Coastal Mesozooplankton Assemblages during Spring Bloom in the Eastern Barents Sea
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skjoldal, H.R.; Gjøsæter, H.; Loeng, H. The Barents Sea ecosystem in the 1980s: Ocean cimate, plankton and capelin growth. ICES Mar. Sci. Symp. 1992, 195, 278–290. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Commercial fish and shellfish in the Barents Sea: Have introduced crab species affected the population trajectories of commercial fish? Rev. Fish Biol. Fish. 2015, 25, 297–322. [Google Scholar] [CrossRef]
- Hop, H.; Assmy, P.; Wold, A.; Sundfjord, A.; Daase, M.; Duarte, P.; Kwasniewski, S.; Gluchowska, M.; Wiktor, J.M.; Tatarek, A.; et al. Pelagic ecosystem characteristics across the Atlantic Water boundary current from Rijpfjorden, Svalbard, to the Arctic Ocean during summer (2010–2014). Front. Mar. Sci. 2019, 6, 181. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Cucumaria in Russian waters of the Barents Sea: Biological aspects and aquaculture potential. Front. Mar. Sci. 2021, 8, 613453. [Google Scholar] [CrossRef]
- Svensen, C.; Halvorsen, E.; Vernet, M.; Franzè, G.; Dmoch, K.; Lavrentyev, P.J.; Kwasniewski, S. Zooplankton communities associated with new and regenerated primary production in the Atlantic Inflow north of Svalbard. Front. Mar. Sci. 2019, 6, 293. [Google Scholar] [CrossRef]
- Wassmann, P.; Reigstad, M.; Haug, T.; Rudels, B.; Carroll, M.L.; Hop, H.; Gabrielsen, G.W.; Falk-Petersen, S.; Denisenko, S.G.; Arashkevich, E.; et al. Food webs and carbon flux in the Barents Sea. Progr. Oceanogr. 2006, 71, 232–287. [Google Scholar] [CrossRef]
- Jakobsen, T.; Ozhigin, V.K. (Eds.) The Barents Sea: Ecosystem, Resources, Management: Half a Century of Russian-Norwegian Cooperation; Tapir Academic Press: Trondheim, Norway, 2011. [Google Scholar]
- Sakshaug, E.; Johnsen, G.; Kovacs, K. (Eds.) Ecosystem Barents Sea; Tapir Academic Press: Trondheim, Norway, 2009. [Google Scholar]
- Lind, S.; Ingvaldsen, R.B.; Furevik, T. Arctic warming hotspot in the northern Barents Sea linked to declining sea-ice import. Nat. Clim. Chang. 2018, 8, 634–639. [Google Scholar] [CrossRef]
- Polyakov, I.V.; Pnyushkov, A.; Alkire, M.; Ashik, I.M.; Baumann, T.M.; Carmack, E.C.; Goszczko, I.; Guthrie, J.D.; Ivanov, V.V.; Kanzow, T.; et al. Greater role for Atlantic inflows on sea-ice loss in the Eurasian Basin of the Arctic Ocean. Science 2017, 356, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Wassmann, P.; Slagstad, D.; Ellingsen, I. Advection of mesozooplankton into the northern Svalbard shelf region. Front. Mar. Sci. 2019, 6, 458. [Google Scholar] [CrossRef] [Green Version]
- Oziel, L.; Baudena, A.; Ardyna, M.; Massicotte, P.; Randelhoff, A.; Sallee, J.-B.; Ingvaldsen, R.B.; Devred, E.; Babin, M. Faster Atlantic currents drive poleward expansion of temperate phytoplankton in the Arctic Ocean. Nat. Commun. 2020, 11, 1705. [Google Scholar] [CrossRef] [Green Version]
- Gluchowska, M.; Kwasniewski, S.; Prominska, A.; Olszewska, A.; Goszczko, I.; Falk-Petersen, S.; Hop, H.; Weslawski, J.M. Zooplankton in Svalbard fjords on the Atlantic–Arctic boundary. Polar Biol. 2016, 39, 1785–1802. [Google Scholar] [CrossRef] [Green Version]
- Gluchowska, M.; Dalpadado, P.; Beszczynska-Möller, A.; Olszewska, A.; Ingvaldsen, R.B.; Kwasniewski, S. Interannual zooplankton variability in the main pathways of the Atlantic water flow into the Arctic Ocean (Fram Strait and Barents Sea branches). ICES J. Mar. Sci. 2017, 74, 1921–1936. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Inter-annual dynamics of the Barents Sea red king crab (Paralithodes camtschaticus) stock indices in relation to environmental factors. Polar Sci. 2016, 10, 541–552. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Effects of environmental factors on the abundance, biomass, and individual weight of juvenile red king crabs in the Barents Sea. Front. Mar. Sci. 2020, 7, 726. [Google Scholar] [CrossRef]
- Dalpadado, P.; Arrigo, K.R.; van Dijken, G.L.; Skjoldal, H.R.; Bagøien, E.; Dolgov, A.V.; Prokopchuk, I.P.; Sperfeld, E. Climate effects on temporal and spatial dynamics of phytoplankton and zooplankton in the Barents Sea. Progr. Oceanogr. 2020, 182, 102320. [Google Scholar] [CrossRef]
- Johannesen, E.; Ingvaldsen, R.B.; Bogstad, B.; Dalpadado, P.; Eriksen, E.; Gjøsæter, H.; Knutsen, T.; Skern-Mauritzen, M.; Stiansen, J.E. Changes in Barents Sea ecosystem state, 1970–2009: Climate fluctuations, human impact, and trophic interactions. ICES J. Mar. Sci. 2012, 69, 880–889. [Google Scholar] [CrossRef] [Green Version]
- Dalpadado, P.; Ingvaldsen, R.; Hassel, A. Zooplankton biomass variation in relation to climatic conditions in the Barents Sea. Polar Biol. 2003, 26, 233–241. [Google Scholar] [CrossRef]
- Raymont, J.E.G. Plankton and Productivity in the Oceans, 2nd ed.; Pergamon Press: Southampton, UK, 1983; Volume 2. [Google Scholar]
- Hodal, H.; Falk-Petersen, S.; Hop, H.; Kristiansen, S.; Reigstad, M. Spring bloom dynamics in Kongsfjorden, Svalbard: Nutrients, phytoplankton, protozoans and primary production. Polar Biol. 2012, 35, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Hegseth, E.N.; Tverberg, V. Effect of Atlantic water inflow on timing of the phytoplankton spring bloom in a high Arctic fjord (Kongsfjorden, Svalbard). J. Mar. Syst. 2013, 113, 94–105. [Google Scholar] [CrossRef]
- Asch, R.G.; Stock, C.A.; Sarmiento, J.L. Climate change impacts on mismatches between phytoplankton blooms and fish spawning phenology. Glob. Change Biol. 2019, 25, 2544–2559. [Google Scholar] [CrossRef]
- Makarevich, P.R.; Vodopianova, V.V.; Bulavina, A.S.; Vashchenko, P.S.; Ishkulova, T.G. Features of the distribution of chlorophyll-a concentration along the western coast of the Novaya Zemlya Archipelago in spring. Water 2021, 13, 3648. [Google Scholar] [CrossRef]
- Wassmann, P.; Ratkova, T.; Andreassen, I.; Vernet, M.; Pedersen, G.; Rey, F. Spring bloom development in the Marginal Ice Zone and the Central Barents Sea. Mar. Ecol. 1999, 20, 321–346. [Google Scholar] [CrossRef] [Green Version]
- Dvoretsky, V.G.; Dvoretsky, A.G. Summer-fall macrozooplankton assemblages in a large Arctic estuarine zone (south-eastern Barents Sea): Environmental drivers of spatial distribution. Mar. Environ. Res. 2022, 173, 105498. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Epiplankton in the Barents Sea: Summer variations of mesozooplankton biomass, community structure and diversity. Cont. Shelf Res. 2013, 52, 1–11. [Google Scholar] [CrossRef]
- Box, J.E. Key indicators of Arctic climate change. Environ. Res. Lett. 2019, 14, 045010. [Google Scholar] [CrossRef]
- Kravtsova, L.; Vorobyeva, S.; Naumova, E.; Izhboldina, L.; Mincheva, E.; Potemkina, T.; Pomazkina, G.; Rodionova, E.; Onishchuk, N.; Sakirko, M.; et al. Response of aquatic organisms communities to global climate changes and anthropogenic impact: Evidence from Listvennichny Bay of Lake Baikal. Biology 2021, 10, 904. [Google Scholar] [CrossRef]
- Falk-Petersen, S.; Pedersen, G.; Kwasniewski, S.; Hegseth, E.N.; Hop, H. Spatial distribution and life-cycle timing of zooplankton in the marginal ice zone of the Barents Sea during the summer melt season in 1995. J. Plankton Res. 1999, 21, 1249–1264. [Google Scholar] [CrossRef] [Green Version]
- Blachowiak-Samolyk, K.; Kwasniewski, S.; Hop, H.; Falk-Petersen, S. Magnitude of mesozooplankton variability: A case study from the Marginal Ice Zone of the Barents Sea in spring. J. Plankton Res. 2008, 30, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Arashkevich, E.; Wassmann, P.; Pasternak, A.; Wexels Riser, C. Seasonal and spatial changes in biomass, structure, and development progress of the zooplankton community in the Barents Sea. J. Mar. Syst. 2002, 38, 125–145. [Google Scholar] [CrossRef]
- Aarflot, J.M.; Skjoldal, H.R.; Dalpadado, P.; Skern-Mauritzen, M. Contribution of Calanus species to the mesozooplankton biomass in the Barents Sea. ICES J. Mar. Sci. 2018, 75, 2342–2354. [Google Scholar] [CrossRef]
- Stige, L.C.; Dalpadado, P.; Orlova, E.; Boulay, A.C.; Durant, J.M.; Ottersen, G.; Stenseth, N.C. Spatiotemporal statistical analyses reveal predator–driven zooplankton fluctuations in the Barents Sea. Progr. Oceanogr. 2014, 120, 243–253. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Summer mesozooplankton distribution near Novaya Zemlya (eastern Barents Sea). Polar Biol. 2009, 32, 719–731. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Summer mesozooplankton community of Moller Bay (Novaya Zemlya Archipelago, Barents Sea). Oceanologia 2013, 55, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Falk-Petersen, S.; Timofeev, S.; Pavlov, V.; Sargent, J.R. Climate variability and possible effects on arctic food chains: The role of Calanus. In Arctic Alpine Ecosystems and People in a Changing Environment; Orbok, J.B., Tombre, T., Kallenborn, R., Hegseth, E., Falk-Petersen, S., Hoel, A.H., Eds.; Springer: Berlin, Germany, 2007; pp. 147–166. [Google Scholar]
- Dvoretsky, V.G.; Dvoretsky, A.G. Mesozooplankton in the Kola Transect (Barents Sea): Autumn and winter structure. J. Sea Res. 2018, 142, 18–22. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Structure of mesozooplankton community in the Barents Sea and adjacent waters in August 2009. J. Nat. Hist. 2013, 47, 2095–2114. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Estimated copepod production rate and structure of mesozooplankton communities in the coastal Barents Sea during summer–autumn 2007. Polar Biol. 2012, 35, 1321–1342. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Life cycle of Oithona similis (Copepoda: Cyclopoida) in Kola Bay (Barents Sea). Mar. Biol. 2009, 156, 1433–1446. [Google Scholar] [CrossRef]
- Matishov, G.G. (Ed.) General Physical and Geographical Characteristics. Environments and Ecosystems of Novaya Zemlya (Archipelago and Shelf); KSC RAS Press: Apatity, Russia, 1995. (In Russian) [Google Scholar]
- Zelickman, E.A.; Golovkin, A.N. Composition, structure and productivity of neritic plankton communities near the bird colonies of the northern shores of Novaya Zemlya. Mar. Biol. 1972, 17, 265–274. [Google Scholar] [CrossRef]
- Koptev, A.V.; Nesterova, V.N. Latitudinal distribution of the summer plankton in the eastern Barents Sea. In Studies on Biology, Morphology, and Physiology of Hydrobionts; Matishov, G.G., Ed.; KSC AN USSR Press: Apatity, Russia, 1983; pp. 22–28. (In Russian) [Google Scholar]
- Matishov, G.G.; Dzhenyuk, S.L.; Denisov, V.V.; Zhichkin, A.P.; Moiseev, D.V. Climate and oceanographic processes in the Barents Sea. Ber. Polarforsch. 2012, 640, 63–73. [Google Scholar]
- Dvoretsky, V.G.; Dvoretsky, A.G. Regional differences of mesozooplankton communities in the Kara Sea. Cont. Shelf Res. 2015, 105, 26–41. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Macrozooplankton of the Arctic—The Kara Sea in relation to environmental conditions. Estuar. Coast. Shelf Sci. 2017, 88, 38–55. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Summer macrozooplankton assemblages of Arctic shelf: A latitudinal study. Cont. Shelf Res. 2019, 188, 103967. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Arctic marine mesozooplankton at the beginning of the polar night: A case study for southern and south-western Svalbard waters. Polar Biol. 2020, 43, 71–79. [Google Scholar] [CrossRef]
- Kwasniewski, S.; Hop, H.; Falk-Petersen, S.; Pedersen, G. Distribution of Calanus species in Kongsfjorden, a glacial fjord in Svalbard. J. Plankton Res. 2003, 25, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Chislenko, L.L. Nomogrammes to Determine Weights of Aquatic Organisms Based on the Size and Form of Their Bodies (Marine Mesobenthos and Plankton); Nauka Press: Leningrad, Russia, 1968. (In Russian) [Google Scholar]
- Deibel, D. Feeding mechanism and house of the appendicularian Oikopleura vanhoeffeni. Mar. Biol. 1986, 93, 429–436. [Google Scholar] [CrossRef]
- Berestovskij, E.G.; Anisimova, N.A.; Denisenk, C.G.; Luppowa, E.N.; Savinov, V.M.; Timofeev, S.F. Relationship between Size and Body Mass of Some Invertebrates and Fish of the North-East Atlantic; Academy of Sciences of the USSR, Murmansk Marine Biological Institute: Apatity, Russia, 1989. (In Russian) [Google Scholar]
- Percy, J.A. Abundance, biomass, and size frequency distribution of an arctic ctenophore, Mertensia ovum (Fabricius) from Frobisher Bay, Canada. Sarsia 1989, 74, 95–105. [Google Scholar] [CrossRef]
- Mumm, N. On the summer distribution of mesozooplankton in the Nansen Basin, Arctic Ocean. Ber. Polarforsch. 1991, 92, 1–146. [Google Scholar]
- Richter, C. Regional and seasonal variability in the vertical distribution of mesozooplankton in the Greenland Sea. Ber. Polarforsch. 1994, 154, 1–90. [Google Scholar]
- Hanssen, H. Mesozooplankton of the Laptev Sea and the adjacent eastern Nansen Basin–distribution and community structure in late summer. Ber. Polarforsch. 1997, 229, 1–131. [Google Scholar]
- Matthews, J.B.L.; Hestad, L. Ecological studies on the deep-water pelagic community of Korsfjorden, Western Norway. Length/weight relationships for some macroplanktonic organisms. Sarsia 1977, 63, 57–63. [Google Scholar] [CrossRef]
- Ashjian, C.J.; Campbell, R.G.; Welch, H.E.; Butler, M.; Keuren, D.V. Annual cycle in abundance, distribution, and size in relation to hydrography of important copepod species in the western Arctic Ocean. Deep-Sea Res. I 2003, 50, 1235–1261. [Google Scholar] [CrossRef]
- Böer, M.; Gannefors, C.; Kattner, G.; Graeve, M.; Hop, H.; Falk-Petersen, S. The Arctic pteropod Clione limacina: Seasonal lipid dynamics and life-strategy. Mar. Biol. 2005, 147, 707–717. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.P.; Wiebe, P.H.; Lenz, J.; Skjoldal, H.R.; Huntley, M. (Eds.) ICES Zooplankton Methodology Manual; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation; Plymouth Marine Laboratory, NERC: Plymouth, UK, 1994. [Google Scholar]
- Shannon, C.B.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F. Canonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef] [Green Version]
- Matishov, G.; Zuyev, A.; Golubev, V.; Adrov, N.; Timofeev, S.; Karamusko, O.; Pavlova, L.; Fadyakin, O.; Buzan, A.; Braunstein, A.; et al. Climatic Atlas of the Arctic Seas 2004: Part I. Database of the Barents, Kara, Laptev, and White Seas—Oceanography and Marine Biology; NOAA Atlas NESDIS 58; U.S. Government Printing Office: Washington, DC, USA, 2004. [Google Scholar]
- Hodal, H.; Kristiansen, S. The importance of small-celled phytoplankton in spring blooms at the marginal ice zone in the northern Barents Sea. Deep-Sea Res. II 2008, 55, 2176–2185. [Google Scholar] [CrossRef] [Green Version]
- Makarevich, P.; Druzhkova, E.; Larionov, V. Primary producers of the Barents Sea. In Diversity of Ecosystems; Mahamane, A., Ed.; In Tech: Rijeka, Croatia, 2012; pp. 367–392. [Google Scholar]
- Dvoretsky, V.G.; Dvoretsky, A.G. Checklist of fauna found in zooplankton samples from the Barents Sea. Polar Biol. 2010, 33, 991–1005. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. The biodiversity of zooplankton communities of the West Arctic seas. Russ. J. Mar. Biol. 2014, 40, 95–99. [Google Scholar] [CrossRef]
- Lischka, S.; Hagen, W. Seasonal dynamics of mesozooplankton in the Arctic Kongsfjord (Svalbard) during year-round observations from August 1998 to July 1999. Polar Biol. 2016, 39, 1859–1878. [Google Scholar] [CrossRef]
- Daase, M.; Eiane, K. Mesozooplankton distribution in northern Svalbard waters in relation to hydrography. Polar Biol. 2007, 30, 969–981. [Google Scholar] [CrossRef]
- Trudnowska, E.; Sagan, S.; Kwasniewski, S.; Darecki, M.; Blachowiak-Samolyk, K. Fine-scale zooplankton vertical distribution in relation to hydrographic and optical characteristics of the surface waters on the Arctic shelf. J. Plankton Res. 2015, 37, 120–133. [Google Scholar] [CrossRef] [Green Version]
- Trudnowska, E.; Stemmann, L.; Błachowiak-Samołyk, K.; Kwasniewski, S. Taxonomic and size structures of zooplankton communities in the fjords along the Atlantic water passage to the Arctic. J. Mar. Syst. 2020, 204, 103306. [Google Scholar] [CrossRef]
- Timofeev, S.F. Ecology of the Marine Zooplankton; Murmansk State Pedagogical Institute Press: Murmansk, Russia, 2000. (In Russian) [Google Scholar]
- Dvoretsky, V.G.; Dvoretsky, A.G. Mesozooplankton structure in the northern White Sea in July 2008. Polar Biol. 2011, 34, 469–474. [Google Scholar] [CrossRef]
- Polyakov, I.V.; Alkire, M.B.; Bluhm, B.A.; Brown, K.A.; Carmack, E.C.; Chierici, M.; Danielson, S.L.; Ellingsen, I.; Ershova, E.A.; Gårdfeldt, K.; et al. Borealization of the Arctic Ocean in response to anomalous advection from sub-arctic seas. Front. Mar. Sci. 2020, 7, 491. [Google Scholar] [CrossRef]
- Hays, G.C.; Richardson, A.J.; Robinson, C. Climate change and marine plankton. Trends Ecol. Evol. 2005, 20, 337–344. [Google Scholar] [CrossRef] [PubMed]
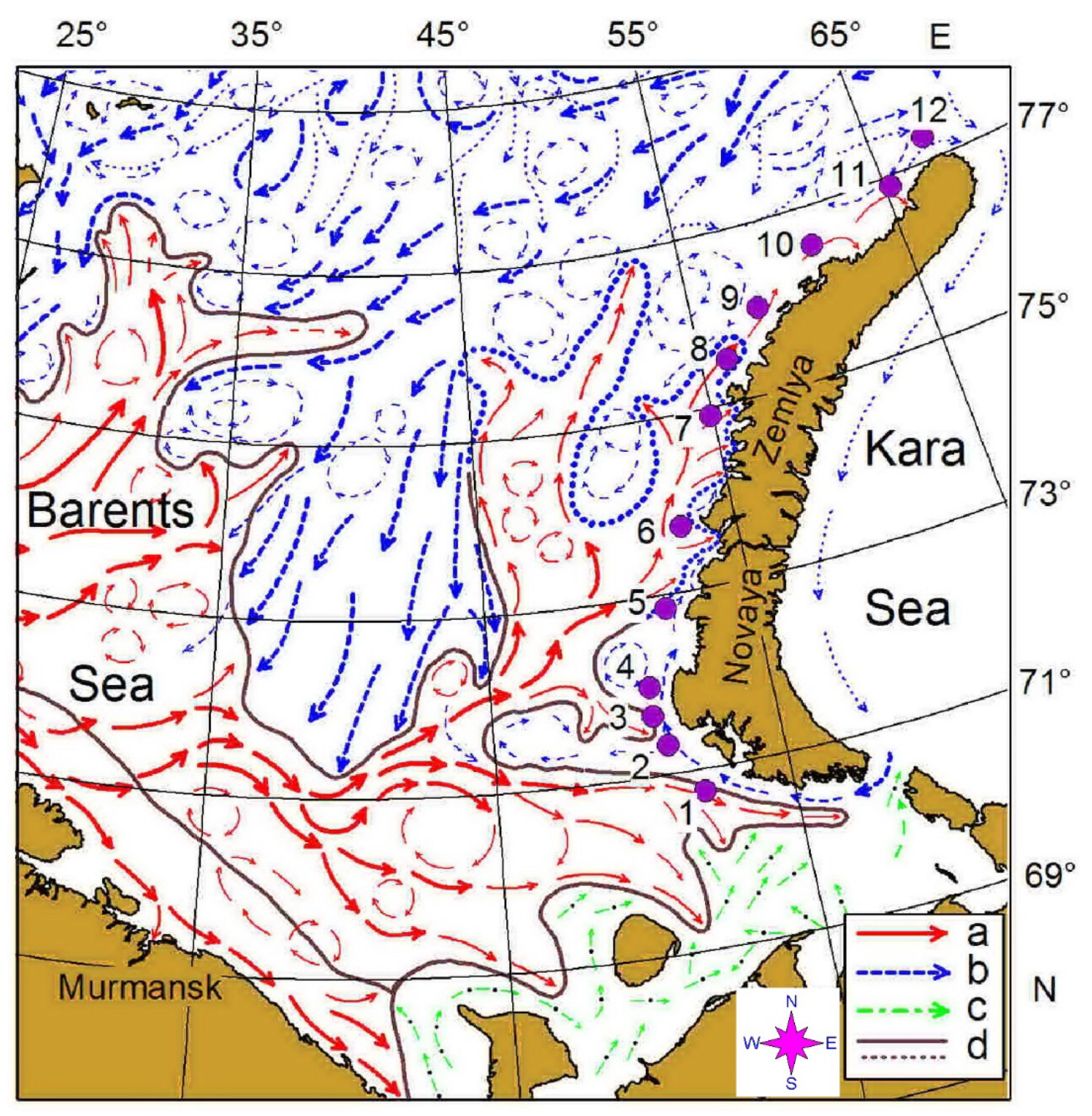





| ID | Date (May 2016) | Latitude (N) | Longitude (E) | Local Time | Depth, m | Temp | Sal | Chla | Ab | Biom |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 70°45′ | 51°60′ | 8:10 | 156 | 0.9 | 34.66 | 2.35 | 84/597 | 0.65/5 |
| 2 | 10 | 71°20′ | 51°00′ | 13:45 | 130 | 0.2 | 34.67 | 1.49 | 126/968 | 1.49/11 |
| 3 | 10 | 71°40′ | 50°39′ | 18:18 | 114 | 0.3 | 34.66 | 1.15 | 74/741 | 1.06/11 |
| 4 | 10 | 71°60′ | 50°41′ | 21:30 | 122 | 0.7 | 34.70 | 1.76 | 82/745 | 1.1/10 |
| 5 | 11 | 72°51′ | 51°43′ | 19:20 | 85 | 1.6 | 34.74 | 4.11 | 42/531 | 0.41/5 |
| 6 | 12 | 73°44′ | 52°50′ | 2:05 | 90 | 1.7 | 34.78 | 3.58 | 29/364 | 0.38/5 |
| 7 | 12 | 74°55′ | 54°54′ | 12:35 | 149 | 0.6 | 34.77 | 1.95 | 134/954 | 1.23/9 |
| 8 | 12 | 75°30′ | 56°07′ | 19:21 | 169 | 0.4 | 34.78 | 0.92 | 133/833 | 1.00/6 |
| 9 | 13 | 76°00′ | 57°55′ | 0:20 | 94 | −0.2 | 34.74 | 0.53 | 271/3009 | 1.79/20 |
| 10 | 13 | 76°32′ | 61°01′ | 7:32 | 86 | −0.2 | 34.77 | 1.57 | 108/1345 | 1.18/15 |
| 11 | 13 | 76°53′ | 65°17′ | 15:45 | 227 | 0.3 | 34.90 | 1.23 | 195/929 | 2.82/13 |
| 12 | 13 | 77°17′ | 67°30′ | 20:27 | 231 | 0.3 | 34.88 | 1.04 | 188/819 | 9.59/42 |
| Taxon/Parameter | Trophic Status | Biogeography | Cluster 1 | Cluster 2 | ANOVA or Kruskall–Wallis Test (p-Level) |
|---|---|---|---|---|---|
| Copepoda | 49,086 ± 14,379 | 118,331 ± 32,689 | <0.05 | ||
| Calanus finmarchicus (Gunner, 1765) | He | Bor | 2472 ± 567 | 3243 ± 1225 | 0.581 |
| Calanus glacialis Jaschnov, 1955 | He | Ar | 1925 ± 430 | 5396 ± 1485 | <0.05 |
| Calanus hyperboreus Krøyer, 1838 | He | Ar | 6 ± 5 | 2400 ± 1518 | <0.05 |
| Centropages hamatus (Lilljeborg, 1853) | Om | Bor-Ar | 34 ± 24 | 14 ± 10 | 0.818 |
| Copepoda ova | Bor/Bor-Ar | 13,315 ± 2602 | 8308 ± 1234 | 0.113 | |
| Copepoda nauplii | Om | Bor/Bor-Ar | 13,263 ± 4304 | 27,933 ± 8075 | 0.140 |
| Gaetanus tenuispinu (Sars G.O., 1900) | Om | Ar | 14 ± 11 | 6 ± 4 | 0.937 |
| Metridia longa (Lubbock, 1854) | Om | Ar | 122 ± 52 | 7039 ± 5037 | <0.05 |
| Microcalanus pusillus Sars G.O., 1903 | Om | Bor-Ar | 312 ± 111 | 1473 ± 282 | <0.05 |
| Microcalanus pygmaeus (Sars G.O., 1900) | Om | Ar | 5433 ± 2239 | 22,941 ± 2287 | <0.001 |
| Microsetella norvegica (Boeck, 1865) | Om | Cs | 20 ± 9 | 9 ± 9 | 0.485 |
| Oithona atlantica Farran, 1908 | Om | Bor | 31 ± 15 | 239 ± 90 | 0.093 |
| Oithona similis Claus, 1866 | Om | Cs | 11,884 ± 3925 | 33,218 ± 8691 | 0.132 |
| Triconia borealis (Sars G.O., 1918) | Om | Cs | - | 67 ± 48 | na |
| Parathalestris croni (Krøyer, 1842) | Om | Bor-Ar | 3 ± 3 | 5 ± 5 | 0.937 |
| Pseudocalanus spp. I–IV | He | Bor-Ar | 33 ± 33 | 3933 ± 1991 | <0.05 |
| Pseudocalanus minutus (Krøyer, 1845) V–VI | He | Bor-Ar | 152 ± 32 | 1767 ± 626 | <0.05 |
| Pseudocalanus acuspes (Giesbrecht, 1881) V–VI | He | Bor-Ar | 67 ± 17 | 332 ± 64 | <0.05 |
| Scolecithricella minor (Brady, 1883) | Om | Bor-Ar | - | 8 ± 8 | na |
| Medusae | 8 ± 5 | 31 ± 12 | 0.562 | ||
| Aeginopsis laurentii Brandt, 1838 | Cr | Ar | - | 2 ± 2 | na |
| Aglantha digitale (Müller, 1776) | Cr | Bor | - | 6 ± 3 | na |
| Euphysa flammea (Linko, 1905) | Cr | Bor | 5 ± 3 | 9 ± 3 | 0.341 |
| Euphysa spp. juv. | Cr | Bor | 3 ± 2 | 14 ± 4 | <0.05 |
| Meroplanktonic larvae | |||||
| Cirripedia cypris larvae | Om | Mx | 23 ± 23 | - | na |
| Cirripedia nauplii | Om | Mx | 1332 ± 318 | 19,872 ± 10820 | 0.394 |
| Echinoidea (echinopluteus larvae) | Om | Mx | 432 ± 263 | 524 ± 213 | 0.793 |
| Gastropoda larvae | Om | Mx | - | 236 ± 67 | na |
| Ophiuroidea (ophiopluteus larvae) | Om | Mx | 53 ± 30 | 395 ± 161 | 0.180 |
| Polychaeta larvae | Om | Mx | 1648 ± 484 | 5108 ± 1884 | 0.106 |
| Chionoecetes opilio (Fabricius, 1788) larvae | Om | Bor-Ar | 17 ± 8 | 237 ± 106 | <0.05 |
| Pagurus spp. zoea | Om | Bor-Ar | 14 ± 12 | 2 ± 2 | 0.589 |
| Lithodes maja (Linnaeus, 1758) zoea | Om | Bor-Ar | - | 2 ± 2 | na |
| Sabinea spp. larvae | Om | Bor-Ar | - | 2 ± 2 | na |
| Eualus gaimardi (H. Milne-Edwards, 1837) larvae | Om | Bor-Ar | - | 2 ± 2 | na |
| Euphausiids | 19,802 ± 6908 | 3072 ± 947 | <0.05 | ||
| Meganyctyphanes norvegica (M. Sars, 1857) | He | Bor | - | 2 ± 2 | na |
| Thysanoessa inermis (Krøyer, 1846) | He | Bor | 5 ± 2 | 2 ± 2 | 0.394 |
| Thyssanoessa raschii (M. Sars, 1864) | He | Bor-Ar | 2 ± 2 | 5 ± 3 | 0.589 |
| Thyssanoessa spp. calyptopis | He | Bor-Ar | 636 ± 582 | 40 ± 23 | 0.589 |
| Thyssanoessa spp. nauplii | He | Bor-Ar | 19,159 ± 6322 | 3023 ± 917 | <0.05 |
| Hyperiids | 2 ± 2 | 64 ± 41 | <0.05 | ||
| Themisto abyssorum Boeck, 1870 | Cr | Bor | - | 19 ± 10 | na |
| Themisto libellula Lichtenstein, 1822 | Cr | Ar | - | 36 ± 25 | na |
| Themisto juv. | Cr | 2 ± 2 | 9 ± 6 | 0.589 | |
| Appendicularia | 329 ± 237 | 23,505 ± 7342 | <0.05 | ||
| Fritillaria borealis Lohmann, 1896 | Om | Bor-Ar | 274 ± 182 | 21,050 ± 5987 | <0.05 |
| Oikopleura juv. | Om | Bor-Ar | 33 ± 33 | 2345 ± 1317 | <0.05 |
| Oikopleura vanhoeffeni Lohmann, 1896 | Om | Bor-Ar | 22 ± 22 | 110 ± 38 | 0.069 |
| Ctenophora | 5 ± 3 | 16 ± 10 | 0.634 | ||
| Beroe cucumis Fabricius, 1780 | Cr | Bor-Ar | - | 5 ± 3 | na |
| Mertensia ovum (Fabricius, 1780) | Cr | Ar | 5 ± 3 | 11 ± 7 | 0.699 |
| Others | 120 ± 61 | 60 ± 34 | 0.387 | ||
| Boroecia borealis (Sars, 1866) | Om | 12 ± 12 | - | na | |
| Clione limacina (Phipps, 1774) larvae | Cr | Bor-Ar | - | 3 ± 3 | na |
| Limacina helicina Phipps, 1774 larvae | He | Bor-Ar | - | 8 ± 8 | na |
| Limacina helicina Phipps, 1774 | He | Bor-Ar | 33 ± 19 | 22 ± 9 | 0.616 |
| Parasagitta elegans (Verrill, 1873) | Cr | Bor-Ar | 73 ± 28 | 25 ± 12 | 0.240 |
| Tomopteris spp. | He | Bor | 2 ± 2 | - | na |
| Pisces larvae | Cr | Mx | - | 2 ± 2 | na |
| Parameters | |||||
| Total abundance | 73 ± 14 | 171 ± 24 | <0.05 | ||
| Total biomass | 846 ± 179 | 2935 ± 1358 | <0.05 | ||
| J’ | 0.6 ± 0.02 | 0.64 ± 0.01 | 0.202 | ||
| H’(loge) | 1.85 ± 0.05 | 2.17 ± 0.03 | <0.001 | ||
| H’(log2) | 2.67 ± 0.07 | 3.13 ± 0.05 | <0.001 | ||
| Temp | 0.89 ± 0.27 | 0.22 ± 0.13 | <0.05 | ||
| Sal | 34.70 ± 0.02 | 34.81 ± 0.03 | <0.05 | ||
| Chla | 2.41 ± 0.49 | 1.21 ± 0.20 | <0.05 |
| Group | Lat | Long | Depth | Temp | Sal | Chla |
|---|---|---|---|---|---|---|
| Copepoda | 0.71 | 0.80 | 0.68 | −0.60 | 0.65 | −0.67 |
| Medusae | 0.56 | 0.49 | −0.01 | −0.77 | 0.21 | −0.66 |
| Meroplankon | 0.37 | 0.15 | −0.21 | −0.49 | −0.02 | −0.51 |
| Euphausiids | −0.79 | −0.54 | −0.01 | 0.13 | –0.66 | 0.14 |
| Hyperiids | 0.56 | 0.77 | 0.62 | −0.22 | 0.63 | −0.26 |
| Appendicularia | 0.68 | 0.58 | 0.14 | −0.59 | 0.43 | −0.57 |
| Total | 0.56 | 0.62 | 0.54 | −0.83 | 0.35 | −0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvoretsky, V.G.; Dvoretsky, A.G. Coastal Mesozooplankton Assemblages during Spring Bloom in the Eastern Barents Sea. Biology 2022, 11, 204. https://doi.org/10.3390/biology11020204
Dvoretsky VG, Dvoretsky AG. Coastal Mesozooplankton Assemblages during Spring Bloom in the Eastern Barents Sea. Biology. 2022; 11(2):204. https://doi.org/10.3390/biology11020204
Chicago/Turabian StyleDvoretsky, Vladimir G., and Alexander G. Dvoretsky. 2022. "Coastal Mesozooplankton Assemblages during Spring Bloom in the Eastern Barents Sea" Biology 11, no. 2: 204. https://doi.org/10.3390/biology11020204
APA StyleDvoretsky, V. G., & Dvoretsky, A. G. (2022). Coastal Mesozooplankton Assemblages during Spring Bloom in the Eastern Barents Sea. Biology, 11(2), 204. https://doi.org/10.3390/biology11020204







