Cardiac Magnetic Resonance Findings in Patients Recovered from COVID-19 Pneumonia and Presenting with Persistent Cardiac Symptoms: The TRICITY-CMR Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Endpoints
2.3. CMR Scanning Protocol and Image Analysis
2.4. Lung Assessment with Computed Tomography
2.5. Laboratory Measurements of Cardiac and Inflammatory Biomarkers
2.6. Statistical Analysis
3. Results
3.1. Study Course and Participant Characteristics
3.2. Laboratory Tests
3.3. CMR Findings in Hospitalized vs. Non-Hospitalized Post-COVID-19 Patients
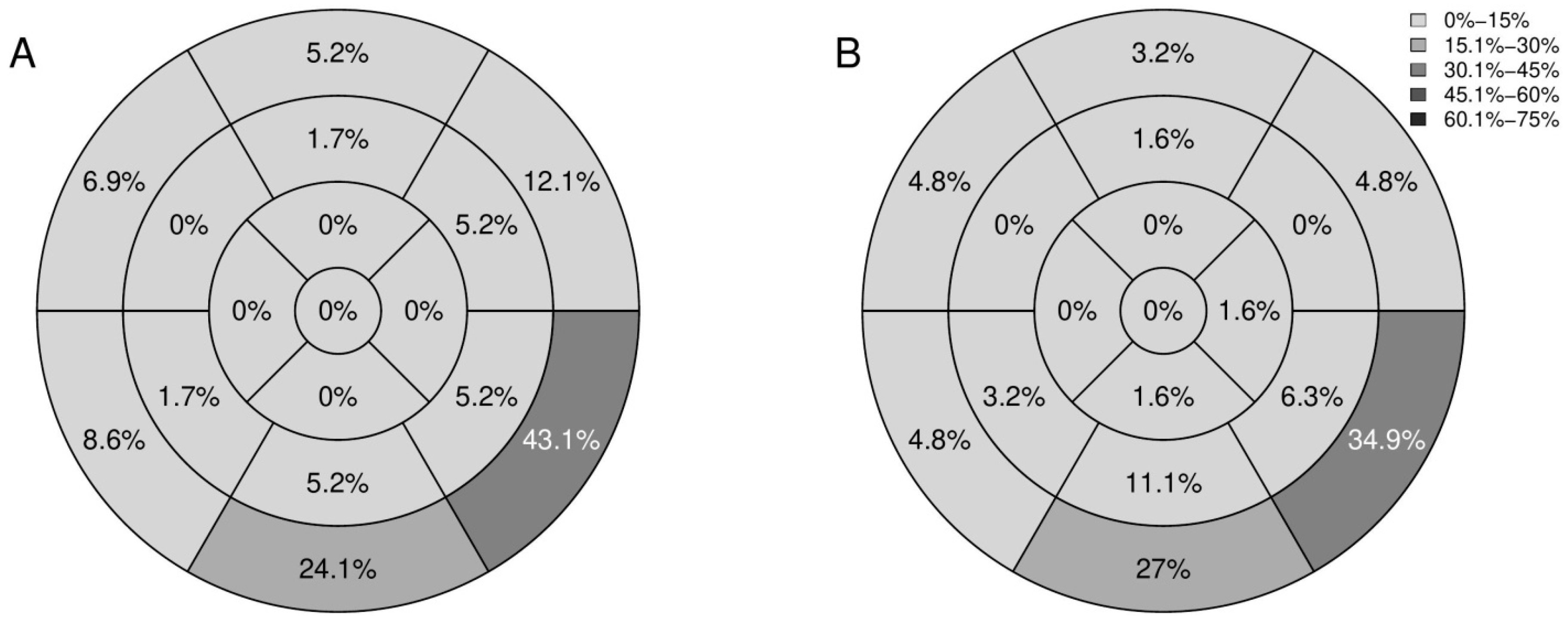
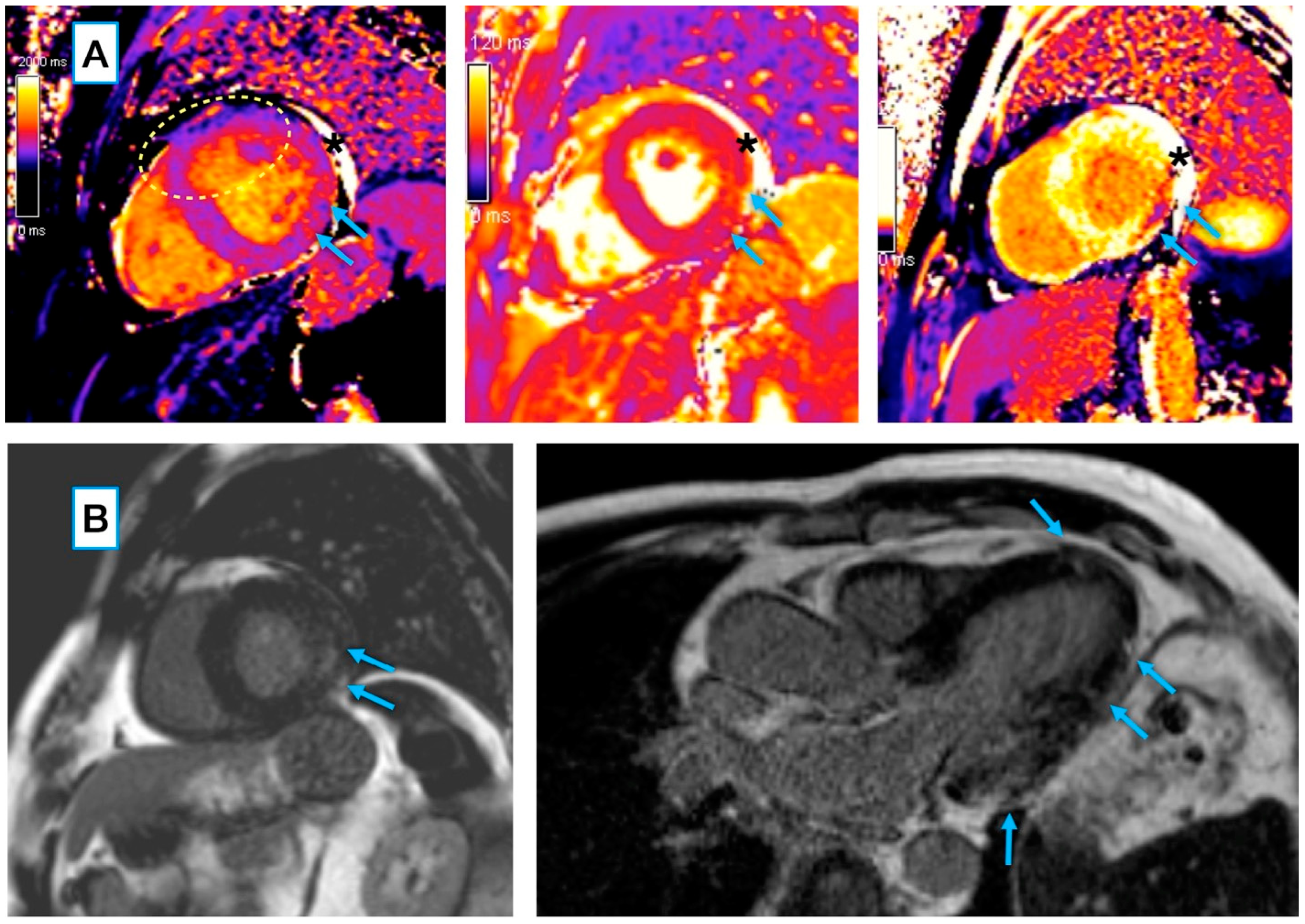
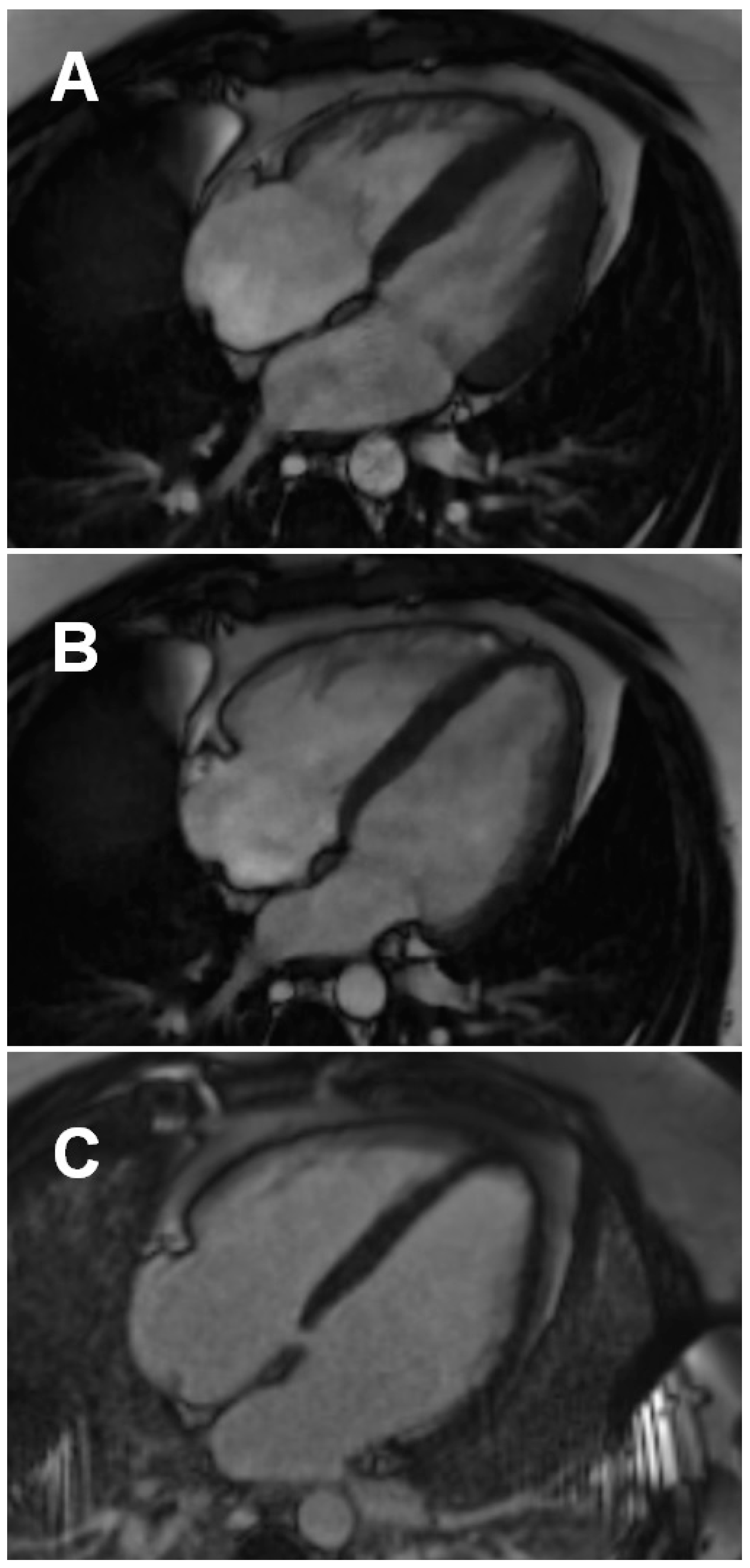
3.3.1. Conventional and LGE Sequences
3.3.2. Mapping Sequences
3.4. Comparison of Post-COVID-19 Patients with and without Non-Ischemic Cardiac Injury
4. Discussion
4.1. Major Study Findings
4.2. COVID-19 Myocarditis
4.3. Left and Right Ventricular Function
4.4. Management of Post-COVID-19 Cardiac Complications
4.5. Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Yee, N.T.S.; Liu, C.; Nerurkar, S.N.; Kai, J.C.Y.; et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Zhao, L.; Yang, X.C.; Wang, P. Cardiovascular complications of SARS-CoV-2 infection (COVID-19): A systematic review and meta-analysis. Rev. Cardiovasc. Med. 2021, 22, 159–165. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Wong, C.K.; Lam, C.W.; Wu, A.K.; Ip, W.K.; Lee, N.L.; Chan, I.H.; Lit, L.C.; Hui, D.S.; Chan, M.H.; Chung, S.S.; et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004, 136, 95–103. [Google Scholar] [CrossRef]
- Dherange, P.; Lang, J.; Qian, P.; Oberfeld, B.; Sauer, W.H.; Koplan, B.; Tedrow, U. Arrhythmias and COVID-19: A Review. JACC Clin. Electrophysiol. 2020, 6, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Gauchotte, G.; Venard, V.; Segondy, M.; Cadoz, C.; Esposito-Fava, A.; Barraud, D.; Louis, G. SARS-Cov-2 fulminant myocarditis: An autopsy and histopathological case study. Int. J. Legal. Med. 2021, 135, 577–581. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, P.; Tang, D.; Zhu, T.; Han, R.; Zhan, C.; Liu, W.; Zeng, H.; Tao, Q.; Xia, L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2020, 13, 2330–2339. [Google Scholar] [CrossRef]
- Kelle, S.; Bucciarelli-Ducci, C.; Judd, R.M.; Kwong, R.Y.; Simonetti, O.; Plein, S.; Raimondi, F.; Weinsaft, J.W.; Wong, T.C.; Carr, J. Society for Cardiovascular Magnetic Resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID-19 infection. J. Cardiovasc. Magn. Reson. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, D.; Dorniak, K.; Ławrynowicz, M.; Rejszel-Baranowska, J.; Fijałkowska, J.; Kulawiak-Gałąska, D.; Szurowska, E.; Koziński, M. Spectrum of lesions visualized in cardiac magnetic resonance imaging in COVID-19-related myocarditis: Findings from a pilot study of the TRICITY-CMR trial. Cardiol. J. 2021, 28, 976–978. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 17 September 2022).
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Kawel-Boehm, N.; Maceira, A.; Valsangiacomo-Buechel, E.R.; Vogel-Claussen, J.; Turkbey, E.B.; Williams, R.; Plein, S.; Tee, M.; Eng, J.; Bluemke, D.A. Normal values for cardiovascular magnetic resonance in adults and children. J. Cardiovasc. Magn. Reson. 2015, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Kellman, P.; Larson, A.C.; Hsu, L.-Y.; Chung, Y.-C.; Simonetti, O.P.; McVeigh, E.R.; Arai, A.E. Motion-corrected free-breathing delayed enhancement imaging of myocardial infarction. Magn. Reson. Med. 2005, 53, 194–200. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging; et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar] [PubMed]
- Bajaj, R.; Sinclair, H.C.; Patel, K.; Low, B.; Pericao, A.; Manisty, C.; Guttmann, O.; Zemrak, F.; Miller, O.; Longhi, P.; et al. Delayed-onset myocarditis following COVID-19. Lancet Respir. Med. 2021, 9, e32–e34. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.; Cacoub, L.; Baudet, M.; Nahmani, Y.; Cacoub, P.; Cohen-Solal, A.; Henry, P.; Adle-Biassette, H.; Logeart, D. Delayed acute myocarditis and COVID-19-related multisystem inflammatory syndrome. ESC Heart Fail. 2020, 7, 4371–4376. [Google Scholar] [CrossRef]
- Piccirillo, F.; Watanabe, M.; Di Sciascio, G. Diagnosis, treatment and predictors of prognosis of myocarditis. A narrative review. Cardiovasc. Pathol. 2021, 54, 107362. [Google Scholar] [CrossRef]
- Chang, J.J.; Lin, M.S.; Chen, T.H.; Chen, D.Y.; Chen, S.W.; Hsu, J.T.; Wang, P.C.; Lin, Y.S. Heart Failure and Mortality of Adult Survivors from Acute Myocarditis Requiring Intensive Care Treatment—A Nationwide Cohort Study. Int. J. Med. Sci. 2017, 14, 1241–1250. [Google Scholar] [CrossRef]
- Gulati, A.; Jabbour, A.; Ismail, T.F.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.; Ismail, N.A.; Dweck, M.R.; et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013, 309, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Lønborg, J.; Vejlstrup, N.; Kelbæk, H.; Holmvang, L.; Jørgensen, E.; Helqvist, S.; Saunamäki, K.; Ahtarovski, K.A.; Bøtker, H.E.; Kim, W.Y.; et al. Final infarct size measured by cardiovascular magnetic resonance in patients with ST elevation myocardial infarction predicts long-term clinical outcome: An observational study. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Ghebru Habtemicael, Y.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Lanzillo, C.; Scatteia, A.; Di Roma, M.; Pontone, G.; et al. “Cardiac Magnetic Resonance” Working Group of the Italian Society of Cardiology. Prognostic Value of Repeating Cardiac Magnetic Resonance in Patients with Acute Myocarditis. J. Am. Coll. Cardiol. 2019, 74, 2439–2448. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Strohm, O.; Schulz-Menger, J.; Marciniak, H.; Luft, F.C.; Dietz, R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation 1998, 97, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Omori, T.; Kurita, T.; Dohi, K.; Takasaki, A.; Nakata, T.; Nakamori, S.; Fujimoto, N.; Kitagawa, K.; Hoshino, K.; Tanigawa, T.; et al. Prognostic impact of unrecognized myocardial scar in the non-culprit territories by cardiac magnetic resonance imaging in patients with acute myocardial infarction. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 108–116. [Google Scholar] [CrossRef]
- Małek, Ł.A.; Barczuk-Falęcka, M.; Werys, K.; Czajkowska, A.; Mróz, A.; Witek, K.; Burrage, M.; Bakalarski, W.; Nowicki, D.; Roik, D.; et al. Cardiovascular magnetic resonance with parametric mapping in long-term ultra-marathon runners. Eur. J. Radiol. 2019, 117, 89–94. [Google Scholar] [CrossRef]
- Banks, L.; Altaha, M.A.; Yan, A.T.; Dorian, P.; Konieczny, K.; Deva, D.P.; Gerche, A.L.A.; Akhavein, F.; Bentley, R.F.; Connelly, K.A.; et al. Left Ventricular Fibrosis in Middle-Age Athletes and Physically Active Adults. Med. Sci. Sports Exerc. 2020, 52, 2500–2507. [Google Scholar] [CrossRef]
- Chen, B.H.; Shi, N.N.; Wu, C.W.; An, D.A.; Shi, Y.X.; Wesemann, L.D.; Hu, J.; Xu, J.R.; Shan, F.; Wu, L.M. Early cardiac involvement in patients with acute COVID-19 infection identified by multiparametric cardiovascular magnetic resonance imaging. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 844–851. [Google Scholar] [CrossRef]
- Ng, M.Y.; Ferreira, V.M.; Leung, S.T.; Yin Lee, J.C.; Ho-Tung Fong, A.; To Liu, R.W.; Man Chan, J.W.; Wu, A.K.L.; Lung, K.C.; Crean, A.M.; et al. Patients Recovered from COVID-19 Show Ongoing Subclinical Myocarditis as Revealed by Cardiac Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2020, 13, 2476–2478. [Google Scholar] [CrossRef]
- Li, Y.L.; Zheng, J.B.; Jin, Y.; Tang, R.; Li, M.; Xiu, C.H.; Dai, Q.Q.; Zuo, S.; Wang, H.Q.; Wang, H.L.; et al. Acute right ventricular dysfunction in severe COVID-19 pneumonia. Rev. Cardiovasc. Med. 2020, 21, 635–641. [Google Scholar]
- Isgro, G.; Yusuff, H.O.; Zochios, V.; Protecting the Right Ventricle Network. The Right Ventricle in COVID-19 Lung Injury: Proposed Mechanisms, Management, and Research Gaps. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Zhu, S.; Xie, Y.; Wang, B.; He, L.; Zhang, D.; Zhang, Y.; Yuan, H.; Wu, C.; et al. Prognostic Value of Right Ventricular Longitudinal Strain in Patients with COVID-19. JACC Cardiovasc. Imaging 2020, 13, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Paternoster, G.; Bertini, P.; Innelli, P.; Trambaiolo, P.; Landoni, G.; Franchi, F.; Scolletta, S.; Guarracino, F. Right Ventricular Dysfunction in Patients with COVID-19: A Systematic Review and Meta-analysis. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3319–3324. [Google Scholar] [CrossRef] [PubMed]
- Raman, B.; Bluemke, D.A.; Luscher, T.F.; Neubauer, S. Lonf COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, T.J.; Bhave, N.M.; Allen, L.A.; Chung, E.H.; Spatz, E.S.; Ammirati, E.; Baggish, A.L.; Bozkurt, B.; Cornwell, W.K., 3rd; Harmon, K.G.; et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 79, 1717–1756. [Google Scholar] [PubMed]
| All Post-COVID-19 Patients (n = 121) | Hospitalized Post-COVID-19 Patients (n = 58) | Non-Hospitalized Post-COVID-19 Patients (n = 63) | p-Value (Hospitalized vs. Non-Hospitalized Post-COVID-19 Patients) | |
|---|---|---|---|---|
| Age, years; Me (IQR) | 46 (40; 57) | 56.5 (44; 66) | 44 (37; 47) | <0.001 w |
| Female gender; n (%) | 55 (45.5) | 18 (31.0) | 37 (58.7) | 0.004 c |
| Time between COVID-19 diagnosis and CMR examination, days; Me (IQR) | 41 (25; 61) | 28 (20; 43) | 47 (34; 75) | <0.001 w |
| BMI, kg/m2; Me (IQR) | 27.2 (25.2; 30.0) | 27.9 (26.4; 31.1) | 26.3 (23.5; 29.3) | 0.008 w |
| Obesity; n (%) | 33 (27.3) | 19 (32.8) | 14 (22.2) | 0.237 c |
| Hypertension; n (%) | 36 (29.8) | 24 (41.4) | 12 (19.1) | 0.013 c |
| Diabetes; n (%) | 15 (12.4) | 13 (22.4) | 2 (3.2) | 0.003 c |
| Hyperlipidemia; n (%) | 32 (26.4) | 13 (22.4) | 19 (30.2) | 0.448 c |
| Chronic kidney disease; n (%) | 2 (1.6) | 1 (1.7) | 1 (1.6%) | 1 f |
| Chronic respiratory disease *; n (%) | 16 (13.2) | 11 (19.0) | 5 (7.9) | 0.128 f |
| Clinical severity of COVID-19 pneumonia; n (%) | ||||
| Mild illness | 64 (52.9) | 1 (1.7) | 63 (100) | <0.001 f |
| Moderate illness | 14 (11.6) | 14 (24.1) | 0 (0) | |
| Severe illness | 40 (33.0) | 40 (69.0) | 0 (0) | |
| Critical illness | 3 (2.5) | 3 (5.2) | 0 (0) | |
| Extent of the involved lung tissue on computed tomography, %; Me (IQR) | N/A | 22.7 (9.8; 35.0) | NR | N/A |
| Laboratory parameters assessed on the day of CMR examination | ||||
| White blood cell count (×103/μL); Me (IQR) | 7.0 (5.6; 8.3) | 7.0 (5.5; 8.3) | 7.0 (6.0; 8.2) | 0.829 w |
| Lymphocyte count (×103/μL); Me (IQR) | 2.2 (1.7; 2.5) | 2.0 (1.7; 2.5) | 2.2 (1.7; 2.6) | 0.485 w |
| Hemoglobin, g/dL; Me (IQR) | 14.2 (13.4; 15.1) | 14.3 (13.7; 15.3) | 14.0 (13.3; 15.0) | 0.416 t |
| CRP, mg/L; Me (IQR) | 2.1 (1.0; 4.2) | 3.3 (1.7; 6.8) | 1.3 (1.0; 2.8) | <0.001 w |
| Creatinine, mg/dL; Me (IQR) | 0.80 (0.74; 0.89) | 0.82 (0.75; 0.94 | 0.78 (0.74; 0.87) | 0.058 w |
| hs-cTnI, ng/mL; Me (IQR) | 0.002 (0.002; 0.002) | 0.002 (0.002; 0.003) | 0.002 (0.002; 0.002) | 0.114 w |
| NT-proBNP, pg/mL; Me (IQR) | 56 (36; 100) | 69 (41; 122) | 49 (34; 80) | 0.063 w |
| Predominant cardiac complaint after recovery from COVID-19 pneumonia; n (%) | ||||
| Chest pain | 10 (8.3) | 1 (1.7) | 9 (14.3) | 0.011 f |
| Palpitations | 6 (4.9) | 1 (1.7) | 5 (7.9) | |
| Dyspnea | 53 (43.8) | 31 (53.5) | 22 (34.9) | |
| Fatigue | 52 (43.0) | 25 (43.1) | 27 (42.9) | |
| All Post-COVID-19 Patients (n = 121) | Hospitalized Post-COVID-19 Patients (n = 58) | Non-Hospitalized Post-COVID-19 Patients (n = 63) | p-Value (Hospitalized vs. Non-Hospitalized Post-COVID-19 Patients) | |
|---|---|---|---|---|
| Non-ischemic cardiac injury #; n (%) | 64 (54.5) | 35 (60.3) | 29 (46.0) | 0.163 c |
| LGE lesion; n (%) | 63 (52.1) | 35 (60.3) | 28 (44.4) | 0.117 c |
| Active myocarditis; n (%) | 10 (8.3) | 6 (10.3) | 4 (6.3) | 0.517 f |
| LVEF, %; Me (IQR) | 59 (55; 63) | 59 (53; 63) | 60 (55; 63) | 0.198 w |
| Reduced LVEF *; n (%) | 47 (38.8) | 24 (41.4) | 23 (36.5) | 0.717 c |
| LVESV, mL; Me (IQR) | 62 (51; 77) | 63 (51; 82) | 62 (51; 70) | 0.297 w |
| LVEDV, mL; Me (IQR) | 156 (135; 186) | 148.5 (133; 180) | 145 (132; 170) | 0.301 w |
| LVSV, mL; Me (IQR) | 86 (76; 101) | 87 (79; 100) | 86 (74; 104) | 0.775 w |
| Myocardial mass, g; Me (IQR) | 113 (93; 141) | 122 (103; 147) | 103 (87; 120) | <0.001 w |
| Global T1, ms; Me (IQR) | 1012 (994; 1031) | 1017 (1001; 1033) | 1009.5 (991; 1024) | 0.133 t |
| Global T1 > 1035 ms; n (%) | 22 (18.8) | 13 (23.6) | 9 (14.5) | 0.306 c |
| Global T2, ms; Me (IQR) | 46 (44; 48) | 47 (45; 48) | 46 (44; 48) | 0.411 w |
| Global T2 > 49 ms; n (%) | 12 (10.1) | 9 (15.8) | 3 (4.8) | 0.093 c |
| Global ECV, %; Me (IQR) | 25 (24; 28) | 25 (24; 28) | 25 (24; 27) | 0.687 w |
| RVEDV, mL; Me (IQR) | 137 (111; 164) | 141 (123; 166) | 130 (107; 154) | 0.029 w |
| RVEF, %; Me (IQR) | 52 (47; 56) | 49.5 (44; 54) | 53 (50; 58) | 0.001 w |
| Reduced RVEF **; n (%) | 56 (46.3) | 35 (60.3) | 21 (33.3) | 0.005 c |
| Pericardial effusion; n (%) | 2 (1.7%) | 1 (1.7%) | 1 (1.6%) | 1 f |
| Post-COVID-19 Patients with Non-Ischemic Cardiac Injury Using CMR (n = 64) | Post-COVID-19 Patients without Non-Ischemic Cardiac Injury Using CMR (n = 57) | p-Value for the Comparison between the Groups | |
|---|---|---|---|
| Age, years; Me (IQR) | 48 (44; 62) | 44 (37; 50) | 0.008 w |
| Female gender; n (%) | 26 (40.6) | 29 (50.9) | 0.343 c |
| Time between COVID-19 diagnosis and CMR examination, days; Me (IQR) | 41 (26; 48) | 42 (25; 67 | 0.459 w |
| BMI, kg/m2; Me (IQR) | 28.1 (25.4; 30.8) | 27 (24.3; 30.0) | 0.181 w |
| Obesity; n (%) | 19 (29.7) | 14 (24.6) | 0.669 c |
| Hypertension; n (%) | 24 (37.5) | 12 (21.1) | 0.076 c |
| Diabetes; n (%) | 11 (17.2) | 4 (7) | 0.156 c |
| Hyperlipidemia; n (%) | 24 (37.5) | 8 (14.0) | 0.007 c |
| Chronic kidney disease; n (%) | 1 (1.6) | 1 (1.8) | 1 f |
| Chronic respiratory disease *; n (%) | 7 (10.9) | 9 (15.8) | 0.605 c |
| Clinical severity of COVID-19 pneumonia; n (%) | |||
| Mild illness | 30 (46.9%) | 34 (59.6%) | |
| Moderate illness | 5 (7.8%) | 9 (15.8%) | 0.035 f |
| Severe illness | 28 (43.8%) | 12 (21.1%) | |
| Critical illness | 1 (1.6%) | 2 (3.5%) | |
| Extent of the involved lung tissue on computed tomography (%) ^; Me (IQR) | 24 (11; 49) | 22 (10; 30) | 0.309 w |
| White blood cell count (×103/μL); Me (IQR) | 7.0 (5.5; 8.3) | 7.0 (5.8; 8.2) | 0.939 t |
| Lymphocyte count (×103/μL); Me (IQR) | 2.0 (1.7; 2.5) | 2.3 (1.6; 2.5) | 0.869 t |
| Hemoglobin, g/dL; Me (IQR) | 14.4 (13.3; 15.4) | 13.9 (13.4; 14.8) | 0.161 t |
| CRP, mg/L; Me (IQR) | 2.4 (1.0; 4.2) | 1.9 (1.0; 4.1) | 0.989 w |
| Creatinine, mg/dL; Me (IQR) | 0.82 (0.76; 0.92) | 0.79 (0.73; 0.86) | 0.023 w |
| hs-cTnI, ng/mL; Me (IQR) | 0.002 (0.002; 0.003) | 0.002 (0.002; 0.002) | 0.009 w |
| NT-proBNP, pg/mL; Me (IQR) | 58 (40; 106) | 52 (34; 96) | 0.549 w |
| Predominant cardiac complaint after recovery from COVID-19 pneumonia; n (%) | |||
| Chest pain | 3 (4.7) | 7 (12.3) | |
| Palpitations | 2 (3.1) | 4 (7) | 0.237 f |
| Dyspnea | 32 (50) | 21 (36.8) | |
| Fatigue | 27 (42.2) | 25 (43.9) | |
| Post-COVID-19 Patients with Non-Ischemic Cardiac Injury Using CMR (n = 64) | Post-COVID-19 Patients without Non-Ischemic Cardiac Injury Using CMR (n = 57) | p-Value for the Comparison between the Groups | |
|---|---|---|---|
| LGE lesion; n (%) | 63 (98.4) | 0 (0) | <0.001 c |
| Active myocarditis; n (%) | 10 (15.6) | 0 (0) | 0.001 f |
| LVEF, %; Me (IQR) | 57 (52; 62) | 61 (56; 64) | <0.001 t |
| Reduced LVEF *; n (%) | 32 (50%) | 15 (26.3%) | 0.013 c |
| LVESV, mL; Me (IQR) | 64 (53; 83) | 59 (49; 68) | 0.046 w |
| LVEDV, mL; Me (IQR) | 147 (134; 177) | 148 (128; 170) | 0.289 w |
| LVSV, mL; Me (IQR) | 85 (76; 101) | 89 (76; 102) | 0.605 w |
| Myocardial mass, g; Me (IQR) | 117 (99; 143) | 103 (87; 132) | 0.033 w |
| Global T1, ms; Me (IQR) | 1019 (996; 1037) | 1008 (994; 1024) | 0.097 w |
| Global T1 > 1035 ms; n (%) | 16 (25.8) | 6 (10.9) | 0.068 c |
| Global T2, ms; Me (IQR) | 47 (44; 49) | 46 (45; 48) | 0.336 w |
| Global T2 > 49 ms; n (%) | 9 (14.1) | 3 (5.5) | 0.211 c |
| Global ECV, %; Me (IQR) | 26 (24; 28) | 25 (24; 27) | 0.326 w |
| RVEDV, mL; Me (IQR) | 143 (117; 165) | 127 (107; 158) | 0.098 t |
| RVEF, %; Me (IQR) | 52 (46; 55) | 54 (49; 58) | 0.236 w |
| Reduced RVEF **; n (%) | 30 (46.9) | 26 (45.6) | 1 c |
| Pericardial effusion; n (%) | 0 (0%) | 2 (3.5%) | 0.220 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtowicz, D.; Dorniak, K.; Ławrynowicz, M.; Wąż, P.; Fijałkowska, J.; Kulawiak-Gałąska, D.; Rejszel-Baranowska, J.; Knut, R.; Haberka, M.; Szurowska, E.; et al. Cardiac Magnetic Resonance Findings in Patients Recovered from COVID-19 Pneumonia and Presenting with Persistent Cardiac Symptoms: The TRICITY-CMR Trial. Biology 2022, 11, 1848. https://doi.org/10.3390/biology11121848
Wojtowicz D, Dorniak K, Ławrynowicz M, Wąż P, Fijałkowska J, Kulawiak-Gałąska D, Rejszel-Baranowska J, Knut R, Haberka M, Szurowska E, et al. Cardiac Magnetic Resonance Findings in Patients Recovered from COVID-19 Pneumonia and Presenting with Persistent Cardiac Symptoms: The TRICITY-CMR Trial. Biology. 2022; 11(12):1848. https://doi.org/10.3390/biology11121848
Chicago/Turabian StyleWojtowicz, Dagmara, Karolina Dorniak, Marzena Ławrynowicz, Piotr Wąż, Jadwiga Fijałkowska, Dorota Kulawiak-Gałąska, Joanna Rejszel-Baranowska, Robert Knut, Maciej Haberka, Edyta Szurowska, and et al. 2022. "Cardiac Magnetic Resonance Findings in Patients Recovered from COVID-19 Pneumonia and Presenting with Persistent Cardiac Symptoms: The TRICITY-CMR Trial" Biology 11, no. 12: 1848. https://doi.org/10.3390/biology11121848
APA StyleWojtowicz, D., Dorniak, K., Ławrynowicz, M., Wąż, P., Fijałkowska, J., Kulawiak-Gałąska, D., Rejszel-Baranowska, J., Knut, R., Haberka, M., Szurowska, E., & Koziński, M. (2022). Cardiac Magnetic Resonance Findings in Patients Recovered from COVID-19 Pneumonia and Presenting with Persistent Cardiac Symptoms: The TRICITY-CMR Trial. Biology, 11(12), 1848. https://doi.org/10.3390/biology11121848










