Habitat and Season Effects on Small Mammal Bycatch in Live Trapping
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sites
2.2. Trapping
2.3. Data Analyses
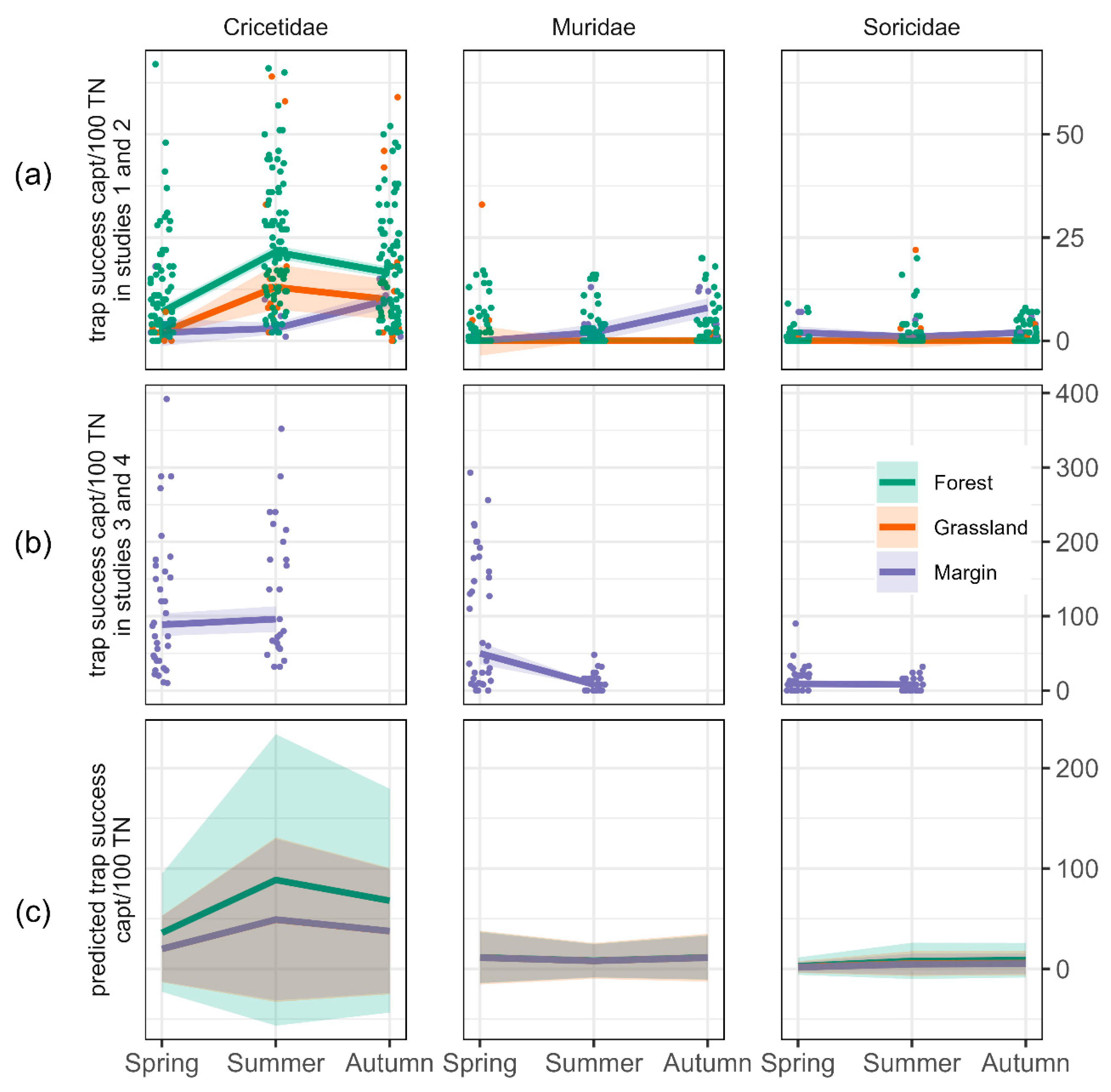
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sullivan, T.P.; Sullivan, D.S.; Ransome, D.B.; Lindgren, P.M.F. Impact of removal-trapping on abundance and diversity attributes in small-mammal communities. Wildl. Soc. Bull. 2003, 31, 464–474. [Google Scholar]
- Baker, S.; Shaw, R.; Atkinson, R.; West, P.; Macdonald, D. Potential welfare impacts of kill-trapping European moles (Talpa europaea) using scissor traps and Duffus traps: A post mortem examination study. Anim. Welf. 2015, 24, 1–14. [Google Scholar] [CrossRef]
- European Union–Canada–Russian Federation. Agreement on international humane trapping standards between the European Community, Canada and the Russian Federation. Off. J. Eur. Communities 1998, L42, 43–57. [Google Scholar]
- Fall, M.W. The search for acceptable animal traps. In Proceedings of the 20th Vertebrate Pest Conference, Reno, NV, USA, 4–7 March 2002; pp. 371–377. [Google Scholar]
- Stephens, R.B.; Anderson, E.M. Effects of trap type on small mammal richness, diversity, and mortality. Wildl. Soc. Bull. 2014, 38, 619–627. [Google Scholar] [CrossRef]
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How many species of mammals are there? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef]
- Gruber, K. Rodent meat—a sustainable way to feed the world?: Using rodents as food has a long tradition in many parts of the world. EMBO Rep. 2016, 17, 630–633. [Google Scholar] [CrossRef]
- Assogbadjo, A.E.; Codjia, J.T.C.; Sinsin, B.; Ekue, M.R.M.; Mensah, G.A. Importance of rodents as a human food source in Benin. Belg. J. Zool. 2005, 135, 11–15. [Google Scholar]
- Kerr, K.R.; Kappen, K.L.; Garner, L.M.; Swanson, K.S. Commercially available avian and mammalian whole prey diet items targeted for consumption by managed exotic and domestic pet felines: Macronutrient, mineral, and long-chain fatty acid composition. Zoo Biol. 2014, 33, 327–335. [Google Scholar] [CrossRef]
- Strege, G. Fur as fashion in America. Fash. Style Pop. Cult. 2014, 1, 413–432. [Google Scholar] [CrossRef]
- Hitchens, P.L.; Booth, R.H.; Stevens, K.; Murphy, A.; Jones, B.; Hemsworth, L.M. The welfare of animals in Australian filmed media. Animals 2021, 11, 1986. [Google Scholar] [CrossRef]
- Brown, C.J.; Donnelly, T.M. Rodent husbandry and care. Vet. Clin. Exot. Anim. Pract. 2004, 7, 201–225. [Google Scholar] [CrossRef]
- Grant, R.A.; Montrose, V.T.; Wills, A.P. ExNOTic: Should we be keeping exotic pets? Animals 2017, 7, 47. [Google Scholar] [CrossRef]
- DFG. Animal Experimentation in Research; Deutsche Forschungsgemeinschaft (DFG, German Research Foundation): Bonn, Germany, 2016; p. 76. [Google Scholar]
- Carbone, L. Estimating mouse and rat use in American laboratories by extrapolation from animal welfare act-regulated species. Sci. Rep. 2021, 11, 493. [Google Scholar] [CrossRef]
- Buckle, A.P.; Smith, R.H. Rodent Pests and Their Control, 2nd ed.; CAB International: Wallingford, Oxon, UK, 2015; p. 432. [Google Scholar]
- Battersby, S.A. Rodents as carriers of disease. In Rodent Pests and Their Control, 2nd ed.; CAB International: Wallingford, Oxon, UK, 2015; pp. 81–100. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species; Version 2014.3; IUCN: Gland, Switzerland, 2014. [Google Scholar]
- Jedrzejewska, B.; Jedrzejewski, W. Antipredatory behaviour of bank voles and prey choice of weasels—enclosure experiments. Ann. Zool. Fenn. 1990, 27, 321–328. Available online: https://www.jstor.org/stable/23734571 (accessed on 20 December 2021).
- Mazza, V.; Eccard, J.A.; Zaccaroni, M.; Jacob, J.; Dammhahn, M. The fast and the flexible: Cognitive style drives individual variation in cognition in a small mammal. Anim. Behav. 2018, 137, 119–132. [Google Scholar] [CrossRef]
- Edge, W.D.; Schauber, E.M. Factors affecting risk assessment of small mammals to pesticides. Environ. Toxicol. Chem. 2000, 19, 2735–2741. [Google Scholar] [CrossRef]
- Sheffield, S.R.; Lochmiller, R.L. Effects of field exposure to diazinon on small mammals inhabiting a semienclosed prairie grassland ecosystem. I. Ecological and reproductive effects. Environ. Toxicol. Chem. 2001, 20, 284–296. [Google Scholar] [CrossRef]
- Singleton, G.R. Population dynamics of an outbreak of house mice (Mus domesticus) in the mallee wheatlands of Australia—hypothesis of plague formation. J. Zool. 1989, 219, 495–515. [Google Scholar] [CrossRef]
- Hein, S.; Jacob, J. Population recovery of a common vole population (Microtus arvalis) after population collapse. Pest Manag. Sci. 2018, 75, 908–914. [Google Scholar] [CrossRef]
- Flowerdew, J.R.; Shore, R.F.; Poulton, S.M.; Sparks, T.H. Live trapping to monitor small mammals in Britain. Mammal Rev. 2004, 34, 31–50. [Google Scholar] [CrossRef]
- Bagamian, K.H.; Towner, J.S.; Kuenzi, A.J.; Douglass, R.J.; Rollin, P.E.; Waller, L.A.; Mills, J.N. Transmission ecology of Sin Nombre hantavirus in naturally infected North American deermouse populations in outdoor enclosures. PLoS ONE 2012, 7, e47731. [Google Scholar] [CrossRef] [PubMed]
- Douglass, R.J.; Vadell, M.R. How much effort is required to accurately describe the complex ecology of a rodent-borne viral disease? Ecosphere 2016, 7, e01368. [Google Scholar] [CrossRef] [PubMed]
- Peitz, D.G.; Tappe, P.A.; Thill, R.E.; Perry, R.W.; Melchiors, M.A.; Wigley, T.B. Non-target captures during small mammal trapping with snap traps. Proc. Annu. Conf. Southeast. Assoc. Fish Wildl. Agencies 2001, 55, 382–388. [Google Scholar]
- Walther, B.; Geduhn, A.; Schenke, D.; Jacob, J. Exposure of passerine birds to brodifacoum during management of Norway rats on farms. Sci. Total Environ. 2021, 762, 144160. [Google Scholar] [CrossRef]
- Karraker, N.E. String theory: Reducing mortality of mammals in pitfall traps. Wildl. Soc. Bull. 2001, 29, 1158–1162. Available online: https://www.jstor.org/stable/3784139 (accessed on 20 December 2021).
- Cook, W.M. Inadvertent bird captures in Sherman small mammal traps in an old field mosaic. Trans. Kans. Acad. Sci. 2004, 107, 170–172. [Google Scholar] [CrossRef]
- Montgomery, W.I. Mortality of small rodents captured in live-traps. Acta Theriol. 1980, 25, 277–294. [Google Scholar] [CrossRef]
- Corke, D. The deaths of small mammals in live-traps. J. Zool. 1967, 153, 552. [Google Scholar]
- Kalinowska, A. Trapping of Apodemus flavicollis and Clethrionomys glareolus into a double trap. Acta Theriol. 1971, 16, 73–78. [Google Scholar] [CrossRef]
- Waudby, H.P.; Petit, S.; Gill, M.J. The scientific, financial and ethical implications of three common wildlife-trapping designs. Wildl. Res. 2019, 46, 690–700. [Google Scholar] [CrossRef]
- Schlötelburg, A.; Jakob, G.; Bellingrath-Kimura, S.; Jacob, J. Natural bait additives improve trapping success of common voles, Microtus arvalis. Appl. Anim. Behav. Sci. 2018, 208, 75–81. [Google Scholar] [CrossRef]
- Takács, S.; Musso, A.E.; Gries, R.; Rozenberg, E.; Borden, J.H.; Brodie, B.; Gries, G. New food baits for trapping house mice, black rats and brown rats. Appl. Anim. Behav. Sci. 2017, 200, 130–135. [Google Scholar] [CrossRef]
- Jacob, J.; Ylönen, H.; Hodkinson, C.G. Trapping efficiency of Ugglan traps and Longworth traps for house mice in southeastern Australia. Wildl. Res. 2002, 29, 101–103. [Google Scholar] [CrossRef]
- Jung, T.S. Comparative efficacy of Longworth, Sherman, and Ugglan live-traps for capturing small mammals in the Nearctic boreal forest. Mammal Res. 2015, 61, 57–64. [Google Scholar] [CrossRef]
- Cunningham, R.B.; Lindenmayer, D.B.; MacGregor, C.; Barry, S.; Welsh, A. Effects of trap position, trap history, microhabitat and season on capture probabilities of small mammals in a wet eucalypt forest. Wildl. Res. 2005, 32, 657–671. [Google Scholar] [CrossRef]
- Weihong, J.; Veitch, C.R.; Craig, J.L. An evaluation of the efficiency of rodent trapping methods: The effect of trap arrangement, cover type, and bait. N. Zealand J. Ecol. 1999, 23, 45–51. [Google Scholar]
- Tew, T.E.; Todd, I.A.; MacDonald, D.W. The effects of trap spacing on population estimation of small mammals. J. Zool. 1994, 233, 340–344. [Google Scholar] [CrossRef]
- Bovendorp, R.S.; Mccleery, R.A.; Galetti, M. Optimising sampling methods for small mammal communities in neotropical rainforests. Mammal Rev. 2017, 47, 148–158. [Google Scholar] [CrossRef]
- Notz, E.; Imholt, C.; Reil, D.; Jacob, J. Testing automated sensor traps for mammal field studies. Wildl. Res. 2017, 44, 72–77. [Google Scholar] [CrossRef]
- Lane, V.R.; Miller, K.V.; Castleberry, S.B.; Miller, D.A.; Wigley, T.B. Methods to reduce avian bycatch in small mammal studies using snap traps. J. Wildl. Manag. 2010, 74, 595–599. [Google Scholar] [CrossRef]
- Gurnell, J.; Flowerdew, J.R. Live trapping small mammals. A practical guide. Publ. Mammal Soc. 1982, Occasional Publication no. 3, 1–24. [Google Scholar]
- Little, J.; Gurnell, J. Shrew Captures and Rodent Field Studies. J. Zool. 1989, 218, 329–331. [Google Scholar] [CrossRef]
- Eccard, J.A.; Klemme, I. Reducing mortality of shrews in rodent live trapping—a method increasing live-trap selectivity with shrew exits. Ann. Zool. Fenn. 2013, 50, 371–376. [Google Scholar] [CrossRef]
- Chiron, F.; Hein, S.; Chargé, R.; Julliard, R.; Martin, L.; Roguet, A.; Jacob, J. Validation of hair tubes for small mammal population studies. J. Mammal. 2018, 99, 478–485. [Google Scholar] [CrossRef]
- Barja, I.; Navarro-Castilla, Á.; Pérez, L. Effectiveness and applications of hair traps for the study of wild mammal populations. Pol. J. Ecol. 2016, 64, 409–419. [Google Scholar] [CrossRef]
- Tennant, E.N.; Cypher, B.L.; Saslaw, L.R.; Westall, T.L.; Mohay, J.L.; Kelly, E.C.; Job, C.L.V. Evaluation of noninvasive survey methods for detecting endangered shrews. Wildl. Soc. Bull. 2020, 44, 610–616. [Google Scholar] [CrossRef]
- Rendall, A.R.; Sutherland, D.R.; Cooke, R.; White, J. Camera trapping: A contemporary approach to monitoring invasive rodents in high conservation priority ecosystems. PLoS ONE 2014, 9, e86592. [Google Scholar] [CrossRef]
- Fauteux, D.; Gauthier, G.; Mazerolle, M.J.; Coallier, N.; Bety, J.; Berteaux, D. Evaluation of invasive and non-invasive methods to monitor rodent abundance in the arctic. Ecosphere 2018, 9, e02124. [Google Scholar] [CrossRef]
- Carey, A.B.; Witt, J.W. Track counts as indexes to abundances of arboreal rodents. J. Mammal. 1991, 72, 192–194. [Google Scholar] [CrossRef]
- Whisson, D.A.; Engeman, R.M.; Collins, K. Developing relative abundance techniques (RATs) for monitoring rodent populations. Wildl. Res. 2005, 32, 239–244. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Sabino-Marques, H.; Barbosa, S.; Costa, P.; Encarnacao, C.; Alpizar-Jara, R.; Pita, R.; Beja, P.; Mira, A.; Searle, J.B.; et al. Genetic non-invasive sampling (gNIS) as a cost-effective tool for monitoring elusive small mammals. Eur. J. Wildl. Res. 2018, 64, 64. [Google Scholar] [CrossRef]
- Sweetapple, P. Chew-track-cards: A multiple-species small mammal detection device. N. Zealand J. Ecol. 2011, 35, 153–162. [Google Scholar]
- Hansson, L.; Hoffmeyer, I. Snap and live trap efficiency for small mammals. Oikos 1973, 24, 477–478. [Google Scholar] [CrossRef]
- Lee, L.-L. Effectiveness of live traps and snap traps in trapping small mammals in kinmen. Acta Zool. Taiwanica 1997, 8, 79–85. [Google Scholar]
- Niethammer, J.; Krapp, F. Handbuch der Säugetiere Europas Band 2/I Nagetiere II; Akademische Verlagsgesellschaft: Wiesbaden, Germany, 1982. [Google Scholar]
- Jacob, J.; Tkadlec, E. Rodent outbreaks in Europe: Dynamics and damage. In Rodent Outbreaks—Ecology and Impacts; Singleton, G.R., Belmain, S., Brown, P.R., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philippines, 2010; pp. 207–223. [Google Scholar]
- Jacob, J.; Manson, P.; Barfknecht, R.; Fredricks, T. Common vole (Microtus arvalis) ecology and management: Implications for risk assessment of plant protection products. Pest Manag. Sci. 2014, 70, 869–878. [Google Scholar] [CrossRef]
- Reil, D.; Imholt, C.; Eccard, J.A.; Jacob, J. Beech fructification and bank vole population dynamics—combined analyses of promoters of human Puumala virus infections in Germany. PLoS ONE 2015, 10, e0134124. [Google Scholar] [CrossRef]
- Tersago, K.; Verhagen, R.; Vapalahti, O.; Heyman, P.; Ducoffre, G.; Leirs, H. Hantavirus outbreak in Western Europe: Reservoir host infection dynamics related to human disease patterns. Epidemiol. Infect. 2011, 139, 381–390. [Google Scholar] [CrossRef]
- Grinnell, J. An Account of the Mammals and Birds of the Lower Colorado Valley, with Especial Reference to the Distributional Problems Presented; University of California Press: Berkeley, CA, USA, 1914; Volume 12, pp. 51–294. [Google Scholar]
- Meinig, H.U.; Boye, P.; Hutterer, R.; Dähne, M. Rote Liste und Gesamtartenliste der Säugetiere (Mammalia) Deutschlands. Nat. Biol. Vielfalt 2020, 170, 1–673. [Google Scholar] [CrossRef]
- RCoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; Volume 1, p. 3. [Google Scholar]
- Braun, M.; Dieterlen, F. Die Säugetiere Baden-Württembergs, 2nd ed.; Ulmer Verlag: Stuttgart, Germany, 2005. [Google Scholar]
- Niedzialkowska, M.; Konczak, J.; Czarnomska, S.; Jedrzejewska, B. Species diversity and abundance of small mammals in relation to forest productivity in northeast poland. Ecoscience 2010, 17, 109–119. [Google Scholar] [CrossRef]
- Suchomel, J.; Purchart, L.; Cepelka, L. Structure and diversity of small-mammal communities of lowland forests in the rural central European landscape. Eur. J. For. Res. 2012, 131, 1933–1941. [Google Scholar] [CrossRef]
- Bryja, J.; Rehak, Z. Community of small terrestrial mammals (Insectivora, Rodentia) in dominant habitats of the Protected Landscape Area of Poodri (Czech Republic). Folia Zool. 1998, 47, 249–260. [Google Scholar]
- Heroldova, M.; Bryja, J.; Zejda, J.; Tkadlec, E. Structure and diversity of small mammal communities in agriculture landscape. Agric. Ecosyst. Environ. 2007, 120, 206–210. [Google Scholar] [CrossRef]
- Pearce, J.L.; Schuurman, D.; Barber, K.N.; Larrivee, M.; Venier, L.A.; McKee, J.; McKenney, D. Pitfall trap designs to maximize invertebrate captures and minimize captures of nontarget vertebrates. Can. Entomol. 2005, 137, 233–250. [Google Scholar] [CrossRef]
- Weary, B.P.; Mendez, P.K.; Lew, S.E.; Nyman, B.L.; Will, K.W. Performance of ramp and pitfall traps for arthropods and non-target vertebrates in californian oak woodland and chaparral habitats. Pan-Pac. Entomol. 2019, 95, 21–32. [Google Scholar] [CrossRef]
- McLeod, M.A.; Koronkiewicz, T.J. Comparison of capture and escape rates between different types of portable cowbird traps. Wildl. Soc. Bull. 2014, 38, 611–618. [Google Scholar] [CrossRef]
- Cha, D.H.; Hesler, S.P.; Park, S.; Adams, T.B.; Zack, R.S.; Rogg, H.; Loeb, G.M.; Landolt, P.J. Simpler is better: Fewer non-target insects trapped with a four-component chemical lure vs. a chemically more complex food-type bait for Drosophila suzukii. Entomol. Exp. Appl. 2015, 154, 251–260. [Google Scholar] [CrossRef]
- Campbell, S.; Cook, S.; Mortimer, L.; Palmer, G.; Sinclair, R.; Woolnough, A.P. To catch a starling: Testing the effectiveness of different trap and lure types. Wildl. Res. 2012, 39, 183–191. [Google Scholar] [CrossRef]
- Anthony, N.M.; Ribic, C.A.; Bautz, R.; Garland, T. Comparative effectiveness of longworth and sherman live traps. Wildl. Soc. Bull. 2005, 33, 1018–1026. [Google Scholar] [CrossRef]
- Von Blanckenhagen, F.; Städtler, T. Small mammal communities in agricultural landscapes in Germany: Review of field data over the last decade. Jul. -Kühn-Arch. 2011, 432, 75. [Google Scholar] [CrossRef]
- Boyard, C.; Vourc’h, G.; Barnouin, J. The relationships between Ixodes ricinus and small mammal species at the woodland-pasture interface. Exp. Appl. Acarol. 2008, 44, 61–76. [Google Scholar] [CrossRef] [PubMed]
- DWD. Nationaler Klimareport, 4th ed.; Deutscher Wetterdienst (DWD): Potsdam, Germany, 2020. [Google Scholar]
- Eccard, J.A.; Ylönen, H. Initiation of breeding after winter in bank voles: Effects of food and population density. Can. J. Zool. 2001, 79, 1743–1753. [Google Scholar] [CrossRef]
- Jacob, J. Body weight dynamics of common voles in agro-ecosystems. Mammalia 2003, 67, 559–566. [Google Scholar] [CrossRef]
- Tkadlec, E.; Zejda, J. Density-dependent life histories in female bank voles from fluctuating populations. J. Anim. Ecol. 1998, 67, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Gurnell, J.; Little, J. The influence of trap residual odour on catching woodland rodents. Anim. Behav. 1992, 43, 623–632. [Google Scholar] [CrossRef]
- Brouard, M.J.; Coulson, T.; Newman, C.; Macdonald, D.W.; Buesching, C.D. Analysis on population level reveals trappability of wild rodents is determined by previous trap occupant. PLoS ONE 2015, 10, e0145006. [Google Scholar] [CrossRef]
- Jacob, J.; Brown, J.S. Microhabitat use, giving-up densities and temporal activity as short and long term anti-predator behaviors in common voles. Oikos 2000, 91, 131–138. [Google Scholar] [CrossRef]
- Yletyinen, S.; Norrdahl, K. Habitat use of field voles (Microtus agrestis) in wide and narrow buffer zones. Agric. Ecosyst. Environ. 2008, 123, 194–200. [Google Scholar] [CrossRef]
- Clapperton, B.K. A Review of the Current Knowledge of Rodent Behaviour in Relation to Control Devices; Science & Technical Publication: Wellington, New Zealand, 2006; Volume 263, pp. 1–55. [Google Scholar]
- Kok, A.D.; Parker, D.M.; Barker, N.P. Rules of attraction: The role of bait in small mammal sampling at high altitude in South Africa. Afr. Zool. 2013, 48, 84–95. [Google Scholar] [CrossRef]
- Hansen, S.C.; Stolter, C.; Jacob, J. Effect of plant secondary metabolites on feeding behavior of microtine and arvicoline rodent species. J. Pest Sci. 2016, 89, 955–963. [Google Scholar] [CrossRef]





| Study | Years | Federal State | No. Study Sites | No. Trap Nights | No. Captures | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forest | Grassland | Margin | Total | Forest | Grassland | Margin | Total | Forest | Grassland | Margin | Total | |||
| # 1 | 16 | 8 | 2 | 26 | 39,788 | 11,172 | 4067 | 55,027 | 9851 | 1482 | 519 | 11,852 | ||
| 2010–2014 | BAW | 4 | 3 | 0 | 7 | 9898 | 3675 | 0 | 13,573 | 2778 | 612 | 0 | 3390 | |
| 2010–2013 | MWP | 3 | 1 | 2 | 6 | 8330 | 2107 | 4067 | 14,503 | 2036 | 318 | 519 | 2873 | |
| 2010–2015 | NRW | 4 | 3 | 0 | 7 | 9506 | 3675 | 0 | 13,181 | 2074 | 181 | 0 | 2255 | |
| 2010–2013 | THU | 5 | 1 | 0 | 6 | 12,054 | 1715 | 0 | 13,769 | 2963 | 371 | 0 | 3334 | |
| # 2 | 2019–2021 | NRW | 12 | 0 | 0 | 12 | 27,500 | 0 | 0 | 27,500 | 5166 | 0 | 0 | 5166 |
| # 3 | 2020 | BAV | 0 | 0 | 9 | 9 | 0 | 0 | 430 | 430 | 0 | 0 | 937 | 937 |
| # 4 | 0 | 0 | 12 | 12 | 0 | 0 | 448.5 | 448.5 | 0 | 0 | 503 | 503 | ||
| 2020 | BAV | 0 | 0 | 4 | 4 | 0 | 0 | 148.5 | 148.5 | 0 | 0 | 254 | 254 | |
| 2020 | LOS | 0 | 0 | 8 | 8 | 0 | 0 | 300 | 300 | 0 | 0 | 249 | 249 | |
| Total | 28 | 8 | 23 | 59 | 67,538 | 8109 | 4945.5 | 83,355.5 | 15,017 | 1482 | 1959 | 18,458 | ||
| Taxon Species | All Habitats | Forest | Grassland | Margin | |||||
|---|---|---|---|---|---|---|---|---|---|
| N 100 TN | Mean (±SE) | N 100 TN | Mean (±SE) | N 100 TN | Mean (±SE) | N 100 TN | Mean (±SE) | ||
| Cricetidae | 16.65 | 32.38 (±0.14) | 17.00 | 17.21 (±0.05) | 11.26 | 12.11 (±0.40) | 24.04 | 90.46 (±1.09) | |
| Bank vole (Clethrionomys glareolus) | 13.66 | 29.46 (±0.15) | 15.81 | 16.59 (±0.06) | 0.33 | 1.68 (±0.28) | 14.50 | 80.50 (±1.24) | |
| Common vole (Microtus arvalis) | 2.82 | 23.71 (±0.3) | 1.09 | 13.12 (±0.48) | 10.56 | 12.86 (±0.45) | 8.91 | 35.03 (±0.79) | |
| Field vole (Microtus agrestis) | 0.15 | 2.27 (±0.12) | 0.09 | 1.61 (±0.07) | 0.3 | 4.63 (±2.32) | 0.63 | 3.16 (±0.77) | |
| Microtus spp. | 0.01 | 0.75 (±0.11) | 0.01 | 0.55 (±0.08) | 0.06 | 0.95 (±0.31) | 0 | 0 | |
| Edible dormouse (Glis glis) | <0.01 | 0.41 | 0.00 | 0.41 | 0 | 0 | 0 | 0 | |
| Muridae | 2.62 | 21.61 (±0.25) | 2.2 | 4.65 (±0.04) | 1.08 | 4.49 (±0.89) | 11.97 | 59.12 (±1.26) | |
| Yellow-necked mouse (Apodemus flavicollis) | 1.77 | 21.46 (±0.31) | 1.59 | 4.24 (±0.05) | 0.07 | 1.09 (±0.39) | 8.09 | 54.81 (±1.35) | |
| Long-tailed field mouse (Apodemus sylvaticus) | 0.25 | 11.9 (±0.33) | 0.18 | 1.58 (±0.05) | 0.08 | 1.84 (±1.01) | 1.49 | 23.72 (±0.85) | |
| Striped field mouse (Apodemus agrarius) | 0.24 | 3.97 (±0.21) | 0.14 | 2.64 (±0.24) | 0 | 0 | 2.17 | 7.3 (±0.86) | |
| Apodemus spp. | 0.22 | 2.35 (±0.08) | 0.26 | 2.24 (±0.07) | 0.04 | 0.55 (±0.08) | 0.02 | 11.11 | |
| Eurasian harvest mouse * (Micromys minutus) | 0.14 | 3.24 (±0.51) | 0.01 | 0.68 (±0.08) | 0.90 | 6.80 (±1.94) | 0.18 | 1.23 (±0.36) | |
| House mouse (Mus musculus) | <0.01 | 1.22 | <0.01 | 1.22 | 0 | 0 | 0 | 0 | |
| House rat (Rattus rattus) | <0.01 | 6.67 | 0 | 0 | 0 | 0 | 0.02 | 6.67 | |
| Muste- linae | Least weasel (Mustela nivalis) | 0.02 | 0.53 (±0.04) | 0.02 | 0.55 (±0.05) | 0 | 0 | 0.02 | 0.41 |
| Soricidae | 1.19 | 6.65 (±0.06) | 1.03 | 2.88 (±0.03) | 0.92 | 2.64 (±0.33) | 3.98 | 14.46 (±0.26) | |
| Common shrew (Sorex araneus) | 0.05 | 19.14 (±0.93) | 0 | 0 | 0 | 0 | 0.92 | 19.14 (±0.93) | |
| Eurasian pygmy shrew (Sorex minutus) | 0.03 | 10.26 (±0.34) | 0 | 0.41 | 0 | 0 | 0.41 | 10.92 (±0.32) | |
| Sorex spp. | 1.08 | 2.88 (±0.03) | 1.03 | 2.87 (±0.03) | 0.92 | 2.64 (±0.33) | 2.10 | 3.15 (±0.17) | |
| Greater white-toothed shrew (Crocidura russula) | |||||||||
| 0.03 | 17.33 (±0.59) | 0 | 0 | 0 | 0 | 0.49 | 17.33 (±0.59) | ||
| Eurasian water shrew * (Neomys fodiens) | 0.01 | 5.05 (±0.81) | < 0.01 | 0.62 (±0.14) | 0 | 0 | 0.06 | 8.00 (±0.00) | |
| Not determined | 1.67 | 6.79 (±0.10) | 2.07 | 6.79 (±0.10) | 0 | 0 | 0 | 0 | |
| All species | 22.14 | 46.42 (±0.20) | 22.32 | 22.05 (±0.06) | 13.27 | 13.31 (±0.38) | 40.02 | 142.05 (±1.51) | |
| Taxon | Season | All Habitats | Forest | Grassland | Margins | ||||
|---|---|---|---|---|---|---|---|---|---|
| N 100 TN | Mean (±SE) | N 100 TN | Mean (±SE) | N 100 TN | Mean (±SE) | N 100 TN | Mean (±SE) | ||
| Cricetidae | Spring | 11.20 | 36.79 (±0.49) | 10.78 | 10.87 (±0.15) | 1.94 | 2.12 (±0.18) | 33.05 | 97.94 (±2.24) |
| Summer | 22.25 | 40.91 (±0.41) | 22.94 | 22.68 (±0.17) | 16.20 | 17.28 (±1.3) | 26.49 | 95.34 (±2.52) | |
| Autumn | 15.93 | 16.50 (±0.12) | 16.8 | 17.44 (±0.14) | 13.13 | 14.01 (±1.07) | 9.84 | 9.27 (±0.84) | |
| Muridae | Spring | 3.88 | 45.73 (±0.97) | 2.37 | 4.79 (±0.13) | 3.64 | 10.92 (±3.8) | 23.44 | 99.58 (±2.69) |
| Summer | 1.95 | 7.77 (±0.14) | 2.03 | 4.38 (±0.11) | 0.05 | 0.41 (±0.00) | 4.98 | 14.61 (±0.5) | |
| Autumn | 2.17 | 4.71 (±0.10) | 2.20 | 4.78 (±0.13) | 0.28 | 0.98 (±0.17) | 7.65 | 7.23 (±0.93) | |
| Mustelinae | Spring | <0.01 | 0.41 | <0.01 | 0.41 | 0 | 0 | 0 | 0 |
| Summer | 0.01 | 0.41 (±0.00) | 0.02 | 0.41 (±0.00) | 0 | 0 | 0 | 0 | |
| Autumn | 0.03 | 0.65 (±0.11) | 0.03 | 0.72 (±0.15) | 0 | 0 | 0.07 | 0.41 | |
| Soricidae | Spring | 0.94 | 11.51 (±0.28) | 0.62 | 2.31 (±0.11) | 0.10 | 1.22 | 6.40 | 18.91 (±0.58) |
| Summer | 1.42 | 6.18 (±0.12) | 1.26 | 3.43 (±0.13) | 1.81 | 4.83 (±1.39) | 2.62 | 11.27 (±0.43) | |
| Autumn | 1.19 | 2.55 (±0.04) | 1.19 | 2.76 (±0.05) | 0.67 | 1.35 (±0.15) | 2.92 | 2.86 (±0.35) | |
| Incognita | Spring | 2.02 | 10.34 (±0.55) | 2.46 | 10.34 (±0.55) | 0 | 0 | 0 | 0 |
| Summer | 1.05 | 4.62 (±0.23) | 1.31 | 4.62 (±0.23) | 0 | 0 | 0 | 0 | |
| Autumn | 1.98 | 6.38 (±0.21) | 2.46 | 6.38 (±0.21) | 0 | 0 | 0 | 0 | |
| All species | Spring | 18.03 | 67.25 (±0.81) | 16.23 | 15.80 (±0.18) | 5.68 | 5.68 (±0.79) | 62.90 | 191.92 (±3.43) |
| Summer | 26.68 | 47.34 (±0.44) | 27.55 | 27.32 (±0.19) | 18.06 | 18.06 (±1.26) | 34.09 | 107.61 (±2.58) | |
| Autumn | 21.29 | 21.16 (±0.14) | 22.67 | 22.59 (±0.18) | 14.08 | 14.17 (±0.99) | 20.48 | 19.43 (±1.39) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotopp, I.; Walther, B.; Fuelling, O.; Reil, D.; Hesse, C.; Below, D.A.; Imholt, C.; Jacob, J. Habitat and Season Effects on Small Mammal Bycatch in Live Trapping. Biology 2022, 11, 1806. https://doi.org/10.3390/biology11121806
Hotopp I, Walther B, Fuelling O, Reil D, Hesse C, Below DA, Imholt C, Jacob J. Habitat and Season Effects on Small Mammal Bycatch in Live Trapping. Biology. 2022; 11(12):1806. https://doi.org/10.3390/biology11121806
Chicago/Turabian StyleHotopp, Ines, Bernd Walther, Olaf Fuelling, Daniela Reil, Christin Hesse, Diana Alexandra Below, Christian Imholt, and Jens Jacob. 2022. "Habitat and Season Effects on Small Mammal Bycatch in Live Trapping" Biology 11, no. 12: 1806. https://doi.org/10.3390/biology11121806
APA StyleHotopp, I., Walther, B., Fuelling, O., Reil, D., Hesse, C., Below, D. A., Imholt, C., & Jacob, J. (2022). Habitat and Season Effects on Small Mammal Bycatch in Live Trapping. Biology, 11(12), 1806. https://doi.org/10.3390/biology11121806







