Impacts of Ecological Shading by Roadside Trees on Tea Foliar Nutritional and Bioactive Components, Community Diversity of Insects and Soil Microbes in Tea Plantation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
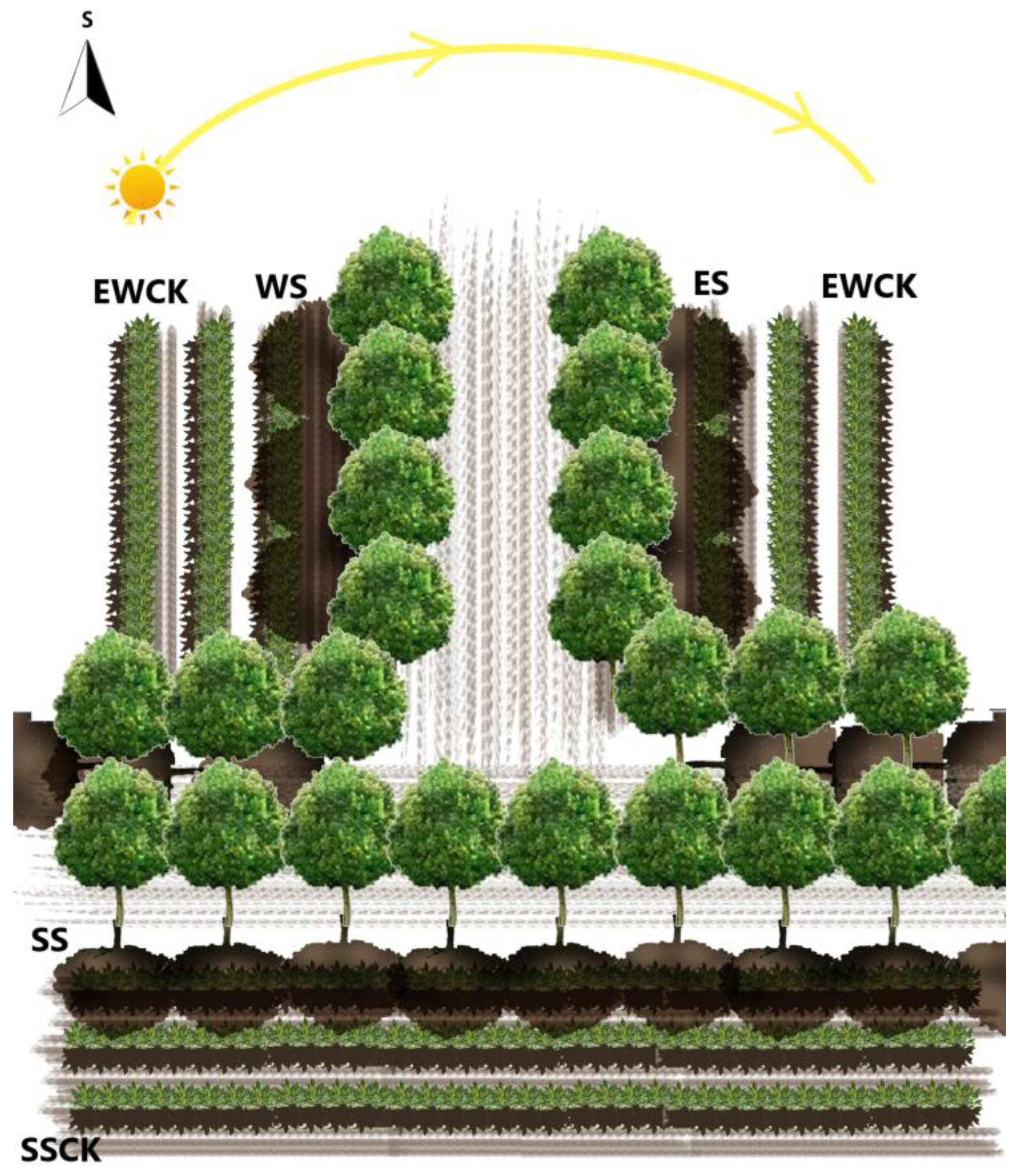
2.1. Experimental Site Description
2.2. Determination of Foliar Nutritional and Bioactive Components of Plants
2.2.1. Foliar Nutrient Contents
2.2.2. Foliar Bioactive Component Contents
2.2.3. Leaf Quality Index
2.3. Insect Investigation
2.4. Composition and Diversity of Soil Microbial Community
2.5. Data Analysis
3. Results
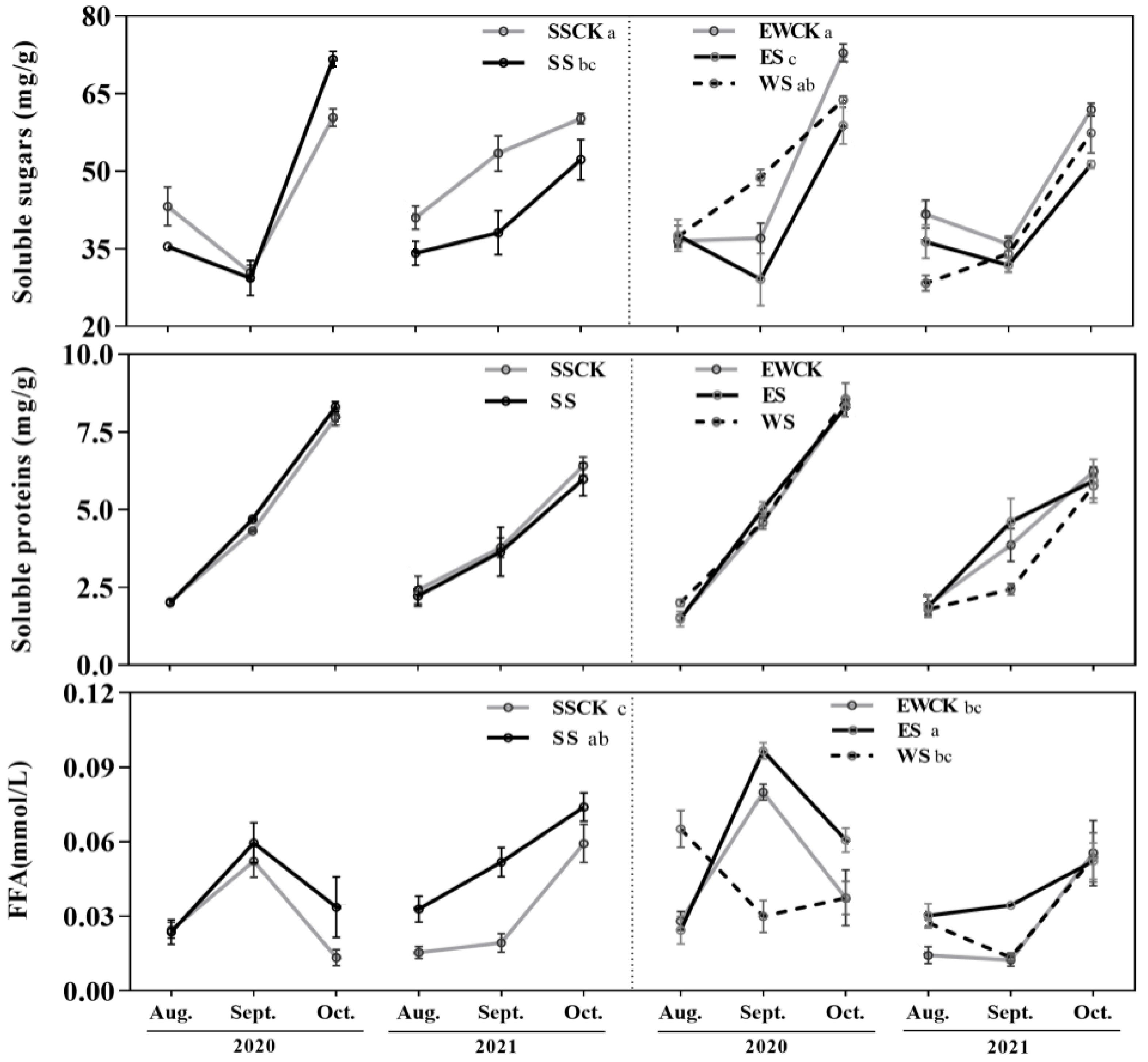
3.1. Effects of Roadside Trees Ecological Shading on the Foliar Soluble Nutrients of Tea Plants
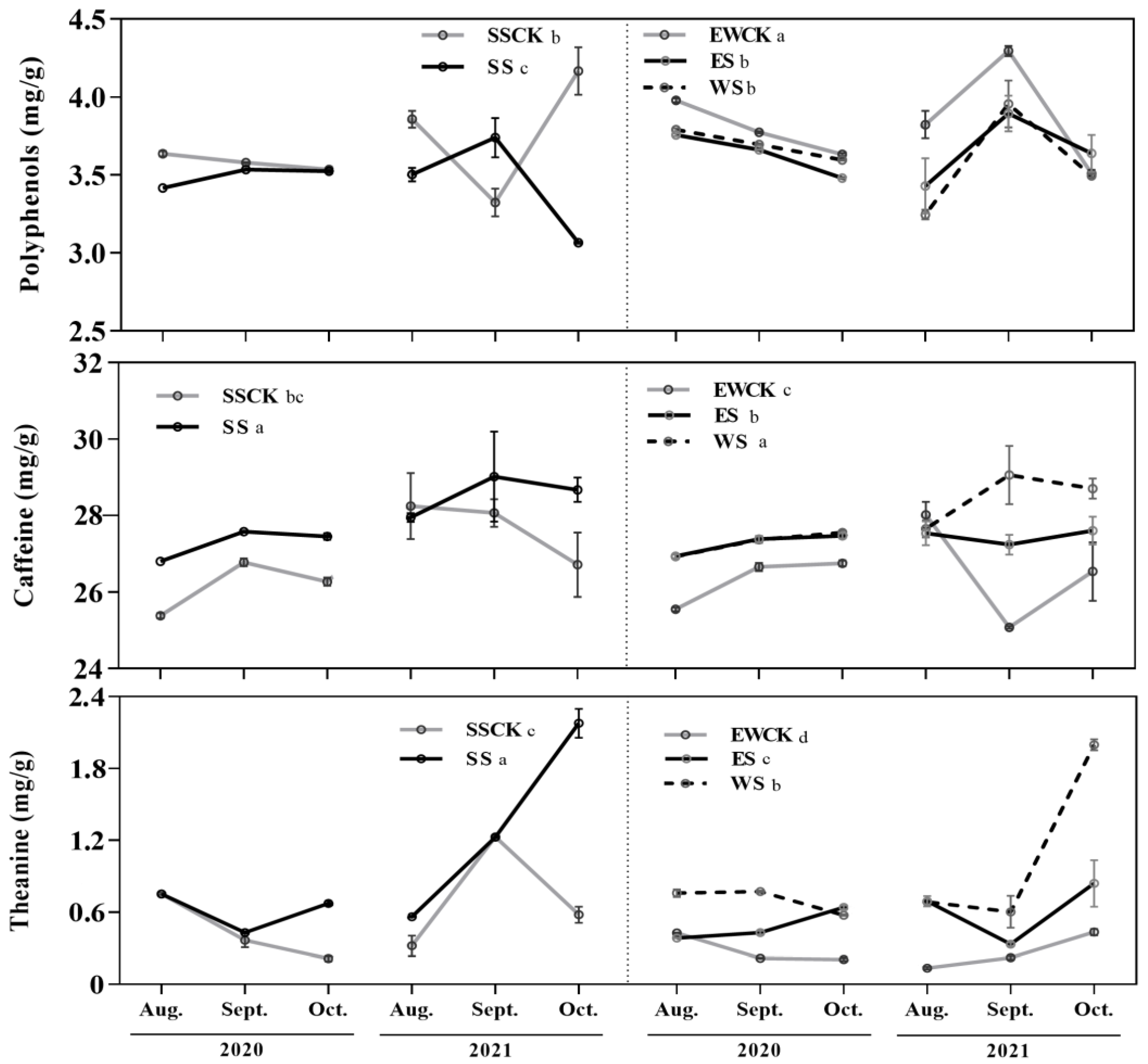
3.2. Effects of Roadside Tree Ecological Shading on the Bioactive Components of Tea Plants
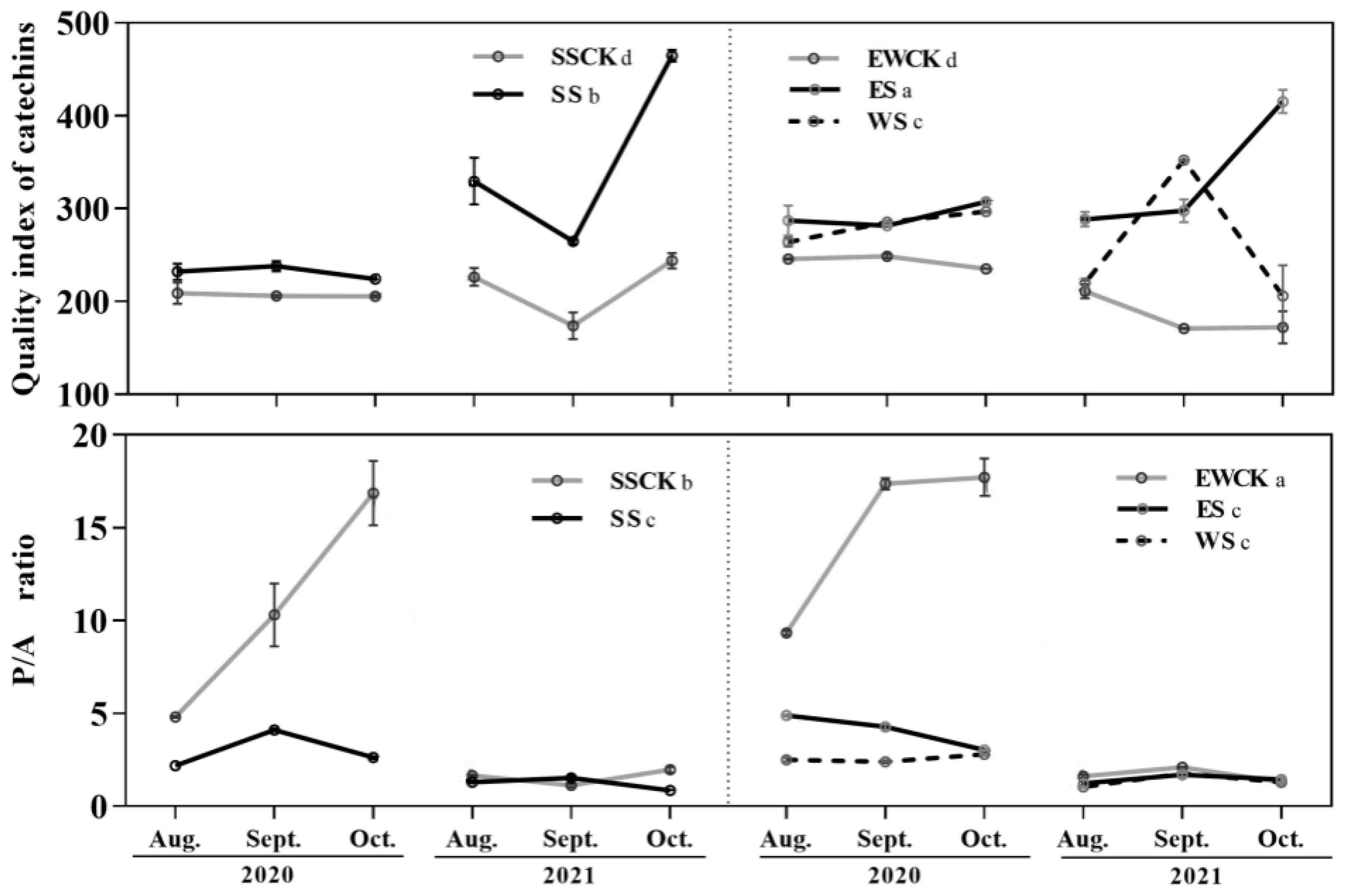
3.3. Effects of Roadside Tree Ecological Shading on the Leaf Quality Indices of Tea Plants
3.4. Effects of Roadside Tree Ecological Shading on Population Occurrence and Community Diversity of Insects in the Tea Plantation
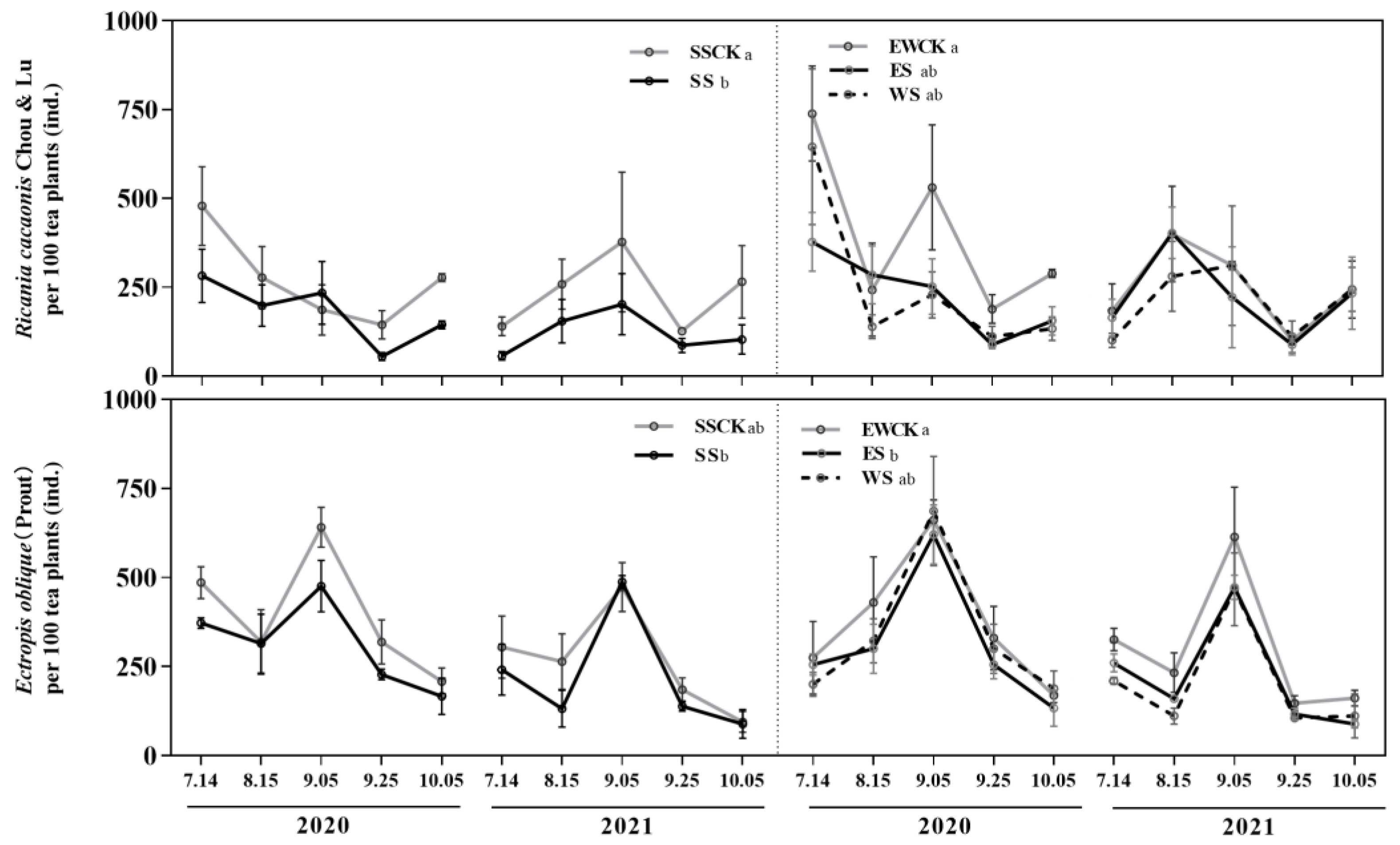
3.4.1. Population Occurrence of Two Key Species of Pests
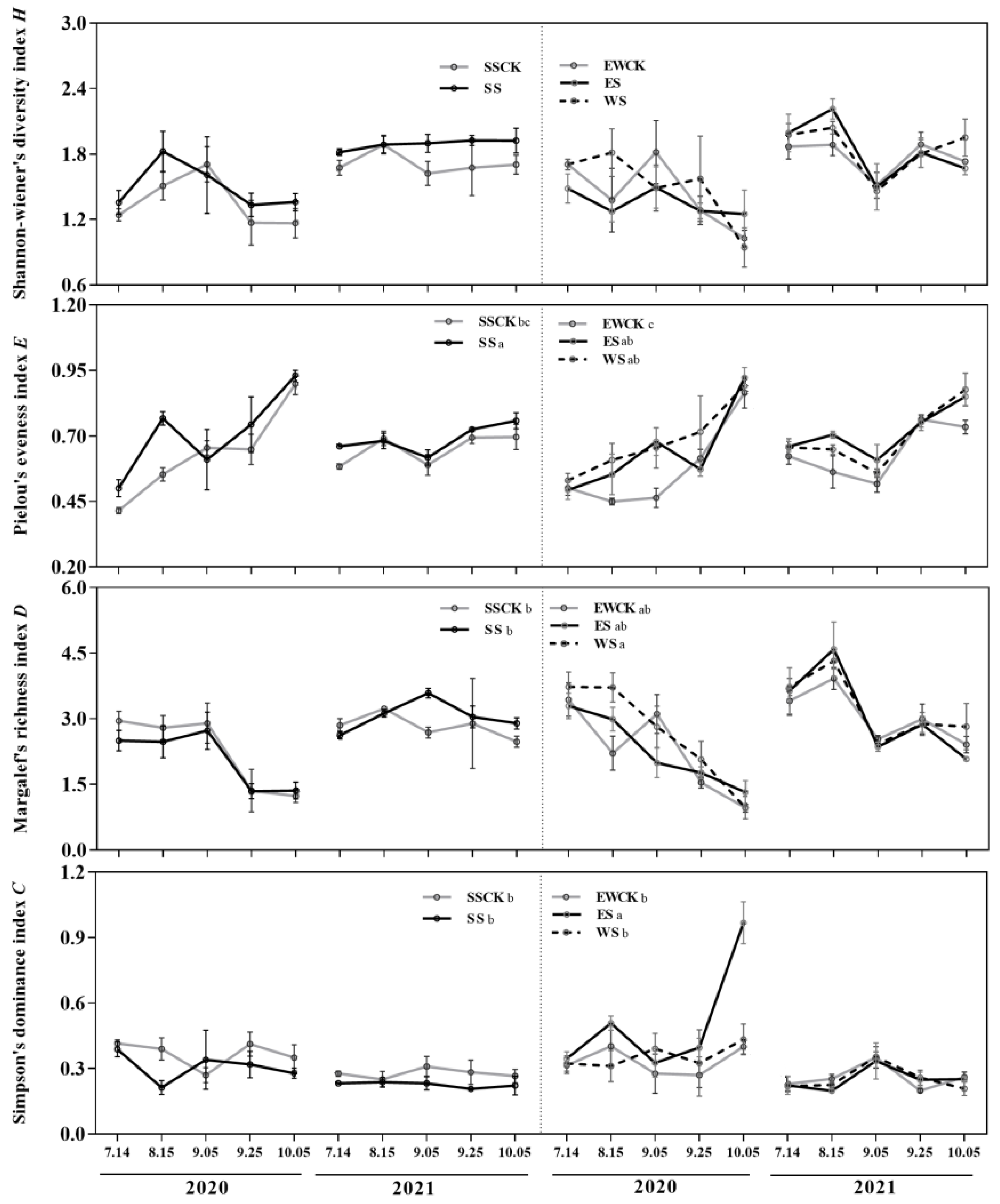
3.4.2. Community Diversity of Insects
3.5. Effects of Roadside Tree Ecological Shading on the Diversity of Soil Microbes
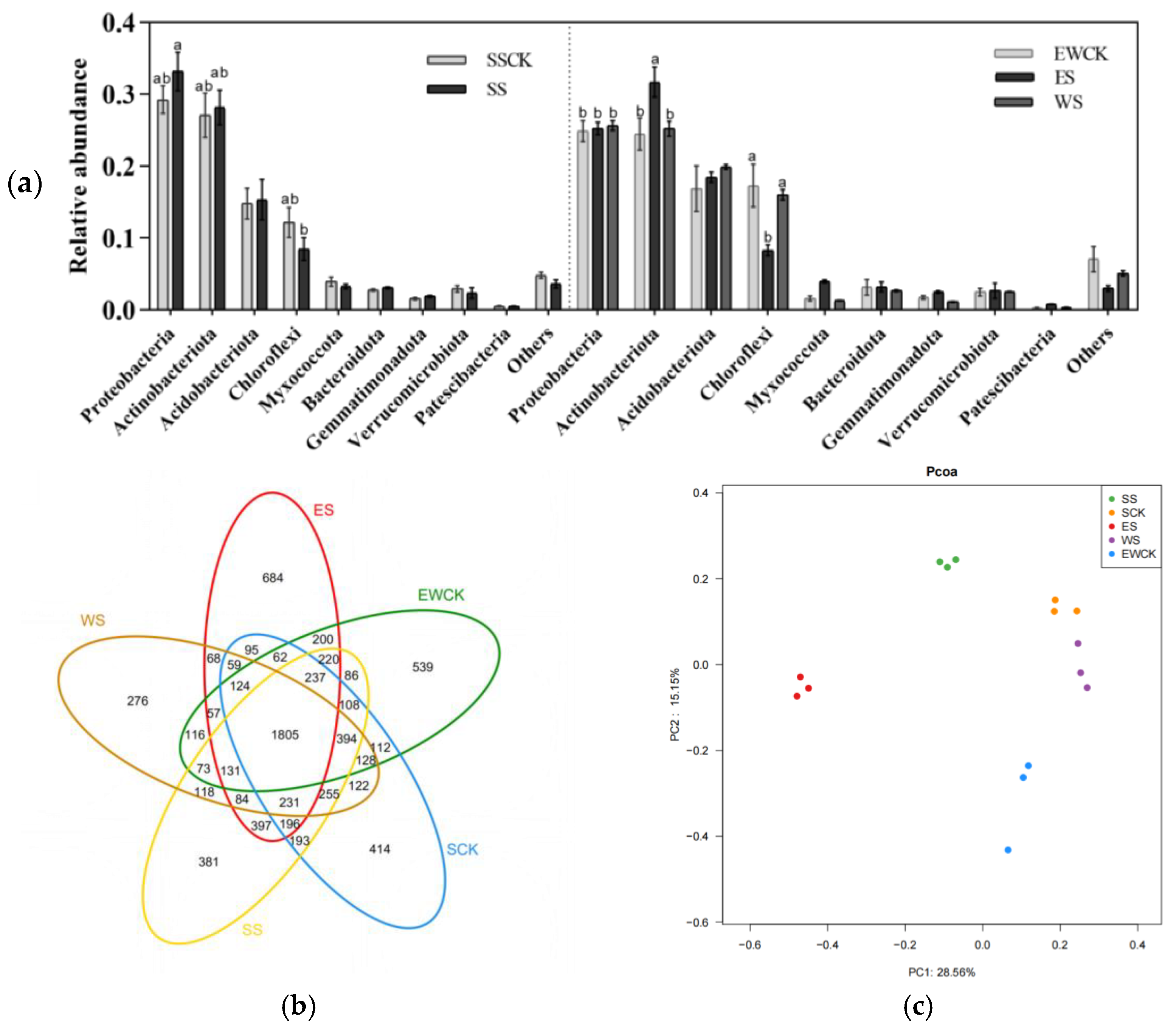
3.5.1. Taxonomic Composition of Soil Microbes
3.5.2. Community Diversity of Soil Microbes
4. Discussion
4.1. Effects of Roadside Tree Ecological Shading on the Foliar Nutrients, Bioactive Compounds, and Quality Indexes of Tea
4.2. Effects of Roadside Tree Ecological Shading on the Population Dynamics and Community Diversity of Insects in Tea Plantations
4.3. Effects of Roadside Tree Ecological Shading on the Soil Microbial Diversity in Tea Plantations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Treatments | Ecological Shading (S) | Sampling Years (Y) | S × Y |
|---|---|---|---|
| ES vs. EWCK | 1739.2/<0.001 *** | 18.05/<0.001 *** | 2.05/0.16 |
| WS vs. EWCK2 | 3322.8/<0.001 *** | 8.22/0.007 ** | 0.07/0.79 |
| SS vs. SSCK | 3472.8/<0.001 *** | 5.70/0.02 * | 0.06/0.81 |
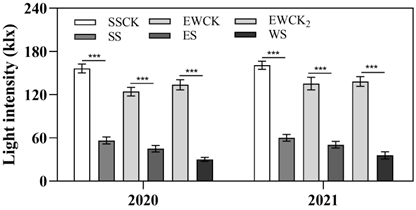 | Note: Effects of ecological shading from roadside trees on the light intensity on the tea canopy.(SS and SSCK: 9:00 am–3:00 pm; ES and EWCK: 6:00 am–12:00 am; WS and EWCK2: 12:00 am–6:00 pm. *** indicated significantly different among the 3 ecological shading treatments and the 2 controls SSCK and EWCK using an LSD test at p < 0.05. Bars represent standard deviation). | ||
Appendix B
| Measured Indexes | SS/ SSCK | ES/ EWCK | WS/ EWCK | SSCK/ EWCK | SS/ES | SS/WS | ES/WS | Ecological-Shade Effects | Rowing-Direction Effects | |
|---|---|---|---|---|---|---|---|---|---|---|
| Foliar soluble nutrients | Soluble sugars | −(SS & ES); WS>SS>ES | / | |||||||
| Soluble proteins | / | / | ||||||||
| Free fatty acids | +(SS & ES); ES>SS>WS | / | ||||||||
| Foliar bioactive components | Polyphenols | −(ES & WS>SS); ES&WS>SS | +(EWCK) | |||||||
| Caffeine | +(ES & WS>SS); ES & WS>SS | / | ||||||||
| Theanine | +(SS>WS>ES); SS>WS>ES | −(EWCK) | ||||||||
| Leaf quality | Catechin quality index | +(ES>SS>WS); ES>WS>SS | / | |||||||
| Phenol/ammonia ratio | −(ES&WS&SS); ES,WS,SS | +(EWCK) | ||||||||
| Population dynamics of insect pests | R. cacaonis | −(SS); ES & WS>SS | / | |||||||
| E. bolique | −(ES); WS>ES & SS | / | ||||||||
| Community diversity of insects | H | / | / | |||||||
| E | +(SS, ES, WS); SS>ES&WS | / | ||||||||
| D | /;WS>ES>SS | / | ||||||||
| C | +(ES); ES>SS & WS | / | ||||||||
| Community diversity of soil microbial microorganisms | Chao1 | +(SS & ES); SS>ES>WS | / | |||||||
| H | +(WS); WS>SS & WS | −(EWCK) | ||||||||
| E | +(ES); ES & SS>WS | / | ||||||||
| C | +(ES); ES & SS>WS | −(EWCK) | ||||||||
 Significant decrease;
Significant decrease;  Significant increase;
Significant increase;  No significant difference; + Increase; − Decrease; / no effect.
No significant difference; + Increase; − Decrease; / no effect.References
- Giacinto, J.J.; Fricker, G.A.; Ritter, M.; Yost, J.; Doremus, J. Urban forest biodiversity and cardiovascular disease: Potential health benefits from California’s street trees. PLoS ONE 2021, 16, e0254973. [Google Scholar] [CrossRef] [PubMed]
- Dumbaugh, E. Safe streets, livable streets. J. Am. Plan. Assoc. 2005, 71, 283–298. [Google Scholar] [CrossRef]
- McPherson, E.G.; Simpson, J.R.; Peper, P.J.; Maco, S.E.; Xiao, Q. Municipal forest benefits and costs in five U.S. cities. J. For. 2005, 103, 411–416. [Google Scholar] [CrossRef]
- Grote, M.; Williams, I.; Preston, J.; Kemp, S. Including congestion effects in urban road traffic co2 emissions modelling: Do local government authorities have the right options? Transp. Res Part D-Transp. Environ. 2016, 43, 95–106. [Google Scholar] [CrossRef]
- Vailshery, L.S.; Jaganmohan, M.; Nagendra, H. Effect of street trees on microclimate and air pollution in a tropical city. Urban For. Urban Green. 2013, 12, 408–415. [Google Scholar] [CrossRef]
- Ozdemir, H. Mitigation impact of roadside trees on fine particle pollution. Sci. Total Environ. 2019, 659, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.; Alagaraj, P.; Arumugam, B. A review on pharmacological activities and active phytoconstituents of roadside trees of tamil nadu. Cardiovasc. Hematol. Agents Med. Chem. 2021, 19, 150–163. [Google Scholar] [CrossRef]
- Bennett, A.F. Linkages in the Landscape. The Role of Corridors and Connectivity in Wildlife Conservation. In Pacific Conservation Biology IUCN; Thanet Press Ltd.: Margate, UK, 2003. [Google Scholar] [CrossRef]
- Fischer, C.; Hanslin, H.M.; Hovstad, K.A.; D’Amico, M.; Kollmann, J.; Kroeger, S.B.; Bastianelli, G.; Habel, J.C.; Rygne, H.; Lennartsson, T. The contribution of roadsides to connect grassland habitat patches for butterflies in landscapes of contrasting permeability. J. Environ. Manage 2022, 311, 114846. [Google Scholar] [CrossRef]
- Forfert, N.; Troxler, A.; Retschnig, G.; Gauthier, L.; Straub, L.; Moritz, R.F.A.; Neumann, P.; Williams, G.R. Neonicotinoid pesticides can reduce honeybee colony genetic diversity. PLoS ONE 2017, 12, e0186109. [Google Scholar] [CrossRef]
- Muturi, E.J.; Donthu, R.K.; Fields, C.J.; Moise, I.K.; Kim, C.H. Effect of pesticides on microbial communities in container aquatic habitats. Sci. Rep. 2017, 7, 44565. [Google Scholar] [CrossRef]
- Thomine, E.; Mumford, J.; Rusch, A.; Desneux, N. Using crop diversity to lower pesticide use: Socio-ecological approaches. Sci. Total Environ. 2022, 804, 150156. [Google Scholar] [CrossRef] [PubMed]
- Pena, J.C.; Martello, F.; Ribeiro, M.C.; Armitage, R.A.; Young, R.J.; Rodrigues, M. Street trees reduce the negative effects of urbanization on birds. PLoS ONE 2017, 12, e0174484. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Eldridge, D.J. Roadside verges support greater ecosystem functions than adjacent agricultural land in a grassy woodland. J. Environ. Manage 2022, 308, 114625. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, D.J.; Delgado-Baquerizo, M.; Quero, J.L.; Ochoa., V.; Maestre., F.T. Surface indicators are correlated with soil multifunctionality in global drylands. J. Appl. Ecol. 2019, 57, 424–435. [Google Scholar] [CrossRef]
- Ding, J.; Travers, S.K.; Eldridge, D.J. Microbial communities are associated with indicators of soil surface condition across a continental gradient. Geoderma 2022, 405, 115439. [Google Scholar] [CrossRef]
- Wood, E.M.; Esaian, S. The importance of street trees to urban avifauna. Ecol. Appl. 2020, 30, e02149. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Nimmo, D.; Bennett, A.F. At the crossroads: Does the configuration of roadside vegetation affect woodland bird communities in rural landscapes? PLoS ONE 2016, 11, e0155219. [Google Scholar] [CrossRef]
- Morrison, E.B.; Lindell, C.A. Birds and bats reduce insect biomass and leaf damage in tropical forest restoration sites. Ecol. Appl. 2012, 22, 1526–1534. [Google Scholar] [CrossRef]
- Napoli, M.; Massetti, L.; Brandani, G.; Petralli, M.; Orlandini, S. Modeling tree shade effect on urban ground surface temperature. J. Environ. Qual. 2016, 45, 146–156. [Google Scholar] [CrossRef]
- Fu, J.; Luo, Y.; Sun, P.; Gao, J.; Zhao, D.; Yang, P.; Hu, T. Effects of shade stress on turfgrasses morphophysiology and rhizosphere soil bacterial communities. BMC Plant Biol. 2020, 20, 92. [Google Scholar] [CrossRef]
- Chen, J.; Wu, S.; Dong, F.; Li, J.; Zeng, L.; Tang, J.; Gu, D. Mechanism underlying the shading-induced chlorophyll accumulation in tea leaves. Front. Plant Sci. 2021, 12, 779819. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Tanaka, Y.; Umetsu, K.; Morita, S.; Ono, Y.; Suzuki, T.; Takemoto, T.; Morita, A.; Ikka, T. Phenotypic markers reflecting the status of overstressed tea plants subjected to repeated shade cultivation. Front. Plant Sci. 2020, 11, 556476. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Takemoto, T.; Ogihara, A.; Suzuki, K.; Masumura, T.; Satoh, S.; Takano, K.; Mimura, Y.; Morita, S. Stress responses of shade-treated tea leaves to high light exposure after removal of shading. Plants 2020, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, T.; Hayashi, K. Effect of the difference in the covering methods on growth of tea tree and the canopy surface temperature in summer based on thermal images. Tea Res. J. 2019, 127, 1–10, (In Japanese with English Abstract). [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sano, H. Pathogen resistance of transgenic tobacco plants producing caffeine. Phytochemistry 2008, 69, 882–888. [Google Scholar] [CrossRef]
- Su, Y.L.; Leung, L.K.; Huang, Y.; Chen, Z.Y. Stability of tea theaflavins and catechins. Food Chem. 2003, 83, 189–195. [Google Scholar] [CrossRef]
- Wang, H.F.; Tsai, Y.S.; Lin, M.L.; Ou, A.S.M. Comparison of bioactive components in GABA tea and green tea produced in Taiwan. Food Chem. 2006, 96, 648–653. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Nguyen, M.; Roach, P.D. Extraction and isolation of catechins from tea. J. Sep. Sci. 2010, 33, 3415–3428. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Li, Z.X.; Zhang, L.; Wei, J.P.; Shen, C.; Yan, P.; Zhang, L.P.; Han, W.Y. Brassinosteroids improve quality of summer tea (Camellia sinensis L.) by balancing biosynthesis of polyphenols and amino Acids. Front. Plant Sci. 2016, 7, 1304. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, D141-5. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yang, Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function function. Crit. Rev. Food Sci. Nutr. 2020, 60, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, Y.; Mei, X.; Katsuno, T.; Kobayashi, E.; Dong, F.; Watanabe, N.; Yang, Z. Regulation of formation of volatile compounds of tea (Camellia sinensis) leaves by single light wavelength. Sci. Rep. 2015, 5, 16858. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, X.; Mei, X.; Zhou, Y.; Cheng, S.; Zeng, L.; Dong, F.; Yang, Z. Proteolysis of chloroplast proteins is responsible for accumulation of free amino acids in dark-treated tea (Camellia sinensis) leaves. J. Proteomics 2017, 157, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liao, Y.; Zeng, L.; Dong, F.; Watanabe, N.; Yang, Z. Transformation of catechins into theaflavins by upregulation of CsPPO3 in preharvest tea (Camellia sinensis) leaves exposed to shading treatment. Food Res. Int. 2020, 129, 108842. [Google Scholar] [CrossRef]
- Bamba, T.; Fukusaki, E. Quality evaluation of green tea leaf cultured under artificial light condition using gas chromatography/mass spectrometry. J. Biosci. Bioeng. 2017, 123, 197–202. [Google Scholar] [CrossRef]
- Wit, M.; Galvão, V.C.; Fankhauser, C. Light-mediated hormonal regulation of plant growth and development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef]
- Deng, W.W.; Fei, Y.; Wang, S.; Wan, X.C.; Zhang, Z.Z.; Hu, X.Y. Effect of shade treatment on theanine biosynthesis in Camellia sinensis seedlings. Plant Growth Regul. 2013, 71, 295–299. [Google Scholar] [CrossRef]
- Mohotti, A.J.; Pushpakumara, G.; Singh, V.P. Shade in Tea Plantations: A new dimension with an agroforestry approach for a climate-smart agricultural landscape system. In Agricultural Research for Sustainable Food Systems in Sri Lanka; De Silva, R.P., Pushpakumara, G., Prasada, P., Weerahewa, J., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Crozier, A.; Yokota, T.; Jaganath, I.B.; Marks, S.C.; Saltmarsh, M.; Clifford, M.N. Secondary metabolites in fruits, vegetables, beverages and other plant-based dietary components. Blackwell Oxford 2006, 299, 208–302. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health benefits and chemical composition of matcha green tea: A review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Zeng, L.; Watanabe, N.; Yang, Z. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit Rev Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Juneja, L.R.; Chu, D.C.; Okubo, T.; Nagato, Y.; Yokogoshi, H. L-theanine-A unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci. Technol. 1999, 10, 199–204. [Google Scholar] [CrossRef]
- Dong, C.; Li, F.; Yang, T.; Feng, L.; Zhang, S.; Li, F.; Li, W.; Xu, G.; Bao, S.; Wan, X.; et al. Theanine transporters identified in tea plants (Camellia sinensis L.). Plant J. 2020, 101, 57–70. [Google Scholar] [CrossRef]
- Ai, S.; Wu, R.; Yan, L.; Wu, Y. Measurement of the ratio of tea polyphenols to amino acids in green tea infusion based on near infrared spectroscopy. Adv. Mater. Res. 2011, 301, 1093–1097. [Google Scholar] [CrossRef]
- Ruan, J.; Haerdter, R.; Gerendás, J. Impact of nitrogen supply on carbon/nitrogen allocation: A case study on amino acids and catechins in green tea [Camellia sinensis (L.) O. Kuntze] plants. Plant Biol. 2010, 12, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bi, G.; Li, T.; Wang, Q.; Xing, Z.; LeCompte, J.; Harkess, R.L. Color shade nets affect plant growth and seasonal leaf quality of Camellia sinensis grown in mississippi, the United States. Front. Nutr. 2022, 9, 786421. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.G.; Lee, Y.R.; Lee, M.S.; Hwang, K.H.; Park, C.Y.; Kim, E.H.; Park, J.S.; Hong, Y.S. Diverse metabolite variations in tea (Camellia sinensis L.) leaves grown under various shade conditions revisited: A metabolomics study. J. Agric. Food Chem. 2018, 66, 1889–1897. [Google Scholar] [CrossRef]
- Mohotti, A.J.; Lawlor, D.W. Diurnal variation of photosynthesis and photoinhibition in tea: Effects of irradiance and nitrogen supply during growth in the field. J. Exp. Bot. 2002, 53, 313–322. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, B.J.; Chung, J.O.; Hwang, J.A.; Lee, S.J.; Lee, C.H.; Hong, Y.S. Geographical and climatic dependencies of green tea (Camellia sinensis) metabolites: A 1h NMR-based metabolomics study. J. Agric. Food Chem. 2010, 58, 1058–1059. [Google Scholar] [CrossRef]
- Li, N.; Taylor, L.S.; Mauer, L.J. Degradation kinetics of catechins in green tea powder: Effects of temperature and relative humidity. J. Agric. Food Chem. 2011, 59, 6082–6090. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Dai, X.; Zhu, M.; Zhang, S.; Dai, Q.; Jiang, X.; Liu, Y.; Gao, L.; Xia, T. Evaluation of astringent taste of green tea through mass spectrometry-based targeted metabolic profiling of polyphenols. Food Chem. 2020, 305, 125507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, M.; Mumm, R.; Vos, R.C.H.; Ruan, J. Metabolomics reveals the within-plant spatial effects of shading on tea plants. Tree Physiol. 2021, 41, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Piato, K.; Subía, C.; Pico, J.; Calderón, D.; Norgrove, L.; Lefort, F. Organic farming practices and shade trees reduce pest infestations in robusta coffee systems in Amazonia. Life 2021, 11, 413. [Google Scholar] [CrossRef]
- Bisseleua, D.H.B.; Fotio, D.; Yede; Missoup, A.D.; Vidal, S. Shade tree diversity, cocoa pest damage, yield compensating inputs and farmers’ net returns in West Africa. PLoS ONE 2013, 8, e56115. [Google Scholar] [CrossRef]
- Railsback, S.F.; Johnson, M.D. Effects of land use on bird populations and pest control services on coffee farms. Proc. Natl. Acad. Sci. USA 2014, 111, 6109–6114. [Google Scholar] [CrossRef]
- Kroeger, S.B.; Hanslin, H.M.; Lennartsson, T.; D’Amico, M.; Kollmann, J.; Fischer, C.; Albertsen, E.; Speed, J.D.M. Impacts of roads on bird species richness: A meta-analysis considering road types, habitats and feeding guilds. Sci. Total Environ. 2022, 812, 151478. [Google Scholar] [CrossRef]
- Beillouin, D.; Ben-Ari, T.; Malézieux, E.; Seufert, V.; Makowski, D. Positive but variable effects of crop diversification on biodiversity and ecosystem services. Glob. Chang. Biol. 2021, 27, 4697–4710. [Google Scholar] [CrossRef]
- Das, S.; Sarker, M.; Mukhopadhyay, A. Changing diversity of hymenopteran parasitoids from organically and conventionally managed tea-ecosystem of North Bengal, India. J. Environ. Biol. 2005, 26, 505–509. [Google Scholar]
- Rojas, L.; Godoy, C.; Hanson, P.; Hilje, L. A survey of homopteran species (Auchenorrhyncha) from coffee shrubs and poró and laurel trees in shaded coffee plantations, in Turrialba, Costa Rica. Rev. Biol. Trop. 2001, 49, 1057–1065. Available online: https://pubmed.ncbi.nlm.nih.gov/12189787/ (accessed on 10 October 2022). [PubMed]
- Li, J.; Zhou, Y.; Zhou, B.; Tang, H.; Chen, Y.; Qiao, X.; Tang, J. Habitat management as a safe and effective approach for improving yield and quality of tea (Camellia sinensis) leaves. Sci. Rep. 2019, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, J.; Chen, F. Azotobacter chroococcum inoculation can improve plant growth and resistance of maize to armyworm, Mythimna separata even under reduced nitrogen fertilizer application. Pest. Manag. Sci. 2020, 76, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor--a fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon-a review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef] [PubMed]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharids in the characterization of microbial communities in soil, a review. Biol. Fert. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, U.B.; Sahu, P.K.; Paul, S.; Kumar, A.; Malviya, D.; Singh, S.; Kuppusamy, P.; Singh, P.; Paul, D.; et al. Linking soil microbial diversity to modern agriculture practices: A review. Int. J. Environ. Res. Public Health 2022, 19, 3141. [Google Scholar] [CrossRef]
- Neal, A.L.; Hughes, D.; Clark, I.M.; Jansson, J.K.; Hirsch, P.R. Microbiome aggregated traits and assembly are more sensitive to soil management than diversity. mSystems 2021, 6, e0105620. [Google Scholar] [CrossRef]
- Shigyo, N.; Umeki, K.; Hirao, T. Plant functional diversity and soil properties control elevational diversity gradients of soil bacteria. Fems. Microbiol. Ecol. 2019, 95, fiz025. [Google Scholar] [CrossRef]
- Rao, M.V.; Rice, R.A.; Fleischer, R.C.; Muletz-Wolz, C.R. Soil fungal communities differ between shaded and sun-intensive coffee plantations in El Salvador. PLoS ONE 2020, 15, e0231875. [Google Scholar] [CrossRef]
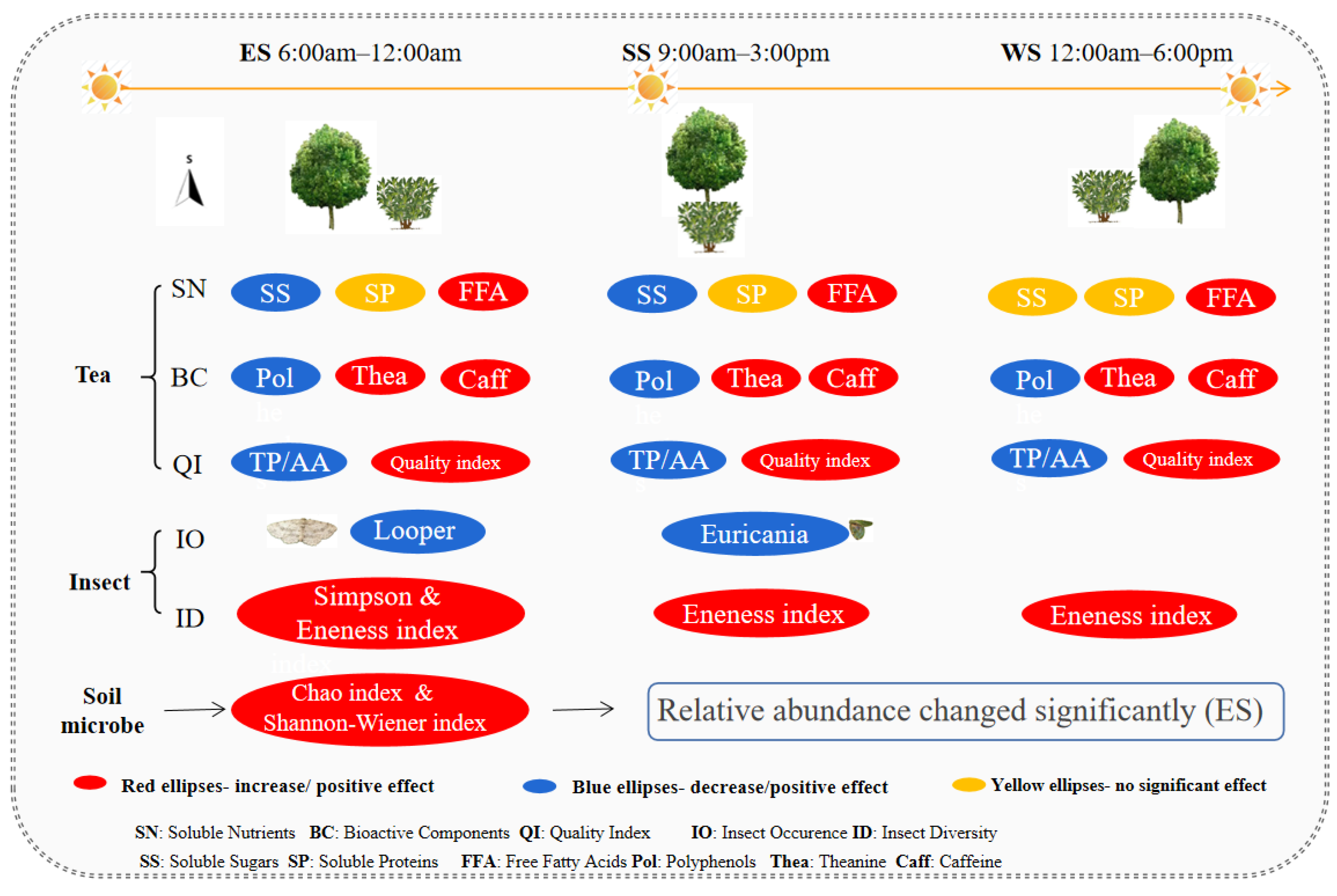
| Measured Indexes | Ecological Shading (S) | Sampling Years (Y) | S×Y | |
|---|---|---|---|---|
| Foliar soluble nutrients | Soluble sugars (mg/g) | 6.04/0.002 ** | 4.40/0.049 * | 6.14/0.002 ** |
| Soluble proteins (mg/g) | 0.91/0.48 | 61.91/<0.001 *** | 2.47/0.08 | |
| Free fatty acids (mmol/L) | 7.10/0.001 ** | 10.13/0.005 ** | 7.44/0.001 ** | |
| Foliar bioactive components | Polyphenols (mg/g) | 34.14/<0.001 *** | 1.34/0.26 | 7.76/<0.001 *** |
| Caffeine (mg/g) | 15.36/<0.001 *** | 37.06/<0.001 *** | 3.72/0.02 * | |
| Theanine (μg/g) | 159.03/<0.001 *** | 216.95/<0.001 *** | 37.36<0.001 *** | |
| Leaf quality | Catechin quality index | 88.73/<0.001 *** | 16.89/0.001 ** | 49.3/<0.001 *** |
| P/A ratio | 103.85/<0.001 *** | 508.74/<0.001 *** | 92.39/<0.001 *** | |
| Population dynamics of two key species | Ricania cacaonis | 4.09/0.01 * | 5.02/0.04 * | 0.78/0.55 |
| Ectropis oblique | 2.94/0.04 * | 32.64/<0.001 *** | 0.40/0.80 | |
| Community diversity of insects | H | 1.20/0.34 | 53.84/<0.001 *** | 0.27/0.90 |
| E | 5.03/0.006 ** | 3.86/0.063 | 1.25/0.32 | |
| D | 2.57/0.07 | 69.41/<0.001 *** | 0.73/0.58 | |
| C | 5.22/0.005 ** | 54.68/<0.001 *** | 4.38/0.01 * | |
| Diversity Indices | SSCK | SS | EWCK | ES | WS | F/p |
|---|---|---|---|---|---|---|
| Chao1 | 4086.3 ± 352.2 a | 4477.9 ± 72.2 a | 3448.0 ± 315.7 b | 4126.7 ± 361.8 a | 3356.8 ± 318.5 b | 7.48/0.005 ** |
| H | 9.95 ± 0.15 ab | 10.12 ± 0.14 a | 9.71 ± 0.26 b | 10.11 ± 0.10 a | 9.69 ± 0.06 b | 5.42/0.01 * |
| E | 0.8449 ± 0.0038 | 0.8539 ± 0.0128 | 0.8423 ± 0.0181 | 0.8557 ± 0.0033 | 0.8428 ± 0.0036 | 1.16/0.38 |
| C | 0.0027 ± 0.0004 | 0.0025 ± 0.0004 | 0.0034 ± 0.0011 | 0.0023 ± 0.0002 | 0.0030 ± 0.0002 | 1.87/0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Zhong, Y.; Yu, H.; Pokharel, S.S.; Fang, W.; Chen, F. Impacts of Ecological Shading by Roadside Trees on Tea Foliar Nutritional and Bioactive Components, Community Diversity of Insects and Soil Microbes in Tea Plantation. Biology 2022, 11, 1800. https://doi.org/10.3390/biology11121800
Zou Y, Zhong Y, Yu H, Pokharel SS, Fang W, Chen F. Impacts of Ecological Shading by Roadside Trees on Tea Foliar Nutritional and Bioactive Components, Community Diversity of Insects and Soil Microbes in Tea Plantation. Biology. 2022; 11(12):1800. https://doi.org/10.3390/biology11121800
Chicago/Turabian StyleZou, Yan, Yanni Zhong, Han Yu, Sabin Saurav Pokharel, Wanping Fang, and Fajun Chen. 2022. "Impacts of Ecological Shading by Roadside Trees on Tea Foliar Nutritional and Bioactive Components, Community Diversity of Insects and Soil Microbes in Tea Plantation" Biology 11, no. 12: 1800. https://doi.org/10.3390/biology11121800
APA StyleZou, Y., Zhong, Y., Yu, H., Pokharel, S. S., Fang, W., & Chen, F. (2022). Impacts of Ecological Shading by Roadside Trees on Tea Foliar Nutritional and Bioactive Components, Community Diversity of Insects and Soil Microbes in Tea Plantation. Biology, 11(12), 1800. https://doi.org/10.3390/biology11121800








