Genetic Stability Assessment of Six Cryopreserved Strawberry (Fragaria × ananassa Duch.) Accessions by Phenotypic and Molecular Studies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Material
2.2. Cryopreservation Using Droplet Vitrification
2.3. Acclimatization of Regrown Plants and Evaluation of Vegetative Characteristics
2.4. Evaluation of Fruit Characteristics and Quality Traits under Greenhouse Conditions
2.5. Data Analysis
2.6. Detection of Genetic Variation Using ISSR
2.7. Sequencing Analysis
3. Results
3.1. Greenhouse Performance
3.2. DNA Analysis Using ISSR Marker
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biswas, M.K.; Dutt, M.; Roy, U.K.; Islam, R.; Hossain, M. Development and evaluation of in vitro somaclonal variation in strawberry for improved horticultural traits. Sci. Hortic. 2009, 122, 409–416. [Google Scholar] [CrossRef]
- Keiko, O.; Shigeru, A.; Hiroshi, A. Effect of cytokinin on strawberry [Fragaria] plantlets micropropagated by axillary buds. Bull Nara Prefect Agric. Exp. Stat. 2003, 34, 15–24. [Google Scholar]
- Kumar, M.B.; Barker, R.E.; Reed, B.M. Morphological and molecular analysis of genetic stability in micropropagated Fragaria ananassa cv. pocahontas. In Vitro Cell. Dev. Biol. Plant 1999, 35, 254–258. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, S.H.; Chung, M.Y.; Park, S.K.; Kim, C.K. In vitro propagation method for production of morphologically and genetically stable plants of different strawberry cultivars. Plant Mehods 2019, 15, 36–45. [Google Scholar] [CrossRef]
- Nehra, N.S.; Kartha, K.K.; Stushnott, C.; Giles, K.L. The influence of plant growth regulator concentrations and callus age on somaclonal variation in callus culture regenerants of strawberry. Plant Cell Tissue Organ Cult. 1992, 29, 257–268. [Google Scholar] [CrossRef]
- Popescu, A.N.; Isac, V.S.; Coman, M.S. MSR. Somaclonal variation in plants regenerated by organogenesis from callus cultures of strawberry (Fragaria ananassa). Acta Hortic. 1997, 439, 89–96. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Kim, H.; Popova, E.; Shin, D.; Yi, J.; Kim, C.H.; Lee, J.; Yoon, M.; Engelmann, F. Cryobanking of Korean allium germplasm collections: Results from a 10 year experience. CryoLetters 2012, 33, 45–57. [Google Scholar]
- Hassan, S.; Bhat, K.M.; Jan, A.; Mehraj, S.; Wani, S.A.; Khanday, M.U.D.; Bisati, I.A. Managing genetic resources in temperate fruit crops. Econ. Aff. 2018, 63, 987–996. [Google Scholar] [CrossRef]
- Kaviani, B.; Kulus, D. Cryopreservation of endangered ornamental plants and fruit crops from tropical and subtropical regions. Biology 2022, 11, 847. [Google Scholar] [CrossRef]
- Yi, J.; Balaraju, K.; Baek, H.; Yoon, M.; Kim, H.; Lee, Y. A successful regeneration from shoot tips of Chrysanthemum morifolium (Ramat.) following cryopreservation by droplet-vitrification. Korean J. Plant Res. 2018, 31, 675–683. [Google Scholar]
- Bae, J.; Lee, S.; Song, J.; Lee, J.; Yoon, M.; Yi, J.; Kim, H.; Lee, Y. Efficient cryopreservation of in vitro grown shoot tips of strawberry (Fragaria × ananassa Duch.) germplasm using droplet-vitrification. Korean J. Plant Res. 2021, 34, 600–607. [Google Scholar]
- Halmagyi, A.; Deliu, C. Cryopreservation of strawberry shoot tips by encapsulation-dehydration. Not. Bot. Hort. Agrobot. 2006, 34, 28–33. [Google Scholar]
- Lee, Y.; Balaraju, K.; Song, J.; Yi, J.; Lee, S.; Lee, J.; Yoon, M.; Kim, H. Cryopreservation of in vitro grown shoot tips of strawberry (Fragaria × ananassa Duch.) genetic resources by droplet-vitrification. Korean J. Plant Res. 2019, 32, 689–697. [Google Scholar]
- Niino, T.; Tanaka, D.; Ichikawa, S.; Takano, J.; Ivette, S.; Shirata, K.; Uemura, M. Cryopreservation of in vitro-grown apical shoot tips of strawberry by vitrification. Plant Biotechnol. 2003, 20, 75–80. [Google Scholar] [CrossRef]
- Yamamoto, S.; Fukui, K.; Rafique, T.; Khan, N.I.; Castillo, M.; Carlos, R.; Sekizawa, K.; Matsumoto, T.; Niino, T. Cryopreservation of in vitro-grown shoot tips of strawberry by the vitrification method using aluminum cryo-plates. Plant Gen. Res. 2012, 10, 14–19. [Google Scholar] [CrossRef]
- Akdemir, H.; Süzerer, V.; Tilkat, E.; Yildirim, H.; Onay, A.; Çiftçi, Y.O. In vitro conservation and cryopreservation of mature pistachio (Pistacia vera L.) germplasm. J. Plant Biochem. Biotechnol. 2013, 22, 43–51. [Google Scholar] [CrossRef]
- Hao, Y.J.; You, C.X.; Deng, X.X. Analysis of ploidy and the patterns of amplified fragment length polymorphism and methylation sensitive amplified polymorphism in strawberry plants recovered from cryopreservation. CryoLetters 2002, 23, 37–46. [Google Scholar] [PubMed]
- Pinker, I.; Halmagyi, A.; Olbricht, K. Effect of sucrose preculture on cryopreservation by droplet-vitrification of strawberry cultivars and morphological stability of cryopreserved plants. CryoLetters 2009, 30, 202–211. [Google Scholar]
- Kim, H.; Lee, Y.; Shin, D.; Ko, H.; Gwag, J.; Cho, E.; Engelmann, F. Development of alternative plant vitrification solutions in droplet-vitrification procedures. CryoLetters 2009, 30, 320–334. [Google Scholar] [CrossRef]
- Cordeiro, L.S.; Simões-Gurgel, C.; Albarello, N. Cryopreservation of adventitious roots of Cleome rosea Vahl (Cleomaceae) using a vitrification technique and assessment of genetic stability. CryoLetters 2016, 37, 231–242. [Google Scholar]
- Agrawal, A.; Sanayaima, R.; Singh, R.; Tandon, R.; Verma, S.; Tyagi, R.K. Phenotypic and molecular studies for genetic stability assessment of cryopreserved banana meristems derived from field and in vitro explant sources. In Vitro Cell. Dev. Biol. Plant 2014, 50, 345–356. [Google Scholar] [CrossRef]
- Harding, K. Genetic integrity of cryopreserved plant cells: A Review. CryoLetters 2004, 22, 3–22. [Google Scholar]
- Augusto, R.C.; Kulus, D.; Souza, A.V.; Kaviani, B.; Vicente, E.F. Cryopreservation of agronomic plant germplasm using vitrification-based methods: An overview of selected case studies. Int. J. Mol. Sci. 2021, 22, 6157. [Google Scholar] [CrossRef]
- Medina, J.J.; Clavero-Ramírez, I.; González-Benito, M.E.; Gálvez-Farfán, J.; Manuel López-Aranda, J.M.; Soria, C. Field performance characterization of strawberry (Fragaria × ananassa Duch.) plants derived from cryopreserved apices. Sci. Hortic. 2007, 113, 28–32. [Google Scholar] [CrossRef]
- Arnau, G.; Lallemand, J.; Bourgoin, M. Fast and reliable strawberry cultivar identification using inter simple sequence repeat (ISSR) amplification. Euphytica 2002, 129, 69–79. [Google Scholar] [CrossRef]
- Daryono, B.S.; Subiastuti, A.S.; Fatmadanni, A.; Sartika, D. Phenotypic and gentic stability of new Indonesian melon cultivar (Cucumis melo L.) ‘Melonia’ based on ISSR markers. Biodiversitas J. Biol. Div. 2019, 21, 1069–1075. [Google Scholar]
- Debnath, S.C.; Khanizadeh, S.; Jamieson, A.R.; Kempler, C. Inter Simple Sequence Repeat (ISSR) markers to assess genetic diversity and relatedness within strawberry genotypes. Can. J. Plant Sci. 2008, 88, 313–322. [Google Scholar] [CrossRef]
- Song, J.; Yi, J.; Bae, J.; Lee, J.; Yoon, M.; Lee, Y. Genetic stability of cryopreserved ornamental Lilium germplasm. Plant Gen. Res. 2022, 20, 66–68. [Google Scholar] [CrossRef]
- Villalobos-Olivera, A.; Ferreira, C.F.; Yanes-Paz, E.; Lorente, G.Y.; Souza, F.V.; Engelmann, F.; Martínez-Montero, M.E.; Lorenzo, J.C. Inter simple sequence repeat (ISSR) markers reveal DNA stability in pineapple plantlets after shoot tip cryopreservation. Vegetos 2022, 35, 360–366. [Google Scholar] [CrossRef]
- Wang, M.R.; Wenlu, B.; Mukund, R.; Shukla, L.R.; Zhibo, H.; Dag-Ragnar, B.; Saxena, P.K.; Wang, Q.C. Epigenetic and genetic integrity, metabolic stability, and field performance of cryopreserved plant. Plants 2021, 10, 1889. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Tikunov, Y.M.; Khrustaleva, L.I.; Karlov, G.I. Application of ISSR markers in the genus Lycopersicon. Euphytica 2003, 131, 71–80. [Google Scholar] [CrossRef]
- Nei, M.; Tajima, F.; Tateno, Y. Accuracy of estimated phylogenetic trees from molecular data. J. Mol. Evol. 1983, 19, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Kamvar, Z.N.; Tabima, J.F.; Grunwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef]
- Debnath, S.C. Propagation strategies and genetic fidelity in strawberries. Int. J. Fruit Sci. 2013, 13, 3–18. [Google Scholar] [CrossRef]
- EL-Sayed, S.F.; EL-Sawy, A.M.; Taha, S.S.; Gomah, M.S. Effect of Benzylaminopurine concentration and number of subcultures on behavior of some strawberry cultivars in vitro. Egypt J. Plant Breed. 2017, 21, 1–12. [Google Scholar] [CrossRef]
- Żebrowska, J.; Kaczmarska, E.; Gawroński, J. Comparative studies on the agronomic value of in vitro and conventionally propagated strawberry (Fragaria × ananassa Duch.) plants. Acta Sci. Pol. Hortorum Cultus 2015, 14, 25–35. [Google Scholar]
- Boxus, P.H.; Jemmali, A.; Terzi, J.M.; Arezki, O. Drift in genetic stability in micrporopagation: The case of strawberry. Acta Hort. 2000, 530, 155–162. [Google Scholar] [CrossRef]
- Zebrowska, J.I.; Czernas, J.; Gawronski, J.; Hortynski, J.A. Suitability of strawberry (Fragaria × ananassa Duch.) microplants to the field cultivation. Food Agr. Environ. 2003, 1, 190–193. [Google Scholar]
- Nehra, N.S.; Kartha, K.K.; Stushnoff, C.; Giles, K.L. Effect of in vitro propagation methods on field performance of two strawberry cultivars. Euphytica 1994, 76, 107–115. [Google Scholar] [CrossRef]
- Waithaka, K.; Hildebrandt, A.C.; Dana, M.N. Hormonal control of strawberry axillary bud development in vitro. J. Amer. Soc. Hort. Sci. 1980, 105, 428–430. [Google Scholar] [CrossRef]
- Martinez-Montero, M.E.; Ojeda, E.; Espinosa, A.; Sa´nchez, M.; Castillo, R.; Gonzalez-Arnao, M.T.; Engelmann, F.; Lorenzo, J.C. Field performance of sugarcane (Saccharum sp.) plants derived from cryopreserved calluses. CryoLetters 2002, 23, 21–26. [Google Scholar]
- Cote, F.X.; Goue, O.; Domergue, R.; Panis, B.J.; Jennny, C. In-field behaviour of banana plants (Musa AA sp.) obtained after regeneration of cryopreserved embryogenic cell suspensions. CryoLetters 2000, 21, 19–24. [Google Scholar] [PubMed]
- Sen, S.; Dhawan, V. Molecular analysis of micropropagated strawberry plants using ISSR (Inter Simple Sequence Repeats) markers for ascertaining clonal fidelity. In IV International Symposium on Acclimatization and Establishment of Micropropagated. Plants 2008, 865, 345–348. [Google Scholar]
- Kaya, E.; Souza, F.V.D. Comparison of two PVS2-based procedures for cryopreservation of commercial sugarcane (Saccharum spp.) germplasm and confirmation of genetic stability after cryopreservation using ISSR markers. In Vitro Cell. Dev. Biol. Plant 2017, 53, 410–417. [Google Scholar] [CrossRef]
- Saker, M.M.; Adawy, S.S.; Mohamed, A.A.; El-Itriby, H.A. Monitoring of cultivar identity in tissue culture-derived date palms using RAPD and AFLP analysis. Biol. Plant. 2006, 50, 198–204. [Google Scholar] [CrossRef]
- Godwin, I.D.; Sangduen, N.; Kunanuvatchaidach, R.; Piperidis, G.; Adkins, S.W. RAPD polymorphisms among variant and phenotypically normal rice (Oryza sativa var. indica) somaclonal progenies. Plant Cell Rep. 1997, 16, 320–324. [Google Scholar] [CrossRef]
- Gaafar, R.M.; Saker, M.M. Monitoring of cultivars identity and genetic stability in strawberry varieties grown in Egypt. World J Agric Sci. 2006, 2, 29–36. [Google Scholar]
- Kaity, A.; Ashmore, S.E.; Drew, R.A.; Dulloo, M.E. Assessment of genetic and epigenetic changes following cryopreservation in papaya. Plant Cell Rep. 2008, 27, 1529–1539. [Google Scholar] [CrossRef]


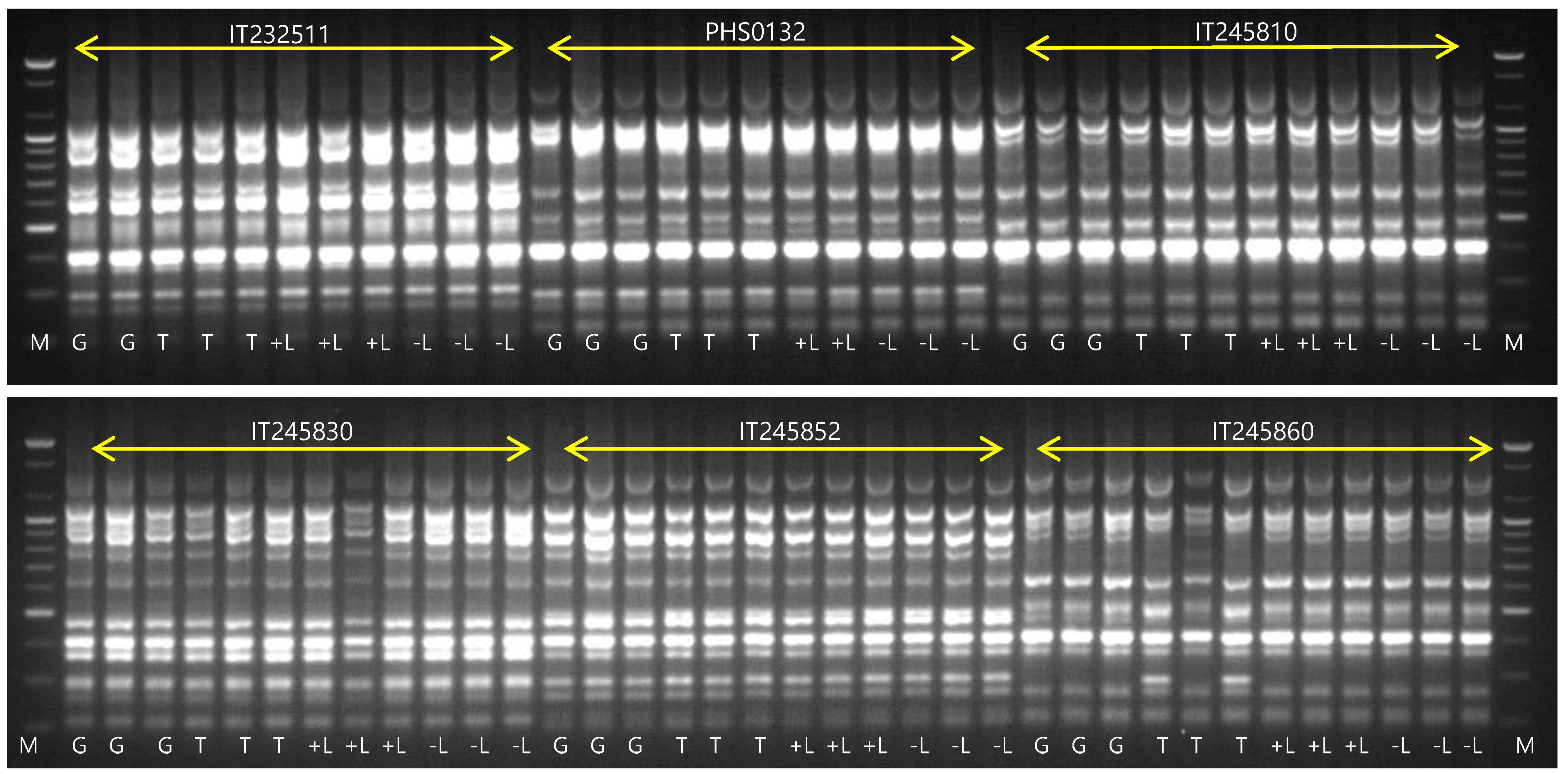
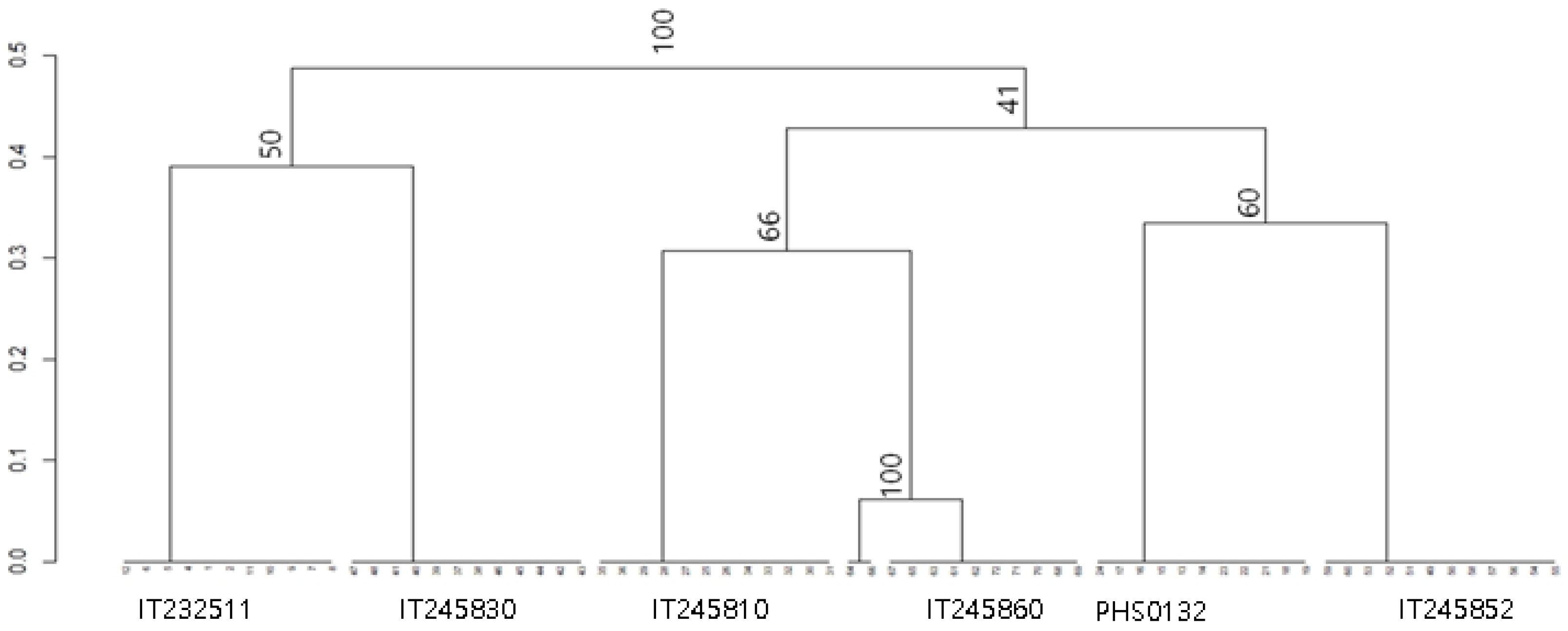
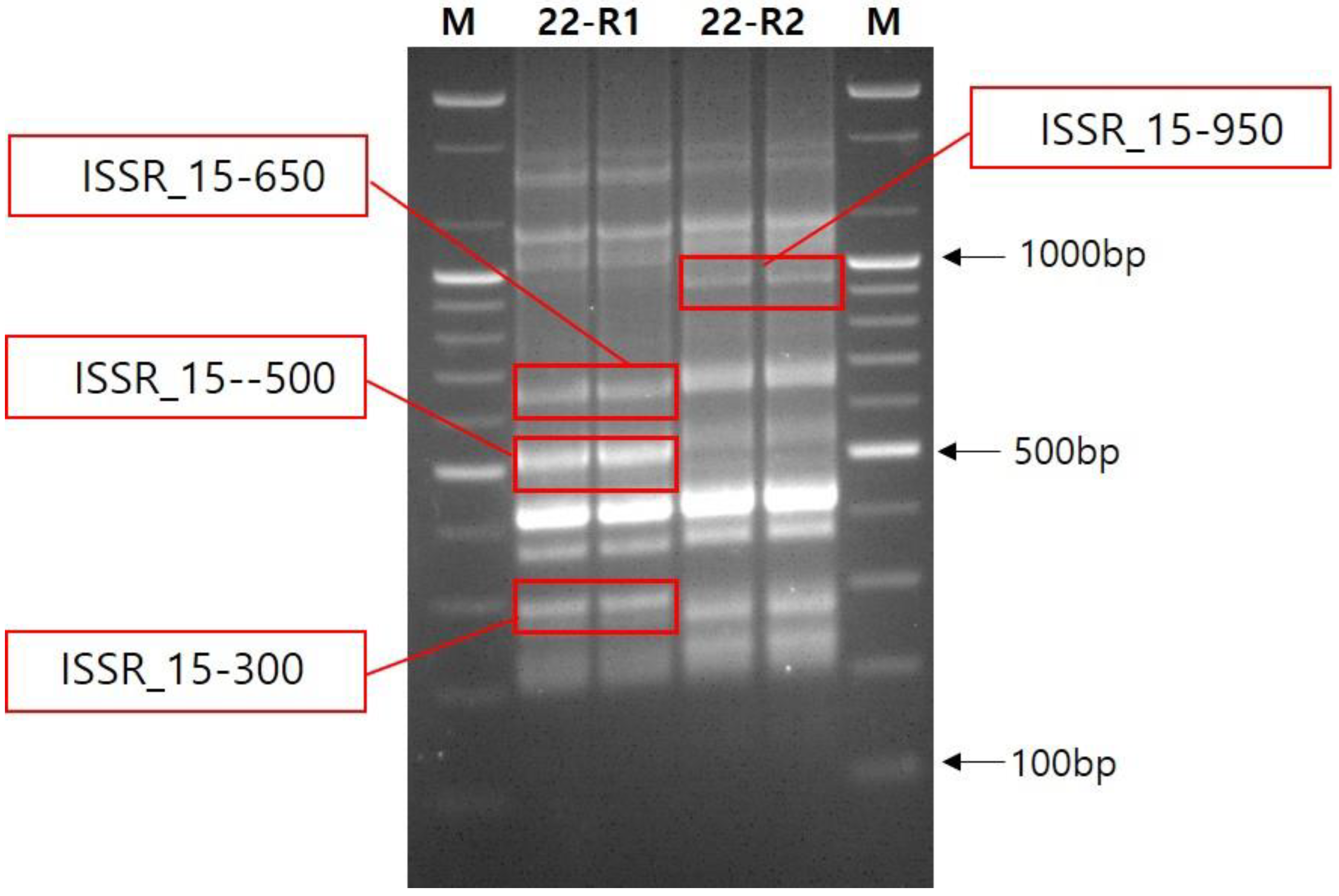
| Genebank No. | Name | Status | Origin |
|---|---|---|---|
| IT232511 | Pink paend | Wild relatives | USA |
| PHS0132 | Gorella | Developed Varieties | USA |
| IT245810 | NY1406 | Developed Line | USA |
| IT245830 | Merrimack | Developed Varieties | unknown |
| IT245852 | Tangi | Developed Varieties | unknown |
| IT245860 | Tufts | Developed Varieties | unknown |
| No | Marker | Primer Sequence a | Tm (°C) | References |
|---|---|---|---|---|
| 1 | ISSR_1 | VBV(AC)7 | 51 | [26] |
| 2 | ISSR_2 | BDB(CA)7 | 51 | [26] |
| 3 | ISSR_3 | HBH(CT)7 | 47 | [26] |
| 4 | ISSR_4 | GCV(TC)7 | 49 | [26] |
| 5 | ISSR_5 | BDV(AG)7 | 47 | [26] |
| 6 | ISSR_6 | (AG)8C | 45 | [28] |
| 7 | ISSR_7 | (GA)8T | 45 | [28] |
| 8 | ISSR_8 | (CA)8A | 45 | [28] |
| 9 | ISSR_9 | (AC)8G | 45 | [28] |
| 10 | ISSR_10 | (AG)8YA | 45 | [28] |
| 11 | ISSR_11 | (GATA)2(GACA)2 | 45 | [28] |
| 12 | ISSR_12 | (AC)8YG | 55 | [33] |
| 13 | ISSR_13 | (AG)8YT | 55 | [33] |
| 14 | ISSR_14 | (CA)8RC | 55 | [33] |
| 15 | ISSR_15 | (GA)8C | 55 | [33] |
| Accession | Treatment z | Plant Length y (cm) | Leaf Length (cm) | Leaf Width (cm) | Petiole Length (cm) | No. of Leaves | No. of Runners |
|---|---|---|---|---|---|---|---|
| IT232511 | GH | 12.00 NS | 4.60 NS | 4.00 NS | 6.63 NS | 7.25 b | 4.25 b |
| TC | 12.10 | 4.37 | 4.16 | 6.17 | 10.20 a | 7.67 a | |
| +LN | 12.03 | 4.43 | 4.27 | 6.10 | 9.80 a | 7.73 a | |
| −LN | 11.86 | 4.18 | 3.98 | 6.00 | 9.36 a | 7.27 a | |
| PHS0132 | GH | 21.38 NS | 7.50 NS | 6.12 NS | 12.38 NS | 6.25 b | 3.00 b |
| TC | 21.53 | 7.68 | 6.19 | 11.89 | 7.43 a | 4.43 a | |
| +LN | 20.17 | 7.67 | 6.10 | 10.83 | 7.67 a | 5.67 a | |
| −LN | 21.91 | 7.91 | 6.53 | 12.37 | 7.93 a | 5.40 a | |
| IT245810 | GH | 21.20 NS | 6.44 b | 6.40 NS | 12.70 NS | 6.00 b | 2.80 b |
| TC | 21.20 | 6.95 a | 6.75 | 12.27 | 7.60 a | 4.47 a | |
| +LN | 20.25 | 7.10 a | 6.57 | 11.80 | 7.73 a | 5.20 a | |
| −LN | 20.25 | 7.38 a | 6.25 | 12.13 | 7.50 a | 4.75 a | |
| IT245830 | GH | 21.50 NS | 7.40 NS | 5.10 NS | 12.10 NS | 6.20 b | 3.20 b |
| TC | 20.56 | 7.24 | 4.84 | 11.66 | 8.36 a | 5.00 a | |
| +LN | 20.92 | 7.42 | 5.10 | 11.17 | 8.27 a | 4.80 a | |
| −LN | 20.43 | 7.53 | 5.13 | 11.10 | 9.00 a | 4.30 a | |
| IT245852 | GH | 14.17 b | 3.82 b | 2.90 b | 9.42 b | 5.00 b | 2.83 b |
| TC | 20.19 a | 6.35 a | 4.96 a | 12.23 a | 8.00 a | 5.08 a | |
| +LN | 19.93 a | 6.75 a | 5.17 a | 11.85 a | 8.00 a | 5.14 a | |
| −LN | 20.31 a | 6.29 a | 4.88 a | 12.00 a | 8.62 a | 5.00 a | |
| IT245860 | GH | 21.04 NS | 6.58 NS | 6.10 NS | 12.94 NS | 7.20 NS | 5.80 NS |
| TC | 21.75 | 6.34 | 5.37 | 13.47 | 7.67 | 5.93 | |
| +LN | 20.50 | 6.32 | 5.43 | 12.68 | 7.93 | 6.07 | |
| −LN | 21.98 | 6.23 | 5.51 | 13.50 | 8.00 | 6.33 |
| Accession | Treatment z | Fruit Fresh Weight (g) y | Fruit Width (mm) | Fruit Length (mm) | Sugar Content (Brix) | pH |
|---|---|---|---|---|---|---|
| PHS0132 | GH | 2.28 NS | 17.08 NS | 22.21 NS | 8.54 NS | 3.28 b |
| TC | 2.09 | 15.66 | 22.58 | 8.90 | 3.25 b | |
| +LN | 2.13 | 16.23 | 22.30 | 9.07 | 3.48 a | |
| −LN | 2.10 | 15.74 | 21.63 | 8.69 | 3.40 a | |
| IT245810 | GH | 7.31 NS | 24.69 NS | 29.91 NS | 7.28 NS | 3.82 NS |
| TC | 7.90 | 25.37 | 29.80 | 7.36 | 3.78 | |
| +LN | 7.27 | 24.16 | 29.57 | 7.15 | 3.78 | |
| −LN | 6.12 | 23.39 | 29.38 | 7.56 | 3.88 | |
| IT245830 | GH | 3.36 NS | 20.37 NS | 19.66 NS | 7.10 NS | 3.35 NS |
| TC | 3.82 | 20.53 | 21.53 | 7.11 | 3.26 | |
| +LN | 3.46 | 19.90 | 20.84 | 7.41 | 3.31 | |
| −LN | 3.51 | 20.59 | 20.87 | 7.15 | 3.31 | |
| IT245852 | GH | 5.93 NS | 22.33 NS | 30.82 a | 6.22 c | 3.42 NS |
| TC | 5.41 | 21.70 | 27.68 b | 6.68 bc | 3.37 | |
| +LN | 5.56 | 21.81 | 28.45 b | 8.28 a | 3.35 | |
| −LN | 5.57 | 21.89 | 27.89 b | 7.21 b | 3.37 | |
| IT245860 | GH | 5.11 NS | 22.98 NS | 21.80 NS | 6.62 c | 3.50 b |
| TC | 4.70 | 22.55 | 21.84 | 7.82 b | 3.62 a | |
| +LN | 4.33 | 21.53 | 20.83 | 8.76 ab | 3.71 a | |
| −LN | 4.25 | 22.12 | 21.91 | 9.02 a | 3.67 a |
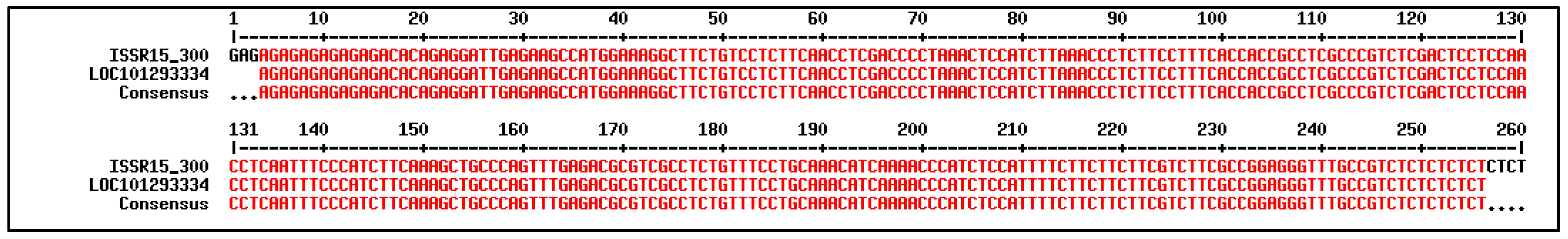
| No | PCR Band | Blastn z | |||
|---|---|---|---|---|---|
| Organism | Type | Query Cover (%) | Organism | ||
| 1 | ISSR15_300 | Fragaria vesca subsp. vesca | mRNA | 96 | XM_004287017.2 |
| 2 | ISSR15_500 | - | - | - | - |
| 3 | ISSR15_650 | Gossypium hirsutum | gDNA | 4 | CP023744.1 |
| 4 | ISSR15-950 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.; Choi, Y.; Song, J.-Y.; Lee, J.-R.; Yoon, M.; Lee, Y.-Y. Genetic Stability Assessment of Six Cryopreserved Strawberry (Fragaria × ananassa Duch.) Accessions by Phenotypic and Molecular Studies. Biology 2022, 11, 1746. https://doi.org/10.3390/biology11121746
Bae J, Choi Y, Song J-Y, Lee J-R, Yoon M, Lee Y-Y. Genetic Stability Assessment of Six Cryopreserved Strawberry (Fragaria × ananassa Duch.) Accessions by Phenotypic and Molecular Studies. Biology. 2022; 11(12):1746. https://doi.org/10.3390/biology11121746
Chicago/Turabian StyleBae, Jinjoo, Yunseo Choi, Jae-Young Song, Jung-Ro Lee, Munsup Yoon, and Young-Yi Lee. 2022. "Genetic Stability Assessment of Six Cryopreserved Strawberry (Fragaria × ananassa Duch.) Accessions by Phenotypic and Molecular Studies" Biology 11, no. 12: 1746. https://doi.org/10.3390/biology11121746
APA StyleBae, J., Choi, Y., Song, J.-Y., Lee, J.-R., Yoon, M., & Lee, Y.-Y. (2022). Genetic Stability Assessment of Six Cryopreserved Strawberry (Fragaria × ananassa Duch.) Accessions by Phenotypic and Molecular Studies. Biology, 11(12), 1746. https://doi.org/10.3390/biology11121746






