Bacillus spp. as Bioagents: Uses and Application for Sustainable Agriculture
Abstract
:Simple Summary
Abstract
1. Introduction
2. Limitations and Challenges in the Use of Conventional Pesticides
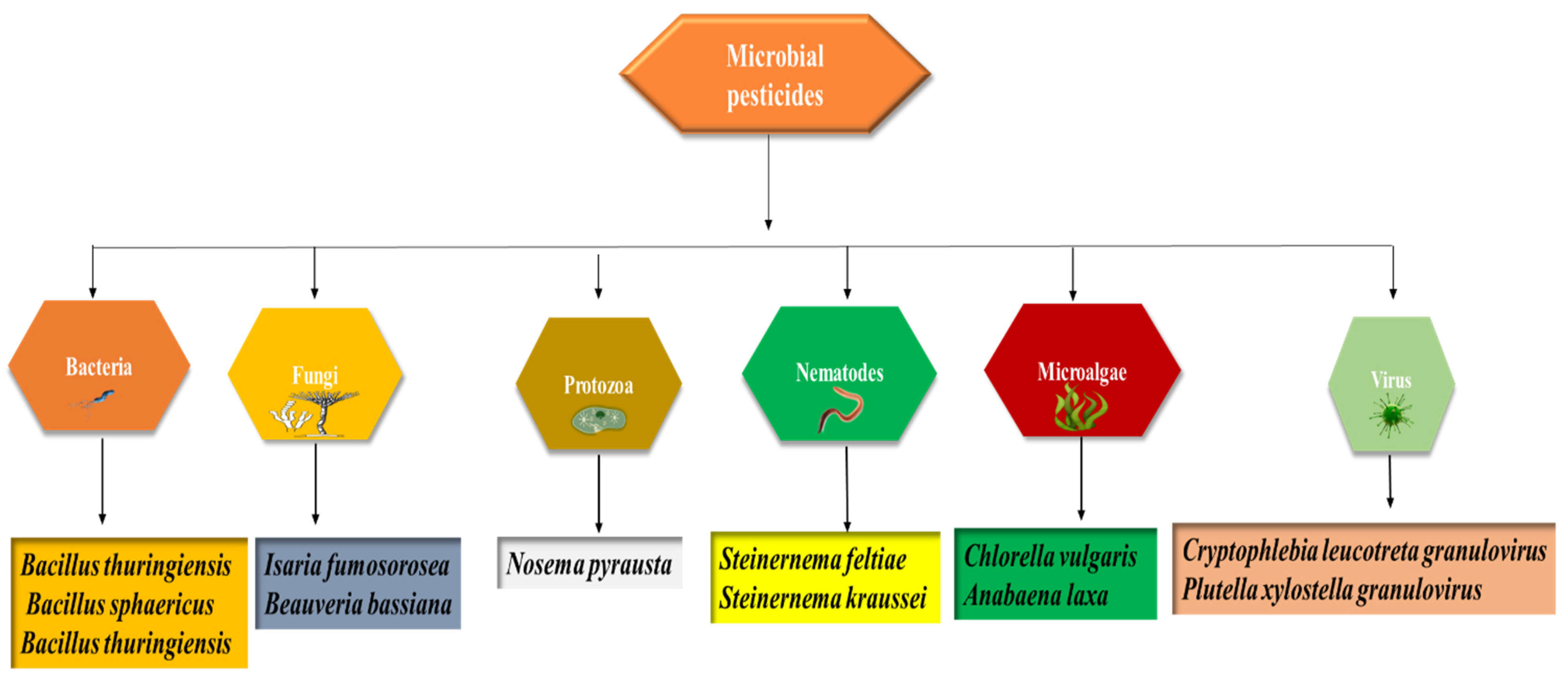
3. Microbes as Sources of Biopesticides in Sustainable Agriculture (Biopesticides and Sustainable Agriculture)
4. Diversity of Species of the Genus Bacillus Existing in Agriculture Soil
5. Bacillus spp. as PGPR (Plant Growth Promoting Rhizobacteria)
6. Mechanisms of PGPR
6.1. Direct Mechanism of PGPR
6.1.1. Nitrogen Fixation
6.1.2. Phosphate Solubilization
6.1.3. Potassium Solubilization
6.1.4. Phytohormones Production
6.2. Indirect Mechanism of PGPR
6.2.1. Siderophore Production by Bacillus spp.
6.2.2. Induced Systemic Resistance—ISR
6.2.3. Production of Lytic Enzymes
7. Plant Protection Activity Stimulated by Bacillus spp.
7.1. Quorum Quenching
7.2. Production of Volatile Organic Compounds (VOCs)
7.3. Antibiotic Compounds
7.4. Biofilm Formation by Bacillus spp.
8. Multifaceted Role of Bacillus thuringiensis as a Biocontrol Agent
9. Biosynthesis of Metallic Nanoparticles by Bacillus spp.
10. Effect of Bacillus spp. on Uptake of Nutrients and Crop Yield
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Savary, S.; Ficke, A.; Aubertot, J.-N.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Ab Rahman, S.F.S.; Singh, E.; Pieterse, C.M.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.d.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Lazarovits, G.; Turnbull, A.; Johnston-Monje, D. Plant Health Management: Biological Control of Plant Pathogens. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 388–399. [Google Scholar] [CrossRef]
- Mnif, I.; Ghribi, D. Potential of bacterial derived biopesticides in pest management. Crop Prot. 2015, 77, 52–64. [Google Scholar] [CrossRef]
- Nemutanzhela, M.E.; Roets, Y.; Gardiner, N.; Lalloo, R. The use and benefits of Bacillus based biological agents in aquaculture. Sustain. Aquac. Tech. 2014, 19, 1–34. [Google Scholar]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Wang, C.; Song, X.; Ding, X.; Wu, L.; Wu, H.; Gao, X.; Borriss, R. Bacillus velezensis FZB42 in 2018: The Gram-Positive Model Strain for Plant Growth Promotion and Biocontrol. Front. Microbiol. 2018, 9, 2491. [Google Scholar] [CrossRef] [Green Version]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clement, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aloo, B.N.; Makumba, B.A.; Mbega, E.R. The potential of Bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol. Res. 2019, 219, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of Pesticides on Environment. In Plant, Soil and Microbes; Springer International Publishing: Amsterdam, The Netherlands, 2016; pp. 253–269. [Google Scholar]
- Pelaez, V.; Mizukawa, G. Diversification strategies in the pesticide industry: From seeds to biopesticides. Ciência Rural 2017, 47, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Atreya, K.; Sitaula, B.K.; Johnsen, F.H.; Bajracharya, R.M. Continuing Issues in the Limitations of Pesticide Use in Developing Countries. J. Agric. Environ. Ethics 2010, 24, 49–62. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, J.; Tang, L.; Li, H.; Zhu, Y.; Jiang, D.; Song, B.; Chen, M.; Zeng, G. Nano-pesticides: A great challenge for biodiversity? Nano Today 2019, 28, 100757. [Google Scholar] [CrossRef]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil biological activity as an indicator of soil pollution with pesticides—A review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. The influence of chlorothalonil on the activity of soil microorganisms and enzymes. Ecotoxicology 2018, 27, 1188–1202. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, J.S.; Meena, V.S. Rhizospheric Microbes for Sustainable Agriculture: An Overview. In Role of Rhizospheric Microbes in Soil; Springer: Singapore, 2018; pp. 1–31. [Google Scholar]
- Kogan, M. Integrated pest management: Historical perspectives and contemporary developments. Annu. Rev. Entomol. 1998, 43, 243–270. [Google Scholar] [CrossRef]
- Yadouleton, A.W.; Padonou, G.; Asidi, A.; Moiroux, N.; Bio-Banganna, S.; Corbel, V.; N’Guessan, R.; Gbenou, D.; Yacoubou, I.; Gazard, K.; et al. Insecticide resistance status in Anopheles gambiae in southern Benin. Malar J. 2010, 9, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhakal, R. Biopesticides: A Key to Sustainable Agriculture. Int. J. Pure Appl. Biosci. 2019, 7, 391–396. [Google Scholar] [CrossRef]
- Azizoglu, U. Bacillus thuringiensis as a Biofertilizer and Biostimulator: A Mini-Review of the Little-Known Plant Growth-Promoting Properties of Bt. Curr. Microbiol. 2019, 76, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Kamarulzaman, P.S.D.; Yusup, S.; Osman, N.; Ramli@Yusof, N.H.; Kueh, B.W.B.; Talib, R. Effectiveness of neem based biopesticide to enhance rice (Oryza sativa) productivity. Sustain. Chem. Pharm. 2018, 7, 36–40. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in Sustainable Agriculture: Current Status and Future Prospects. In New and Future Development in Biopesticide Research: Biotechnological Exploration; Springer Nature: Singapore, 2022; pp. 1–53. [Google Scholar]
- Mishra, J.; Prakash, J.; Arora, N.K. Role of Beneficial Soil Microbes in Sustainable Agriculture and Environmental Management. Clim. Chang. Environ. Sustain. 2016, 4, 137. [Google Scholar] [CrossRef]
- Youssef, M.M.A.; Eissa, M.F.M. Biofertilizers and their role in management of plant parasitic nematodes. J. Biotechnol. Pharm. Res. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Kumar, V.V. Biofertilizers and Biopesticides in Sustainable Agriculture. In Role of Rhizospheric Microbes in Soil; Springer: Singapore, 2018; pp. 377–398. [Google Scholar]
- Meena, R.K.; Singh, R.K.; Singh, N.P.; Meena, S.K.; Meena, V.S. Isolation of low temperature surviving plant growth–promoting rhizobacteria (PGPR) from pea (Pisum sativum L.) and documentation of their plant growth promoting traits. Biocatal. Agric. Biotechnol. 2015, 4, 806–811. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Meena, S.K.; Meena, R.K.; Kumar, A.; Verma, J.P.; Singh, N.P. Can Bacillus Species Enhance Nutrient Availability in Agricultural Soils? In Bacilli and Agrobiotechnology; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 367–395. [Google Scholar] [CrossRef]
- Yi, Y.; Luan, P.; Liu, S.; Shan, Y.; Hou, Z.; Zhao, S.; Jia, S.; Li, R. Efficacy of Bacillus subtilis XZ18-3 as a Biocontrol Agent against Rhizoctonia cerealis on Wheat. Agriculture 2022, 12, 258. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Lu, Y.; Zhang, J.; Sun, Y.; Zhou, J.; Tu, T.; Gong, W.; Sun, W.; Wang, Y. Characterization of Bacillus velezensis E2 with abilities to degrade ochratoxin A and biocontrol against Aspergillus westerdijkiae fc-1. Toxicon 2022, 216, 125–131. [Google Scholar] [CrossRef]
- Wang, L.; Hua, X.; Jing, N.; Ji, T.; Zhou, C.; Liu, W.; Lv, B.; Liu, L.; Chen, Y. Isolation and characterization of Bacillus amyloliquefaciens YL-1 with ochratoxin A degradation ability and biocontrol activity against Aspergillus westerdijkiae. Biol. Control 2022, 175, 105052. [Google Scholar] [CrossRef]
- Khedher, S.B.; Mejdoub-Trabelsi, B.; Tounsi, S. Biological potential of Bacillus subtilis V26 for the control of Fusarium wilt and tuber dry rot on potato caused by Fusarium species and the promotion of plant growth. Biol. Control 2021, 152, 104444. [Google Scholar] [CrossRef]
- Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus subtilis MBI600 Promotes Growth of Tomato Plants and Induces Systemic Resistance Contributing to the Control of Soilborne Pathogens. Plants 2021, 10, 1113. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Khan, S.U.; Khan, W.U.; Saleh, T.A.; Khan, M.H.U.; Ullah, S.; Ali, A.; Ikram, M. Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: Alternatives to chemical pesticides. Comptes Rendus Biol. 2019, 342, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Myo, E.M.; Liu, B.; Ma, J.; Shi, L.; Jiang, M.; Zhang, K.; Ge, B. Evaluation of Bacillus velezensis NKG-2 for bio-control activities against fungal diseases and potential plant growth promotion. Biol. Control 2019, 134, 23–31. [Google Scholar] [CrossRef]
- Samaras, A.; Efthimiou, K.; Roumeliotis, E.; Karaoglanidis, G.S. Biocontrol Potential and Plant-Growth-Promoting Effects of Bacillus amyloliquefaciens MBI 600 against Fusarium oxysporum f. sp. radicis-lycopersici on Tomato, 1st ed.; International Society for Horticultural Science (ISHS): Málaga, Spain, 2018; Volume 18, pp. 139–146. [Google Scholar]
- Xu, S.J.; Park, D.H.; Kim, J.-Y.; Kim, B.-S. Biological control of gray mold and growth promotion of tomato using Bacillus spp. isolated from soil. Trop. Plant Pathol. 2016, 41, 169–176. [Google Scholar] [CrossRef]
- Rahman, M.M.E.; Hossain, D.M.; Suzuki, K.; Shiiya, A.; Suzuki, K.; Dey, T.K.; Nonaka, M.; Harada, N. Suppressive effects of Bacillus spp. on mycelia, apothecia and sclerotia formation of Sclerotinia sclerotiorum and potential as biological control of white mold on mustard. Australas. Plant Pathol. 2016, 45, 103–117. [Google Scholar] [CrossRef]
- Sakthivel, K.; Manigundan, K.; Gautam, R.; Singh, P.; Nakkeeran, S.; Sharma, S.K. Bacillus spp. for suppression of eggplant bacterial wilt pathogen in Andaman Islands: Isolation and characterization. Indian J. Exp. Biol. 2019, 57, 131–137. [Google Scholar]
- Bhusal, B.; Mmbaga, M.T. Biological control of Phytophthora blight and growth promotion in sweet pepper by Bacillus species. Biol. Control 2020, 150, 104373. [Google Scholar] [CrossRef]
- Adeniji, A.A.; Aremu, O.S.; Babalola, O.O. Selecting lipopeptide-producing, Fusarium-suppressing Bacillus spp.: Metabolomic and genomic probing of Bacillus velezensis NWUMFkBS10.5. Microbiologyopen 2019, 8, e00742. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.-H.; Liao, M.-J.; Wang, H.-K.; Zheng, M.-Z.; Xu, J.-J.; Guo, J.-H. Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis cinerea. Biol. Control 2018, 126, 147–157. [Google Scholar] [CrossRef]
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Kumar, A.; Prakash, A.; Johri, B.N. Bacillus as PGPR in Crop Ecosystem. In Bacteria in Agrobiology: Crop Ecosystems; Springer: Berlin/Heidelberg, Germany, 2011; pp. 37–59. [Google Scholar]
- Govindasamy, V.; Senthilkumar, M.; Magheshwaran, V.; Kumar, U.; Bose, P.; Sharma, V.; Annapurna, K. Bacillus and PaeniBacillus spp.: Potential PGPR for Sustainable Agriculture. In Plant Growth and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 333–364. [Google Scholar]
- Sansinenea, E. Bacillus spp.: As plant growth-promoting bacteria. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Springer International Publishing: Midtown Manhattan, NY, USA, 2019; pp. 225–237. [Google Scholar]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2019, 128, 1583–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lata, C.; Muthamilarasan, M.; Prasad, M. Drought Stress Responses and Signal Transduction in Plants. In Elucidation of Abiotic Stress Signaling in Plants; Springer: New York, NY, USA, 2015; pp. 195–225. [Google Scholar]
- Tiwari, S.; Lata, C.; Chauhan, P.S.; Nautiyal, C.S. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol. Biochem. 2016, 99, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gagné-Bourque, F.; Mayer, B.F.; Charron, J.-B.; Vali, H.; Bertrand, A.; Jabaji, S. Accelerated Growth Rate and Increased Drought Stress Resilience of the Model Grass Brachypodium distachyon Colonized by Bacillus subtilis B26. PLoS ONE 2015, 10, e0130456. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wu, G.; Veronican Njeri, K.; Shen, Q.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016, 158, 34–44. [Google Scholar] [CrossRef]
- Zubair, M.; Hanif, A.; Farzand, A.; Sheikh, T.M.M.; Khan, A.R.; Suleman, M.; Ayaz, M.; Gao, X. Genetic Screening and Expression Analysis of Psychrophilic Bacillus spp. Reveal Their Potential to Alleviate Cold Stress and Modulate Phytohormones in Wheat. Microorganisms 2019, 7, 337. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, P.; Goswami, M.; Bhattacharyya, L. Perspective of beneficial microbes in agriculture under changing climatic scenario: A review. J. Phytol. 2016, 8, 26–41. [Google Scholar] [CrossRef] [Green Version]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus spp. in Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Mus, F.; Crook, M.B.; Garcia, K.; Garcia Costas, A.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.H.; Oldroyd, G.E.D.; Poole, P.S.; et al. Symbiotic Nitrogen Fixation and the Challenges to Its Extension to Nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuan, K.B.; Othman, R.; Abdul Rahim, K.; Shamsuddin, Z.H. Plant Growth-Promoting Rhizobacteria Inoculation to Enhance Vegetative Growth, Nitrogen Fixation and Nitrogen Remobilisation of Maize under Greenhouse Conditions. PLoS ONE 2016, 11, e0152478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spohn, M.; Zeißig, I.; Brucker, E.; Widdig, M.; Lacher, U.; Aburto, F. Phosphorus solubilization in the rhizosphere in two saprolites with contrasting phosphorus fractions. Geoderma 2020, 366, 114245. [Google Scholar] [CrossRef]
- Saeid, A.; Prochownik, E.; Dobrowolska-Iwanek, J. Phosphorus solubilization by Bacillus species. Molecules 2018, 23, 2897. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Shankhdhar, D.; Shankhdhar, S.C. Potassium-Solubilizing Microorganisms: Mechanism and Their Role in Potassium Solubilization and Uptake. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 203–219. [Google Scholar] [CrossRef]
- Sattar, A.; Naveed, M.; Ali, M.; Zahir, Z.A.; Nadeem, S.M.; Yaseen, M.; Meena, V.S.; Farooq, M.; Singh, R.; Rahman, M.; et al. Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Appl. Soil Ecol. 2019, 133, 146–159. [Google Scholar] [CrossRef]
- Raghavendra, M.P.; Chandra Nayaka, S.; Nuthan, B.R. Role of Rhizosphere Microflora in Potassium Solubilization. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 43–59. [Google Scholar] [CrossRef]
- Masood, S.; Bano, A. Mechanism of Potassium Solubilization in the Agricultural Soils by the Help of Soil Microorganisms. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 137–147. [Google Scholar] [CrossRef]
- Ali, A.M.; Awad, M.Y.; Hegab, S.A.; Gawad, A.M.A.E.; Eissa, M.A. Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J. Plant Nutr. 2021, 44, 411–420. [Google Scholar] [CrossRef]
- Han, X.; Zeng, H.; Bartocci, P.; Fantozzi, F.; Yan, Y. Phytohormones and Effects on Growth and Metabolites of Microalgae: A Review. Fermentation 2018, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, M.G.; Akimova, G.P.; Vaishlia, O.B. Effect of phytohormones synthesized by rhizosphere bacteria on plants. Prikl. Biokhim. Mikrobiol. 2011, 47, 302–307. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Patel, K.; Goswami, D.; Dhandhukia, P.; Thakker, J. Techniques to Study Microbial Phytohormones. In Bacterial Metabolites in Sustainable Agroecosystem; Springer International Publishing: Midtown Manhattan, NY, USA, 2015; pp. 1–27. [Google Scholar] [CrossRef]
- Kang, S.-M.; Khan, A.L.; Waqas, M.; Asaf, S.; Lee, K.-E.; Park, Y.-G.; Kim, A.-Y.; Khan, M.A.; You, Y.-H.; Lee, I.-J. Integrated phytohormone production by the plant growth-promoting rhizobacterium Bacillus tequilensis SSB07 induced thermotolerance in soybean. J. Plant Interact. 2019, 14, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Lomax, T.L.; Muday, G.K.; Rubery, P.H. Auxin transport. In Plant Hormones; Springer International Publishing: Midtown Manhattan, NY, USA, 1995; pp. 509–530. [Google Scholar]
- Quint, M.; Gray, W.M. Auxin signaling. Curr. Opin. Plant Biol. 2006, 9, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Hooley, R. Gibberellins: Perception, transduction and responses. In Signals and Signal Transduction Pathways in Plants; Springer: Amsterdam, The Netherlands, 1994; pp. 293–319. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achard, P.; Genschik, P. Releasing the brakes of plant growth: How GAs shutdown DELLA proteins. J. Exp. Bot. 2008, 60, 1085–1092. [Google Scholar] [CrossRef] [Green Version]
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Davière, J.-M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef]
- Singh, B.N.; Hidangmayum, A.; Singh, A.; Shera, S.S.; Dwivedi, P. Synthesis and Application of Hydroxamic Acid: A Key Secondary Metabolite of Piriformospora indica. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Springer: Singapore, 2019; pp. 391–404. [Google Scholar]
- Alina, S.O.; Constantinscu, F.; Petruţa, C.C. Biodiversity of Bacillus subtilis group and beneficial traits of Bacillus species useful in plant protection. Rom. Biotechnol. Lett. 2015, 20, 10737–10750. [Google Scholar]
- Brenner, W.G.; Schmülling, T. Summarizing and exploring data of a decade of cytokinin-related transcriptomics. Front. Plant Sci. 2015, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Li, S.-M.; Zheng, H.-X.; Zhang, X.-S.; Sui, N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 2020, 40, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Kieber, J.J. Ethylene. Arab. Book 2002, 1, e0071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, N.; Trivellini, A.; Masood, A.; Ferrante, A.; Khan, N.A. Current understanding on ethylene signaling in plants: The influence of nutrient availability. Plant Physiol. Biochem. 2013, 73, 128–138. [Google Scholar] [CrossRef]
- Chang, C. Q&A: How do plants respond to ethylene and what is its importance? BMC Biol. 2016, 14, 7. [Google Scholar] [CrossRef] [Green Version]
- Misra, S.; Chauhan, P.S. ACC deaminase-producing rhizosphere competent Bacillus spp. mitigate salt stress and promote Zea mays growth by modulating ethylene metabolism. 3 Biotech 2020, 10, 119. [Google Scholar] [CrossRef]
- Spaepen, S. Plant Hormones Produced by Microbes. In Principles of Plant-Microbe Interactions; Springer International Publishing: Amsterdam, The Netherlands, 2014; pp. 247–256. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.-M.; Lee, I.-J. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ. Exp. Bot. 2017, 136, 68–77. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Zandi, P. Effects of exogenously applied plant growth regulators in combination with PGPR on the physiology and root growth of chickpea (Cicer arietinum) and their role in drought tolerance. J. Plant Interact. 2018, 13, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [Green Version]
- Ramadoss, D.; Lakkineni, V.K.; Bose, P.; Ali, S.; Annapurna, K. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. Springerplus 2013, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Ai, C.; Xin, L.; Zhou, G. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011, 47, 138–145. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Chaabouni, I.; Guesmi, A.; Cherif, A. Secondary Metabolites of Bacillus: Potentials in Biotechnology. In Bacillus thuringiensis Biotechnology; Springer: Amsterdam, The Netherlands, 2012; pp. 347–366. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and plants—With special reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Wang, X.; Wang, Y.; Song, X.; Wang, J.; Guo, J.; Zhao, H. Bacillus cereus AR156 activates PAMP-triggered immunity and induces a systemic acquired resistance through a NPR1 -and SA-dependent signaling pathway. Biochem. Biophys. Res. Commun. 2016, 469, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Chowdappa, P.; Mohan Kumar, S.P.; Jyothi Lakshmi, M.; Upreti, K.K. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol. Control 2013, 65, 109–117. [Google Scholar] [CrossRef]
- Jayapala, N.; Mallikarjunaiah, N.H.; Puttaswamy, H.; Gavirangappa, H.; Ramachandrappa, N.S. Rhizobacteria Bacillus spp. induce resistance against anthracnose disease in chili (Capsicum annuum L.) through activating host defense response. Egypt. J. Biol. Pest Control 2019, 29, 45. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, N.; Wang, X.; Hu, J.; Wang, S. Characterization of two anti-fungal lipopeptides produced by Bacillus amyloliquefaciens SH-B10. Bioresour. Technol. 2010, 101, 8822–8827. [Google Scholar] [CrossRef]
- Jain, D.; Saharan, V.; Pareek, S. Current Status of Bacillus thuringiensis: Insecticidal Crystal Proteins and Transgenic Crops. In Advances in Plant Breeding Strategies: Agronomic, Abiotic and Biotic Stress Traits; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 657–698. [Google Scholar] [CrossRef]
- Xie, J.; Shi, H.; Du, Z.; Wang, T.; Liu, X.; Chen, S. Comparative genomic and functional analysis reveal conservation of plant growth promoting traits in PaeniBacillus polymyxa and its closely related species. Sci. Rep. 2016, 6, 21329. [Google Scholar] [CrossRef] [Green Version]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering with Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Rhizosphere Colonization Determinants by Plant Growth-Promoting Rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef]
- Bodhankar, S.; Grover, M.; Hemanth, S.; Reddy, G.; Rasul, S.; Yadav, S.K.; Desai, S.; Mallappa, M.; Mandapaka, M.; Srinivasarao, C. Maize seed endophytic bacteria: Dominance of antagonistic, lytic enzyme-producing Bacillus spp. 3 Biotech 2017, 7, 232. [Google Scholar] [CrossRef] [PubMed]
- Karthika, S.; Midhun, S.J.; Jisha, M.S. A potential antifungal and growth-promoting bacterium Bacillus sp. KTMA4 from tomato rhizosphere. Microb. Pathog. 2020, 142, 104049. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Behboudi, K.; Ahmadzadeh, M. Quorum quenching by Bacillus cereus U92: A double-edged sword in biological control of plant diseases. Biocontrol Sci. Technol. 2013, 23, 555–573. [Google Scholar] [CrossRef]
- Fincheira, P.; Quiroz, A. Microbial volatiles as plant growth inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef]
- Yi, H.-S.; Ahn, Y.-R.; Song, G.C.; Ghim, S.-Y.; Lee, S.; Lee, G.; Ryu, C.-M. Impact of a Bacterial Volatile 2,3-Butanediol on Bacillus subtilis Rhizosphere Robustness. Front. Microbiol. 2016, 7, 993. [Google Scholar] [CrossRef] [Green Version]
- van Straaten, K.E.; Ko, J.B.; Jagdhane, R.; Anjum, S.; Palmer, D.R.J.; Sanders, D.A.R. The structure of NtdA, a sugar aminotransferase involved in the kanosamine biosynthetic pathway in Bacillus subtilis, reveals a new subclass of aminotransferases. J. Biol. Chem. 2013, 288, 34121–34130. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Mukherjee, A.K. Assessment of mosquito larvicidal potency of cyclic lipopeptides produced by Bacillus subtilis strains. Acta Trop. 2006, 97, 168–173. [Google Scholar] [CrossRef]
- Chan, Y.-K.; Savard, M.E.; Reid, L.M.; Cyr, T.; McCormick, W.A.; Seguin, C. Identification of lipopeptide antibiotics of a Bacillus subtilis isolate and their control of Fusarium graminearum diseases in maize and wheat. BioControl 2009, 54, 567–574. [Google Scholar] [CrossRef]
- Romero, D.; de Vicente, A.; Rakotoaly, R.H.; Dufour, S.E.; Veening, J.-W.; Arrebola, E.; Cazorla, F.M.; Kuipers, O.P.; Paquot, M.; Pérez-García, A. The Iturin and Fengycin Families of Lipopeptides Are Key Factors in Antagonism of Bacillus subtilis Toward Podosphaera Fusca. Mol. Plant-Microb. Interact 2007, 20, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Das, P.; Mukherjee, S.; Sen, R. Improved bioavailability and biodegradation of a model polyaromatic hydrocarbon by a biosurfactant producing bacterium of marine origin. Chemosphere 2008, 72, 1229–1234. [Google Scholar] [CrossRef]
- Rivardo, F.; Turner, R.J.; Allegrone, G.; Ceri, H.; Martinotti, M. Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl. Microbiol. Biotechnol. 2009, 83, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Pandin, C.; Le Coq, D.; Canette, A.; Aymerich, S.; Briandet, R. Should the biofilm mode of life be taken into consideration for microbial biocontrol agents? Microb. Biotechnol. 2017, 10, 719–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi Jouzani, G.; Pourjan Abad, A.; Seifinejad, A.; Marzban, R.; Kariman, K.; Maleki, B. Distribution and diversity of Dipteran-specific cry and cyt genes in native Bacillus thuringiensis strains obtained from different ecosystems of Iran. J. Ind. Microbiol. Biotechnol. 2007, 35, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Jouzani, G.S.; Valijanian, E.; Sharafi, R. Bacillus thuringiensis: A successful insecticide with new environmental features and tidings. Appl. Microbiol. Biotechnol. 2017, 101, 2691–2711. [Google Scholar] [CrossRef]
- Jain, S.; Vaishnav, A.; Kumari, S.; Varma, A.; Tuteja, N.; Choudhary, D.K. Chitinolytic Bacillus-Mediated Induction of Jasmonic Acid and Defense-Related Proteins in Soybean (Glycine max L. Merrill) Plant Against Rhizoctonia solani and Fusarium oxysporum. J. Plant Growth Regul. 2016, 36, 200–214. [Google Scholar] [CrossRef]
- Melo, A.L.d.A.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications: A review. Crit. Rev. Biotechnol. 2014, 36, 317–326. [Google Scholar] [CrossRef]
- de la Fuente-Salcido, N.M.; Casados-Vázquez, L.E.; Barboza-Corona, J.E. Bacteriocins of Bacillus thuringiensis can expand the potential of this bacterium to other areas rather than limit its use only as microbial insecticide. Can. J. Microbiol. 2013, 59, 515–522. [Google Scholar] [CrossRef]
- Park, S.-J.; Park, S.-Y.; Ryu, C.-M.; Park, S.-H.; Lee, J.-K. The role of AiiA, a quorum-quenching enzyme from Bacillus thuringiensis, on the rhizosphere competence. J. Microbiol. Biotechnol. 2008, 18, 1518–1521. [Google Scholar]
- Bora, L.C.; Kataki, L.; Talukdar, K.; Nath, B.C.; Sarkar, R. Molecular characterizations of microbial antagonists and development of bioformulations for management of bacterial wilt of Naga Chilli (Capsicum chinens Jacq.) in Assam. J. Exp. Biol. Agric. Sci. 2015, 3, 109–122. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Nakatani, M.; Nishimura, M.; Arakawa, T.; Shimizu, M.; Hyakumachi, M. Control of tomato bacterial wilt and root-knot diseases by Bacillus thuringiensis CR-371 and Streptomyces avermectinius NBRC14893. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2015, 65, 575–580. [Google Scholar] [CrossRef]
- Crickmore, N. Bacillus thuringiensis Toxin Nomenclature. 2016. Available online: http://www.btnomenclature.Info (accessed on 1 November 2022).
- Murthy, K.S.; Vineela, V.; Devi, P.V. Generation of nanoparticles from technical powder of the insecticidal bacterium Bacillus thuringiensis var. kurstaki for improving efficacy. Int. J. Biomed. Nanosci. Nanotechnol. 2014, 3, 236. [Google Scholar] [CrossRef]
- Vineela, V.; Nataraj, T.; Reddy, G.; Vimala Devi, P.S. Enhanced bioefficacy of Bacillus thuringiensis var. kurstaki against Spodoptera litura (Lepidoptera: Noctuidae) through particle size reduction and formulation as a suspension concentrate. Biocontrol Sci. Technol. 2016, 27, 58–69. [Google Scholar]
- Rao, W.; Zhan, Y.; Chen, S.; Xu, Z.; Huang, T.; Hong, X.; Zheng, Y.; Pan, X.; Guan, X. Flowerlike Mg(OH)2 Cross-Nanosheets for Controlling Cry1Ac Protein Loss: Evaluation of Insecticidal Activity and Biosecurity. J. Agric. Food Chem. 2018, 66, 3651–3657. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.Y.; Choi, J.Y.; Li, M.S.; Jin, B.R.; Je, Y.H. Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J. Microbiol. Biotechnol. 2007, 17, 547–559. [Google Scholar]
- Xu, L.; Zhu, Z.; Sun, D.W. Bioinspired Nanomodification Strategies: Moving from Chemical-Based Agrosystems to Sustainable Agriculture. ACS Nano 2021, 15, 12655–12686. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis of Metal and Metal Oxide Nanoparticles. ChemBioEng Rev. 2016, 3, 55–67. [Google Scholar] [CrossRef]
- Ahmed, T.; Wu, Z.; Jiang, H.; Luo, J.; Noman, M.; Shahid, M.; Manzoor, I.; Allemailem, K.; Alrumaihi, F.; Li, B. Bioinspired Green Synthesis of Zinc Oxide Nanoparticles from a Native Bacillus cereus Strain RNT6: Characterization and Antibacterial Activity against Rice Panicle Blight Pathogens Burkholderia glumae and B. gladioli. Nanomaterials 2021, 11, 884. [Google Scholar] [CrossRef]
- Meena, V.S.; Bahadur, I.; Maurya, B.R.; Kumar, A.; Meena, R.K.; Meena, S.K.; Verma, J.P. Potassium-Solubilizing Microorganism in Evergreen Agriculture: An Overview. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer International Publishing: Midtown Manhattan, NY, USA, 2016; pp. 1–20. [Google Scholar]
- Verma, J.P.; Yadav, J.; Tiwari, K.N. Enhancement of Nodulation and Yield of Chickpea by Co-inoculation of Indigenous Mesorhizobium spp. and Plant Growth–Promoting Rhizobacteria in Eastern Uttar Pradesh. Commun. Soil Sci. Plant Anal. 2012, 43, 605–621. [Google Scholar] [CrossRef]
- Čolo, J.O.S.I.P.; Hajnal-Jafari, T.; ĐURIĆ, S.; Stamenov, D.; Hamidović, S.A.U.D. Plant Growth Promotion Rhizobacteria in Onion Production. Pol. J. Microbiol. 2014, 63, 83–88. [Google Scholar] [CrossRef]
- Mukhtar, S.; Shahid, I.; Mehnaz, S.; Malik, K.A. Assessment of two carrier materials for phosphate solubilizing biofertilizers and their effect on growth of wheat (Triticum aestivum L.). Microbiol. Res. 2017, 205, 107–117. [Google Scholar] [CrossRef]
- Vinci, G.; Cozzolino, V.; Mazzei, P.; Monda, H.; Savy, D.; Drosos, M.; Piccolo, A. Effects of Bacillus amyloliquefaciens and different phosphorus sources on Maize plants as revealed by NMR and GC-MS based metabolomics. Plant Soil 2018, 429, 437–450. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Amby, D.B.; Hegelund, J.N.; Fimognari, L.; Großkinsky, D.K.; Westergaard, J.C.; Müller, R.; Moelbak, L.; Liu, F.; Roitsch, T. Bacillus licheniformis FMCH001 Increases Water Use Efficiency via Growth Stimulation in Both Normal and Drought Conditions. Front. Plant Sci. 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Saxena, A.K.; Singh, J.S.; Singh, D.P. Impact of Native ST-PGPR Bacillus pumilus; EU927414) on PGP Traits, Antioxidants Activities, Wheat Plant Growth and Yield under Salinity. Clim. Change Environ. Sustain. 2019, 7, 157. [Google Scholar] [CrossRef]
- Wagi, S.; Ahmed, A. Bacillus spp.: Potent microfactories of bacterial IAA. PeerJ 2019, 7, e7258. [Google Scholar] [CrossRef] [Green Version]
- Al-Ali, A.; Deravel, J.; Krier, F.; Béchet, M.; Ongena, M.; Jacques, P. Biofilm formation is determinant in tomato rhizosphere colonization by Bacillus velezensis FZB42. Environ. Sci. Pollut. Res. 2017, 25, 29910–29920. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, S.; Marefat, A.; Naseri, B.; Hemmati, R. Improvement of bean yield and Fusarium root rot biocontrol using mixtures of Bacillus, Pseudomonas and Rhizobium. Trop. Plant Pathol. 2018, 43, 499–505. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I.; Pichtel, J. Growth stimulation and alleviation of salinity stress to wheat by the biofilm forming Bacillus pumilus strain FAB10. Appl. Soil Ecol. 2019, 143, 45–54. [Google Scholar] [CrossRef]
- Agarwal, M.; Dheeman, S.; Dubey, R.C.; Kumar, P.; Maheshwari, D.K.; Bajpai, V.K. Differential antagonistic responses of Bacillus pumilus MSUA3 against Rhizoctonia solani and Fusarium oxysporum causing fungal diseases in Fagopyrum esculentum Moench. Microbiol. Res. 2017, 205, 40–47. [Google Scholar] [CrossRef]
- Caulier, S.; Gillis, A.; Colau, G.; Licciardi, F.; Liépin, M.; Desoignies, N.; Modrie, P.; Legrève, A.; Mahillon, J.; Bragard, C. Versatile Antagonistic Activities of Soil-Borne Bacillus spp. and Pseudomonas spp. against Phytophthora infestans and Other Potato Pathogens. Front. Microbiol. 2018, 9, 143. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, D.P.; Solanki, M.K.; Singh, P.; Srivastva, A.K.; Kumar, S.; Kashyap, P.L.; Saxena, A.K.; Singhal, P.K.; Arora, D.K. Optimization of media components for chitinase production by chickpea rhizosphere associated LysiniBacillus fusiformis B-CM18. J. Basic Microbiol. 2012, 53, 451–460. [Google Scholar] [CrossRef]
- Wemheuer, F.; Hollensteiner, J.; Poehlein, A.; Liesegang, H.; Daniel, R.; Wemheuer, B. Draft Genome Sequence of the Endophyte Bacillus mycoides Strain GM5LP Isolated from Lolium perenne. Genome Announc. 2018, 6, e00011-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel Motaleb, N.A.; Abd Elhady, S.A.; Ghoname, A.A. AMF and Bacillus megaterium Neutralize the Harmful Effects of Salt Stress On Bean Plants. Gesunde Pflanzen 2019, 72, 29–39. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Zaid, A.; Abo-Baker, A.-B.A.-E.; Salem, W.; Abu Alhmad, M.F. Mitigation of Copper Stress in Maize by Inoculation with Paenibacillus polymyxa and Bacillus circulans. Plants 2020, 9, 1513. [Google Scholar] [CrossRef] [PubMed]
- Kranthi, K.R.; Stone, G.D. Long-term impacts of Bt cotton in India. Nat. Plants 2020, 6, 188–196. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, S.; Chai, Y.; Clardy, J.; Kolter, R.; Guo, J.-H.; Losick, R. A Bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants. Mol. Microbiol. 2012, 85, 418–430. [Google Scholar] [CrossRef] [Green Version]
- de Lima, B.C.; Moro, A.L.; Santos, A.C.P.; Bonifacio, A.; Araujo, A.S.F.; de Araujo, F.F. Bacillus subtilis ameliorates water stress tolerance in maize and common bean. J. Plant Interact. 2019, 14, 432–439. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Lee, I.-J. Gibberellins producing Bacillus methylotrophicus KE2 supports plant growth and enhances nutritional metabolites and food values of lettuce. Plant Physiol. Biochem. 2016, 109, 181–189. [Google Scholar] [CrossRef]
- Park, Y.-G.; Mun, B.-G.; Kang, S.-M.; Hussain, A.; Shahzad, R.; Seo, C.-W.; Kim, A.-Y.; Lee, S.-U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef] [Green Version]
- Woo, O.-G.; Kim, H.; Kim, J.-S.; Keum, H.L.; Lee, K.-C.; Sul, W.J.; Lee, J.-H. Bacillus subtilis strain GOT9 confers enhanced tolerance to drought and salt stresses in Arabidopsis thaliana and Brassica campestris. Plant Physiol. Biochem. 2020, 148, 359–367. [Google Scholar] [CrossRef]
- Thumanu, K.; Sompong, M.; Phansak, P.; Nontapot, K.; Buensanteai, N. Use of infrared microspectroscopy to determine leaf biochemical composition of cassava in response to Bacillus subtilis CaSUT007. J. Plant Interact. 2015, 10, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, S.K.; Arumugam, C.; Sajitha, A.S.; Rao, S.P.; Selvaraj, S.; Vetrivel, R.; Selvarajan, R.; Alosaimi, A.M.; Khan, A.; Hussein, M.A.; et al. Paradisiaca/Solanum Tuberosum Biowaste Composited with Graphene Oxide for Flexible Supercapacitor. J. New Mater. Electrochem. Syst. 2021, 24, 21–28. [Google Scholar] [CrossRef]
- Bahadir, P.S.; Liaqat, F.; Eltem, R. Plant growth promoting properties of phosphate solubilizing Bacillus species isolated from the Aegean Region of Turkey. Turk. J. Bot. 2018, 42, 183–196. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator—Siderophore: A review. Microbiol. Res. 2018, 212–213, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Sood, G.; Kaushal, R.; Sharma, M. Significance of inoculation with Bacillus subtilis to alleviate drought stress in wheat (Triticum aestivum L.). Vegetos 2020, 33, 782–792. [Google Scholar] [CrossRef]
- Shah, R.; Amaresan, N.; Patel, P.; Jinal, H.N.; Krishnamurthy, R. Isolation and Characterization of Bacillus spp. Endowed with Multifarious Plant Growth-Promoting Traits and Their Potential Effect on Tomato (Lycopersicon esculentum) Seedlings. Arab. J. Sci. Eng. 2020, 45, 4579–4587. [Google Scholar] [CrossRef]
- Thilagar, G.; Bagyaraj, D.J.; Podile, A.R.; Vaikuntapu, P.R. Bacillus sonorensis, a Novel Plant Growth Promoting Rhizobacterium in Improving Growth, Nutrition and Yield of Chilly (Capsicum annuum L.). Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2016, 88, 813–818. [Google Scholar] [CrossRef]
- Mendis, H.C.; Thomas, V.P.; Schwientek, P.; Salamzade, R.; Chien, J.-T.; Waidyarathne, P.; Kloepper, J.; De La Fuente, L. Strain-specific quantification of root colonization by plant growth promoting rhizobacteria Bacillus firmus I-1582 and Bacillus amyloliquefaciens QST713 in non-sterile soil and field conditions. PLoS ONE 2018, 13, e0193119. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.J.; Zahir, Z.A.; Naveed, M.; Mitter, B.; Sessitsch, A. Cadmium-tolerant bacteria induce metal stress tolerance in cereals. Environ. Sci. Pollut. Res. 2014, 21, 11054–11065. [Google Scholar] [CrossRef]
- Kaloterakis, N.; van Delden, S.H.; Hartley, S.; De Deyn, G.B. Silicon application and plant growth promoting rhizobacteria consisting of six pure Bacillus species alleviate salinity stress in cucumber (Cucumis sativus L). Sci. Hortic. 2021, 288, 110383. [Google Scholar] [CrossRef]
- Jamil, N.; Hyder, S.; Valipour, M.; Yasir, M.; Iqbal, R.; Roy, R.; Zafar, M.U.; Ahmed, A. Evaluation of the Bioremediation Potential of Staphlococcus lentus Inoculations of Plants as a Promising Strategy Used to Attenuate Chromium Toxicity. Sustainability 2022, 14, 13056. [Google Scholar] [CrossRef]
- Fayaz, S.; Kanth, R.H.; Bhat, T.A.; Valipour, M.; Iqbal, R.; Munir, A.; Nazir, A.; Mir, M.S.; Ahanger, S.A.; Al-Ashkar, I.; et al. Leaf Color Chart (LCC)-Based Precision Nitrogen Management for Assessing Phenology, Agrometeorological In-dices and Sustainable Yield of Hybrid Maize Genotypes under Temperate Climate. Agronomy 2022, 12, 2981. [Google Scholar] [CrossRef]
- Mazlan, N.A.; Zaki, N.A.M.; Narashid, R.H.; Talib, N.; Manokaran, J.; Arshad, F.C.; Fauzi, S.S.M.; Dom, N.C.; Valipour, M.; Dambul, R.; et al. COVID-19 Restriction Movement Control Order (MCO) Impacted Emissions of Peninsular Malaysia Using Sentinel-2a and Sentinel-5p Satellite. Earth Syst. Environ. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.W.; Nafees, M.; Valipour, M.; Iqbal, R.; Ali, S.; Asad, H.U.; Faried, H.N.; Aslam, M.; Iqbal, J.; Shahzad, M.A. Postharvest Eucalyptus Leaf Extract Application Extends the Sustainable Supply of Strawberries by Retaining Physicochemical Quality during Cold Storage. Sustainability 2022, 14, 14822. [Google Scholar] [CrossRef]



| Bacillus Species | Plant Species | Pathogens | References |
|---|---|---|---|
| Bacillus subtilis | Wheat | Rhizoctonia cerealis | [34] |
| Bacillus velezensis | Pear fruits | Apergillus westerdijkiae | [35] |
| Bacillus amyloliquefaciens | Rice grains | Aspergillus westerdijkiae | [36] |
| Bacillus cereus | Potato | Fusarium oxysporum | [37] |
| Bacillus subtilis | Tomato | Pythium ultimum | [38] |
| Bacillus spp. | - | Rhizoctonia solani | [39] |
| Bacillus velezensis | - | Fusarium oxysporum, F. graminearum, Botrytis cinerea, Alternaria alternata, Fulvia fulva, and Ustilaginoidea virens. | [40] |
| Bacillus amyloliquefaciens | Tomato | Fusarium oxysporum | [41] |
| Bacillus amyloliquefaciens and Bacillus subtilis | Tomato | Botrytis cinerea | [42] |
| Bacillus amyloliquefaciens | Mustard | Sclerotinia sclerotiorum | [43] |
| B. vallismortis, B. amyloliquefaciens and B. thuringiensis | Eggplant | Ralstonia solanacearum | [44] |
| Bacillus spp. | Sweet pepper | Phytophthora capsici | [45] |
| Bacillus velezensis | Maize crop | Fusarium graminearum and F. culmorum | [46] |
| Bacillus velezensis | Pepper | Botrytis cinerea | [47] |
| Bacillus spp. | Plant Species | Impact | References |
|---|---|---|---|
| Bacillus licheniformis | Zea mays | Drought tolerance | [145] |
| Bacillus pumilus | Triticum aestivum | PGPR under salinity stress | [146] |
| Bacillus cereus | Solanum nigrum | IAA producer | [147] |
| Bacillus velezensis | Solanum lycopersicum | Biofilm formation | [148] |
| B. subtilis | Phaseolus vulgaris | Bio fertilizer | [149] |
| B. pumilus | Triticum aestivum | Biofilm formation | [150] |
| Bacillus pumilus | Fagopyrum esculentum | Antifungal impact | [151] |
| Bacillus amyloliquefaciens | Solanum tuberosum | Disease management | [152] |
| Lysinibacillus fusiformis | Cicer arietinum | Anti-fungal activity | [153] |
| Bacillus mycoides | Lolium perenne | PGPR | [154] |
| Priestia megaterium | Phaseolus vulgaris L. | Mitigate salinity stress | [155] |
| Paenibacillus polymyxa and Bacillus circulans | Zea mays | Copper stress tolerance | [156] |
| Bacillus thuringiensis | Gossypium herbaceum | Genetically modified crop (insecticide) | [157] |
| Bacillus subtilis | Lycopersicon esculentum, Zea mays | Biofilm formation ameliorates water stress | [158,159] |
| Bacillus methylotrophicus | Lactuca sativa | GAs production | [160] |
| Bacillus pumilus | Zea mays | N2 –fixation | [63] |
| Bacillus aryabhattai | Glycine max | Phytohormones (ABA, IAA, CKs, GAs) production | [161] |
| Bacillus subtilis | Arabidopsis thaliana and Brassia campestris | Drought and salt stresses | [162] |
| B. subtilis | Manihot esculenta | Acts as PGPR and promotes biomass | [163] |
| B. amyloliquefaciens | Musa paradisiaca | IAA | [164] |
| Bacillus megaterium | Solanum melongena | P-Solubilization | [165] |
| Bacillus thuringiensis, P. megaterium and Bacillus subtilis | Cicer arietinum | Drought tolerance | [166] |
| Bacillus subtilis | Triticum aestivum L. | Alleviate drought stress | [167] |
| Bacillus cereus, Bacillus velezensis and Bacillus thuringiensis | Lycopersicon esculentum | PGPR | [168] |
| Bacillus sonorensis | Capsicum annuum L. | P-solubilizer, siderophore, chitinase, IAA, hydrogen cyanide, and biofilm formation | [169]. |
| Bacillus firmus and Bacillus amyloliquefaciens | Zea mays and Glycine max | PGPR | [170] |
| B. thuringiensis | Lavandula dentate | Drought tolerance | [171] |
| Bacillus licheniformis, Bacillus subtilis, Bacillus amyloliquefaciens, Bacillus mycoides, Bacillus methylotropicus | Cucumis sativus L. | Reduce salinity stress | [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.R.; Mustafa, A.; Hyder, S.; Valipour, M.; Rizvi, Z.F.; Gondal, A.S.; Yousuf, Z.; Iqbal, R.; Daraz, U. Bacillus spp. as Bioagents: Uses and Application for Sustainable Agriculture. Biology 2022, 11, 1763. https://doi.org/10.3390/biology11121763
Khan AR, Mustafa A, Hyder S, Valipour M, Rizvi ZF, Gondal AS, Yousuf Z, Iqbal R, Daraz U. Bacillus spp. as Bioagents: Uses and Application for Sustainable Agriculture. Biology. 2022; 11(12):1763. https://doi.org/10.3390/biology11121763
Chicago/Turabian StyleKhan, Aimen Razzaq, Adeena Mustafa, Sajjad Hyder, Mohammad Valipour, Zarrin Fatima Rizvi, Amjad Shahzad Gondal, Zubaida Yousuf, Rashid Iqbal, and Umar Daraz. 2022. "Bacillus spp. as Bioagents: Uses and Application for Sustainable Agriculture" Biology 11, no. 12: 1763. https://doi.org/10.3390/biology11121763
APA StyleKhan, A. R., Mustafa, A., Hyder, S., Valipour, M., Rizvi, Z. F., Gondal, A. S., Yousuf, Z., Iqbal, R., & Daraz, U. (2022). Bacillus spp. as Bioagents: Uses and Application for Sustainable Agriculture. Biology, 11(12), 1763. https://doi.org/10.3390/biology11121763






