Phylogenetic Diversity of Ossification Patterns in the Avian Vertebral Column: A Review and New Data from the Domestic Pigeon and Two Species of Grebes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review
2.1.1. Vertebral Bodies (Corpora Vertebrae)
2.1.2. Vertebral Arches (Arcus Vertebrae)
2.1.3. Vertebral Arch Fusion
2.2. Skeletal Development
2.3. Ancestral State Reconstructions
3. Results
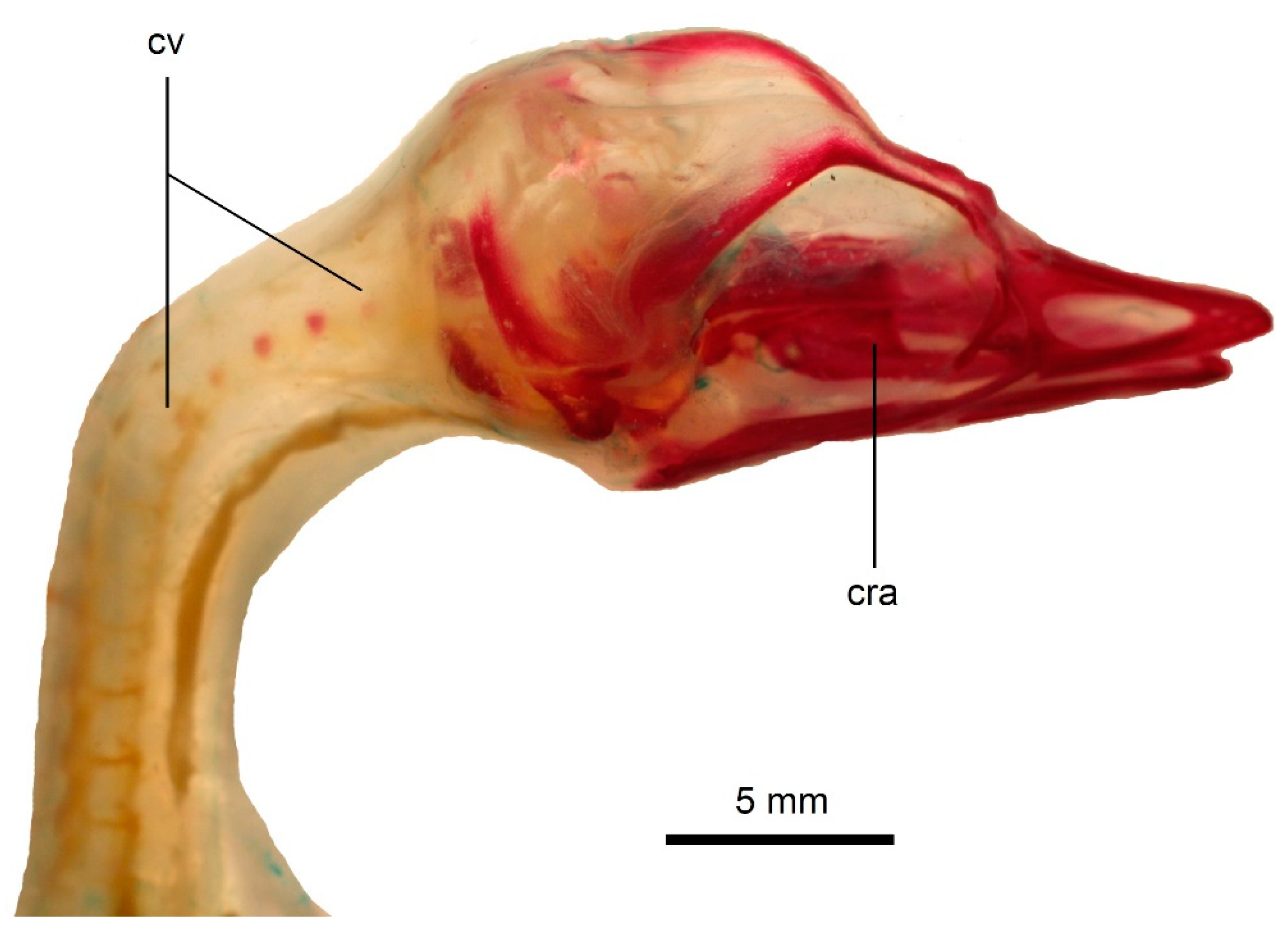
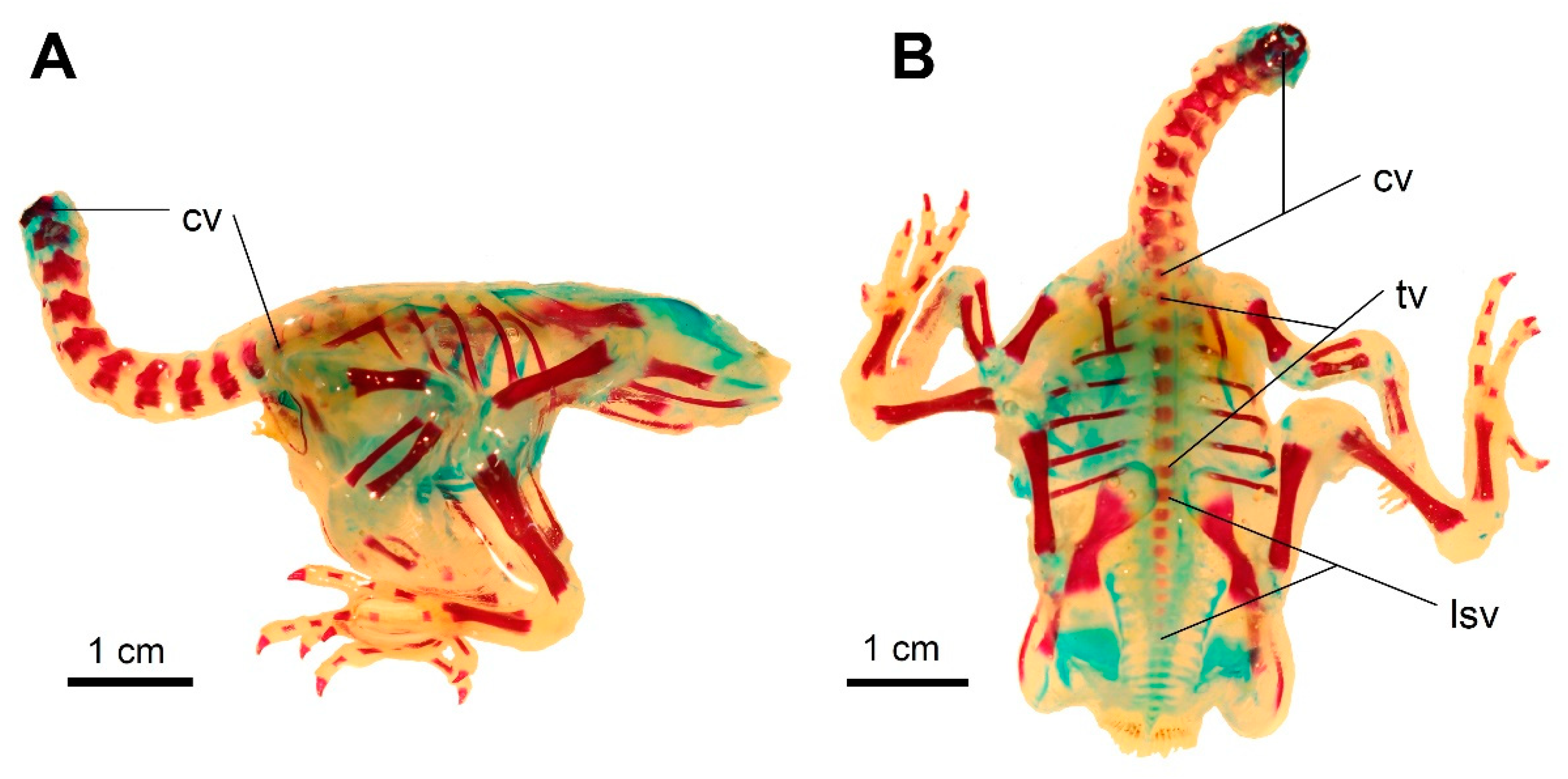
3.1. Development of the Vertebral Column in Podiceps cristatus
3.2. Development of the Vertebral Column in Podiceps grisegena
3.3. Development of the Vertebral Column in Columba livia domestica
3.4. Ancestral State Reconstructions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleming, A.; Kishida, M.G.; Kimmel, C.B.; Keynes, R.J. Building the backbone: The development and evolution of vertebral patterning. Development 2015, 142, 1733–1744. [Google Scholar] [CrossRef] [Green Version]
- Mallo, M. Of necks, trunks and tails: Axial skeletal diversity among vertebrates. Diversity 2021, 13, 289. [Google Scholar] [CrossRef]
- Hautier, L.; Weisbecker, V.; Sánchez-Villagra, M.R.; Goswami, A.; Asher, R.J. Skeletal development in sloths and the evolution of mammalian vertebral patterning. Proc. Natl. Acad. Sci. USA 2010, 107, 18903–18908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, J.; Scheyer, T.M.; Head, J.J.; Barrett, P.M.; Werneburg, I.; Ericson, P.G.P.; Pol, D.; Sánchez-Villagra, M.R. Homeotic effects, somitogenesis and the evolution of vertebral numbers in recent and fossil amniotes. Proc. Natl. Acad. Sci. USA 2010, 107, 2118–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogg, D.A. Fusions occurring in the postcranial skeleton of the domestic fowl. J. Anat. 1982, 135, 501–512. [Google Scholar] [PubMed]
- Rashid, D.J.; Chapman, S.C.; Larsson, H.C.E.; Organ, C.L.; Bebin, A.-G.; Merzdorf, C.S.; Bradley, R.; Horner, J.R. From dinosaurs to birds: A tail of evolution. EvoDevo 2014, 5, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, D.J.; Surya, K.; Chiappe, L.M.; Carroll, N.; Garrett, K.L.; Varghese, B.; Bailleul, A.; O’Connor, J.K.; Chapman, S.C.; Horner, J.R. Avian tail ontogeny, pygostyle formation, and interpretation of juvenile Mesozoic specimens. Sci. Rep. 2018, 8, 9014. [Google Scholar] [CrossRef] [PubMed]
- Skawiński, T.; Borczyk, B.; Hałupka, L. Postnatal ossification sequences in Acrocephalus scirpaceus and Chroicocephalus ridibundus (Aves: Neognathae): The precocial–altricial spectrum and evolution of compound bones in birds. J. Anat. 2021, 238, 349–364. [Google Scholar] [CrossRef]
- Baumel, J.J.; Witmer, L.M. Osteologia. In Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd ed.; Baumel, J.J., King, A.S., Breazile, J.E., Evans, H.E., Vanden Berge, J.C., Eds.; Nuttall Ornithological Club: Cambridge, MA, USA, 1993; pp. 45–132. [Google Scholar]
- Böhmer, C.; Rauhut, O.W.M.; Wörheide, G. Correlation between Hox code and vertebral morphology in archosaurs. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150077. [Google Scholar] [CrossRef] [Green Version]
- Böhmer, C.; Rauhut, O.W.M.; Wörheide, G. New insights into the vertebral Hox code of archosaurs. Evol. Dev. 2015, 17, 258–269. [Google Scholar] [CrossRef]
- Mitgutsch, C.; Wimmer, C.; Sánchez-Villagra, M.R.; Hahnloser, R.; Schneider, R.A. Timing of ossification in duck, quail, and zebra finch: Intraspecific variation, heterochronies, and life history evolution. Zoolog. Sci. 2011, 28, 491–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verrière, A.; Fröbisch, N.B.; Fröbisch, J. Regionalization, constraint, and the ancestral ossification patterns in the vertebral column of amniotes. bioRxiv 2021. [Google Scholar] [CrossRef]
- Carril, J.; Tambussi, C.P. Skeletogenesis of Myiopsitta monachus (Psittaciformes) and sequence heterochronies in Aves. Evol. Dev. 2017, 19, 17–28. [Google Scholar] [CrossRef]
- Weishampel, D.B.; Fastovsky, D.E.; Watabe, M.; Varricchio, D.; Jackson, F.; Tsogtbaatar, K.; Barsbold, R. New oviraptorid embryos from Bugin-Tsav, Nemegt Formation (Upper Cretaceous), Mongolia, with insights into their habitat and growth. J. Vertebr. Paleontol. 2008, 28, 1110–1119. [Google Scholar] [CrossRef]
- Starck, J.M. Evolution of avian ontogenies. In Current Ornithology; Powers, D.M., Ed.; Plenum Press: New York, NY, USA, 1993; Volume 10, pp. 275–366. [Google Scholar] [CrossRef]
- Schinz, H.R.; Zangerl, R. Beiträge zur Osteogenese des Knochensystems beim Haushuhn, bei der Haustaube und beim Haubensteissfuss. Eine vergleichend osteologische Studie. Denkschr. Schweiz. Naturforsch. Ges. 1937, 72, 117–165. (In German) [Google Scholar]
- Starck, J.M. Comparative morphology and cytokinetics of skeletal growth in hatchlings of altricial and precocial birds. Zool. Anz. 1996, 235, 53–75. [Google Scholar]
- Blom, J.; Lilja, C. A comparative study of growth, skeletal development and eggshell composition in some species of birds. J. Zool. 2004, 262, 361–369. [Google Scholar] [CrossRef]
- Maxwell, E.E. Ossification sequence of the avian order Anseriformes, with comparison to other precocial birds. J. Morphol. 2008, 269, 1095–1113. [Google Scholar] [CrossRef]
- Maxwell, E.E. Comparative embryonic development of the skeleton of the domestic turkey (Meleagris gallopavo) and other galliform birds. Zoology 2008, 111, 242–257. [Google Scholar] [CrossRef]
- Maxwell, E.E.; Harrison, L.B. Ossification sequence of the common tern (Sterna hirundo) and its implications for the interrelationships of the Lari (Aves, Charadriiformes). J. Morphol. 2008, 269, 1056–1072. [Google Scholar] [CrossRef]
- Maxwell, E.E. Evolution of Avian Ossification Sequences. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 2008. [Google Scholar]
- Bui, H.N.N.; Larsson, H.C.E. Development and evolution of regionalization within the avian axial column. Zool. J. Linn. Soc. 2021, 191, 302–321. [Google Scholar] [CrossRef]
- Parker, T.J. Observations on the anatomy and development of Apteryx. Philos. Trans. R. Soc. 1891, 182, 25–134. [Google Scholar] [CrossRef]
- Piiper, J. On the evolution of the vertebral column in birds, illustrated by its development in Larus and Struthio. Philos. Trans. R. Soc. B 1928, 216, 285–351. [Google Scholar] [CrossRef]
- Braun, E.L.; Kimball, R.T. Data types and the phylogeny of Neoaves. Birds 2021, 2, 1–22. [Google Scholar] [CrossRef]
- Prum, R.O.; Berv, J.S.; Dornburg, A.; Field, D.J.; Townsend, J.P.; Moriarty Lemmon, E.; Lemmon, A.R. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 2015, 526, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, H.; Frankl-Vilches, C.; Bakker, A.; Mayr, G.; Nikolaus, G.; Boerno, S.T.; Klages, S.; Timmermann, B.; Gahr, M. An unbiased molecular approach using 3’-UTRs resolves the avian family-level tree of life. Mol. Biol. Evol. 2021, 38, 108–127. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.E.; Larsson, H.C.E. Comparative ossification sequence and skeletal development of the postcranium of palaeognathous birds (Aves: Palaeognathae). Zool. J. Linn. Soc. 2009, 157, 169–196. [Google Scholar] [CrossRef] [Green Version]
- Atalgin, S.H.; Kürtül, I. A morphological study of skeletal development in turkey during the pre-hatching stage. Anat. Histol. Embryol. 2009, 38, 23–30. [Google Scholar] [CrossRef]
- Nakane, Y.; Tsudzuki, M. Development of the skeleton in Japanese quail embryos. Dev. Growth Differ. 1999, 41, 523–534. [Google Scholar] [CrossRef]
- Pourlis, A.F.; Antonopoulos, J. The ossification of the vertebral column, thorax and sternum in the quail (Coturnix coturnix japonica). Vet. Res. Forum 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Nakamura, Y.; Nakane, Y.; Tsudzuki, M. Skeletal development in blue-breasted quail embryos. Anim. Sci. J. 2019, 90, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J. Recherches embryologiques sur Catharacta skua Brünn (ptérylose et ossification). Rev. Suisse Zool. 1948, 55, 1–114. (In French) [Google Scholar]
- Rogulska, T. Differences in the process of ossification during the embryonic development of the chick (Gallus domesticus L.), rook (Corvus frugilegus L.) and black-headed gull (Larus ridibundus L.). Zool. Pol. 1962, 12, 223–233. [Google Scholar]
- Schumacher, G.-H.; Wolff, E. Zur vergleichenden Osteogenese von Gallus domesticus L., Larus ridibundus L. und Larus canus L. II. Zeitliches Erscheinen der Ossifikationen bei Larus ridibundus L. und Larus canus L. Gegenbaurs Morphol. Jahrb. 1966, 110, 620–635. (In German) [Google Scholar]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef] [PubMed]
- Dingerkus, G.; Uhler, L.D. Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Technol. 1977, 52, 229–232. [Google Scholar] [CrossRef]
- Maddison, W.; Maddison, D. Mesquite Version 3.61 (Build 927). Available online: https://www.mesquiteproject.org (accessed on 19 January 2022).
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A resource for timelines, timetrees, and divergence dates. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef]
- Mayr, G. The origins of crown group birds: Molecules and fossils. Palaeontology 2014, 57, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Rieppel, O. Studies on skeleton formation in reptiles. v. Patterns of ossification in the skeleton of Alligator mississippiensis DAUDIN (Reptilia, Crocodylia). Zool. J. Linn. Soc. 1993, 109, 301–325. [Google Scholar] [CrossRef]
- Vieira, L.G.; Lima, F.C.; Mendonça, S.H.S.T.; Menezes, L.T.; Hirano, L.Q.L.; Santos, A.L.Q. Ontogeny of the postcranial axial skeleton of Melanosuchus niger (Crocodylia, Alligatoridae). Anat. Rec. 2018, 301, 607–623. [Google Scholar] [CrossRef] [Green Version]
- Elżanowski, A. Embryonic bird skeletons from the Late Cretaceous of Mongolia. Palaeontol. Pol. 1981, 42, 147–179. [Google Scholar]
- Zheng, X.; Wang, X.; O’Connor, J.; Zhou, Z. Insight into the early evolution of the avian sternum from juvenile enantiornithines. Nat. Commun. 2012, 3, 1116. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.K.; Zheng, X.T.; Sullivan, C.; Chuong, C.M.; Wang, X.L.; Li, A.; Zhang, X.M.; Zhou, Z.H. Evolution and functional significance of derived sternal ossification patterns in ornithothoracine birds. J. Evol. Biol. 2015, 28, 1550–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knoll, F.; Chiappe, L.M.; Sanchez, S.; Garwood, R.J.; Edwards, N.P.; Wogelius, R.A.; Sellers, W.I.; Manning, P.L.; Ortega, F.; Serrano, F.J.; et al. A diminutive perinate European Enantiornithes reveals an asynchronous ossification pattern in early birds. Nat. Commun. 2018, 9, 937. [Google Scholar] [CrossRef] [Green Version]
- Balanoff, A.M.; Rowe, T. Osteological description of an embryonic skeleton of the extinct elephant bird, Aepyornis (Palaeognathae: Ratitae). J. Vertebr. Paleontol. 2007, 27, 1–53. [Google Scholar] [CrossRef]
- Foth, C.; Wang, S.; Spindler, F.; Lin, Y.; Yang, R. A juvenile specimen of Archaeorhynchus sheds new light on the ontogeny of basal euornithines. Front. Earth Sci. 2021, 9, 604520. [Google Scholar] [CrossRef]
- Kundrát, M.; Cruickshank, A.R.I.; Manning, T.W.; Nudds, J. Embryos of therizinosauroid theropods from the Upper Cretaceous of China: Diagnosis and analysis of ossification patterns. Acta Zool. 2008, 89, 231–251. [Google Scholar] [CrossRef]
- Pu, H.; Zelenitsky, D.K.; Lü, J.; Currie, P.J.; Carpenter, K.; Xu, L.; Koppelhus, E.B.; Jia, S.; Xiao, L.; Chuang, H.; et al. Perinate and eggs of a giant caenagnathid dinosaur from the Late Cretaceous of central China. Nat. Commun. 2017, 8, 14952. [Google Scholar] [CrossRef] [Green Version]
- Bi, S.; Amiot, R.; Peyre de Fabrègues, C.; Pittman, M.; Lamanna, M.C.; Yu, Y.; Yu, C.; Yang, T.; Zhang, S.; Zhao, Q.; et al. An oviraptorid preserved atop an embryo-bearing egg clutch sheds light on the reproductive biology of non-avialan theropod dinosaurs. Sci. Bull. 2021, 66, 947–954. [Google Scholar] [CrossRef]
- Xing, L.; Niu, K.; Ma, W.; Zelenitsky, D.K.; Yang, T.-R.; Brusatte, S.L. An exquisitely preserved in-ovo theropod dinosaur embryo sheds light on avian-like prehatching postures. iScience 2022, 25, 103516. [Google Scholar] [CrossRef]
- Varricchio, D.J.; Horner, J.R.; Jackson, F.D. Embryos and eggs for the Cretaceous theropod dinosaur Troodon formosus. J. Vertebr. Paleontol. 2002, 22, 564–576. [Google Scholar] [CrossRef]
- Shapiro, M.D.; Kronenberg, Z.; Li, C.; Domyan, E.T.; Pan, H.; Campbell, M.; Tan, H.; Huff, C.D.; Hu, H.; Vickrey, A.I.; et al. Genomic diversity and evolution of the head crest in the rock pigeon. Science 2013, 339, 1063–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Núñez-León, D.; Cordero, G.A.; Schlindwein, X.; Jensen, P.; Stoeckli, E.; Sánchez-Villagra, M.R.; Werneburg, I. Shifts in growth, but not differentiation, foreshadow the formation of exaggerated forms under chicken domestication. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210392. [Google Scholar] [CrossRef] [PubMed]
- Head, J.J.; Polly, P.D. Evolution of the snake body form reveals homoplasy in amniote Hox gene function. Nature 2015, 520, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Moreau, C.; Caldarelli, P.; Rocancourt, D.; Roussel, J.; Denans, N.; Pourquise, O.; Gros, J. Timed collinear activation of Hox genes during gastrulation controls the avian forelimb position. Curr. Biol. 2019, 29, 35–50. [Google Scholar] [CrossRef] [Green Version]
- Hautier, L.; Charles, C.; Asher, R.J.; Gaunt, S.J. Ossification sequence and genetic patterning in the mouse axial skeleton. J. Exp. Zool. B Mol. Dev. Evol. 2014, 322, 631–642. [Google Scholar] [CrossRef]



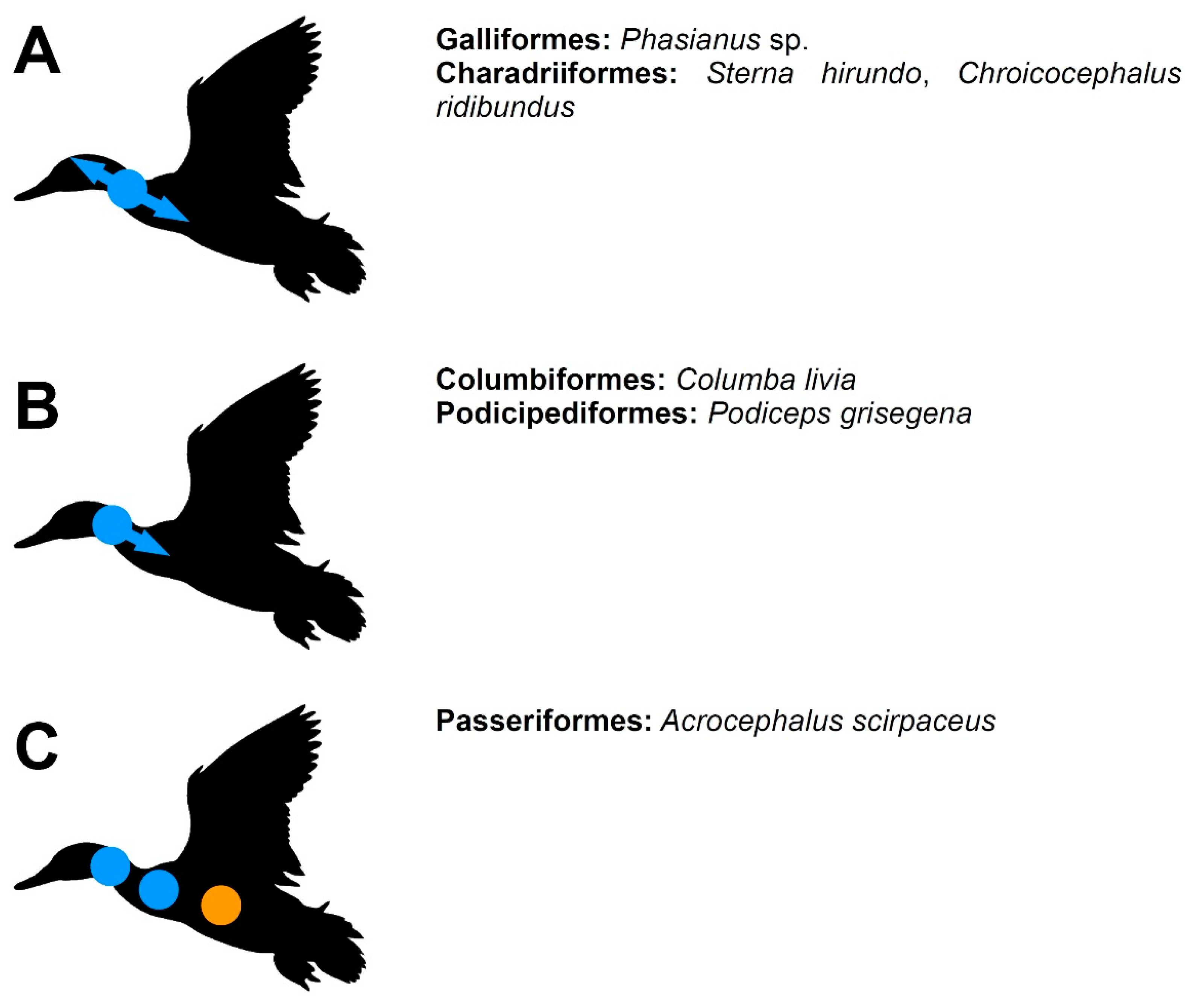








| Species | Sequence | References |
|---|---|---|
| Struthio camelus | Vertebral bodies: thoracic, synsacral → cervical → caudal → pygostyle Vertebral arches: cervical → thoracic → synsacral | [30] |
| Rhea americana | Vertebral bodies: thoracic → cervical → synsacral Vertebral arches: cervical → thoracic → synsacral | [30] |
| Dromaius novaehollandiae | Vertebral bodies: cervical, thoracic → synsacral → caudal → pygostyle Vertebral arches: cervical → thoracic → synsacral | [30] |
| Eudromia elegans | Vertebral bodies: thoracic, synsacral → cervical Vertebral arches: cervical → thoracic → synsacral | [30] |
| Meleagris gallopavo | Vertebral bodies: cervical → thoracic → synsacral → caudal Vertebral arches: cervical → thoracic → synsacral | [21,31] |
| Galllus domesticus | Vertebral bodies: cervical → thoracic → synsacral → caudal Vertebral arches: cervical → thoracic → synsacral | [17,21] |
| Coturnix japonica | Vertebral bodies: cervical → thoracic → synsacral → caudal Vertebral arches: cervical → thoracic → synsacral | [21,32,33] |
| Synoicus chinensis | Vertebral bodies: anterior cervical, thoracic → mid- and posterior cervical, synsacral → caudal Vertebral arches: cervical → thoracic → synsacral | [34] |
| Anas platyrhynchos | Vertebral bodies: cervical → thoracic, synsacral → caudal → pygostyle Vertebral arches: cervical → thoracic → synsacral | [12,20] |
| Cairina moschata | Vertebral bodies: cervical, thoracic, synsacral → caudal → pygostyle Vertebral arches: cervical → thoracic | [20] |
| Somateria mollissima | Vertebral bodies: cervical → thoracic, synsacral → caudal → pygostyle Vertebral arches: cervical → thoracic → synsacral | [20] |
| Podiceps cristatus | Vertebral bodies: thoracic → synsacral → cervical → caudal → pygostyle Vertebral arches: cervical → thoracic → synsacral | this work |
| Columba livia domestica | Vertebral bodies: cervical → thoracic, synsacral → caudal Vertebral arches: cervical → thoracic | this work |
| Sterna hirundo | Vertebral bodies: cervical, thoracic, synsacral → caudal Vertebral arches: cervical → thoracic or thoracic → cervical | [22] |
| Stercorarius skua | Vertebral bodies: cervical, thoracic → synsacral → caudal Vertebral arches: cervical → thoracic | [22,35] |
| Chroicocephalus ridibundus | Vertebral bodies: cervical → thoracic → synsacral → caudal Vertebral arches: cervical → thoracic | [22,36,37] |
| Larus canus | Vertebral bodies: cervical, thoracic, synsacral → caudal Vertebral arches: thoracic → cervical | [22,37] |
| Myiopsitta monachus | Vertebral bodies: cervical → thoracic → synsacral, caudal | [14] |
| Taeniopygia guttata | Vertebral bodies: cervical → thoracic → synsacral, caudal | [12] |
| Corvus frugilegus | Vertebral bodies: cervical → thoracic → synsacral → caudal | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skawiński, T.; Kuziak, P.; Kloskowski, J.; Borczyk, B. Phylogenetic Diversity of Ossification Patterns in the Avian Vertebral Column: A Review and New Data from the Domestic Pigeon and Two Species of Grebes. Biology 2022, 11, 180. https://doi.org/10.3390/biology11020180
Skawiński T, Kuziak P, Kloskowski J, Borczyk B. Phylogenetic Diversity of Ossification Patterns in the Avian Vertebral Column: A Review and New Data from the Domestic Pigeon and Two Species of Grebes. Biology. 2022; 11(2):180. https://doi.org/10.3390/biology11020180
Chicago/Turabian StyleSkawiński, Tomasz, Piotr Kuziak, Janusz Kloskowski, and Bartosz Borczyk. 2022. "Phylogenetic Diversity of Ossification Patterns in the Avian Vertebral Column: A Review and New Data from the Domestic Pigeon and Two Species of Grebes" Biology 11, no. 2: 180. https://doi.org/10.3390/biology11020180







