High Resilience and Fast Acclimation Processes Allow the Antarctic Moss Bryum argenteum to Increase Its Carbon Gain in Warmer Growing Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
- Acclimation processes in Antarctic mosses are fast: short-term, ‘pulse’ chamber experiments can be used to simulate seasonal changes.
- Acclimation processes in Antarctic mosses mitigate negative effects of increased temperatures through thermal acclimation of DR.
- Physiological plasticity and acclimation potential will provide insights into the general inherent resilience of Antarctic mosses and into their adaptability to longer-term threats and stressors.
2. Materials and Methods
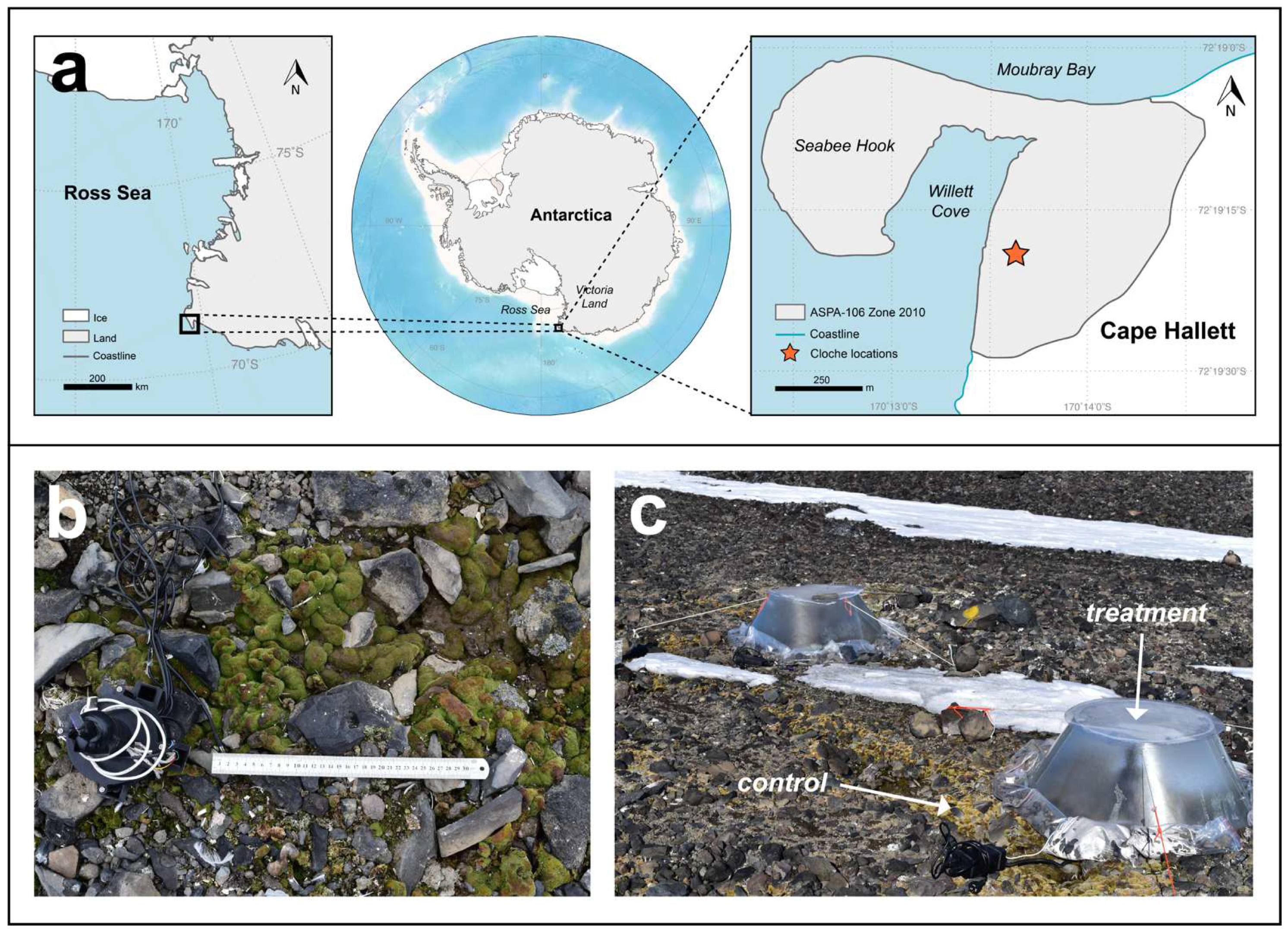
2.1. Study Site
2.2. Climate
2.3. Investigated Species
2.4. Pulse Warming Experiment
2.4.1. Gas-Exchange Measurements
2.4.2. Pulse Warming Event Measurements
2.5. Seasonal Measurements
2.6. Measurements of Chlorophyll Contents
2.7. Calculations and Statistical Analysis
2.7.1. Cardinal Points of Photosynthesis and Respiration
2.7.2. Carbon Use Efficiency
2.7.3. Acclimation Potential
3. Results
3.1. Pulse Warming Experiment
3.1.1. Conditions
3.1.2. Physiological Responses
3.2. Seasonal Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chown, S.L.; Lee, J.E.; Hughes, K.A.; Barnes, J.; Barrett, P.J.; Bergstrom, D.M.; Convey, P.; Cowan, D.A.; Crosbie, K.; Dyer, G.; et al. Challenges to the Future Conservation of the Antarctic. Science 2012, 337, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Jump, A.; Peñuelas, J. Running to Stand Still: Adaptation and the Response of Plants to Rapid Climate Change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.A.; Klekociuk, A.R.; King, D.H.; Rojas, M.P.; Zúñiga, G.E.; Bergstrom, D.M. The 2019/2020 Summer of Antarctic Heatwaves. Glob. Change Biol. 2020, 26, 3178–3180. [Google Scholar] [CrossRef]
- Convey, P.; Peck, L.S. Antarctic Environmental Change and Biological Responses. Sci. Adv. 2019, 5, eaaz0888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colesie, C.; Walshaw, C.V.; Sancho, L.G.; Davey, M.P.; Gray, A. Antarctica’s Vegetation in a Changing Climate. WIREs Clim. Change 2022, e810. [Google Scholar] [CrossRef]
- Siegert, M.; Atkinson, A.; Banwell, A.; Brandon, M.; Convey, P.; Davies, B.; Downie, R.; Edwards, T.; Hubbard, B.; Marshall, G.; et al. The Antarctic Peninsula Under a 1.5°C Global Warming Scenario. Front. Environ. Sci. 2019, 7. [Google Scholar] [CrossRef]
- Reed, A.J.; Thatje, S. Long-Term Acclimation and Potential Scope for Thermal Resilience in Southern Ocean Bivalves. Mar. Biol. 2015, 162, 2217–2224. [Google Scholar] [CrossRef]
- Molina-Montenegro, M.A.; Naya, D.E. Latitudinal Patterns in Phenotypic Plasticity and Fitness-Related Traits: Assessing the Climatic Variability Hypothesis (CVH) with an Invasive Plant Species. PLoS ONE 2012, 7, e47620. [Google Scholar] [CrossRef] [Green Version]
- Pannewitz, S.; Green, T.G.A.; Maysek, K.; Schlensog, M.; Seppelt, R.D.; Sancho, L.G.; Türk, R.; Schroeter, B. Photosynthetic Responses of Three Common Mosses from Continental Antarctica. Antarct. Sci. 2005, 17, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Marshall, G.J. Temperature. In Encyclopedia of the Antarctic; Riffenburgh, B., Ed.; Routledge: New York, NY, USA, 2007; pp. 986–988. ISBN 978-0-203-94318-2. [Google Scholar]
- Sun, B.; Williams, C.M.; Li, T.; Speakman, J.R.; Jin, Z.; Lu, H.; Luo, L.; Du, W. Higher Metabolic Plasticity in Temperate Compared to Tropical Lizards Suggests Increased Resilience to Climate Change. Ecol. Monogr. 2022, 92, e1512. [Google Scholar] [CrossRef]
- Peralta, G.; Godoy, O.; Egea, L.G.; de Los Santos, C.B.; Jiménez-Ramos, R.; Lara, M.; Brun, F.G.; Hernández, I.; Olivé, I.; Vergara, J.J.; et al. The Morphometric Acclimation to Depth Explains the Long-Term Resilience of the Seagrass Cymodocea Nodosa in a Shallow Tidal Lagoon. J. Environ. Manag. 2021, 299, 113452. [Google Scholar] [CrossRef]
- Visram, S.; Douglas, A.E. Resilience and Acclimation to Bleaching Stressors in the Scleractinian Coral Porites Cylindrica. J. Exp. Mar. Biol. Ecol. 2007, 349, 35–44. [Google Scholar] [CrossRef]
- Culumber, Z.; Monks, S. Resilience to Extreme Temperature Events: Acclimation Capacity and Body Condition of a Polymorphic Fish in Response to Thermal Stress. Biol. J. Linn. Soc. 2014, 111, 504–510. [Google Scholar] [CrossRef] [Green Version]
- King, A.W.; Gunderson, C.A.; Post, W.M.; Weston, D.J.; Wullschleger, S.D. Plant Respiration in a Warmer World. Science 2006, 312, 536–537. [Google Scholar] [CrossRef]
- Körner, C. Significance of Temperature in Plant Life. In Plant Growth and Climate Change; Morison, J.I.L., Morecroft, M.D., Eds.; Blackwell Publishing Ltd: Hoboken, NJ, USA, 2006; Volume 100, pp. 48–66. ISBN 978-0-470-99418-4. [Google Scholar]
- Vanderwel, M.C.; Slot, M.; Lichstein, J.W.; Reich, P.B.; Kattge, J.; Atkin, O.K.; Bloomfield, K.J.; Tjoelker, M.G.; Kitajima, K. Global Convergence in Leaf Respiration from Estimates of Thermal Acclimation across Time and Space. New Phytol. 2015, 207, 1026–1037. [Google Scholar] [CrossRef]
- Oechel, W.C.; Vourlitis, G.L.; Hastings, S.J.; Zulueta, R.C.; Hinzman, L.; Kane, D. Acclimation of Ecosystem CO2 Exchange in the Alaskan Arctic in Response to Decadal Climate Warming. Nature 2000, 406, 978–981. [Google Scholar] [CrossRef]
- Luo, Y.; Wan, S.; Hui, D.; Wallace, L.L. Acclimatization of Soil Respiration to Warming in a Tall Grass Prairie. Nature 2001, 413, 622–625. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature Sensitivity of Soil Carbon Decomposition and Feedbacks to Climate Change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Wythers, K.R.; Reich, P.B.; Tjoelker, M.G.; Bolstad, P.B. Foliar Respiration Acclimation to Temperature and Temperature Variable Q10 Alter Ecosystem Carbon Balance. Glob. Change Biol. 2005, 11, 435–449. [Google Scholar] [CrossRef]
- Larigauderie, A.; Körner, C. Acclimation of Leaf Dark Respiration to Temperature in Alpine and Lowland Plant Species. Ann. Bot. 1995, 76, 245–252. [Google Scholar] [CrossRef]
- Lange, O.L.; Green, T.G.A. Lichens Show That Fungi Can Acclimate Their Respiration to Seasonal Changes in Temperature. Oecologia 2005, 142, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Hurry, V. A Plant for All Seasons: Alterations in Photosynthetic Carbon Metabolism during Cold Acclimation in Arabidopsis. Curr. Opin. Plant Biol. 2002, 5, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, S.J.; Hajela, R.K.; Thomashow, M.F. Cold Acclimation in Arabidopsis Thaliana. Plant Physiol. 1988, 87, 745–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, D.W.; Gifford, R.M.; Hilbert, D.; Luo, Y. Integration of Photosynthetic Acclimation to CO2 at the Whole-Plant Level. Glob. Change Biol. 1998, 4, 879–893. [Google Scholar] [CrossRef] [Green Version]
- Gauslaa, Y.; Alam, M.A.; Solhaug, K.A. How to Optimize Lichen Relative Growth Rates in Growth Cabinets. Lichenol. 2016, 48, 305–310. [Google Scholar] [CrossRef]
- Kennedy, A.D. Antarctic Fellfield Response to Climate Change: A Tripartite Synthesis of Experimental Data. Oecologia 1996, 107, 141–150. [Google Scholar] [CrossRef]
- Sáez, P.L.; Cavieres, L.A.; Galmés, J.; Gil-Pelegrín, E.; Peguero-Pina, J.J.; Sancho-Knapik, D.; Vivas, M.; Sanhueza, C.; Ramírez, C.F.; Rivera, B.K.; et al. In Situ Warming in the Antarctic: Effects on Growth and Photosynthesis in Antarctic Vascular Plants. New Phytol. 2018, 218, 1406–1418. [Google Scholar] [CrossRef] [Green Version]
- Wynn-Williams, D.D. Response of Pioneer Soil Microalgal Colonists to Environmental Change in Antarctica. Microb. Ecol. 1996, 31, 177–188. [Google Scholar] [CrossRef]
- Smith, R.I.L. Plant Colonisation Response to Climate Change in the Antarctic. Folia Fac. Sci. Nat. Univ. Masaryk. Brun. Geogr. 2001, 25, 19–33. [Google Scholar]
- Kennedy, A.D. Simulated Climate Change: Are Passive Greenhouses a Valid Microcosm for Testing the Biological Effects of Environmental Perturbations? Glob. Change Biol. 1995, 1, 29–42. [Google Scholar] [CrossRef]
- Aronson, E.; Mcnulty, S. Appropriate Experimental Ecosystem Warming Methods by Ecosystem, Objective, and Practicality. Agric. For. Meteorol. 2009, 149, 1791–1799. [Google Scholar] [CrossRef]
- Frei, E.R.; Schnell, L.; Vitasse, Y.; Wohlgemuth, T.; Moser, B. Assessing the Effectiveness of In-Situ Active Warming Combined With Open Top Chambers to Study Plant Responses to Climate Change. Front. Plant Sci. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Ochyra, R.; Lewis Smith, R.I.; Bednarek-Ochyra, H. The Illustrated Moss Flora of Antarctica. Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Perera-Castro, A.V.; González-Rodríguez, Á.M.; Fernández-Marín, B. When Time Is Not of the Essence: Constraints to the Carbon Balance of Bryophytes. J. Exp. Bot. 2022, 73, 4562–4575. [Google Scholar] [CrossRef]
- Perera-Castro, A.V.; Waterman, M.J.; Turnbull, J.D.; Ashcroft, M.B.; McKinley, E.; Watling, J.R.; Bramley-Alves, J.; Casanova-Katny, A.; Zuniga, G.; Flexas, J.; et al. It Is Hot in the Sun: Antarctic Mosses Have High Temperature Optima for Photosynthesis Despite Cold Climate. Front. Plant Sci. 2020, 11, 1178. [Google Scholar] [CrossRef]
- Perera-Castro, A.V.; Flexas, J.; González-Rodríguez, Á.M.; Fernández-Marín, B. Photosynthesis on the Edge: Photoinhibition, Desiccation and Freezing Tolerance of Antarctic Bryophytes. Photosynth. Res. 2021, 149, 135–153. [Google Scholar] [CrossRef]
- Folgar-Cameán, Y.; Barták, M. Evaluation of Photosynthetic Processes in Antarctic Mosses and Lichens Exposed to Controlled Rate Cooling: Species-Specific Responses. Czech Polar Rep. 2019, 9, 114–124. [Google Scholar] [CrossRef]
- Schroeter, B.; Green, T.G.A.; Pintado, A.; Türk, R.; Sancho, L.G. Summer Activity Patterns for a Moss and Lichen in the Maritime Antarctic with Respect to Altitude. Polar Biol. 2021, 44, 2117–2137. [Google Scholar] [CrossRef]
- Green, T.G.A.; Sancho, L.G.; Pintado, A.; Schroeter, B. Functional and Spatial Pressures on Terrestrial Vegetation in Antarctica Forced by Global Warming. Polar Biol. 2011, 34, 1643. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Yu, Q.; Fan, F.; Liu, S.; Feng, G.; Zhang, P. A CPD Photolyase Gene PnPHR1 from Antarctic Moss Pohlia Nutans Is Involved in the Resistance to UV-B Radiation and Salinity Stress. Plant Physiol. Biochem. 2021, 167, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Waterman, M.J.; Bramley-Alves, J.; Miller, R.E.; Keller, P.A.; Robinson, S.A. Photoprotection Enhanced by Red Cell Wall Pigments in Three East Antarctic Mosses. Biol. Res. 2018, 51, 49. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Zhang, W.; Chen, K.; Zhang, P. Transcriptional Profiling and Physiological Analysis Reveal the Critical Roles of ROS-Scavenging System in the Antarctic Moss Pohlia Nutans under Ultraviolet-B Radiation. Plant Physiol. Biochem. 2019, 134, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, J.D.; Robinson, S.A. Accumulation of DNA Damage in Antarctic Mosses: Correlations with Ultraviolet-B Radiation, Temperature and Turf Water Content Vary among Species. Glob. Change Biol. 2009, 15, 319–329. [Google Scholar] [CrossRef]
- Pizarro, M.; Contreras, R.A.; Köhler, H.; Zúñiga, G.E. Desiccation Tolerance in the Antarctic Moss Sanionia Uncinata. Biol. Res. 2019, 52, 46. [Google Scholar] [CrossRef] [Green Version]
- Takezawa, D. Mechanisms Underlying Freezing and Desiccation Tolerance in Bryophytes. Adv. Exp. Med. Biol. 2018, 1081, 167–187. [Google Scholar] [CrossRef]
- Roads, E.; Longton, R.E.; Convey, P. Millennial Timescale Regeneration in a Moss from Antarctica. Curr. Biol. 2014, 24, 222–223. [Google Scholar] [CrossRef] [Green Version]
- Pisa, S.; Biersma, E.M.; Convey, P.; Patiño, J.; Vanderpoorten, A.; Werner, O.; Ros, R.M. The Cosmopolitan Moss Bryum Argenteum in Antarctica: Recent Colonisation or in Situ Survival? Polar Biol. 2014, 37, 1469–1477. [Google Scholar] [CrossRef] [Green Version]
- Green, T.G.A.; Seppelt, R.D.; Brabyn, L.R.; Beard, C.; Türk, R.; Lange, O.L. Flora and Vegetation of Cape Hallett and Vicinity, Northern Victoria Land, Antarctica. Polar Biol. 2015, 38, 1825–1845. [Google Scholar] [CrossRef]
- Hofstee, E.H.; Balks, M.R.; Petchey, F.; Campbell, D.I. Soils of Seabee Hook, Cape Hallett, Northern Victoria Land, Antarctica. Antarct. Sci. 2006, 18, 473–486. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, B.J.; Jaco Klok, C.; Scott, M.B.; Terblanche, J.S.; Chown, S.L. Diurnal Variation in Supercooling Points of Three Species of Collembola from Cape Hallett, Antarctica. J. Insect Physiol. 2003, 49, 1049–1061. [Google Scholar] [CrossRef]
- Brabyn, L.; Beard, C.; Seppelt, R.D.; Rudolph, E.D.; Türk, R.; Green, T.G.A. Quantified Vegetation Change over 42 Years at Cape Hallett, East Antarctica. Antarct. Sci. 2006, 18, 561–572. [Google Scholar] [CrossRef] [Green Version]
- Colesie, C.; Pan, Y.; Cary, S.C.; Gemal, E.; Brabyn, L.; Kim, J.-H.; Green, T.G.A.; Lee, C.K. The Longest Baseline Record of Vegetation Dynamics in Antarctica Reveals Acute Sensitivity to Water Availability. Earth’s Future 2022, 10, e2022EF002823. [Google Scholar] [CrossRef]
- Guthridge, G.G. Hallett Station, Antarctica, 1956–1973. Antarct. J. U.S. 1983, 18, 1–8. [Google Scholar]
- Campbell, D.; MacCulloch, R.J.L.; Campbell, I. Thermal Regimes of Some Soils in the McMurdo Sound Region, Antarctica. In Ecosystem Processes in Antarctic Ice-Free Landscapes; CRC Press: Boca Raton, FL, USA, 1997; pp. 45–55. [Google Scholar]
- Ellis, L.T.; Bednarek-Ochyra, H.; Cykowska, B.; Ochyra, R.; Garcia, C.; Sérgio, C.; Lebouvier, M.; Manolaki, P.; Giannouris, E.; Kadis, C.; et al. New National and Regional Bryophyte Records, 30. J. Bryol. 2012, 34, 45–51. [Google Scholar] [CrossRef]
- Seppelt, R.D.; Green, T.G.A. A Bryophyte Flora for Southern Victoria Land, Antarctica. N. Z. J. Bot. 1998, 36, 617–635. [Google Scholar] [CrossRef]
- Secretariat of the Antarctic Treaty. Management Plan for Antarctic Specially Protected Area (ASPA) No. 106—Cape Hallett, Northern Victoria Land, Ross Sea (170° 14′ E, 72° 19′ S); The Antarctic Treaty System: Sofia, Bulgaria, 2015; pp. 1–17. [Google Scholar]
- Green, T.G.A.; Kulle, D.; Pannewitz, S.; Sancho, L.G.; Schroeter, B. UV-A Protection in Mosses Growing in Continental Antarctica. Polar Biol. 2005, 28, 822–827. [Google Scholar] [CrossRef]
- Hills, S.F.K.; Stevens, M.I.; Gemmill, C.E.C. Molecular Support for Pleistocene Persistence of the Continental Antarctic Moss Bryum Argenteum. Antarct. Sci. 2010, 22, 721–726. [Google Scholar] [CrossRef]
- Pannewitz, S.; Green, T.G.A.; Scheidegger, C.; Schlensog, M.; Schroeter, B. Activity Pattern of the Moss Hennediella Heimii (Hedw.) Zand. in the Dry Valleys, Southern Victoria Land, Antarctica during the Mid-Austral Summer. Polar Biol. 2003, 26, 545–551. [Google Scholar] [CrossRef]
- Segreto, R.; Hassel, K.; Bardal, R.; Stenøien, H.K. Desiccation Tolerance and Natural Cold Acclimation Allow Cryopreservation of Bryophytes without Pretreatment or Use of Cryoprotectants. Bryologist 2010, 113, 760–769. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S. Modelling of Photosynthetic Response to Environmental Conditions. In Physiological Plant Ecology II: Water Relations and Carbon Assimilation; Encyclopedia of Plant Physiology; Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 549–587. ISBN 978-3-642-68150-9. [Google Scholar]
- de Lobo, F.A.; de Barros, M.P.; Dalmagro, H.J.; Dalmolin, Â.C.; Pereira, W.E.; de Souza, É.C.; Vourlitis, G.L.; Ortíz, C.E. Fitting Net Photosynthetic Light-Response Curves with Microsoft Excel—A Critical Look at the Models. Photosynthetica 2013, 51, 445–456. [Google Scholar] [CrossRef]
- Ronen, R.; Galun, M. Pigment Extraction from Lichens with Dimethyl Sulfoxide (DMSO) and Estimation of Chlorophyll Degradation. Environ. Exp. Bot. 1984, 24, 239–245. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Sterratt, D.C.; Lyngholm, D.; Willshaw, D.J.; Thompson, I.D. Standard Anatomical and Visual Space for the Mouse Retina: Computational Reconstruction and Transformation of Flattened Retinae with the Retistruct Package. PLoS Comput. Biol. 2013, 9, e1002921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95364-9. [Google Scholar]
- Ise, T.; Litton, C.M.; Giardina, C.P.; Ito, A. Comparison of Modeling Approaches for Carbon Partitioning: Impact on Estimates of Global Net Primary Production and Equilibrium Biomass of Woody Vegetation from MODIS GPP. J. Geophys. Res. Biogeosciences 2010, 115, 1–11. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Ågren, G.I. Environmental and Stoichiometric Controls on Microbial Carbon-Use Efficiency in Soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Street, L.E.; Subke, J.-A.; Sommerkorn, M.; Sloan, V.; Ducrotoy, H.; Phoenix, G.K.; Williams, M. The Role of Mosses in Carbon Uptake and Partitioning in Arctic Vegetation. New Phytol. 2013, 199, 163–175. [Google Scholar] [CrossRef]
- Way, D.A.; Yamori, W. Thermal Acclimation of Photosynthesis: On the Importance of Adjusting Our Definitions and Accounting for Thermal Acclimation of Respiration. Photosynth. Res. 2014, 119, 89–100. [Google Scholar] [CrossRef]
- Atkin, O.K.; Bloomfield, K.J.; Reich, P.B.; Tjoelker, M.G.; Asner, G.P.; Bonal, D.; Bönisch, G.; Bradford, M.G.; Cernusak, L.A.; Cosio, E.G.; et al. Global Variability in Leaf Respiration in Relation to Climate, Plant Functional Types and Leaf Traits. New Phytol. 2015, 206, 614–636. [Google Scholar] [CrossRef] [Green Version]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Photosynthesis. In Plant Physiological Ecology; Springer: New York, NY, USA, 2008; ISBN 978-0-387-78341-3. [Google Scholar]
- Slot, M.; Kitajima, K. General Patterns of Acclimation of Leaf Respiration to Elevated Temperatures across Biomes and Plant Types. Oecologia 2015, 177, 885–900. [Google Scholar] [CrossRef] [Green Version]
- Seneviratne, S.I.; Nicholls, N.; Easterling, D.; Goodess, C.M.; Kanae, S.; Kossin, J.; Luo, Y.; Marengo, J.; McInnes, K.; Rahimi, M.; et al. Changes in Climate Extremes and Their Impacts on the Natural Physical Environment. In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation; Field, C.B., Barros, V., Stocker, T.F., Dahe, Q., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 109–230. ISBN 978-1-139-17724-5. [Google Scholar]
- Sancho, L.G.; Allan Green, T.G.; Pintado, A. Slowest to Fastest: Extreme Range in Lichen Growth Rates Supports Their Use as an Indicator of Climate Change in Antarctica. Flora—Morphol. Distrib. Funct. Ecol. Plants 2007, 202, 667–673. [Google Scholar] [CrossRef]
- Schroeter, B.; Green, T.G.A.; Kulle, D.; Pannewitz, S.; Schlensog, M.; Sancho, L.G. The Moss Bryum Argenteum Var. Muticum Brid. Is Well Adapted to Cope with High Light in Continental Antarctica. Antarct. Sci. 2012, 24, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Boardman, N.K. Comparative Photosynthesis of Sun and Shade Plants. Annu. Rev. Plant Physiol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Green, T.G.A.; Schroeter, B.; Seppelt, R. Effect of Temperature, Light and Ambient UV on the Photosynthesis of the Moss Bryum Argenteum Hedw. In Continental Antarctica. In Antarctic Ecosystems: Models for Wider Understanding; William Davison, C., Howard-Williams, P.B., Eds.; New Zealand Natural Sciences: Canterbury, New Zealand, 2000; pp. 165–170. [Google Scholar]
- Bokhorst, S.; Convey, P.; Huiskes, A.; Aerts, R. Usnea Antarctica, an Important Antarctic Lichen, Is Vulnerable to Aspects of Regional Environmental Change. Polar Biol. 2016, 39, 511–521. [Google Scholar] [CrossRef]
- Newsham, K.K.; Hodgson, D.A.; Murray, A.W.A.; Peat, H.J.; Smith, R.I.L. Response of Two Antarctic Bryophytes to Stratospheric Ozone Depletion. Glob. Change Biol. 2002, 8, 972–983. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.; Colwell, S.R.; Marshall, G.J.; Lachlan-Cope, T.A.; Carleton, A.M.; Jones, P.D.; Lagun, V.; Reid, P.A.; Iagovkina, S. Antarctic Climate Change during the Last 50 Years. Int. J. Climatol. 2005, 25, 279–294. [Google Scholar] [CrossRef]
- Feron, S.; Cordero, R.R.; Damiani, A.; Malhotra, A.; Seckmeyer, G.; Llanillo, P. Warming Events Projected to Become More Frequent and Last Longer across Antarctica. Sci. Rep. 2021, 11, 19564. [Google Scholar] [CrossRef]
- Meredith, M.; Sommerkorn, M.; Cassotta, S.; Derksen, C.; Ekaykin, A.; Hollowed, A.; Kofinas, G.; Mackintosh, A.; Melbourne-Thomas, J.; Muelbert, M.M.C.; et al. Polar Regions. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019; pp. 203–320. [Google Scholar]
- González-Herrero, S.; Barriopedro, D.; Trigo, R.M.; López-Bustins, J.A.; Oliva, M. Climate Warming Amplified the 2020 Record-Breaking Heatwave in the Antarctic Peninsula. Commun. Earth Environ. 2022, 3, 122. [Google Scholar] [CrossRef]
- Royles, J.; Griffiths, H. Invited Review: Climate Change Impacts in Polar Regions: Lessons from Antarctic Moss Bank Archives. Glob. Change Biol. 2015, 21, 1041–1057. [Google Scholar] [CrossRef] [Green Version]
- Guglielmin, M.; Fratte, M.D.; Cannone, N. Permafrost Warming and Vegetation Changes in Continental Antarctica. Environ. Res. Lett. 2014, 9, 045001. [Google Scholar] [CrossRef]



| CUE | Significance | |||
|---|---|---|---|---|
| Temperature (°C) | Control | Treatment | p | t |
| 2 | 78.7 ± 2.1 | 85.1 ± 3.3 | 0.19 | −1.65 |
| 5 | 64.1 ± 6.2 | 73.6 ± 6.8 | 0.33 | −1.03 |
| 10 | 42.20 ± 9.6 | 60.2 ± 7.3 | 0.17 | −1.49 |
| 15 | 38.0 ± 5.5 | 53.7 ± 6.8 | 0.10 | −1.81 |
| 20 | 28.8 ± 3.14 | 32.6 ± 9.9 | 0.73 | −0.36 |
| 25 | 21.7 ± 6.4 | 21.3 ± 5.7 | 0.97 | 0.38 |
| 30 | 25.7 ± 7.2 | 12.9 ± 9.3 | 0.35 | 1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gemal, E.L.; Green, T.G.A.; Cary, S.C.; Colesie, C. High Resilience and Fast Acclimation Processes Allow the Antarctic Moss Bryum argenteum to Increase Its Carbon Gain in Warmer Growing Conditions. Biology 2022, 11, 1773. https://doi.org/10.3390/biology11121773
Gemal EL, Green TGA, Cary SC, Colesie C. High Resilience and Fast Acclimation Processes Allow the Antarctic Moss Bryum argenteum to Increase Its Carbon Gain in Warmer Growing Conditions. Biology. 2022; 11(12):1773. https://doi.org/10.3390/biology11121773
Chicago/Turabian StyleGemal, Emma L., T. G. Allan Green, S. Craig Cary, and Claudia Colesie. 2022. "High Resilience and Fast Acclimation Processes Allow the Antarctic Moss Bryum argenteum to Increase Its Carbon Gain in Warmer Growing Conditions" Biology 11, no. 12: 1773. https://doi.org/10.3390/biology11121773
APA StyleGemal, E. L., Green, T. G. A., Cary, S. C., & Colesie, C. (2022). High Resilience and Fast Acclimation Processes Allow the Antarctic Moss Bryum argenteum to Increase Its Carbon Gain in Warmer Growing Conditions. Biology, 11(12), 1773. https://doi.org/10.3390/biology11121773





