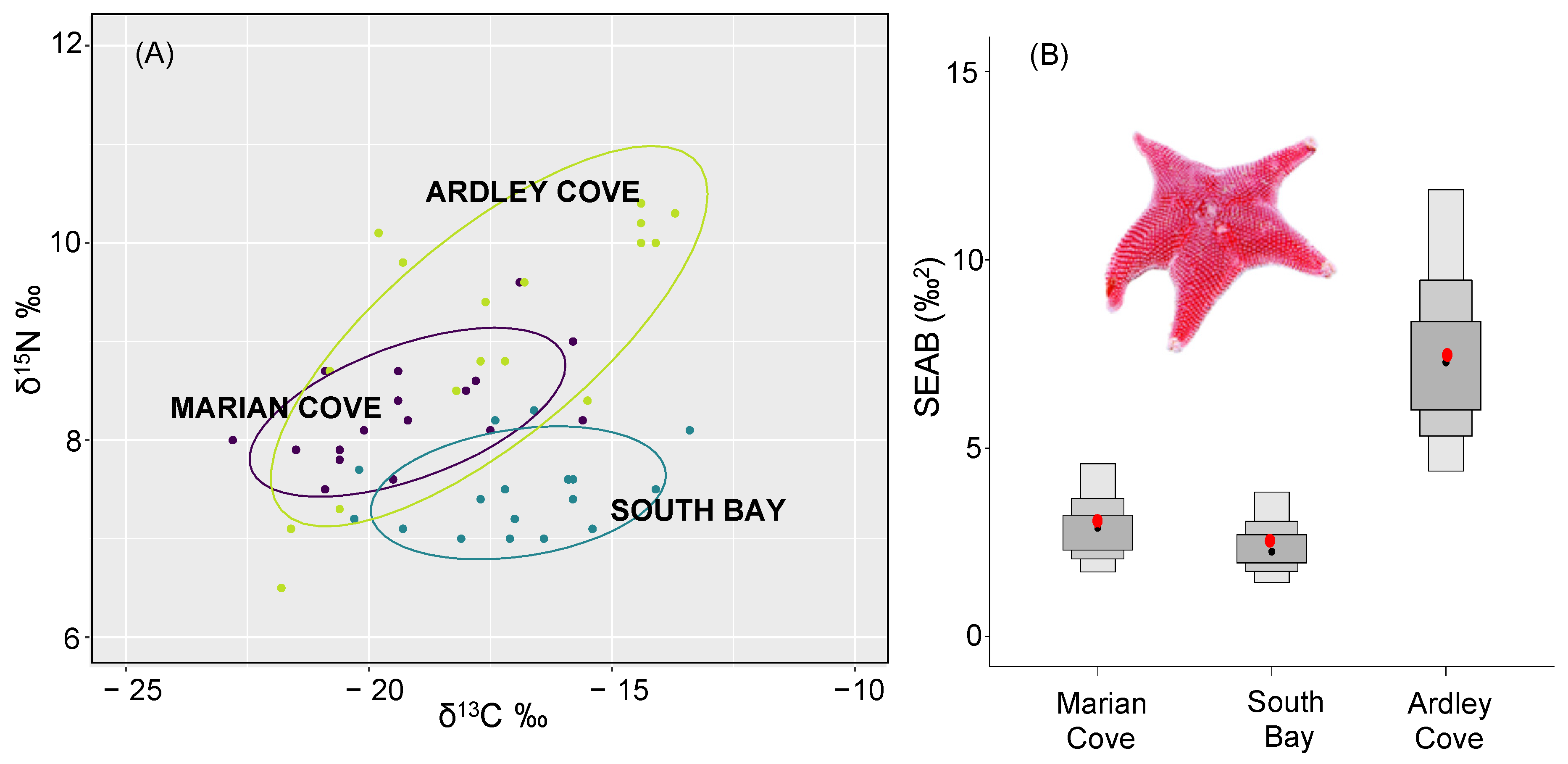
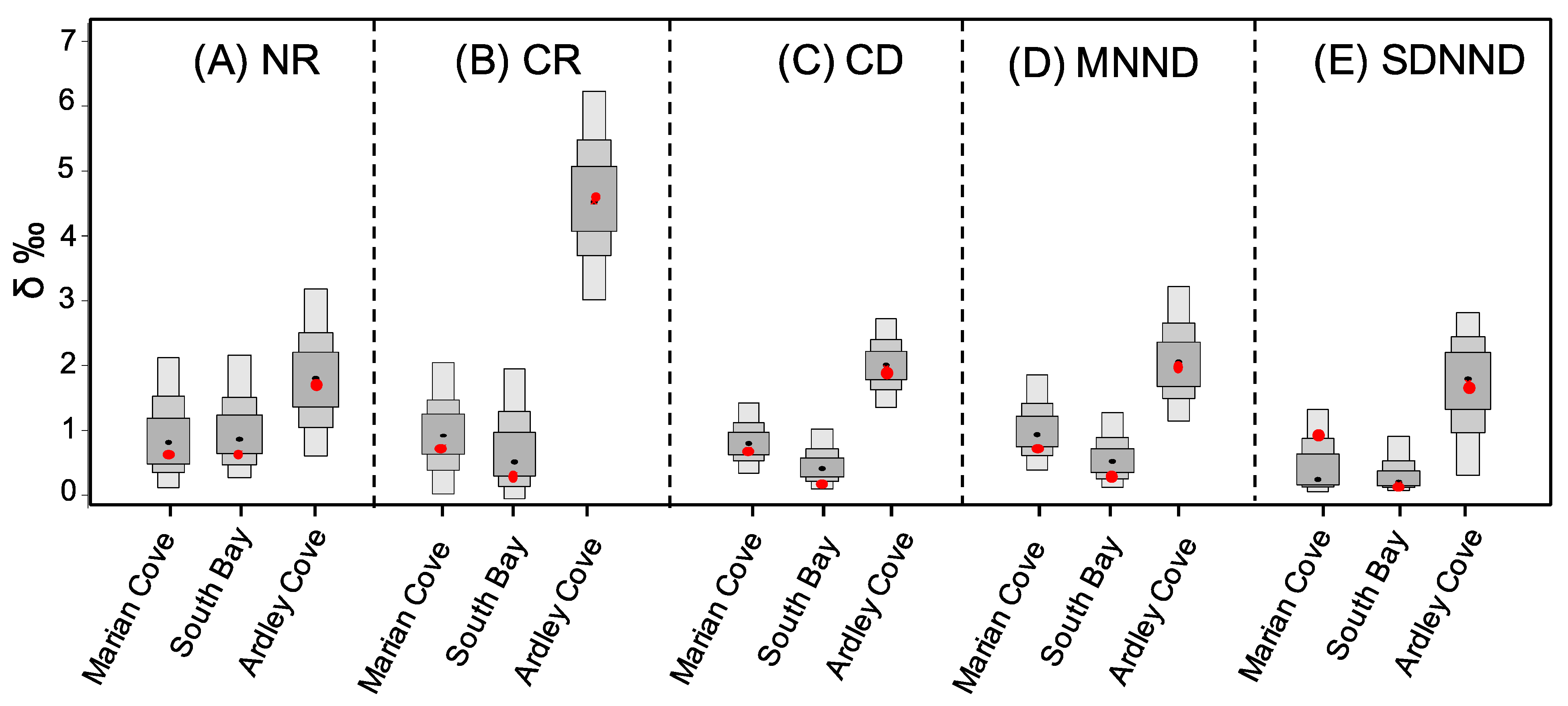
3.2. Isotopic Niche and Trophic Structure of O. validus Populations among Regions
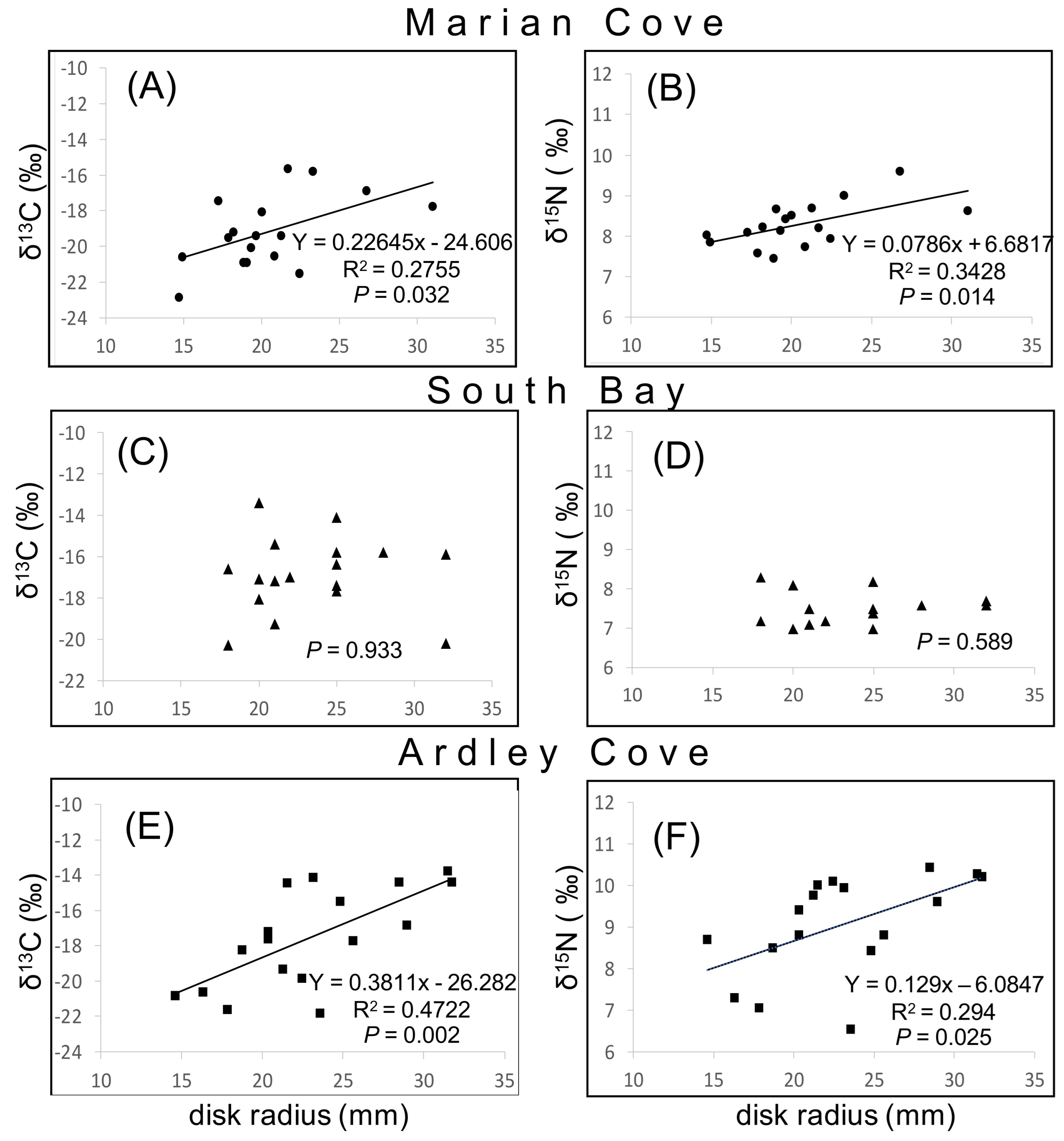
3.3. Exploring O. validus δ15N–δ13C Values with Disk Radius
3.4. Observing In Situ Cardiac Stomach Everted Contents of O. validus from Ardley Bay
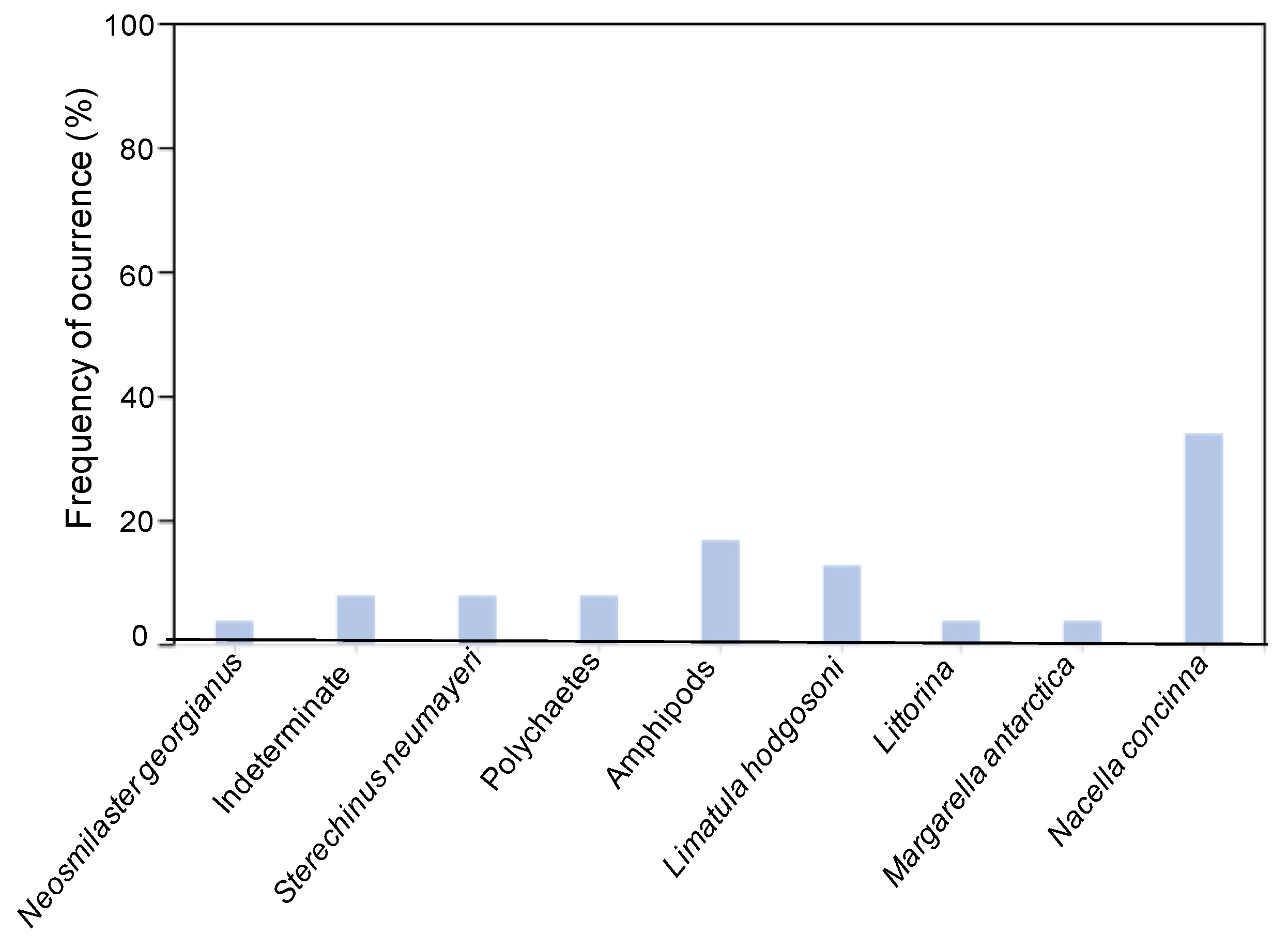
3.5. Relative Contribution of Four Potential Prey to O. validus Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearse, J.S. Slow developing demersal embryos and larvae of the Antarctic sea star Odontaster validus. Mar. Biol. 1969, 3, 110–116. [Google Scholar] [CrossRef]
- McClintock, J.B.; Pearse, J.S.; Bosch, I. Population structure an energetics of the shallow-water Antarctic sea star Odontaster validus in contrasting habitats. Mar. Biol. 1988, 99, 235–246. [Google Scholar] [CrossRef]
- Pearse, J.S. Reproductive periodicities in several contrasting populations of Odontaster validus Koehler, a common Antarctic asteroid. Biology of the Antarctic Seas II. Antarct. Res. Ser. 1965, 5, 39–85. [Google Scholar]
- Stanwell-Smith, D.; Clarke, A. Seasonality of reproduction in the cushion star Odontaster validus at Signy Island, Antarctica. Mar. Biol. 1998, 131, 479–487. [Google Scholar] [CrossRef]
- Dayton, P.K.; Robilliard, G.A.; Paine, R.T.; Dayton, L.B. Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecol. Monogr. 1974, 44, 105–128. [Google Scholar] [CrossRef]
- Dearborn, J.H. Foods and feeding characteristics of Antarctic asteroids and ophiuroids. In Proceedings of the 3rd SCAR Symposium on Antarctic Biology, Washington, DC, USA, 26–30 August 1974; pp. 293–326. [Google Scholar]
- Zamorano, J.H. Zonación y biomasa de la macrofauna bentónica en Bahía South, Archipiélago de Palmer, Antártica. INACH Serie Científica 1973, 20, 27–38. [Google Scholar]
- Brueggeman, P. Underwater Field Guide to Ross Islandand McMurdo Sound, Antarctica. Echinodermata—Asteroidea. 1998. Available online: http://www.peterbrueggeman.com/nsf/fguide/ (accessed on 2 October 2022).
- McClintock J Trophic biology of Antarctic shallow-water echinoderms. Mar. Ecol. Prog. Ser. 1994, 111, 191–202. [CrossRef]
- Núñez-Pons, L.; Work, T.M.; Angulo-Preckler, C.; Moles, J.; Avila, C. Exploring the pathology of an epidermal disease affecting a circum-Antarctic sea star. Sci. Rep. 2018, 8, 11353. [Google Scholar] [CrossRef]
- Peck, L.S.; Webb, K.E.; Clark, M.S.; Miller, A.; Hill, T. Temperature limits to activity, feeding and metabolism in the Antarctic starfish Odontaster validus. Mar. Ecol. Prog. Ser. 2008, 381, 181–189. [Google Scholar] [CrossRef]
- Kidawa, A.; Potocka, M.; Janecki, T. The effects of temperature on the behaviour of the Antarctic sea star Odontaster validus. Pol. Polar Res. 2010, 31, 273–284. [Google Scholar] [CrossRef]
- Morley, S.A.; Martin, S.M.; Bates, A.E.; Clark, M.S.; Ericson, J.; Lamare, M.; Peck, L.S. Spatial and temporal variation in the heat tolerance limits of two abundant Southern Ocean invertebrates. Mar. Ecol. Prog. Ser. 2012, 450, 81–92. [Google Scholar] [CrossRef]
- Agüera, A.; Collard, M.; Jossart, Q.; Moreau, C.; Danis, B. Parameter Estimations of Dynamic Energy Budget (DEB) Model over the Life History of a Key Antarctic Species: The Antarctic Sea Star Odontaster validus Koehler, 1906. PLoS ONE 2015, 10, e0140078. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.N.; Danis, B.; Dubois, P.; Eleaume, M.; Fournier, J.; Gallut, C.; Jane, P.; Lepoint, G. Increased sea ice cover alters food web structure in east Antarctica. Sci. Rep. 2019, 9, 8062. [Google Scholar] [CrossRef] [PubMed]
- Le Bourg, B.; Kuklinski, P.; Balazy, P.; Lepoint, G.; Michel, L.N. Interactive effects of body size and environmental gradient on the trophic ecology of sea stars in an Antarctic fjord. Mar. Ecol. Prog. Ser. 2021, 674, 189–202. [Google Scholar] [CrossRef]
- Van den Hoff, J.; McMahon, C.R.; Simpkins, G.R.; Hindell, M.A.; Alderman, R.; Burton, H.R. Bottom-up regulation of a pole-ward migratory predator population. Proc. R. Soc. B 2014, 281, 20132842. [Google Scholar] [CrossRef]
- Baum, J.K.; Worm, B. Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 2009, 78, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Thurber, A.; Hammerstrom, K.; Conlan, K. Seastar response to organic enrichment in an oligotrophic polar habitat. J. Exp. Mar. Biol. Ecol. 2007, 346, 66–75. [Google Scholar] [CrossRef]
- Barros, N.B.; Clarke, M.R. Diet. In Encyclopedia of Marine Mammals; Perrin, W.F., Wursig, B., Thewissen, J.G.M., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 323–327. [Google Scholar]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Evol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Wada, E.; Mizutani, H.; Minagawa, M. The use of stable isotopes for food web analysis. Crit. Rev. Food Sci. Nutr. 1991, 30, 361–372. [Google Scholar] [CrossRef]
- Montoya, J.P. Natural abundance of 15N in marine planktonic ecosystems. In Stable Isotopes in Ecology and Environmental Science, 2nd ed.; Michener, R., Lajtha, K., Eds.; Blackwell Publishing Ltd.: Malden, MA, USA, 2007; pp. 176–201. [Google Scholar]
- Graham, B.S.; Koch, P.L.; Newsome, S.D.; McMahon, K.W.; Aurioles, D. Using Isoscapes to Trace the Movements and Foraging Behavior of Top Predators in Oceanic Ecosystems. In Isoscapes; West, J., Bowen, G., Dawson, T., Tu, K., Eds.; Springer: Dordrecht, The Nederlands, 2010. [Google Scholar]
- Caut, S.; Angulo, E.; Courchamp, F. Variation in discrimination factors (∆15N and ∆13C): The effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Bearhop, S.; Adams, C.E.; Waldron, S.; Fuller, R.A.; Macleod, H. Determining trophic niche width: A novel approach using stable isotope analysis. J. Anim. Ecol. 2004, 73, 1007–1012. [Google Scholar] [CrossRef]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—stable isotope Bayesian ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Newsome, S.D.; del Rio Martinez, C.; Bearhop, S.; Phillips, D.L. A niche for isotopic ecology. Front. Ecol. Environ. 2007, 5, 429–436. [Google Scholar] [CrossRef]
- Layman, C.A.; Arrington, D.A.; Montaña, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Colwell, S.R.; Marshall, G.J.; Lachlan-Cope, T.A.; Carleton, A.M.; Jones, P.D.; Lagun, V.; Reid, P.A.; Lagovkina, S. Antarctic climate change during the last 50 years. Int. J. Climatol. 2005, 25, 279–294. [Google Scholar] [CrossRef]
- Stammerjohn, S.; Massom, R.; Rind, D.; Martinson, D. Regions of rapid sea ice change: An inter-hemispheric seasonal comparison. Geophys. Res. Lett. 2012, 39, L06501. [Google Scholar] [CrossRef]
- Sahade, R.; Lagger, C.; Torre, L.; Momo, F.; Monien, P.; Schloss, I.; Barnes, D.K.A.; Servetto, N.; Tarantelli, S.; Tatián, M.; et al. Climate change and glacier retreat drive shifts in an Antarctic benthic ecosystem. Sci. Adv. 2015, 1, e1500050. [Google Scholar] [CrossRef]
- Vaughan, D.G.; Marshall, G.J.; Connolley, W.M.; Parkinson, C.; Mulvaney, R.; Hodgson, D.A.; King, J.C.; Pudsey, C.J.; Turner, J. Recent rapid regional climate warming on the Antarctic Peninsula. Clim. Chang. 2003, 60, 243–274. [Google Scholar] [CrossRef]
- Meredith, M.P.; King, J.C. Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys. Res. Lett. 2005, 32, L19604. [Google Scholar] [CrossRef]
- Kim, D.U.; Khim, J.S.; Ahn, I.Y. Patterns, drivers and implications of ascidian distributions in a rapidly deglaciating fjord, King George Island, West Antarctic Peninsula. Ecol. Indic. 2021, 125, 107467. [Google Scholar] [CrossRef]
- Shichao, T.; Haiyan, J.; Shengquan, G.; Yanpei, Z.; Yang, Z.; Bin, W.; Jianfang, C. Sources and distribution of particulate organic carbon in Great Wall Cove and Ardley Cove, King George Island, West Antarctica. Adv. Polar Sci. 2015, 26, 55–62. [Google Scholar]
- Krause, J.; Hopwood, M.J.; Höfer, J.; Krisch, S.; Achterberg, E.P.; Alarcón, E.; Carroll, D.; González, H.E.; Juul-Pedersen, T.; Liu, T.; et al. Trace Element (Fe, Co, Ni and Cu) Dynamics Across the Salinity Gradient in Arctic and Antarctic Glacier Fjords. Front. Earth Sci. 2021, 9, 725279. [Google Scholar] [CrossRef]
- Cárdenas, C.A.; González-Aravena, M.; Santibañez, P.A. The importance of local settings: Within-year variability in seawater temperature at South Bay, Western Antarctic Peninsula. PeerJ 2018, 6, e4289. [Google Scholar] [CrossRef]
- Höfer, J.; Giesecke, R.; Hopwood, M.J.; Carrera, V.; Alarcón, E.; González, H.E. The role of water column stability and wind mixing in the production/export dynamics of two bays in the Western Antarctic Peninsula. Prog. Oceanogr. 2019, 174, 105–116. [Google Scholar] [CrossRef]
- Bae, H.; Ahn, I.Y.; Park, J.; Song, S.J.; Noh, J.; Kim, H.; Khim, J.S. Shift in polar benthic community structure in a fast retreating glacial area of Marian Cove, West Antarctica. Sci. Rep. 2021, 11, 241. [Google Scholar] [CrossRef]
- Yoon, H.; Park, B.K.; Domack, E.W.; Kim, Y. Distribution and dispersal pattern of suspended particulate matter in Maxwell Bay and its tributary, Marian Cove, in the South Shetland Islands, West Antarctica. Mar. Geol. 1998, 152, 261–275. [Google Scholar] [CrossRef]
- Ahn, I.Y.; Chung, K.H.; Choi, H.J. Influence of glacial runoff on baseline metal accumulation in the Antarctic limpet Nacella concinna from King George Island. Mar. Pollut. Bull. 2004, 49, 119–127. [Google Scholar] [CrossRef]
- Yoo, K.C.; Lee, M.K.; Yoon, H.I.; Lee, Y.I.; Kang, C.Y. Hydrography of Marian Cove, King George Island, West Antarctica: Implications for ice-proximal sedimentation during summer. Antarct. Sci. 2015, 27, 185–196. [Google Scholar] [CrossRef]
- Ahn, I.-Y.; Moon, H.-W.; Jeon, M.; Kang, S.-H. First record of massive blooming of benthic diatoms and their association with megabenthic filter feeders on the shallow seafloor of an Antarctic Fjord: Does glacier melting fuel the bloom? Ocean Sci. J. 2016, 51, 273–279. [Google Scholar] [CrossRef]
- Ha, S.-Y.; Ahn, I.-Y.; Moon, H.-W.; Choi, B.; Shin, K.-H. Tight trophic association between benthic diatom blooms and shallow-water megabenthic communities in a rapidly deglaciated Antarctic fjord. Estuar. Coast. Shelf Sci. 2019, 218, 258–267. [Google Scholar] [CrossRef]
- Valdivia, N.; Diaz, M.J.; Holtheuer, J.; Garrido, I.; Huovinen, P.; Gomez, I. Up, down, and all around: Scale dependent spatial variation in rocky-shore communities of Fildes Peninsula, King George Island, Antarctica. PLoS ONE 2014, 23, e100714. [Google Scholar] [CrossRef] [PubMed]
- Aghmich, A.; Taboada, S.; Toll, L.; Ballesteros, M. First assessment of the rocky intertidal communities of Fildes Bay, King George Island (South Shetland Islands, Antarctica). Polar Biol. 2016, 39, 189–198. [Google Scholar] [CrossRef]
- Schloss, I.R.; Wasilowska, A.; Dumont, D.; Almandoz, G.O.; Hernando, M.P.; Michaud-Tremblay, C.-A.; Saravia, L.; Rzepecki, M.; Monien, P.; Monien, D.; et al. On the Phytoplankton Bloom in Coastal Waters of Southern King George Island (Antarctica) in January 2010: An Exceptional Feature? Limnol. Oceanogr. 2014, 59, 195–210. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.H.; Lim, J.H.; Jeong, J.H.; Heo, J.M.; Kim, I.N. Distribution and Control of Bacterial Community Composition in Marian Cove Surface Waters, King George Island, Antarctica during the Summer of 2018. Microorganisms 2020, 8, 1115. [Google Scholar] [CrossRef]
- Rovelli, L.; Attard, K.M.; Cárdenas, C.A.; Ronnie, N.G. Benthic primary production and respiration of shallow rocky habitats: A case study from South Bay (Doumer Island, Western Antarctic Peninsula). Polar Biol. 2019, 42, 1459–1474. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Deniro, M.J.; Epstein, S. Influence of diet on distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach contents analysis: A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley Statsref: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; University of Auckland: Auckland, New Zealand, 2017. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial, 3rd ed.; PRIMER-E: Plymouth, UK, 2015. [Google Scholar]
- Jackson, M.C.; Donohue, I.; Jackson, A.L.; Britton, J.R.; Harper, D.M.; Grey, J. Population-level metrics of trophic structure based on stable isotopes and their application to invasion ecology. PLoS ONE 2012, 7, e31757. [Google Scholar] [CrossRef]
- Parnell, A.C. Simmr: A Stable Isotope Mixing Model, R Package Version 0.3; 2016. Available online: https://cran.r-project.org/package=simmr (accessed on 7 September 2022).
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source partitioning using stable isotopes: Coping with too much variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef] [PubMed]
- McCutchan, J.H.; Lewis, W.M.; Kendall, C.; McGrath, C.C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- Ahn, I.Y.; Elias-Piera, F.; Ha, S.Y.; Rossi, S.; Kim, D.U. Seasonal Dietary Shifts of the Gammarid Amphipod Gondogeneia antarctica in a Rapidly Warming Fjord of the West Antarctic Peninsula. J. Mar. Sci. Eng. 2021, 9, 1447. [Google Scholar] [CrossRef]
- Choy, E.J.; Park, H.; Kim, J.H.; Ahn, I.Y.; Kang, C.K. Isotopic shift for defining habitat exploitation by the Antarctic limpet Nacella concinna from rocky coastal habitats (Marian Cove, King George Island). Estuar Coast. Shelf Sci. 2011, 92, 339–346. [Google Scholar] [CrossRef]
- Pasotti, F.; Saravia, L.A.; De Troch, M.; Tarantelli, M.S.; Sahade, R.; Vanreusel, A. Benthic trophic interactions in an Antarctic shallow water ecosystem affected by recent glacier retreat. PLoS ONE 2015, 10, e0141742. [Google Scholar] [CrossRef] [PubMed]
- Chown, S.L.; Clarke, A.; Fraser, C.I.; Cary, S.C.; Moon, K.L.; McGeoch, M.A. The changing form of Antarctic biodiversity. Nature 2015, 522, 431–438. [Google Scholar] [CrossRef]
- Clark, G.F.; Stark, J.S.; Johnston, E.; Runcie, J.W.; Goldsworthy, P.M.; Raymond, B.; Riddle, M.J. Light-driven tipping points in polar ecosystems. Glob. Chang. Biol. 2013, 19, 3749–3761. [Google Scholar] [CrossRef]
- Rossi, L.; Sporta Caputi, S.; Calizza, E.; Careddu, G.; Oliverio, M.; Schiaparelli, S.; Costantini, M.L. Antarctic food web architecture under varying dynamics of sea ice cover. Sci. Rep. 2019, 9, 12454. [Google Scholar] [CrossRef]
- Zenteno, L.; Cárdenas, L.; Valdivia, N.; Gómez, I.; Höfer, J.; Garrido, I.; Pardo, L.M. Unraveling the multiple bottom-up supplies of an Antarctic nearshore benthic community. Prog. Oceanogr. 2019, 174, 55–63. [Google Scholar] [CrossRef]
- Bathmann, U.; Fischer, G.; Müller, P.J.; Gerdes, D. Short-term variations in particulate matter sedimentation off Kapp Norvegia, Weddell Sea, Antarctica: Relation to water mass advection, ice cover, plankton biomass and feeding activity. Polar Biol. 1991, 11, 185–195. [Google Scholar] [CrossRef]
- Henley, S.F.; Annett, A.L.; Ganeshram, R.S.; Carson, D.S.; Weston, K.; Crosta, X.; Tait, A.; Dougans, J.; Fallick, A.E.; Clarke, A. Factors influencing the stable carbon isotopic composition of suspended and sinking organic matter in the coastal Antarctic sea ice environment. Biogeosciences 2012, 9, 1137–1157. [Google Scholar] [CrossRef]
- Pimm, S.L.; Lawton, J.H. Are food webs divided into compartments? J. Anim. Ecol. 1980, 49, 879–898. [Google Scholar] [CrossRef]
- Kratina, P.; Lecraw, R.M.; Ingram, T.; Anholt, B.R. Stability and persistence of food webs with omnivory: Is there a general pattern? Ecosphere 2012, 3, 50. [Google Scholar] [CrossRef]
- McCann, K.; Hastings, A. Re-evaluating the omnivory-stability relationship in food webs. Proc. R. Soc. B 1997, 264, 1249–1254. [Google Scholar] [CrossRef]
- Holyoak, M.; Sachdev, S. Omnivory and the stability of simple food webs. Oecologia 1998, 117, 413–419. [Google Scholar] [CrossRef]
- Fox, M.D.; Williams, G.J.; Johnson, M.D.; Radice, V.Z.; Zgliczynski, B.J.; Kelly, E.L.A.; Rohwer, F.L.; Sandin, S.A.; Smith, J.E. Gradients in primary production predict trophic strategies of mixotrophic corals across spatial scales. Curr. Biol. 2018, 28, 3355–3363. [Google Scholar] [CrossRef]
- Smale, D.A.; Barnes, D.K.A.; Fraser, K.P.P.; Peck, L.S. Benthic community response to iceberg scouring at an intensely disturbed shallow water site at Adelaide Island, Antarctica. Mar. Ecol. Progr. Ser. 2008, 355, 85–94. [Google Scholar] [CrossRef]
- Gutt, J. On the direct impact of ice on marine benthic communities, a review. Polar Biol. 2001, 24, 553–564. [Google Scholar] [CrossRef]
- Dayton, P.K. Polar benthos. In Polar Oceanography, Part B, Chemistry, Biology and Geology; Smith, W.O., Ed.; Academic Press: New York, NY, USA, 1990; pp. 631–685. [Google Scholar]

| Maxwell Bay | South Bay | ||
|---|---|---|---|
| Marian Cove | Ardley Cove | ||
| Salinity gradient (psu) | 33.8–34.1 [35] | 33.97–34.14 [36] | 34.2–34.7 [37] |
| Seawater temperature (°C) | −1.8–1.5 [35] | −1.4–1.8 [36] | −1.7–3.0 [38] |
| Physical disturbances (runoff) | High [35] | Low [36] | Low [37] |
| Total chlorophyll maxima (mg Chl-a m−3) | 29.2 [39] | 19.7 [39] | |
| Annual sea ice extent | June–July [37] | June–November [37] | |
| PERMANOVA Test | |||
| F | p | ||
| δ15N | 16.695 | 0.001 | |
| δ13C | 4.5288 | 0.016 | |
| Post Hoc Pairwise Comparisons | |||
| Group | t | p | |
| δ15N | Marian Cove, South Bay | 5.0445 | 0.001 |
| Marian Cove, Ardley Cove | 2.3037 | 0.034 | |
| South Bay, Ardley Cove | 5.1102 | 0.001 | |
| δ13C | Marian Cove, South Bay | 3.3621 | 0.002 |
| Marian Cove, Ardley Cove | 2.0963 | 0.037 | |
| South Bay, Ardley Cove | 0.6094 | 0.560 | |
| δ13C Range (CR) | |||
| Marian Cove | South Bay | Ardley Cove | |
| Marian Cove | 0.8870 | 0.0088 | |
| South Bay | 0.1130 | 0.0018 | |
| Ardley Cove | 0.9913 | 0.9983 | |
| δ15N Range (NR) | |||
| Marian Cove | South Bay | Ardley Cove | |
| Marian Cove | 0.5418 | 0.2105 | |
| South Bay | 0.45825 | 0.1853 | |
| Ardley Cove | 0.7895 | 0.81475 | |
| Mean Distance to Centroid (CD) | |||
| Marian Cove | South Bay | Ardley Cove | |
| Marian Cove | 0.8373 | 0.0009 | |
| South Bay | 0.1628 | ||
| Ardley Cove | 0.9913 | 0.9968 | 0.0033 |
| Mean Nearest Neighbor Distance (MNND) | |||
| Marian Cove | South Bay | Ardley Cove | |
| Marian Cove | 0.7988 | 0.0365 | |
| South Bay | 0.2013 | 0.0085 | |
| Ardley Cove | 0.9635 | 0.9915 | |
| Standard Deviation of the Nearest Neighbor Distance (SDNND) | |||
| Marian Cove | South Bay | Ardley Cove | |
| Marian Cove | 0.6910 | 0.0943 | |
| South Bay | 0.3090 | 0.0470 | |
| Ardley Cove | 0.9058 | 0.9530 | |
| Standard Ellipse Area Bayesian Estimations (SEAB) | |||
| Marian Cove | South Bay | Ardley Cove | |
| Marian Cove | 0.6935 | 0.0028 | |
| South Bay | 0.3065 | 0.0013 | |
| Ardley Cove | 0.9973 | 0.9988 | |
| Study Site | Species/Source | δ13C (‰) | δ15N (‰) | N |
|---|---|---|---|---|
| Marian Cove | P. decipiens [61] | −23.1 ± 0.9 | 3.3 ± 0.8 | 2 |
| G. antarctica [61] | −19.2 ± 0.4 | 6.5 ± 0.4 | 6 | |
| N. concinna [62] | −16.5 ± 1.5 | 5.0 ± 0.6 | 27 | |
| Suspended POM [62] | −24.8 ± 1.2 | 3.3 ± 0.3 | 2 | |
| O. validus | −19.2 ± 2.0 | 8.3 ± 0.5 | 17 | |
| South Bay | P. decipiens | −20.6 ± 0.4 | 4.5 ± 0.3 | 5 |
| G. antarctica | −18.8 ± 0.6 | 5.1 ± 0.4 | 5 | |
| N. concinna | −14.1 ± 0.4 | 6.6 ± 0.2 | 5 | |
| Suspended POM | −20.4 ± 1.2 | 3.3 ± 0.8 | 4 | |
| O. validus | −16.9 ± 1.9 | 7.5 ± 0.4 | 17 | |
| Ardley Cove | P. decipiens | −18.5 ± 0.5 | 2.4 ± 0.0 | 5 |
| G. antarctica | −19.5 ± 0.2 | 5.8 ± 0.3 | 5 | |
| N. concinna | −13.9 ± 0.7 | 6.9 ± 0.2 | 5 | |
| Suspended POM | −19.8 ± 0.1 | 2.4 ± 0.0 | 2 | |
| O. validus | −17.5 ± 2.8 | 9.1 ± 1.2 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenteno-Devaud, L.; Aguirre-Martinez, G.V.; Andrade, C.; Cárdenas, L.; Pardo, L.M.; González, H.E.; Garrido, I. Feeding Ecology of Odontaster validus under Different Environmental Conditions in the West Antarctic Peninsula. Biology 2022, 11, 1723. https://doi.org/10.3390/biology11121723
Zenteno-Devaud L, Aguirre-Martinez GV, Andrade C, Cárdenas L, Pardo LM, González HE, Garrido I. Feeding Ecology of Odontaster validus under Different Environmental Conditions in the West Antarctic Peninsula. Biology. 2022; 11(12):1723. https://doi.org/10.3390/biology11121723
Chicago/Turabian StyleZenteno-Devaud, Lisette, Gabriela V. Aguirre-Martinez, Claudia Andrade, Leyla Cárdenas, Luis Miguel Pardo, Humberto E. González, and Ignacio Garrido. 2022. "Feeding Ecology of Odontaster validus under Different Environmental Conditions in the West Antarctic Peninsula" Biology 11, no. 12: 1723. https://doi.org/10.3390/biology11121723
APA StyleZenteno-Devaud, L., Aguirre-Martinez, G. V., Andrade, C., Cárdenas, L., Pardo, L. M., González, H. E., & Garrido, I. (2022). Feeding Ecology of Odontaster validus under Different Environmental Conditions in the West Antarctic Peninsula. Biology, 11(12), 1723. https://doi.org/10.3390/biology11121723





