Variability of Bacterial Homopolysaccharide Production and Properties during Food Processing
Abstract
:Simple Summary
Abstract
1. Introduction
2. Variability in Structure and Size
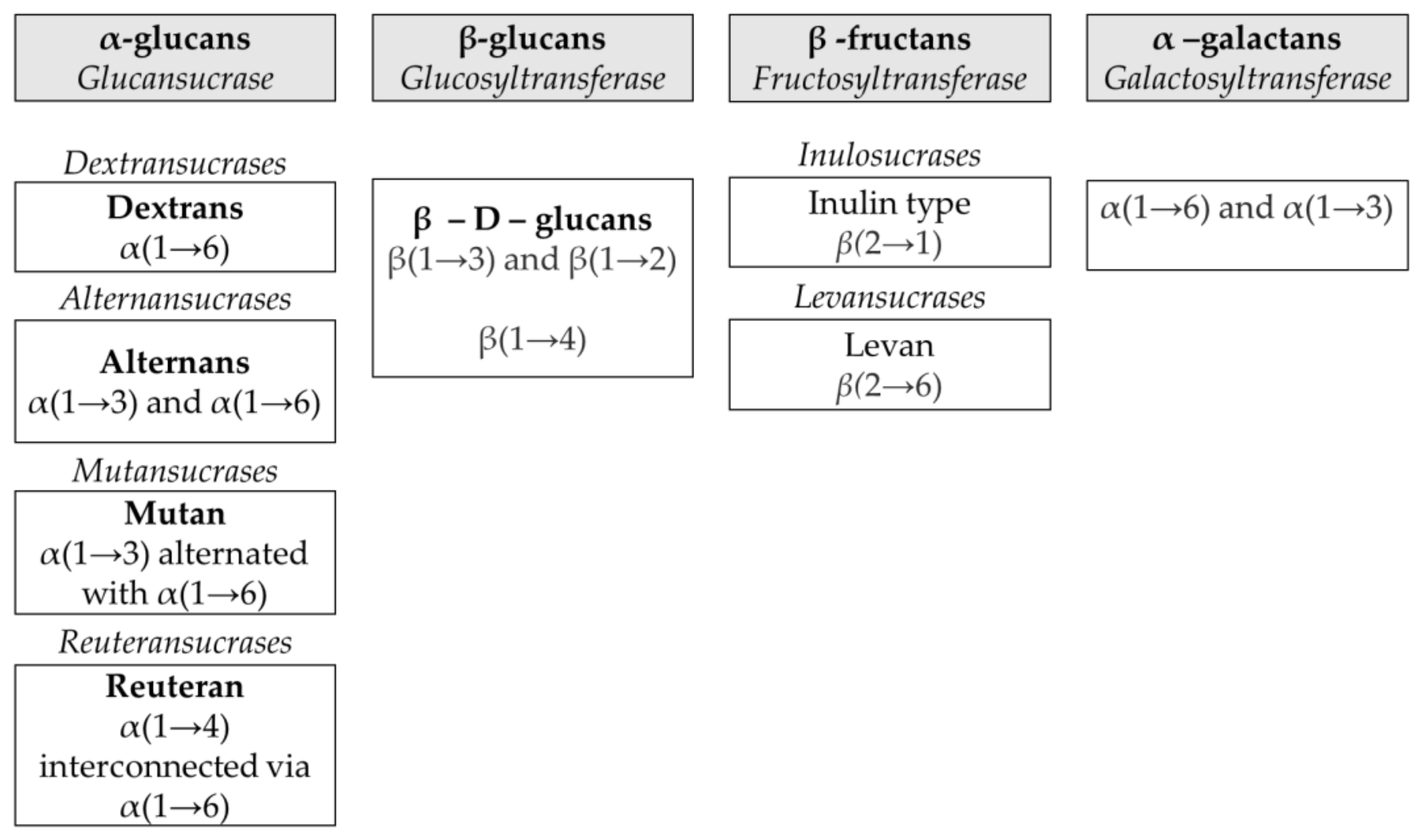
2.1. Different Families of Homopolysaccharides
2.2. α-Glucans
2.3. β-Glucans
2.4. Fructans
2.5. α-Galactans
3. Gene Regulation and Variability of HoPS Production Level
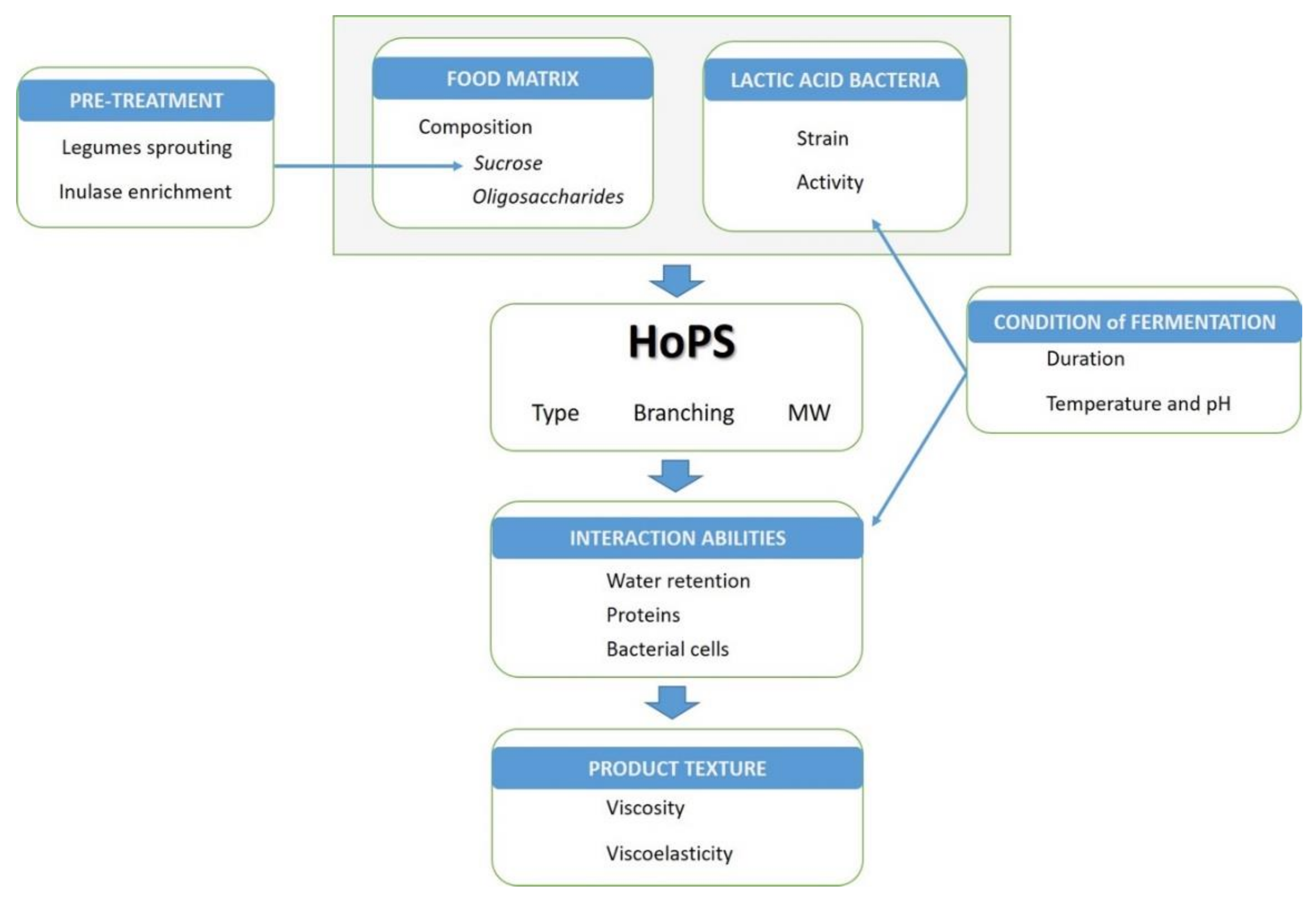
4. Effect of Food Composition and Processing
4.1. Role of the Matrix Composition
4.2. Addition of Ingredients
4.3. Pretreatments
4.4. Conditions of Fermentation
4.5. Traditional (Spontaneous) vs. Inoculated
5. Technological Impact of EPSs in Foods, Depending on Matrix and Processing Conditions
5.1. Applications of EPSs
5.2. In Situ Production versus Ex Situ Addition
5.3. Texture
5.3.1. Rheological Properties
5.3.2. Viscosity
5.3.3. Syneresis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramawat, K.G.; Merillon, J.-M. Polysaccharides: Bioactivity and Biotechnology; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Kim, M.J.; Seo, H.N.; Hwang, T.S.; Lee, S.H.; Park, D.H. Characterization of exopolysaccharide (EPS) produced by Weissella hellenica SKkimchi3 isolated from kimchi. J. Microbiol. 2008, 46, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.-L.; Chen, L.-D.; Wu, J.-Y. Levan production using Bacillus subtilis natto cells immobilized on alginate. Carbohydr. Polym. 2010, 82, 111–117. [Google Scholar] [CrossRef]
- Ye, G.; Chen, Y.; Wang, C.; Yang, R.; Bin, X. Purification and characterization of exopolysaccharide produced by Weissella cibaria YB-1 from pickle Chinese cabbage. Int. J. Biol. Macromol. 2018, 120, 1315–1321. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Ishola, R.; Oyewunmi, T. Characterization, antioxidant and immunomodulatory potential on exopolysaccharide produced by wild type and mutant Weissella confusa strains. Biotechnol. Rep. 2018, 19, e00271. [Google Scholar] [CrossRef]
- Lakra, A.K.; Domdi, L.; Tilwani, Y.M.; Arul, V. Physicochemical and functional characterization of mannan exopolysaccharide from Weissella confusa MD1 with bioactivities. Int. J. Biol. Macromol. 2020, 143, 797–805. [Google Scholar] [CrossRef]
- Rosca, I.; Petrovici, A.R.; Peptanariu, D.; Nicolescu, A.; Dodi, G.; Avadanei, M.; Ivanov, I.C.; Bostanaru, A.C.; Mares, M.; Ciolacu, D. Biosynthesis of dextran by Weissella confusa and its in vitro functional characteristics. Int. J. Biol. Macromol. 2018, 107, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic acid bacteria exopolysaccharides in foods and beverages: Isolation, properties, characterization, and health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef]
- Rana, S.; Upadhyay, L.S.B. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef]
- Duboc, P.; Mollet, B. Applications of exopolysaccharides in the dairy industry. Int. Dairy J. 2001, 11, 759–768. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Dal Bello, F.; Coffey, A.; Gänzle, M.G.; Arendt, E.K. Influence of in-situ synthesized exopolysaccharides on the quality of gluten-free sorghum sourdough bread. Int. J. Food Microbiol. 2012, 155, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Czerny, M.; Arendt, E.K. Influence of dextran-producing Weissella cibaria on baking properties and sensory profile of gluten-free and wheat breads. Int. J. Food Microbiol. 2014, 172, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Mauch, A.; Galle, S.; Gänzle, M.; Coffey, A.; Arendt, E.K.; Taylor, J.P.; Waters, D.M. Barley malt wort fermentation by exopolysaccharide-forming Weissella cibaria MG1 for the production of a novel beverage. J. Appl. Microbiol. 2013, 115, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Datta, S.; Biswas, D. Towards a better production of bacterial exopolysaccharides by controlling genetic as well as physico-chemical parameters. Appl. Microbiol. Biotechnol. 2018, 102, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.E.; Figueroa, T.; Navarro, D.; Gómez, M.I.; Font de Valdez, G.; Torino, M.I. Techno-functional properties of HoPS from lactic acid bacteria of different origins as potential food additives. Food Chem. 2021, 356, 129627. [Google Scholar] [CrossRef]
- Van Hijum, S.A.; Kralj, S.; Ozimek, L.K.; Dijkhuizen, L.; Van Geel-Schutten, I.G. Structure-function relationships of glucansucrase and fructansucrase enzymes from lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 157–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kralj, S.; Stripling, E.; Sanders, P.; Van Geel-Schutten, G.H.; Dijkhuizen, L. Highly hydrolytic reuteransucrase from probiotic Lactobacillus reuteri strain ATCC 55730. Appl. Environ. Microbiol. 2005, 71, 3942–3950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hijum, S.A.F.T.; Szalowska, E.; Van der Maarel, M.J.E.C.; Dijkhuizen, L.Y. Biochemical and molecular characterization of a levansucrase from Lactobacillus reuteri. Microbiology 2004, 150, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semor, N.; Azmi, W.; Gautam, M. Characterization and structural analysis of unique dextran synthesized by purified dextransucrase of newly isolated Acetobacter tropicalis. Curr. Biotechnol. 2018, 7, 376–386. [Google Scholar] [CrossRef]
- Vuillemin, M.; Grimaud, F.; Claverie, M.; Rolland-Sabaté, A.; Garnier, C.; Lucas, P.; Monsan, P.; Dols-Lafargue, M.; Remaud-Siméon, M.; Moulis, C. A Dextran with unique rheological properties produced by the dextransucrase from Oenococcus kitaharae DSM 17330. Carbohydr. Polym. 2018, 179, 10–18. [Google Scholar] [CrossRef]
- Polak-Berecka, M.; Choma, A.; Waśko, A.; Górska, S.; Gamian, A.; Cybulska, J. Physicochemical characterization of exopolysaccharides produced by Lactobacillus rhamnosus on various carbon sources. Carbohydr. Polym. 2015, 117, 501–509. [Google Scholar] [CrossRef]
- Du, R.; Xing, H.; Yang, Y.; Jiang, H.; Zhou, Z.; Han, Y. Optimization, purification and structural characterization of a dextran produced by L. mesenteroides isolated from Chinese sauerkraut. Carbohydr. Polym. 2017, 174, 409–416. [Google Scholar] [CrossRef]
- Nácher-Vázquez, M.; Iturria, I.; Zarour, K.; Mohedano, M.L.; Aznar, R.; Pardo, M.Á.; López, P. Dextran production by Lactobacillus sakei MN1 coincides with reduced autoagglutination, biofilm formation and epithelial cell adhesion. Carbohydr. Polym. 2017, 168, 22–31. [Google Scholar] [CrossRef]
- Zarour, K.; Llamas, M.G.; Prieto, A.; Rúas-Madiedo, P.; Dueñas, M.T.; De Palencia, P.F.; Aznar, R.; Kihal, M.; López, P. Rheology and bioactivity of high molecular weight dextrans synthesised by lactic acid bacteria. Carbohydr. Polym. 2017, 174, 646–657. [Google Scholar] [CrossRef]
- Fraunhofer, M.E.; Geissler, A.J.; Wefers, D.; Bunzel, M.; Jakob, F.; Vogel, R.F. Characterization of β-glucan formation by Lactobacillus brevis TMW 1.2112 isolated from slimy spoiled beer. Int. J. Biol. Macromol. 2018, 107, 874–881. [Google Scholar] [CrossRef]
- Polak-Berecka, M.; Waśko, A.; Kubik-Komar, A. Optimization of culture conditions for exopolysaccharide production by a probiotic strain of Lactobacillus rhamnosus E/N. Pol. J. Microbiol. 2014, 63, 253–257. [Google Scholar] [CrossRef]
- Ni, D.; Zhu, Y.; Xu, W.; Bai, Y.; Zhang, T.; Mu, W. Biosynthesis of inulin from sucrose using inulosucrase from Lactobacillus gasseri DSM 20604. Int. J. Biol. Macromol. 2018, 109, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Kavitake, D.; Devi, P.B.; Singh, S.P.; Shetty, P.H. Characterization of a novel galactan produced by Weissella confusa KR780676 from an acidic fermented food. Int. J. Biol. Macromol. 2016, 86, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Vettori, M.H.P.B.; Franchetti, S.M.M.; Contiero, J. Structural characterization of a new dextran with a low degree of branching produced by Leuconostoc mesenteroides FT045B dextransucrase. Carbohydr. Polym. 2012, 88, 1440–1444. [Google Scholar] [CrossRef] [Green Version]
- Oleksy, M.; Klewicka, E. Exopolysaccharides produced by Lactobacillus pp.: Biosynthesis and applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 450–462. [Google Scholar] [CrossRef]
- Meng, X.; Gangoiti, J.; Bai, Y.; Pijning, T.; Van Leeuwen, S.S.; Dijkhuizen, L. Structure–function relationships of family GH70 glucansucrase and 4,6-α-glucanotransferase enzymes, and their evolutionary relationships with family GH13 enzymes. Cell Mol. Life Sci. 2016, 73, 2681–2706. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, H.; Li, M.; Hu, X.; Li, Y. Functional analysis of truncated and site-directed mutagenesis dextransucrases to produce different type dextrans. Enzym. Microb. Technol. 2017, 102, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Claverie, M.; Cioci, G.; Vuillemin, M.; Bondy, P.; Remaud-Simeon, M.; Moulis, C. Processivity of dextransucrases synthesizing very-high-molar-mass dextran is mediated by sugar-binding pockets in domain V. J. Biol. Chem. 2020, 295, 5602–5613. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Dobruchowska, J.M.; Pijning, T.; López, C.A.; Kamerling, J.P.; Dijkhuizen, L. Residue Leu940 has a crucial role in the linkage and reaction specificity of the glucansucrase GTF180 of the probiotic bacterium Lactobacillus reuteri 180. J. Biol. Chem. 2014, 289, 32773–32782. [Google Scholar] [CrossRef] [Green Version]
- Molina, M.; Moulis, C.; Monties, N.; Pizzut-Serin, S.; Guieysse, D.; Morel, S.; Cioci, G.; Remaud-Siméon, M. Deciphering an undecided enzyme: Investigations of the structural determinants involved in the linkage specificity of alternansucrase. ACS Catal. 2019, 9, 2222–2237. [Google Scholar] [CrossRef]
- Badel, S.; Bernardi, T.; Michaud, P. New perspectives for Lactobacilli exopolysaccharides. Biotechnol. Adv. 2011, 29, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Bechtner, J.; Vogel, R.F.; Jakob, F. A systematic approach to study the pH-dependent release, productivity and product specificity of dextransucrases. Microb. Cell Factories 2019, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gänzle, M.G. Effect of temperature on production of oligosaccharides and dextran by Weissella cibaria 10 M. Int. J. Food Microbiol. 2018, 280, 27–34. [Google Scholar] [CrossRef]
- Schmid, J.; Wefers, D.; Vogel, R.F.; Jakob, F. Analysis of structural and functional differences of glucans produced by the natively released dextransucrase of Liquorilactobacillus hordei TMW 1.1822. Appl. Biochem. Biotechnol. 2021, 193, 96–110. [Google Scholar] [CrossRef]
- Schlörmann, W.; Bockwoldt, J.A.; Mayr, M.F.; Lorkowski, S.; Dawczynski, C.; Rohn, S.; Ehrmann, M.A.; Glei, M. Fermentation profile, cholesterol-reducing properties and chemopreventive potential of β-glucans from Levilactobacillus brevis and Pediococcus claussenii—A comparative study with β-glucans from different sources. Food Funct. 2021, 12, 10615–10631. [Google Scholar] [CrossRef]
- Dols Lafargue, M. Polysaccharide production by wine lactic acid bacteria: Negative trait or potential advantage? A review. Appli Micro Open Access 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Walling, E.; Gindreau, E.; Lonvaud-funel, A. A putative glucan synthase gene Dps detected in exopolysaccharide-producing Pediococcus damnosus and Oenococcus oeni strains isolated from wine and cider. Int. J. Food Microbiol. 2005, 98, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-Chasco, M.T.; Rodríguez-Carvajal, M.A.; Tejero-Mateo, P.; Espartero, J.L.; Irastorza-Iribas, A.; Gil-Serrano, A.M. Structural analysis of the exopolysaccharides produced by Lactobacillus spp. G-77. Carbohydr. Res. 1998, 307, 125–133. [Google Scholar] [CrossRef]
- Gullo, M.; La China, S.; Falcone, P.M.; Giudici, P. Biotechnological production of cellulose by acetic acid bacteria: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 6885–6898. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D. Chapter 3—The interaction between insoluble and soluble fiber. In Dietary Fiber for the Prevention of Cardiovascular Disease; Samaan, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 35–59. ISBN 978-0-12-805130-6. [Google Scholar]
- Peesapati, S.; Sajeevan, K.A.; Patel, S.K.; Roy, D. Relation between glycosidic linkage, structure and dynamics of α- and β-glucans in water. Biopolymers 2021, 112, e23423. [Google Scholar] [CrossRef] [PubMed]
- Harutoshi, T. Exopolysaccharides of Lactic Acid Bacteria for Food and Colon Health Applications; IntechOpen: London, UK, 2013; ISBN 978-953-51-0955-6. [Google Scholar]
- Monsan, P.; Bozonnet, S.; Albenne, C.; Joucla, G.; Willemot, R.-M.; Remaud-Siméon, M. Homopolysaccharides from lactic acid bacteria. Int. Dairy J. 2001, 11, 675–685. [Google Scholar] [CrossRef]
- Pomin, V.H.; Mourão, P.A.S. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef] [Green Version]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.F.; Øregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From Genes to industrial applications. FEMS Microbiol. Rev. 2017, 41, S168–S200. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Guérin, M.; Silva, C.R.-D.; Garcia, C.; Remize, F. Lactic acid bacterial production of exopolysaccharides from fruit and vegetables and associated benefits. Fermentation 2020, 6, 115. [Google Scholar] [CrossRef]
- Årsköld, E.; Svensson, M.; Grage, H.; Roos, S.; Rådström, P.; Van Niel, E.W.J. Environmental influences on exopolysaccharide formation in Lactobacillus reuteri ATCC 55730. Int. J. Food Microbiol. 2007, 116, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Ma, Y.; Huang, C.; Jiang, B.; Cui, S.W.; Zhang, T. Physicochemical properties of a water soluble extracellular homopolysaccharide from Lactobacillus reuteri SK24.003. Carbohydr. Polym. 2015, 131, 377–383. [Google Scholar] [CrossRef]
- Looijesteijn, P.J.; Casteren, W.H.M.V.; Tuinier, R.; Doeswijk-Voragen, C.H.L.; Hugenholtz, J. Influence of different substrate limitations on the yield, composition and molecular mass of exopolysaccharides produced by Lactococcus lactis subsp. cremoris in continuous cultures. J. Appl. Microbiol. 2000, 89, 116–122. [Google Scholar] [CrossRef]
- Van Geel-Schutten, G.H.; Flesch, F.; Ten Brink, B.; Smith, M.R.; Dijkhuizen, L. Screening and characterization of Lactobacillus strains producing large amounts of exopolysaccharides. Appl. Microbiol. Biotechnol. 1998, 50, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Galle, S.; Gänzle, M.G.; Waters, D.M.; Arendt, E.K. Evaluation of exopolysaccharide producing Weissella cibaria MG1 strain for the production of sourdough from various flours. Food Microbiol. 2014, 37, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Galle, S.; Schwab, C.; Arendt, E.; Gänzle, M. Exopolysaccharide-forming Weissella strains as starter cultures for sorghum and wheat sourdoughs. J. Agric. Food Chem. 2010, 58, 5834–5841. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.T.; Dertli, E.; Toker, O.S.; Tatlisu, N.B.; Sagdic, O.; Arici, M. Effect of in situ exopolysaccharide production on physicochemical, rheological, sensory, and microstructural properties of the yogurt drink Ayran: An optimization study based on fermentation kinetics. J. Dairy Sci. 2015, 98, 1604–1624. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Coda, R.; Säde, E.; Tuomainen, P.; Tenkanen, M.; Katina, K. In situ synthesis of exopolysaccharides by Leuconostoc spp. and Weissella spp. and their rheological impacts in fava bean flour. Int. J. Food Microbiol. 2017, 248, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Galli, V.; Venturi, M.; Coda, R.; Maina, N.H.; Granchi, L. Isolation and characterization of indigenous Weissella confusa for in situ bacterial exopolysaccharides (EPS) production in chickpea sourdough. Food Res. Int. 2020, 138, 109785. [Google Scholar] [CrossRef]
- Meng, Q.; Lu, C.; Gao, H.; Chen, G.; Wu, L.; Wu, J.; Li, S.; He, B.-F. Efficient biosynthesis of exopolysaccharide from Jerusalem artichoke using a novel strain of Bacillus velezensis LT-2. Bioresour. Technol. 2021, 320, 124346. [Google Scholar] [CrossRef]
- Hilbig, J.; Gisder, J.; Prechtl, R.M.; Herrmann, K.; Weiss, J.; Loeffler, M. Influence of exopolysaccharide-producing lactic acid bacteria on the spreadability of fat-reduced raw fermented sausages (Teewurst). Food Hydrocoll. 2019, 93, 422–431. [Google Scholar] [CrossRef]
- Perri, G.; Coda, R.; Rizzello, C.G.; Celano, G.; Ampollini, M.; Gobbetti, M.; De Angelis, M.; Calasso, M. Sourdough fermentation of whole and sprouted lentil flours: In situ formation of dextran and effects on the nutritional, texture and sensory characteristics of white bread. Food Chem. 2021, 355, 129638. [Google Scholar] [CrossRef]
- Hickisch, A.; Beer, R.; Vogel, R.F.; Toelstede, S. Influence of lupin-based milk alternative heat treatment and exopolysaccharide-producing lactic acid bacteria on the physical characteristics of lupin-based yogurt alternatives. Food Res. Int. 2016, 84, 180–188. [Google Scholar] [CrossRef]
- Düven, G.; Kumcuoğlu, S.; Kışla, D. Ultrasonication-assisted kefir production and its effect on fermentation time and EPS production. Food Biosci. 2021, 42, 101059. [Google Scholar] [CrossRef]
- Dertli, E.; Yilmaz, M.T.; Tatlisu, N.B.; Toker, O.S.; Cankurt, H.; Sagdic, O. Effects of in situ exopolysaccharide production and fermentation conditions on physicochemical, microbiological, textural and microstructural properties of Turkish-type fermented sausage (Sucuk). Meat Sci. 2016, 121, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Abedfar, A.; Hosseininezhad, M.; Sadeghi, A.; Raeisi, M.; Feizy, J. Investigation on “spontaneous fermentation” and the productivity of microbial exopolysaccharides by Lactobacillus plantarum and Pediococcus pentosaceus isolated from wheat bran sourdough. LWT 2018, 96, 686–693. [Google Scholar] [CrossRef]
- Dertli, E.; Mercan, E.; Arıcı, M.; Yılmaz, M.T.; Sağdıç, O. Characterisation of lactic acid bacteria from Turkish sourdough and determination of their exopolysaccharide (EPS) production characteristics. LWT-Food Sci. Technol. 2016, 71, 116–124. [Google Scholar] [CrossRef]
- Katina, K.; Maina, N.H.; Juvonen, R.; Flander, L.; Johansson, L.; Virkki, L.; Tenkanen, M.; Laitila, A. In situ production and analysis of Weissella confusa dextran in wheat sourdough. Food Microbiol. 2009, 26, 734–743. [Google Scholar] [CrossRef]
- Rühmkorf, C.; Rübsam, H.; Becker, T.; Bork, C.; Voiges, K.; Mischnick, P.; Brandt, M.J.; Vogel, R.F. Effect of structurally different microbial homoexopolysaccharides on the quality of gluten-free bread. Eur. Food Res. Technol. 2012, 235, 139–146. [Google Scholar] [CrossRef]
- Xu, Y.; Coda, R.; Holopainen-Mantila, U.; Laitila, A.; Katina, K.; Tenkanen, M. Impact of in situ produced exopolysaccharides on rheology and texture of fava bean protein concentrate. Food Res. Int. 2019, 115, 191–199. [Google Scholar] [CrossRef]
- Lorusso, A.; Coda, R.; Montemurro, M.; Rizzello, C. Use of selected lactic acid bacteria and quinoa flour for manufacturing novel yogurt-like beverages. Foods 2018, 7, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korcz, E.; Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Llamas-Arriba, M.G.; Peirotén, Á.; Puertas, A.I.; Prieto, A.; López, P.; Pardo, M.Á.; Rodríguez, E.; Dueñas, M.T. Heteropolysaccharide-producing bifidobacteria for the development of functional dairy products. LWT 2019, 102, 295–303. [Google Scholar] [CrossRef]
- Nachtigall, C.; Surber, G.; Herbi, F.; Wefers, D.; Jaros, D.; Rohm, H. Production and molecular structure of heteropolysaccharides from two lactic acid bacteria. Carbohydr. Polym. 2020, 236, 116019. [Google Scholar] [CrossRef] [PubMed]
- Kant Bhatia, S.; Gurav, R.; Choi, Y.-K.; Choi, T.-R.; Kim, H.; Song, H.-S.; Mi Lee, S.; Lee Park, S.; Soo Lee, H.; Kim, Y.-G.; et al. Bioprospecting of exopolysaccharide from marine Sphingobium yanoikuyae BBL01: Production, characterization, and metal chelation activity. Bioresour. Technol. 2021, 324, 124674. [Google Scholar] [CrossRef]
- EUR-Lex—32001D0122. Commission Decision of 30 January 2001 on Authorising the Placing on the Market of a Dextran Preparation Produced by Leuconostoc Mesenteroides as a Novel Food Ingredient in Bakery Products under Regulation (EC) No 258/97 of the European Parliament and of the Council (Notified under Document Number C(2001) 174); European Parliament: Strasbourg, France, 2001. [Google Scholar]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef]
- Arendt, E.K.; Ryan, L.A.M.; Dal Bello, F. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef]
- Birch, J.; Van Calsteren, M.-R.; Pérez, S.; Svensson, B. The exopolysaccharide properties and structures database: EPS-DB. Application to bacterial exopolysaccharides. Carbohydr. Polym. 2019, 205, 565–570. [Google Scholar] [CrossRef]
- Mende, S.; Rohm, H.; Jaros, D. Influence of exopolysaccharides on the structure, texture, stability and sensory properties of yoghurt and related products. Int. Dairy J. 2016, 52, 57–71. [Google Scholar] [CrossRef]
- Benigar, E.; Dogsa, I.; Stopar, D.; Jamnik, A.; Cigić, I.K.; Tomšič, M. Structure and dynamics of a polysaccharide matrix: Aqueous solutions of bacterial levan. Langmuir 2014, 30, 4172–4182. [Google Scholar] [CrossRef]
- Xu, Y.; Pitkänen, L.; Maina, N.H.; Coda, R.; Katina, K.; Tenkanen, M. Interactions between fava bean protein and dextrans produced by Leuconostoc pseudomesenteroides DSM 20193 and Weissella cibaria Sj 1b. Carbohydr. Polym. 2018, 190, 315–323. [Google Scholar] [CrossRef]
- Kasaai, M.R. Dilute solution properties and degree of chain branching for dextran. Carbohydr. Polym. 2012, 88, 373–381. [Google Scholar] [CrossRef]
- Hundschell, C.S.; Wagemans, A.M. Rheology of common uncharged exopolysaccharides for food applications. Curr. Opin. Food Sci. 2019, 27, 1–7. [Google Scholar] [CrossRef]
- Bejar, W.; Gabriel, V.; Amari, M.; Morel, S.; Mezghani, M.; Maguin, E.; Fontagné-Faucher, C.; Bejar, S.; Chouayekh, H. Characterization of glucansucrase and dextran from Weissella sp. TN610 with potential as safe food additives. Int. J. Biol. Macromol. 2013, 52, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, Q.; Zhao, F.; Xiao, H.; Zhou, Z.; Han, Y. Purification and characterization of dextran produced by Leuconostoc pseudomesenteroides PC as a potential exopolysaccharide suitable for food applications. Process Biochem. 2019, 87, 187–195. [Google Scholar] [CrossRef]
- Han, M.; Du, C.; Xu, Z.-Y.; Qian, H.; Zhang, W.-G. Rheological properties of phosphorylated exopolysaccharide produced by Sporidiobolus pararoseus JD-2. Int. J. Biol. Macromol. 2016, 88, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. Int. J. Biol. Macromol. 2021, 173, 79–89. [Google Scholar] [CrossRef]
| Strain | HoPS | Molecular Weight Range (Da) | Linkage | Reference |
|---|---|---|---|---|
| Leuconostoc mesenteroides TDS2-19 | Dextran | 9 × 107 | α(1→6) Glc linear | [22] |
| Leu. mesenteroides RTF10 | Dextran | 4 × 108 | α(1→6) Glc and branched | [23] |
| Latilactobacillus sakei MN1 | Dextran | 2 × 108 | α(1→6) Glc and partially branched in the O-3 position by a single α-glucopyranose unit (between 8.5% and 10.3%) | [24] |
| Leu. mesenteroides NRRL | Dextran | 6 × 105 | 95% α(1→6) Glc and 5% α(1→3) Glc | [16] |
| Acetobacter tropicalis | Dextran | 1 × 104 to 4 × 104 | α(1→6) Glc linear | [19] |
| Limosilactobacillus reuteri ML1 | Reuteran | α(1→6) Glc and 4,6-disubstituted α-glucosyl units at the branching points | [17] | |
| Levilactobacillusbrevis TMW 1.2112 | β-Glucan | β(1 → 3) Glc ramified with β-Glc residues at position O2 | [25] | |
| Lim. reuteri 121 | Levan | 2 × 105 to 2 × 106 | 98% β(2→6) Fru and 2% β(1→2) and β(2→6) Fru branched | [18] |
| Bacillus subtilis Natto | Levan | β(2→6) fructofuranoside | [3] | |
| Lactobacillus rhamnosus | Inulin | 11 × 106 | β(2→1) Fru glycosidic and Fru branched at the β(2→6) position | [26] |
| Lactobacillus gasseri DSM 20604 | Inulin | 6 × 106 | β(2→1) Fru glycosidic | [27] |
| Weissella confusa KR780676 | Galactan | α(1 → 6) galactose | [28] |
| Strain | MW (Da) | Optimum Temperature (°C) | pH | Medium Composition | Yield (g L−1) | Application | Reference |
|---|---|---|---|---|---|---|---|
| Leu. mesenteroides TDS2-19 | 8.8 × 107 | 25 | 6.8 | MRS 1 medium | 71.23 | Used in food industries as an emulsifier or as part of starter culture | [22] |
| Leu. mesenteroides RTF10 | 4.4 × 108 | 30 | 4.8 | CDM 2 0.8% sucrose | 1.25 | Used as adjuvant and stabilizer in food industries | [23] |
| Lat. sakei MN1 | 1.7 × 108 | 30 | MRS 2% sucrose | 1.72 | Used for biofilm formation | [24] | |
| W. cibaria MG1 | 7.2 × 108 | 30 | 4.0–4.1 | MRS 10% sucrose | Used in bakery for sorghum bread | [7] | |
| Lim. reuteri ML1 | 35 | 4.7 | Sucrose (purified enzyme) | 5.12 | [17] | ||
| Lim. reuteri 121 | 37 | 4.8 | MRS medium sucrose | 5.2 | [56] | ||
| Lim. reuteri VIP | 1.0 × 107 | 37 | 3.6 | MRS 10% sucrose | Used in bakery for sorghum bread | [7] | |
| B. subtilis Natto | 37 | 5.6–5.8 | SM 3 sucrose 20% | 70.60 | Used in pharmaceutical industries as a commercial spore, on alginate matrix for repeated production of levan | [3] | |
| Weissella hellenica SKkimchi3 | 20.3 × 104 | 20 | 5 | MRS sucrose 30% | 74.00 | [2] | |
| Lim. reuteri 121 | 2.0 × 106 | 37 | 4.5–5.5 | In vitro enzyme | [18] | ||
| L. rhamnosus | 11.1 × 106 | 37 | 5.0 | YNB 4 | 2.10 | [26] | |
| Lim. reuteri Y2 | 8.9 × 106 | 37 | 3.7–3.8 | MRS 2.5% sucrose or raffinose | [7] | ||
| L. gasseri DSM 20604 | 5.8 × 106 | 35 | 5.5 | sucrose | 53.00 | [27] |
| EPS | Strain | Processing Conditions | Effects | Reference |
|---|---|---|---|---|
| Dextran | W. cibaria MG1 | Different fermented flours (buckwheat, quinoa, sorghum and teff) used as the basis for bread recipes using sourdough | Yield depended on the substrate and was highest in buckwheat and quinoa sourdough. The level of maltose in flour influences the MW of synthetized EPSs in sourdoughs (lower maltose in buckwheat resulting in the most high-MW dextran). Bread rheological properties were influenced by the flour matrix in relation to the variability of HoPS: reduction of crumb hardness in buckwheat (−122%), teff and quinoa breads; reduction of the staling rate in buckwheat and teff breads. | [57] |
| Dextran | W. confusa VTT E-90392 | In situ EPS production in wheat sourdoughs, 10% enriched with sucrose or unenriched. Sourdoughs were used in baking at 43% of the dough weight. | W. confusa efficiently produced polymeric dextran (11–16 g/kg DW) from the added sucrose in wheat sourdough without strong acid production. The produced dextran significantly increased the viscosity of the sourdoughs. Application of dextran-enriched sourdoughs in bread baking provided mildly acidic wheat bread with improved volume (up to 10%) and crumb softness (25–40%) during 6 days of storage. | [70] |
| Dextran | W. confusa SLA4 | Lentil flour sprouting | Increase slightly the dextran synthesis in comparison to nonsprouted lentil sourdoughs (9.7% and 9.2% w/w flour weight, respectively). | [64] |
| EPS | Kefir starter culture | Ultrasonic sound waves | The treatment allows kefir production in a shorter time by affecting the growth rate and lactic acid and EPS production rate. | [66] |
| EPS | Latilactobacillus plantarum 162R and Leu. mesenteroides N6 | Ripening period and fermentation temperature | Increase of EPS production level, associated with hardness reduction of the fat-reduced products and lower loss and storage moduli, when the ripening period was prolonged and the fermentation temperature was higher. | [67] |
| Dextran Fructan, glucan Dextran | Latilactobacillus curvatus TMW 1.624 Ligiactobacillus animalis TMW 1.971 Lim. reuteri TMW 1.106 | In situ production of various EPSs compared to the addition of hydroxypropyl-methylcellulose (HPMC) | Only HPMC and the dextran of Lat. curvatus TMW 1.624 retained water. The moisture content, baking loss and crumb firmness were improved the most by dextran of Lat. curvatus TMW 1.624. Structure analysis revealed that this dextran had the highest molecular weight of the analyzed EPSs (118–242 MDa) and was branched in position 3 (8–9%). A structure–function relation was suggested in which high weight, average molar mass and branching at position 3 of the glucose monomer foster a compact conformation of the molecule, enabling an increased water-binding capacity and promoting superior structural effects in gluten-free breads. | [71] |
| Dextran | Leuconostoc pseudomesenteroides DSM 20193 and W. confusa E3403 | In situ production in legume protein-rich foods (fava bean protein concentrate) | Stabilization, prevention of protein aggregation. Improvement in rheological and textural parameters in sucrose-added pastes after fermentation. W. confusa exhibited a higher viscosity index and a more rigid character of formed gel values than Leu. pseudomesenteroides (1441 and 766 g sof viscosity index, 0.39 and 0.23 of relative viscoelasticity index tan δ, respectively). | [72] |
| Dextran | W. confusa DSM 20194, compared to probiotic strains (Lat. plantarum T6B10, L. rhamnosus SP1) | Quinoa flour subjected to desaponification and gelatinization prior to fermentation | The content of 35%, w/w of quinoa flour in water was determined as optimal regarding the viscosity parameter. The viscosity and water holding capacity increased during fermentation with W. confusa, as the consequence of the EPS synthesized, contrary to what observed for other strains (0.7 Pa s and 98% observed for W. confusa versus 0.2 Pa s and 69–70% for the other tested strains, respectively). | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabot, M.; Guérin, M.; Sivakumar, D.; Remize, F.; Garcia, C. Variability of Bacterial Homopolysaccharide Production and Properties during Food Processing. Biology 2022, 11, 171. https://doi.org/10.3390/biology11020171
Nabot M, Guérin M, Sivakumar D, Remize F, Garcia C. Variability of Bacterial Homopolysaccharide Production and Properties during Food Processing. Biology. 2022; 11(2):171. https://doi.org/10.3390/biology11020171
Chicago/Turabian StyleNabot, Marion, Marie Guérin, Dharini Sivakumar, Fabienne Remize, and Cyrielle Garcia. 2022. "Variability of Bacterial Homopolysaccharide Production and Properties during Food Processing" Biology 11, no. 2: 171. https://doi.org/10.3390/biology11020171
APA StyleNabot, M., Guérin, M., Sivakumar, D., Remize, F., & Garcia, C. (2022). Variability of Bacterial Homopolysaccharide Production and Properties during Food Processing. Biology, 11(2), 171. https://doi.org/10.3390/biology11020171









