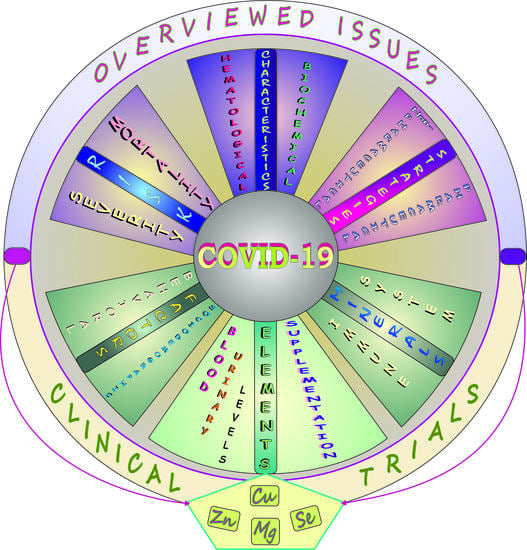
Elements and COVID-19: A Comprehensive Overview of Studies on Their Blood/Urinary Levels and Supplementation with an Update on Clinical Trials
Abstract
:Simple Summary
Abstract
1. Introduction
Methodology
2. COVID-19: Detection and intelligent Models Application—A Brief Note
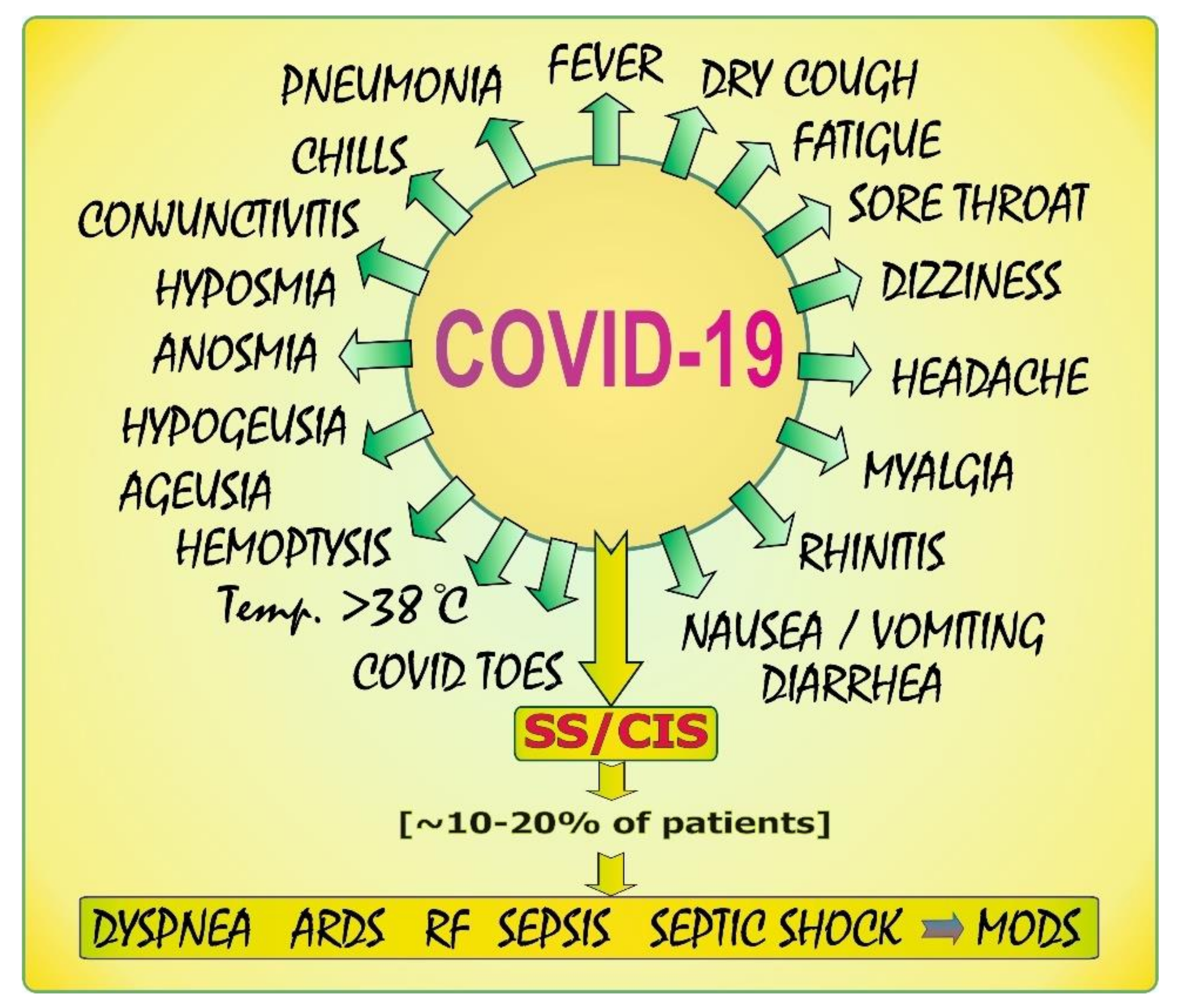
3. COVID-19: Clinical Characteristics—A Brief Summary
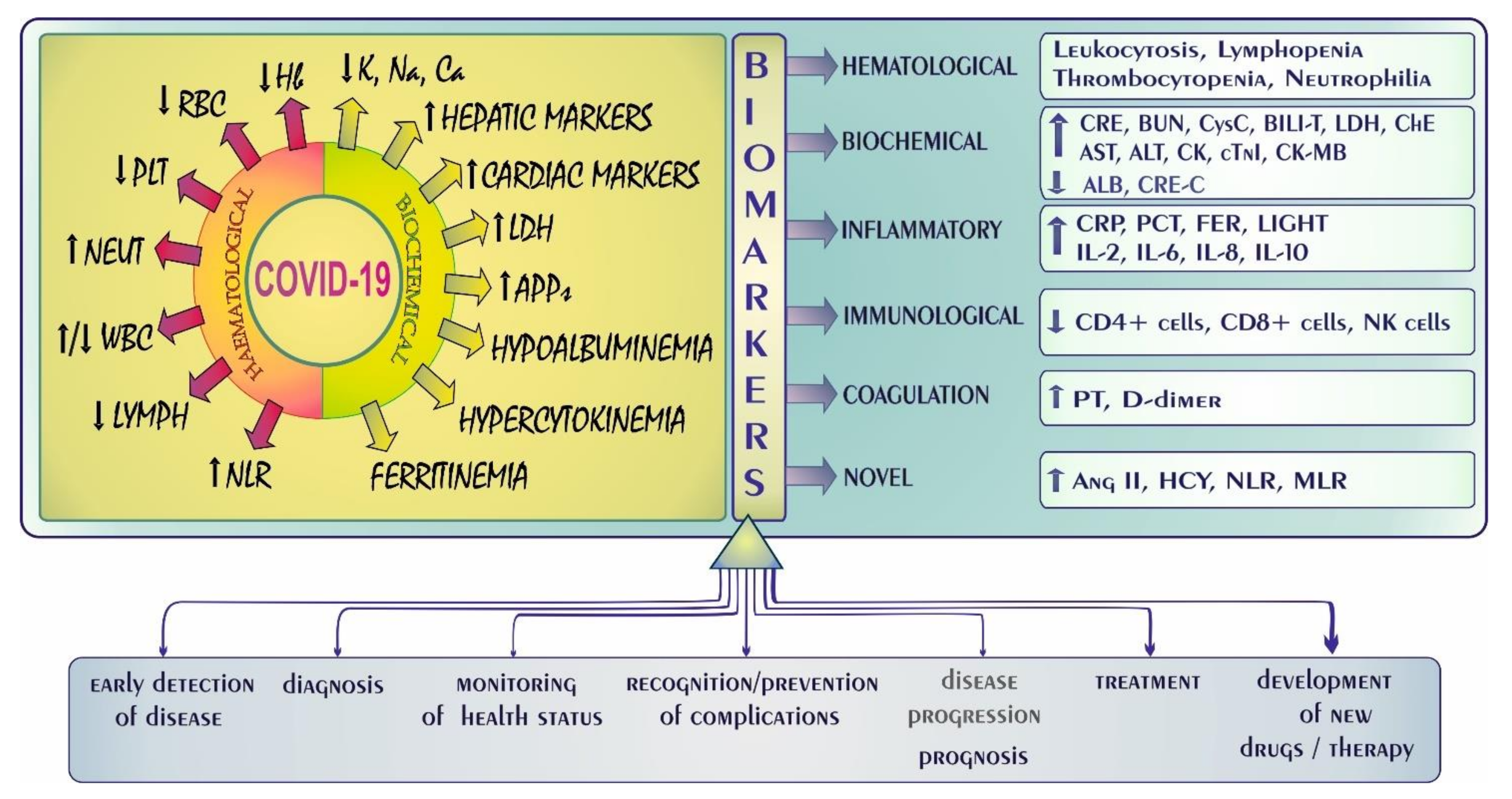
4. COVID-19: Laboratory Abnormalities and Biomarkers—A Quick Overview
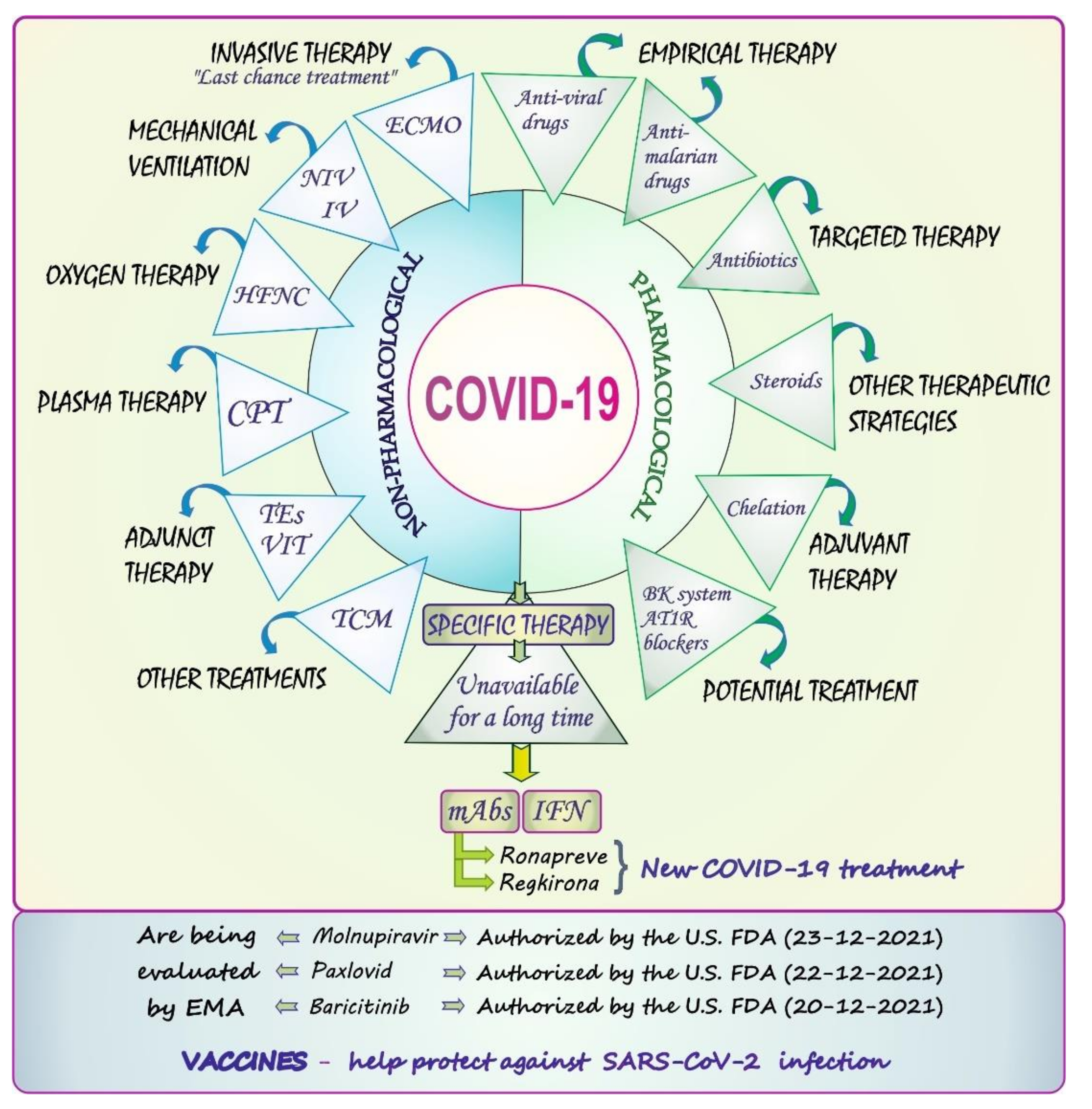
5. COVID-19: Therapeutic Approach—A Brief Outline
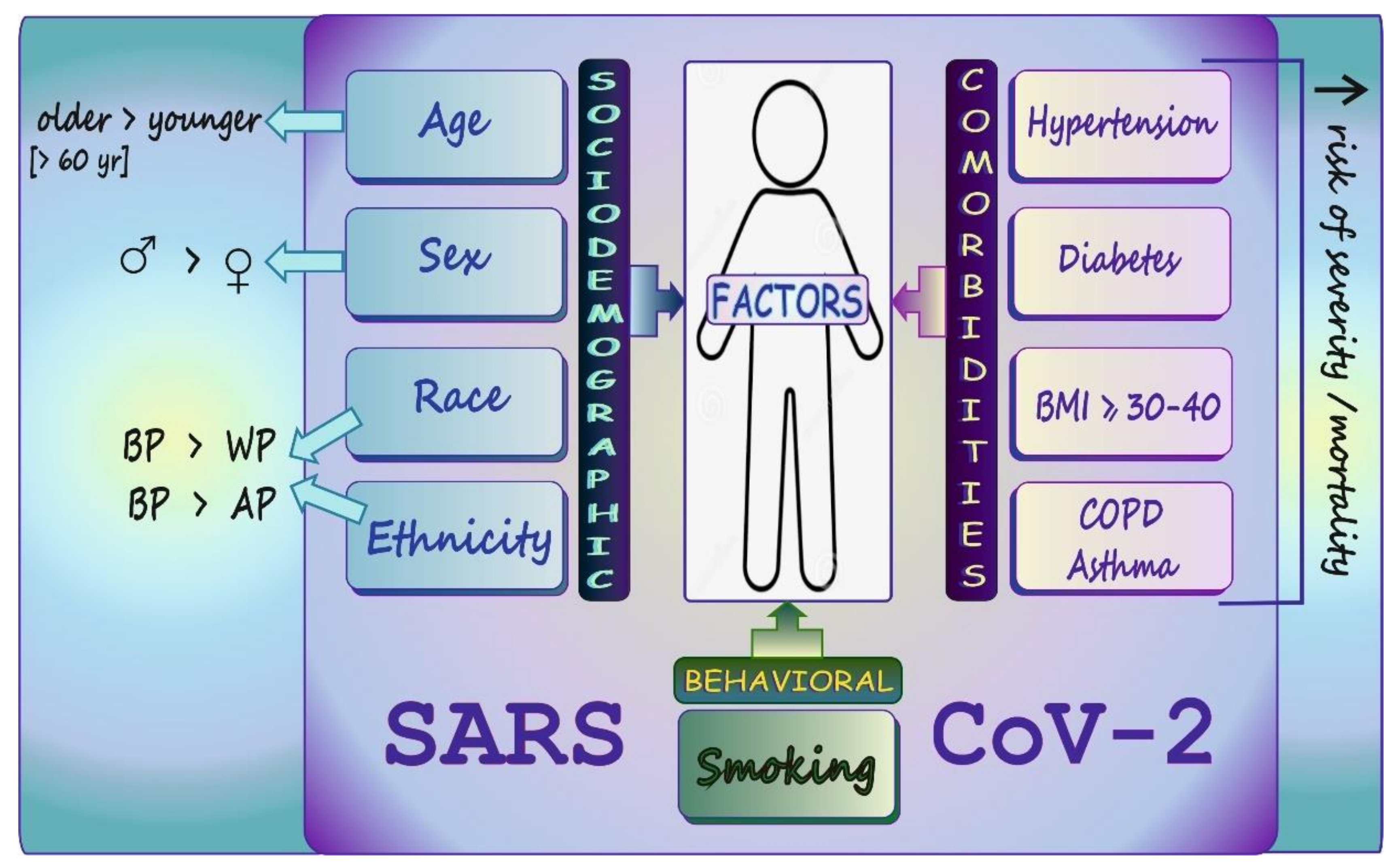
6. COVID-19: Sociodemographic/Behavioral Factors and Comorbid Conditions Affecting Disease Susceptibility, Severity, and Mortality in a Nutshell
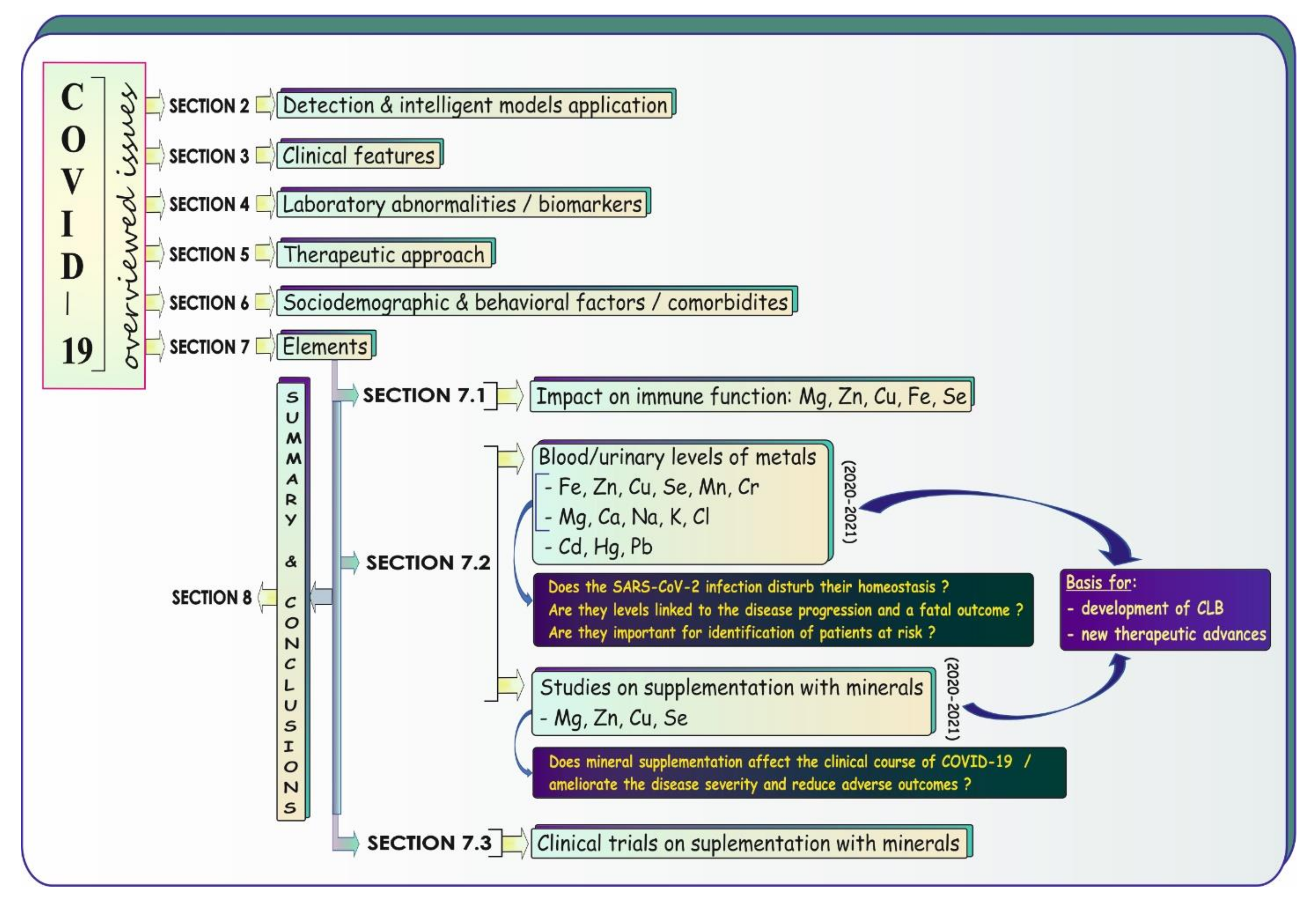
7. COVID-19: Elements
7.1. Impact on Immune Response—A Concise Summary
7.1.1. Magnesium (Mg)
7.1.2. Zinc (Zn)
7.1.3. Copper (Cu)
7.1.4. Iron (Fe)
7.1.5. Selenium (Se)
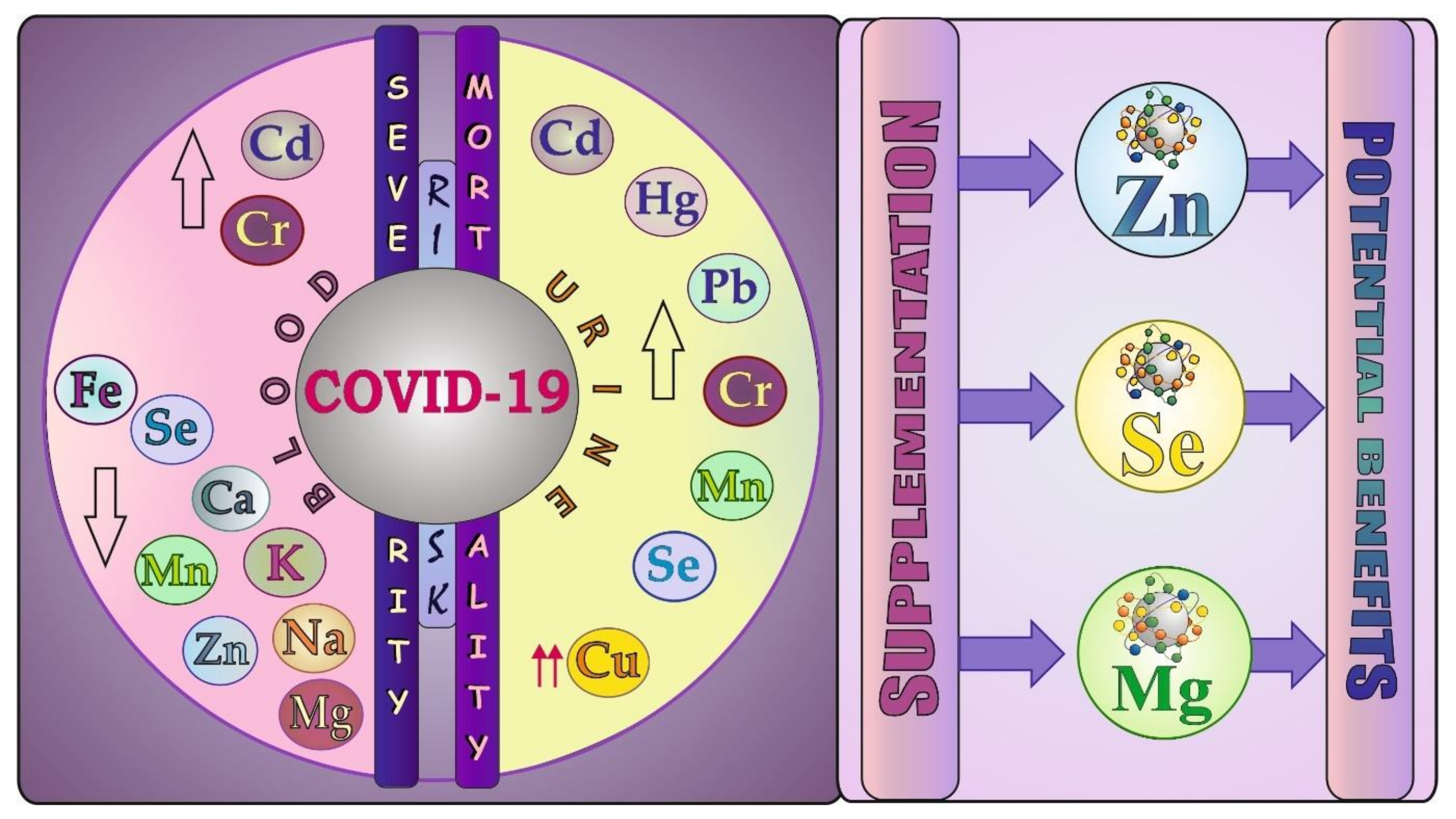
7.2. Blood and Urinary Levels of Elements and Studies on Mineral Supplementation
7.2.1. Essential Trace Elements (Fe, Zn, Cu, Se, Mn, Cr)
Fe
Zn
Cu
Se
Mn and Cr
7.2.2. The Most Toxic Trace Metals (Hg, Pb, Cd)
7.2.3. Macroelements (Mg, Ca, Na, K, Cl)
7.3. COVID-19: Clinical Trials on Supplementation with Minerals—A Brief Update in a Nutshell
7.3.1. Zn
7.3.2. Cu and Se
7.3.3. Mg
8. Summary
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adhikari, S.P.; Meng, S.; Wu, Y.-J.; Mao, Y.-P.; Ye, R.-X.; Wang, Q.-Z.; Sun, C.; Sylvia, S.; Rozelle, S.; Raat, H.; et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: A scoping review. Infect. Dis. Poverty 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Lang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv 2020, 21, 1–9. [Google Scholar] [CrossRef] [Green Version]
- WHO 2021. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 18 January 2022).
- ClinicalTrials.gov. Database. Available online: https://clinicaltrials.gov/ (accessed on 16 January 2022).
- Cochrane COVID-19 Study Register. Available online: https://covid-19.cochrane.org/ (accessed on 16 January 2022).
- WHO 2021. Coronavirus (COVID-19), 13 May 2021. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19 (accessed on 16 January 2022).
- EMA, COVID-19 Treatments: Under Evalution. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/treatments-covid-19/covid-19-treatments-under-evaluation#covid-19-treatments-under-marketing-authorisation-evaluation-section (accessed on 16 January 2022).
- U.S. FDA. Emergency Use Authorization. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs (accessed on 16 January 2022).
- Kalra, M.K.; Homayounieh, F.; Arru, C.; Holmberg, O.; Vassileva, J. Chest CT practice and protocols for COVID-19 from radiation dose management perspective. Eur. Radiol. 2020, 30, 6554–6560. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A.; et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacMullan, M.A.; Ibrayeva, A.; Trettner, K.; Deming, L.; Das, S.; Tran, F.; Moreno, J.R.; Casian, J.G.; Chellamuthu, P.; Kraft, J.; et al. ELISA detection of SARS-CoV-2 antibodies in saliva. Sci. Rep. 2020, 10, 20818. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.-Y.; Wang, X.-T.; Zhang, L.-N.; Chinese Critical Care Ultrasound Study Group (CCUSG). Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. 2020, 46, 849–850. [Google Scholar] [CrossRef] [Green Version]
- Lomoro, P.; Verde, F.; Zerboni, F.; Simonetti, I.; Borghi, C.; Fachinetti, C.; Natalizi, A.; Martegani, A. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: Single-center study and comprehensive radiologic literature review. Eur. J. Radiol. Open 2020, 7, 100231. [Google Scholar] [CrossRef]
- Jamshidi, M.B.; Lalbakhsh, A.; Talla, J.; Peroutka, Z.; Hadjilooei, F.; Lalbakhsh, P.; Jamshidi, M.; La Spada, L.; Mirmozafari, M.; Dehghani, M.; et al. Artificial intelligence and COVID-19: Deep learning approaches for diagnosis and treatment. IEEE Access 2020, 8, 109581–109591. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.R.; Haworth, J.; Lipani, A.; Aslam, N.; Cheng, T.; Christie, N. Variational-LSTM autoencoder to forecast the spread of coronavirus across the globe. PLoS ONE 2021, 16, e0246120. [Google Scholar] [CrossRef]
- Asada, K.; Komatsu, M.; Shimoyama, R.; Takasawa, K.; Shinkai, N.; Sakai, A.; Bolatkan, A.; Yamada, M.; Takahashi, S.; Machino, H.; et al. Application of artificial intelligence in COVID-19 diagnosis and therapeutics. J. Personal. Med. 2021, 11, 886. [Google Scholar] [CrossRef]
- Jamshidi, M.B.; Lalbakhsh, A.; Talla, J.; Peroutka, Z.; Roshani, S.; Matousek, V.; Roshani, S.; Mirmozafari, M.; Malek, Z.; La Spada, L.; et al. Deep learning techniques and COVID-19 drug discovery: Fundamentals, state-of-the-art and future directions. In Emerging Technologies during the Era of COVID-19 Pandemic; Arpaci, I., Al-Emran, M., Al-Sharafi, M.A., Marques, G., Eds.; Springer: Cham, Switzerland, 2021; Volume 348, pp. 9–31. [Google Scholar] [CrossRef]
- Centres for Disease Control and Prevention (CDC). 24/7: Saving Lives, Protecting PeopleTM, 22 February 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed on 25 November 2021).
- Gavriatopoulou, M.; Korompoki, E.; Fotiou, D.; Ntanasis-Stathopoulos, I.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020, 20, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Koschitzky, M.; Oyola, R.R.; Lee-Wong, M.; Abittan, B.; Silverberg, N. Pediatric COVID toes and fingers. Clin. Dermatol. 2021, 39, 84–91. [Google Scholar] [CrossRef]
- Wujtewicz, M.A.; Dylczyk-Sommer, A.; Aszkiełowicz, A.; Zdanowski, S.; Piwowarczyk, S.; Owczuk, R. COVID-19—What should anaethesiologists and intensivists know about it? Anaesthesiol. Intensive Ther. 2020, 52, 34–41. [Google Scholar] [CrossRef]
- Mehta, P.; Bunker, C.B.; Ciurtin, C.; Porter, J.C.; Chambers, R.C.; Papdopoulou, C.; Garthwaite, H.; Hillman, T.; Heightman, M.; Howell, K.J.; et al. Chilblain-like acral lesions in long COVID-19: Management and implications for understanding microangiopathy. Lancet Infect. Dis. 2021, 21, 912. [Google Scholar] [CrossRef]
- Lopes-Pacheco, M.; Silva, P.L.; Cruz, F.F.; Battaglini, D.; Robba, C.; Pelosi, P.; Morales, M.M.; Caruso Neves, C.; Rocco, P.R.M. Pathogenesis of multiple organ injury in COVID-19 and potential therapeutic strategies. Front. Physiol. 2021, 12, 593223. [Google Scholar] [CrossRef]
- Koçak Tufan, Z.; Kayaaslan, B.; Mer, M. COVID-19 and sepsis. Turk. J. Med. Sci. 2021, 51, 3301–3311. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, D.; Wang, Y.; Lu, Y.; Zhu, X.; Li, Y.; Xue, H.; Lin, Y.; Zhang, M.; Sun, Y.; et al. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv 2020, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2020, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Ciaccio, M.; Agnello, L. Biochemical biomarkers alterations in coronavirus disease 2019 (COVID-19). Diagnosis 2020, 7, 365–372. [Google Scholar] [CrossRef]
- Anai, M.; Akaike, K.; Iwagoe, H.; Akasaka, T.; Higuchi, T.; Miyazaki, A.; Naito, D.; Tajima, Y.; Takahashi, H.; Komatsu, T.; et al. Decrease in hemoglobin level predicts increased risk for severe respiratory failure in COVID-19 patients with pneumonia. Respir. Investig. 2021, 59, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, Y.; Tan, G.; Dong, X.; Wang, B.; Lin, J.; Yan, Y.; Liu, G.; Akdis, M.; Akdis, C.A.; et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy 2021, 92, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Edeas, M.; Saleh, J.; Peyssonnaux, C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int. J. Infect. Dis. 2020, 97, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Kałużna-Oleksy, M.; Gackowski, A.; Jankowska, E.A.; Kukulski, T.; Lelonek, M.; Nessler, J.; Pawlak, A.; Rozentryt, P.; Rubiś, P.; Straburzyńska-Migaj, E.; et al. The patient with heart failure in the face of the coronavirus disease 2019 pandemic. Kardiol. Pol. 2020, 78, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Maccaferri, M.; Ruini, C.; Tomasi, A.; Ozben, T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Puchalska-Reglińska, E.; Dziewiatowski, K.; Szabat, K.; Karzyński, P.; Zakrzewski, M.; Moys, K.; Lange, M.; Szczęśniewicz, A.; Lipka, M.; Dębska-Ślizień, A. COVID-19—Przebieg kliniczny u pacjentki przewlekle hemodializowanej—Opis przypadku i przegląd piśmiennictwa. Forum Nefrol. 2020, 13, 142–148. [Google Scholar]
- Sonnweber, T.; Boehm, A.; Sahanic, S.; Pizzini, A.; Aichner, M.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: A prospective observational cohort study. Respir. Res. 2020, 21, 276. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, H. Biomarkers of COVID-19 and technologies to combat SARS-CoV-2. Adv. Biomark. Sci. Technol. 2020, 2, 1–23. [Google Scholar] [CrossRef]
- BDWG, Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020, 100, 343–349. [Google Scholar] [CrossRef]
- Yan, X.; Li, F.; Wang, X.; Yan, J.; Zhu, F.; Tang, S.; Deng, Y.; Wang, H.; Chen, R.; Yu, Z.; et al. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: A retrospective cross-sectional study. J. Med. Virol. 2020, 92, 2573–2581. [Google Scholar] [CrossRef]
- Calabrese, L.H.; Lenfant, T.; Calabrese, C. Interferon therapy for COVID-19 and emerging infections: Prospects and concerns. Cleve. Clin. J. Med. 2020, 1–6. [Google Scholar] [CrossRef]
- Dimitrov, D.S. The secret life of ACE2 as a receptor for the SARS virus. Cell 2003, 115, 652–653. [Google Scholar] [CrossRef] [Green Version]
- Gasmi, A.; Noor, S.; Tippairote, T.; Dadar, M.; Menzel, A.; Bjørklund, G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin. Immunol. 2020, 215, 108409. [Google Scholar] [CrossRef]
- Gurwitz, D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020, 81, 537–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, H.M.; Ibrahim, S.; Zaim, A.; Ibrahim, W.H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharmacother. 2021, 136, 111228. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, S.; Nekhai, S.; Liu, S. Depriving iron supply to the virus represents a promising adjuvant therapeutic against viral survival. Curr. Clin. Microbiol. Rep. 2020, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-X.; Wang, Y.; Lu, Y.-B.; Li, J.-Y.; Song, Y.-J.; Nyamgerelt, M.; Wang, X.-X. Diagnosis and treatment of novel coronavirus pneumonia based on the theory of traditional Chinese medicine. J. Integr. Med. 2020, 18, 275–283. [Google Scholar] [CrossRef]
- Tolouian, R.; Vahed, S.Z.; Ghiyasvand, S.; Tolouian, A.; Ardalan, M. COVID-19 interactions with angiotensin-converting enzyme 2 (ACE2) and the kinin system; looking at a potential treatment. J. Ren. Inj. Prev. 2020, 9, e19. [Google Scholar] [CrossRef]
- Malone, B.; Campbell, E.A. Molnupiravir: Coding for catastrophe. Nat. Struc. Mol. Biol. 2021, 28, 706–711. [Google Scholar] [CrossRef]
- Peiffer-Smadja, N.; Yazdanpanah, Y. Nebulised interferon beta-1a for patients with COVID-19. Lancet Respir. Med. 2021, 9, 122–123. [Google Scholar] [CrossRef]
- Ratre, Y.K.; Kahar, N.; Bhaskar, L.V.K.S.; Bhattacharya, A.; Verma, H.K. Molecular mechanism, diagnosis, and potential treatment for novel coronavirus (COVID-19): A current literature review and perspective. 3 Biotech 2021, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- WHO. Therapeutics and COVID-19: Living Guideline, 20 November 2020. WHO/2019-nCov/Remdesivir/2020.1. Available online: https://apps.who.int/iris/handle/10665/336729 (accessed on 25 November 2021).
- Uzunova, K.; Filipova, E.; Pavlova, V.; Vekov, T. Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Biomed. Pharmacother. 2020, 131, 110668. [Google Scholar] [CrossRef]
- Satarker, S.; Ahuja, T.; Banerjee, M.; Balaji E, V.; Dogra, S.; Agarwal, T.; Nampoothiri, M. Hydroxychloroquine in COVID-19: Potential mechanism of action against SARS-CoV-2. Curr. Pharmacol. Rep. 2020, 6, 203–211. [Google Scholar] [CrossRef]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef]
- WHO. Updates Clinical Care Guidance with Corticosteroid Recommendations, 2 September 2020. Available online: https://www.who.int/news-room/feature-stories/detail/who-updates-clinical-care-guidance-with-corticosteroid-recommendations#:~:text=On%202%20September%2C%20WHO%20published%20guidance%20for%20clinicians,treatment%20of%20patients%20with%20severe%20and%20critical%20COVID-19 (accessed on 25 November 2021).
- Abobaker, A. Can iron chelation as an adjunct treatment of COVID-19 improve the clinical outcome? Eur. J. Clin. Pharmacol. 2020, 76, 1619–1620. [Google Scholar] [CrossRef]
- Garrick, M.D.; Ghio, A.J. Iron chelation may harm patients with COVID-19. Eur. J. Clin. Pharmacol. 2021, 77, 265–266. [Google Scholar] [CrossRef]
- Aleem, A.; Slenker, A.K. Monoclonal antibody therapy for high-risk coronavirus (COVID-19) patients with mild to moderate disease presentations. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Conti, P.; Pregliasco, F.; Calvisi, V.; Caraffa, A.; Gallenga, C.; Kritas, S.; Ronconi, G. Monoclonal therapy in COVID-19. J. Biol. Regul. Homeost. Agents 2021, 35, 423–427. [Google Scholar] [CrossRef]
- Darazam, I.A.; Shokouhi, S.; Pourhoseingholi, M.A.; Naghibi Irvani, S.S.; Mokhtari, M.; Shabani, M.; Amirdosara, M.; Torabinavid, P.; Golmohammadi, M.; Hashemi, S.; et al. Role of interferon therapy in severe COVID-19: The COVIFERON randomized controlled trial. Sci. Rep. 2021, 11, 8059. [Google Scholar] [CrossRef]
- EMA. Product Information as Approved by the CHMP on 11 November 2021, Pending Endorsement by the European Commission. ANNEX I Summary of Product Characteristics. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/ronapreve-epar-product-information_en.pdf (accessed on 25 November 2021).
- EMA. ANNEX I Conditions of Use, Conditions for Distribution and Patients Targeted and Conditions for Safety Monitoring Adressed to Member States for Unauthorised Product Regkirona (Regdanvimab). 2021. Available online: https://www.ema.europa.eu/en/documents/referral/celltrion-use-regdanvimab-treatment-covid-19-article-53-procedure-conditions-use-conditions_en.pdf (accessed on 25 November 2021).
- Gordon, C.J.; Tchesnokov, E.P.; Schinazi, R.F.; Götte, M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J. Biol. Chem. 2021, 297, 100770. [Google Scholar] [CrossRef]
- Kabinger, F.; Stiller, C.; Schmitzová, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Höbartner, C.; Cramer, P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struc. Mol. Biol. 2021, 28, 740–746. [Google Scholar] [CrossRef]
- Fischer, W.; Eron, J.J.; Holman, W.; Cohen, M.S.; Fang, L.; Szewczyk, L.J.; Sheahan, T.P.; Baric, R.; Mollan, K.R.; Wolfe, C.R.; et al. Molnupiravir, an oral antiviral treatment for COVID-19. medRxiv 2021, 1–30. [Google Scholar] [CrossRef]
- Zhou, S.; Hill, C.S.; Sarkar, S.; Tse, L.V.; Woodburn, B.M.D.; Schinazi, R.F.; Sheahan, T.P.; Baric, R.S.; Heise, M.T.; Swanstrom, R. β-D-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but i salso mutagenic to mammalin cells. J. Infect. Dis. 2021, 224, 415–419. [Google Scholar] [CrossRef]
- EMA. EMA Starts Rolling Review of Molnupiravir. 2021. Available online: https://www.ema.europa.eu/en/news/covid-19-ema-starts-rolling-review-molnupiravir (accessed on 25 October 2021).
- EMA Issues Advice on Use of Lagevrio (Molnupiravir) for the Treatment of COVID-19. Available online: https://www.ema.europa.eu/en/news/ema-issues-advice-use-lagevrio-molnupiravir-treatment-covid-19 (accessed on 19 November 2021).
- Shoukri, A.M. High flow nasal cannula oxygen and non-invasive mechanical ventilation in management of COVID-19 patients with acute respiratory failure: A retrospective observational study. Egypt. J. Bronchol. 2021, 15, 17. [Google Scholar] [CrossRef]
- Rajendran, K.; Krishnasamy, N.; Rangarajan, J.; Rathinam, J.; Natarajan, M.; Ramachandran, A. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J. Med. Virol. 2020, 92, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qi, F. Traditional Chinese medicine to treat COVID-19: The importance of evidence-based research. Drug Discov. Ther. 2020, 14, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Tippairote, T.; Mujawdiya, P.K.; Peana, M.; Menzel, A.; Dadar, M.; Gasmi Benahmed, A.; Bjørklund, G. Micronutrients as immunomodulatory tools for COVID-19 management. Clin. Immunol. 2020, 220, 108545. [Google Scholar] [CrossRef]
- Jovic, T.H.; Ali, S.R.; Ibrahim, N.; Jessop, Z.M.; Tarassoli, S.P.; Dobbs, T.D.; Holford, P.; Thornton, C.A.; Whitaker, I.S. Could vitamins help in the fight against COVID-19? Nutrients 2020, 12, 2550. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raha, S.; Mallick, R.; Basak, S.; Duttaroy, A.K. Is copper beneficial for COVID-19 patients? Med. Hypotheses 2020, 142, 109814. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. COVID-19 pandemic: Can maintaining optimal zinc balance enhance host resistance? Tohoku J. Exp. Med. 2020, 251, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Rolles, B.; Rink, L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front. Immunol. 2020, 11, 1712. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Idid, S.Z. Can Zn be a critical element in COVID-19 treatment? Biol. Trace Elem. Res. 2021, 199, 550–558. [Google Scholar] [CrossRef]
- Heller, R.A.; Sun, Q.; Hackler, J.; Seelig, J.; Seibert, L.; Cherkezov, A.; Minich, W.B.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021, 38, 101764. [Google Scholar] [CrossRef]
- Gesesew, H.A.; Koye, D.N.; Fetene, D.M.; Woldegiorgis, M.; Kinfu, Y.; Geleto, A.B.; Melaku, Y.A.; Mohammed, H.; Alene, K.A.; Awoke, M.A.; et al. Risk factors for COVID-19 infection, disease severity and related deaths in Africa: A systematic review. BMJ Open 2021, 11, e044618. [Google Scholar] [CrossRef]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Wolff, D.; Nee, S.; Hickey, N.S.; Marschollek, M. Risk factors for COVID-19 severity and fatality: A structured literature review. Infection 2021, 49, 15–28. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Mughal, M.S.; Kaur, I.P.; Jaffery, A.R.; Dalmacion, D.L.; Wang, C.; Koyoda, S.; Kramer, V.E.; Patton, C.D.; Weiner, S.; Eng, M.H.; et al. COVID-19 patients in a tertiary US hospital: Assessment of clinical course and predictors of the disease severity. Respir. Med. 2020, 172, 106130. [Google Scholar] [CrossRef]
- Izquierdo, J.L.; Almonacid, C.; González, Y.; Del Rio-Bermudez, C.; Ancochea, J.; Cárdenas, R.; Lumbreras, S.; Soriano, J.B. The impact of COVID-19 on patients with asthma. Eur. Respir. J. 2021, 57, 2003142. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-M.; Bai, P.; He, W.; Wu, F.; Liu, X.-F.; Han, D.-M.; Liu, S.; Yang, J.-K. Gender differences in patients with COVID-19: Focus on severity and mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Marin, B.G.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, J.E.; Achamllah, N.; Ji, H.; Claggett, B.L.; Sun, N.; Botting, P.; Nguyen, T.T.; Luong, E.; Kim, E.H.; Park, E.; et al. Pre-existing traits associated with COVID-19 illness severity. PLoS ONE 2020, 15, e0236240. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, R.W.; Lewer, D.; Katikireddi, S.V.; Mathur, R.; Pathak, N.; Burns, R.; Fragaszy, E.B.; Johnson, A.M.; Devakumar, D.; Abubakar, I.; et al. Black, Asian and Minority Ethnic groups in England are at increased risk of death from COVID-19: Indirect standardisation of NHS mortality data. Wellcome Open Res. 2020, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.P.; Essien, U.R.; Pasha, S.; Gross, J.R.; Wang, S.; Nunez-Smith, M. Racial and ethnic disparities in population-level COVID-19 mortality. J. Gen. Intern. Med. 2020, 35, 3097–3099. [Google Scholar] [CrossRef]
- Hu, L.; Chen, S.; Fu, Y.; Gao, Z.; Long, H.; Ren, H.W.; Zuo, Y.; Wang, J.; Li, H.; Xu, Q.; et al. Risk factors associated with clinical outcomes in 323 coronavirus disease 2019 (COVID-19) hospitalized patients in Wuhan, China. Clin. Infect. Dis. 2020, 71, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Umnuaypornlert, A.; Kanchanasurakit, S.; Lucero-Prisno, D.E.I.; Saokaew, S. Smoking and risk of negative outcomes among COVID-19 patients: A systematic review and meta-analysis. Tob. Induc. Dis. 2021, 19, 9. [Google Scholar] [CrossRef]
- Patanavanich, R.; Glantz, S.A. Smoking is associated with worse outcomes of COVID-19 particularly among younger adults: A systematic review and meta-analysis. BMC Public Health 2021, 21, 1554. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Nikitara, K. COVID-19 and smoking: A systematic review of the evidence. Tob. Induc. Dis. 2020, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.J.; Loman, N.; Bogaert, D.; O’Grady, J. Co-infections: Potentially lethal and unexplored in COVID-19. Lancet Microbe 2020, 1, e11. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Goharrizi, M.A.S.B.; Bahardoust, M.; Alvanegh, A.G.; Ataee, M.R.; Bagheri, M.; Navidiyan, E.S.; Zijoud, S.R.H.; Heiat, M. Should all patients with hypertension be worried about developing severe coronavirus disease 2019 (COVID-19)? Clin. Hypertens. 2021, 27, 3. [Google Scholar] [CrossRef]
- Stefan, N.; Birkenfeld, A.L.; Schulze, M.B.; Ludwig, D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020, 16, 341–342. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Chen, F.; Wang, T.; Luo, F.; Liu, X.; Wu, Q.; He, Q.; Wang, Z.; Liu, Y.; Liu, L.; et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care 2020, 43, 1392–1398. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.D.; Heidel, R.E.; Dhand, R. Asthma in adult patients with COVID-19. Prevalence and risk of severe disease. Am. J. Respir. Crit. Care Med. 2021, 203, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Laires, M.J.; Monteiro, C. Exercise, magnesium and immune function. Magnes. Res. 2008, 21, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Tam, M.; Gómez, S.; González-Gross, M.; Marcos, A. Possible roles of magnesium on the immune system. Eur. J. Clin. Nutr. 2003, 57, 1193–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Percival, S.S. Copper and immunity. Am. J. Clin. Nutr. 1998, 67, 1064S–1068S. [Google Scholar] [CrossRef]
- Mattmiller, S.A.; Carlson, B.A.; Sordillo, L.M. Regulation of inflammation by selenium and selenoproteins: Impact on eicosanoid biosynthesis. J. Nutr. Sci. 2013, 2, e28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nairz, M.; Haschka, D.; Demetz, E.; Weiss, G. Iron at the interface of immunity and infection. Front. Pharmacol. 2014, 5, 152. [Google Scholar] [CrossRef] [Green Version]
- Kuvibidila, S.R.; Baliga, S.B.; Chandra, L.C.; French, C.L. The role of iron in immunity and inflammation: Implications for the response to infection. In Diet, Immunity and Inflammation; Calder, P.C., Yaqoob, P., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 193–220. [Google Scholar]
- Ivory, K.; Prieto, E.; Spinks, C.; Armah, C.N.; Goldson, A.J.; Dainty, J.R.; Nicoletti, C. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin. Nutr. 2017, 36, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.H.; Wilkens, M.R.; Möller, B.; Ganter, M.; Breves, G.; Schuberth, H.-J. Blood leukocyte composition and function in periparturient ewes kept on different dietary magnesium supply. BMC Vet. Res. 2020, 16, 484. [Google Scholar] [CrossRef]
- Sugimoto, J.; Romani, A.M.; Valentin-Torres, A.M.; Luciano, A.A.; Ramirez Kitchen, C.M.; Funderburg, N.; Mesiano, S.; Bernstein, H.B. Magnesium decreases inflammatory cytokine production: A novel innate immunomodulatory mechanism. J. Immunol. 2012, 188, 6338–6346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibovitch, C.M.; Cohen, E.G.; Djaldetti, M.; Bessler, H. The role of magnesium as immunomodulator and mediator between immune and colon cancer cells. Arch. Hematol. Blood Dis. 2019, 2, 6–13. [Google Scholar]
- Libako, P.; Miller, J.; Nowacki, W.; Castiglioni, S.; Maier, J.A.; Mazur, A. Extracellular Mg concentration and Ca blockers modulate the initial steps of the response of Th2 lymphocytes in co-culture with macrophages and dendritic cells. Eur. Cytokine Netw. 2015, 26, 1–9. [Google Scholar] [CrossRef]
- Weiss, G.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Werner, E.R.; Werner-Felmayer, G.; Wachter, H. Iron modulates interferon-gamma effects in the human myelomonocytic cell line THP-1. Exp. Hematol. 1992, 20, 605–610. [Google Scholar]
- Kiremidjian-Schumacher, L.; Roy, M.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Supplementation with selenium and human immune cell functions. II. Effect of cytotoxic lymphocytes and natural killer cells. Biol. Trace Elem. Res. 1994, 41, 115–127. [Google Scholar] [CrossRef]
- Roy, M.; Kiremidjian-Schumacher, L.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Supplementation with selenium and human immune cell functions. I. Effect on lymphocyte proliferation and interleukin 2 receptor expression. Biol. Trace Elem. Res. 1994, 41, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Bao, B.; Beck, F.W.J.; Sarkar, F.H. Zinc activates NF-κB in HUT-78 cells. J. Lab. Clin. Med. 2001, 138, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Dyck, J.L.; Galan, P.; Aplogan, A.; Schneider, D.; Traissac, P.; Hercberg, S. Effect of daily iron supplementation on iron status, cell-mediated immunity, and incidence of infections in 6-36 month old Togolese children. Eur. J. Clin. Nutr. 2000, 54, 29–35. [Google Scholar] [CrossRef]
- Weiss, G.; Meusburger, E.; Radacher, G.; Garimorth, K.; Neyer, U.; Mayer, G. Effect of iron treatment on circulating cytokine levels in ESRD patients receiving recombinant human erythropoietin. Kidney Int. 2003, 64, 572–578. [Google Scholar] [CrossRef] [Green Version]
- Broome, C.S.; McArdle, F.; Kyle, J.A.M.; Andrews, F.; Lowe, N.M.; Hart, C.A.; Arthur, J.R.; Jackson, M.J. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J. Clin. Nutr. 2004, 80, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lastra, M.D.; Aguilar, A.E.; Cabañas, M.A.; Hernandez, R.; Humanez, K.; Pastelin, R. Interleukin-1α, tumor necrosis factor-α, and interleukin-12 secreted by zinc-induced murine macrophages in vivo and in vitro. J. Trace Elem. Exp. Med. 2004, 17, 123–135. [Google Scholar] [CrossRef]
- Hurwitz, B.E.; Klaus, J.R.; Llabre, M.M.; Gonzalez, A.; Lawrence, P.J.; Maher, K.J.; Greeson, J.M.; Baum, M.K.; Shor-Posner, G.; Skyler, J.S.; et al. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: A randomized controlled trial. Arch. Intern. Med. 2007, 167, 148–154. [Google Scholar] [CrossRef]
- Muñoz, C.; Rios, E.; Olivos, J.; Brunser, O.; Olivares, M. Iron, copper and immunocompetence. Br. J. Nutr. 2007, 98, S24–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.S.; Beck, F.W.J.; Bao, B.; Fitzgerald, J.T.; Snell, D.C.; Steinberg, J.D.; Cardozo, L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007, 85, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab. 2007, 51, 301–323. [Google Scholar] [CrossRef] [Green Version]
- Das, I.; Saha, K.; Mukhopadhyay, D.; Roy, S.; Raychaudhuri, G.; Chatterjee, M.; Mitra, P.K. Impact of iron deficiency anemia on cell-mediated and humoral immunity in children: A case control study. J. Nat. Sci. Biol. Med. 2014, 5, 158–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.S. Zinc is an antioxidant and anti-inflammatory agent: Its role in human health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Brazão, V.; Caetano, L.C.; Del Vecchio Filipin, M.; Paula Alonso Toldo, M.; Caetano, L.N.; do Prado, J.C. Zinc supplementation increases resistance to experimental infection by Trypanosoma cruzi. Vet. Parasitol. 2008, 154, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sun, L.; Nan, Y.; Zhu, L.-Y. Protection from H1N1 influenza virus infections in mice by supplementation with selenium: A comparison with selenium-deficient mice. Biol. Trace Elem. Res. 2011, 141, 254–261. [Google Scholar] [CrossRef]
- Biesalski, H.K. Nutrition meets the microbiome: Micronutrients and the microbiota. Ann. N. Y. Acad. Sci. 2016, 1372, 53–64. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Li, Y.; Luo, X.; He, J. Effect of different selenium supplementation levels on oxidative stress, cytokines, and immunotoxicity in chicken thymus. Biol. Trace Elem. Res. 2016, 172, 488–495. [Google Scholar] [CrossRef]
- Maywald, M.; Rink, L. Zinc supplementation induces CD4+ CD25+ Foxp3+ antigen-specific regulatory T cells and suppresses IFN-γ production by upregulation of Foxp3 and KLF-10 and downregulation of IRF-1. Eur. J. Nutr. 2017, 56, 1859–1869. [Google Scholar] [CrossRef]
- Bernstein, H.; Sugimoto, J.; Suzuki-Kakisaka, H.; Romani, A. Magnesium decreases inflammatory cytokine production: A novel innate immunomodulatory mechanism. Am. J. Obstet. Gynecol. 2012, 206, S361. [Google Scholar] [CrossRef] [Green Version]
- Díez-Tercero, L.; Delgado, L.M.; Bosch-Rué, E.; Perez, R.A. Evaluation of the immunomodulatory effects of cobalt, copper and magnesium ions in a pro inflammatory environment. Sci. Rep. 2021, 11, 11707. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Bediz, C.S.; Mogulkoc, R.; Kurtoglu, E.; Pekel, A. Effect of zinc and melatonin supplementation on cellular immunity in rats with toxoplasmosis. Biol. Trace Elem. Res. 2003, 96, 237–245. [Google Scholar] [CrossRef]
- Erickson, K.L.; Medina, E.A.; Hubbard, N.E. Micronutrients and innate immunity. J. Infect. Dis. 2000, 182, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Sunzel, B.; Holm, S.; Reuterving, C.-O.; Söderberg, T.; Hallmans, G.; Hänström, L. The effect of zinc on bacterial phagocytosis, killing and cytoprotection in human polymorphonuclear leucocytes. Apmis 1995, 103, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Parent, G.; Chevalier, P.; Zalles, L.; Sevilla, R.; Bustos, M.; Dhenin, J.M.; Jambon, B. In vitro lymphocyte-differentiating effects of thymulin (Zn-FTS) on lymphocyte subpopulations of severely malnourished children. Am. J. Clin. Nutr. 1994, 60, 274–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariani, E.; Neri, S.; Cattini, L.; Mocchegiani, E.; Malavolta, M.; Dedoussis, G.V.; Kanoni, S.; Rink, L.; Jajte, J.; Facchini, A. Effect of zinc supplementation on plasma IL-6 and MCP-1 production and NK cell function in healthy elderly: Interactive influence of +647 MT1a and -174 IL-6 polymorphic alleles. Exp. Gerontol. 2008, 43, 462–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Foster, M.; Samman, S. Zinc and regulation of inflammatory cytokines: Implications for cardiometabolic disease. Nutrients 2012, 4, 676–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De, K.; Pal, S.; Mukherjee, J.; Prasad, S.; Dang, A.K. Effect of in vitro copper and zinc supplementation on neutrophil phagocytic activity and lymphocyte proliferation response of transition dairy cows. Agric. Res. 2015, 4, 388–395. [Google Scholar] [CrossRef]
- Haryanto, B.; Suksmasari, T.; Wintergerst, E.; Maggini, S. Multivitamin supplementation supports immune function and ameliorates conditions triggered by reduced air quality. Vitam. Miner. 2015, 4, 2. [Google Scholar] [CrossRef]
- Muñoz, C.; López, M.; Olivares, M.; Pizarro, F.; Arredondo, M.; Araya, M. Differential response of interleukin-2 production to chronic copper supplementation in healthy humans. Eur. Cytokine Netw. 2005, 16, 261–265. [Google Scholar]
- Asao, H. Interleukin-2. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014; pp. 60–63. [Google Scholar]
- Lahans, T. General pathophysiology and treatment: Conventional and Chinese medicine. In Integrating Conventional and Chinese Medicine in Cancer Care, A Clinical Guide, 1st ed.; Chapter 1; Churchill Livingstone: London, UK, 2007; pp. 1–34. [Google Scholar] [CrossRef]
- Turnlund, J.R.; Jacob, R.A.; Keen, C.L.; Strain, J.J.; Kelley, D.S.; Domek, J.M.; Keyes, W.R.; Ensunsa, J.L.; Lykkesfeldt, J.; Coulter, J. Long-term high copper intake: Effects on indexes of copper status, antioxidant status, and immune function in young men. Am. J. Clin. Nutr. 2004, 79, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Fattori, E.; Cappelletti, M.; Costa, P.; Sellitto, C.; Cantoni, L.; Carelli, M.; Faggioni, R.; Fantuzzi, G.; Ghezzi, P.; Poli, V. Defective inflammatory response in interleukin 6-deficient mice. J. Exp. Med. 1994, 180, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M. Reactive oxygen species: A novel antimicrobial. Int. J. Antimicrob. Agents 2018, 51, 299–303. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Qin, J.; Sun, H.; He, H. Induced susceptibility of host is associated with an impaired antioxidant system following infection with Cryptosporidium parvum in Se-deficient mice. PLoS ONE 2009, 4, e4628. [Google Scholar] [CrossRef] [PubMed]
- van Kempen, T.A.T.G.; Deixler, E. SARS-CoV-2: Influence of phosphate and magnesium, moderated by vitamin D, on energy (ATP) metabolism and on severity of COVID-19. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E2–E6. [Google Scholar] [CrossRef]
- Cappellini, F.; Brivio, R.; Casati, M.; Cavallero, A.; Contro, E.; Brambilla, P. Low levels of total and ionized calcium in blood of COVID-19 patients. Clin. Chem. Lab. Med. 2020, 58, e171–e173. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, X.; Song, Q.; Hu, C.; Su, F.; Dai, J.; Ye, Y.; Huang, J.; Zhang, X. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw. Open 2020, 3, e2011122. [Google Scholar] [CrossRef]
- Lippi, G.; South, A.M.; Henry, B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann. Clin. Biochem. 2020, 57, 262–265. [Google Scholar] [CrossRef] [Green Version]
- Alkattan, A.; Alabdulkareem, K.; Kamel, A.; Abdelseed, H.; Almutairi, Y.; Alsalameen, E. Correlation between micronutrient plasma concentration and disease severity in COVID-19 patients. Alexandria J. Med. 2021, 57, 21–27. [Google Scholar] [CrossRef]
- Dubourg, G.; Lagier, J.-C.; Brouqui, P.; Casalta, J.-P.; Jacomo, V.; La Scola, B.; Rolain, J.-M.; Raoult, D. Low blood zinc concentrations in patients with poor clinical outcome during SARS-CoV-2 infection: Is there a need to supplement with zinc COVID-19 patients? J. Microbiol. Immunol. Infect. 2021, 54, 997–1000. [Google Scholar] [CrossRef]
- Zhao, K.; Huang, J.; Dai, D.; Feng, Y.; Liu, L.; Nie, S. Serum iron level as a potential predictor of COVID-19 severity and mortality: A retrospective study. Open Forum Infect. Dis. 2020, 7, ofaa250. [Google Scholar] [CrossRef]
- Gonçalves, T.J.M.; Gonçalves, S.E.A.B.; Guarnieri, A.; Risegato, R.C.; Guimarães, M.P.; de Freitas, D.C.; Razuk-Filho, A.; Junior, P.B.B.; Parrillo, E.F. Association between low zinc levels and severity of acute respiratory distress syndrome by new coronavirus SARS-CoV-2. Nutr. Clin. Pract. 2021, 36, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Vogel-González, M.; Talló-Parra, M.; Herrera-Fernández, V.; Pérez-Vilaró, G.; Chillón, M.; Nogués, X.; Gómez-Zorrilla, S.; López-Montesinos, I.; Arnau-Barrés, I.; Sorli-Redó, M.L.; et al. Low zinc levels at admission associates with poor clinical outcomes in SARS-CoV-2 infection. Nutrients 2021, 13, 562. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Yasui, H.; Suzuki, K.; Saitou, T.; Yamamoto, Y.; Ishizaka, T.; Nishida, K.; Yoshihara, S.; Gohma, I.; Ogawa, Y. Analysis of the predictive factors for a critical illness of COVID-19 during treatment—Relationship between serum zinc level and critical illness of COVID-19. Int. J. Infect. Dis. 2020, 100, 230–236. [Google Scholar] [CrossRef]
- Zeng, H.L.; Yang, Q.; Yuan, P.; Wang, X.; Cheng, L. Associations of essential and toxic metals/metalloids in whole blood with both disease severity and mortality in patients with COVID-19. FASEB J. 2021, 35, e21392. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.L.; Zhang, B.; Wang, X.; Yang, Q.; Cheng, L. Urinary trace elements in association with disease severity and outcome in patients with COVID-19. Environ. Res. 2021, 194, 110670. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, S.R.; Tabriz, H.M.; Togha, M.; Ariyanfar, S.; Ghorbani, Z.; Naeeni, S.; Haghighi, S.; Jazayeri, A.; Montazeri, M.; Talebpour, M.; et al. The correlation between serum selenium, zinc, and COVID-19 severity: An observational studies. BMC Infect. Dis. 2021, 21, 899. [Google Scholar] [CrossRef]
- Sun, J.K.; Zhang, W.H.; Zou, L.; Liu, Y.; Li, J.J.; Kan, X.H.; Dai, L.; Shi, Q.K.; Yuan, S.T.; Yu, W.K.; et al. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging 2020, 12, 11287–11295. [Google Scholar] [CrossRef]
- Abdelmaksoud, A.A.; Ghweil, A.A.; Hassan, M.H.; Rashad, A.; Khodeary, A.; Aref, Z.F.; Sayed, M.A.A.; Elsamman, M.K.; Bazeed, S.E.S. Olfactory disturbances as presenting manifestation among egyptian patients with COVID-19: Possible role of zinc. Biol. Trace Elem. Res. 2021, 7, 4101–4108. [Google Scholar] [CrossRef]
- Arrieta, F.; Martinez-Vaello, V.; Bengoa, N.; Jiménez-Mendiguchia, L.; Rosillo, M.; de Pablo, A.; Voguel, C.; Martinez-Barros, H.; Pintor, R.; Belanger-Quintana, A.; et al. Serum zinc and copper in people with COVID-19 and zinc supplementation in parenteral nutrition. Nutrition 2021, 91–92, 111467. [Google Scholar] [CrossRef]
- Notz, Q.; Herrmann, J.; Schlesinger, T.; Helmer, P.; Sudowe, S.; Sun, Q.; Hackler, J.; Roeder, D.; Lotz, C.; Meybohm, P.; et al. Clinical significance of micronutrient supplementation in critically ill COVID-19 patients with severe ARDS. Nutrients 2021, 13, 2113. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.P.Z.; Teh, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.J.W.; Chandran, M.; Chay, J.W.M.; et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition 2020, 79–80, 111017. [Google Scholar] [CrossRef]
- Skalny, A.V.; Timashev, P.S.; Aschner, M.; Aaseth, J.; Chernova, L.N.; Belyaev, V.E.; Grabeklis, A.R.; Notova, S.V.; Lobinski, R.; Tsatsakis, A.; et al. Serum zinc, copper, and other biometals are associated with COVID-19 severity markers. Metabolites 2021, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Y.; Kani, Y.A.; Iliya, S.; Muhammad, J.B.; Binji, A.; El-Fulaty Ahmad, A.; Kabir, M.B.; Umar Bindawa, K.; Ahmed, A. Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: A cross-sectional comparative study in Jigawa, Northwestern Nigeria. SAGE Open Med. 2021, 9, 2050312121991246. [Google Scholar] [CrossRef] [PubMed]
- Elham, A.S.; Azam, K.; Azam, J.; Mostafa, L.; Nasrin, B.; Marzieh, N. Serum vitamin D, calcium, and zinc levels in patients with COVID-19. Clin. Nutr. ESPEN 2021, 43, 276–282. [Google Scholar] [CrossRef]
- Pour, O.B.; Yahyavi, Y.; Karimi, A.; Khamaneh, A.M.; Milani, M.; Khalili, M.; Sharifi, A. Serum trace elements levels and clinical outcomes among Iranian COVID-19 patients. Int. J. Infect. Dis. 2021, 111, 164–168. [Google Scholar] [CrossRef]
- Hackler, J.; Heller, R.A.; Sun, Q.; Schwarzer, M.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; Schomburg, L. Relation of serum copper status to survival in COVID-19. Nutrients 2021, 13, 1898. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Gowda, S.; Mundkur, L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: The case for adequate selenium status. Nutrition 2021, 82, 111053. [Google Scholar] [CrossRef]
- Carlucci, P.M.; Ahuja, T.; Petrilli, C.; Rajagopalan, H.; Jones, S.; Rahimian, J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized patients. J. Med. Microbiol. 2020, 69, 1228–1234. [Google Scholar] [CrossRef]
- Yao, J.S.; Paguio, J.A.; Dee, E.C.; Tan, H.C.; Moulick, A.; Milazzo, C.; Jurado, J.; Della Penna, N.; Celi, L.A. The minimal effect of zinc on the survival of hospitalized patients with COVID-19: An observational study. Chest 2020, 22, 108–111. [Google Scholar] [CrossRef]
- Derwand, R.; Scholz, M.; Zelenk, V. COVID-19 outpatients: Early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: A retrospective case series study. Int. J. Antimicrob. Agents 2020, 56, 106214. [Google Scholar] [CrossRef]
- Sulaiman, K.A.; Aljuhani, O.; Al Shaya, A.I.; Kharbosh, A.; Kensara, R.; Al Guwairy, A.; Alharbi, A.; Algarni, R.; Al Harbi, S.; Vishwakarma, R.; et al. Evaluation of zinc sulfate as an adjunctive therapy in COVID-19 critically ill patients: A two center propensity-score matched study. Crit. Care 2021, 25, 363. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Hardigan, P.C. A case-control study for the effectiveness of oral zinc in the prevention and mitigation of COVID-19. Front. Med. 2021, 8, 756707. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Rahimian, J.O.; Yaghi, S.; Liu, M.; Lewis, A.; de Havenon, A.; Mainali, S.; Huang, J.; Scher, E.; Wisniewski, T.; et al. Treatment with zinc is associated with reduced in-hospital mortality among COVID-19 patients: A multi-center cohort study. Res. Sq. 2020, rs.3.rs-94509. [Google Scholar] [CrossRef]
- Li, C.; Zhou, H.M. The role of manganese superoxide dismutase in inflammation defense. Enzyme Res. 2011, 2011, 387176. [Google Scholar] [CrossRef] [Green Version]
- Moradi, F.; Maleki, V.; Saleh-Ghadimi, S.; Kooshki, F.; Pourghassem Gargari, B. Potential roles of chromium on inflammatory biomarkers in diabetes: A Systematic. Clin. Exp. Pharmacol. Physiol. 2019, 46, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, K.; Bawazeer, N.; Joy, S.S. Variation in micro and trace elements in progression of type 2 diabetes. Sci. World J. 2014, 2014, 461591. [Google Scholar] [CrossRef] [Green Version]
- Quilliot, D.; Bonsack, O.; Jaussaud, R.; Mazur, A. Dysmagnesemia in COVID-19 cohort patients: Prevalence and associated factors. Magnes. Res. 2020, 33, 114–122. [Google Scholar] [CrossRef]
- Alamdari, N.M.; Afaghi, S.; Rahimi, F.S.; Tarki, F.E.; Tavana, S.; Zali, A.; Fathi, M.; Besharat, S.; Bagheri, L.; Pourmotahari, F.; et al. Mortality risk factors among hospitalized COVID-19 patients in a major referral center in Iran. Tohoku J. Exp. Med. 2020, 252, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.-Q.; Yang, N.-B.; Ding, F.; Ma, A.H.Y.; Wang, Z.-Y.; Shen, Y.-F.; Shi, C.-W.; Lian, X.; Chu, J.-G.; Chen, L.; et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: A retrospective, multi-centre case series. QJM 2020, 113, 474–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennouar, S.; Cherif, A.B.; Kessira, A.; Bennouar, D.E.; Abdi, S. Vitamin D deficiency and low serum calcium as predictors of poor prognosis in patients with severe COVID-19. J. Am. Coll. Nutr. 2021, 40, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Collage, R.D.; Howell, G.M.; Zhang, X.; Stripay, J.L.; Lee, J.S.; Angus, D.C.; Rosengart, M.R. Calcium supplementation during sepsis exacerbates organ failure and mortality via calcium/calmodulin-dependent protein kinase kinase (CaMKK) signaling. Crit. Care Med. 2013, 41, e352–e360. [Google Scholar] [CrossRef] [Green Version]
- Dotson, B.; Larabell, P.; Patel, J.U.; Wong, K.; Qasem, L.; Arthur, W.; Leiberman, C.; Whittaker, P.; Tennenberg, S.D. Calcium administration is associated with adverse outcomes in critically ill patients receiving parenteral nutrition: Results from a natural experiment created by a calcium gluconate shortage. J. Hum. Pharmacol. Drug. Ther. 2016, 36, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Xiang, Y.; Fang, W.; Zheng, Y.; Li, B.; Hu, Y.; Lang, C.; Huang, D.; Sun, Q.; Xiong, Y.; et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020, 92, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, A.; Rysz, S.; Sjöström, H.; Höybe, C. Electrolyte and acid-base imbalance in severe COVID-19. Endocr. Connect. 2021, 10, 805–814. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, H.; Richared, M.C.; Chouihed, T.; Goffinet, N.; Le Bastard, Q.; Freund, Y.; Kratz, A.; Dubroux, M.; Masson, D.; Figueres, L.; et al. Electrolyte imbalance in COVID-19 patients admitted to the Emergency Department: A case-control study. Intern. Emerg. Med. 2021, 16, 1945–1950. [Google Scholar] [CrossRef]
- Gálvez-Barrón, C.; Arroyo-Huidobro, M.; Miňarro, A.; Añaños, G.; Chamero, A.; Martin, M.; Gris, C.; Avalos, J.L.; Capielo, A.M.; Ventosa, E.; et al. COVID-19: Clinical presentation and prognostic factors of severe disease and mortality in the oldest-old population: A cohort study. Gerontology 2022, 68, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Dullaart, R.P.F.; Bakker, S.J.L. Is low sodium intake a risk factor for severe and fatal COVID-19 infection? Eur. J. Intern. Med. 2020, 75, 109. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Dullaart, R.P.F.; Bakker, S.J.L. Sodium status and kidney involvement during COVID-19 infection. Virus Res. 2020, 286, 198034. [Google Scholar] [CrossRef]
- Alfano, G.; Ferrari, A.; Fontana, F.; Perrone, R.; Mori, G.; Ascione, E.; Magistroni, R.; Venturi, G.; Pederzoli, S.; Margiotta, G.; et al. Hypokalemia in patients with COVID-19. Clin. Exp. Nephrol. 2021, 25, 401–409. [Google Scholar] [CrossRef]
- Pal, R.; Ram, S.; Zohmangaihi, D.; Biswas, I.; Suri, V.; Yaddanapudi, L.N.; Malhotra, P.; Soni, S.L.; Puri, G.D.; Bhalla, A.; et al. High prevalence of hypocalcemia in non-severe COVID-19 patients: A retrospective case-control study. Front Med. 2021, 7, 590805. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; II’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptoms length and reduction among ambulatory patients with SARS-CoV-2 infection. The COVID A to Z randomized clinical trial. JAMA Netw. Open 2021, 4, e210369. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Anbarasi, C.; Sathiyarajeswaran, P.; Manickam, P.; Geetha, S.; Kathiravan, R.; Prathiba, P.; Pitchiahkumar, M.; Parthiban, P.; Kanakavalli, K.; et al. Kabasura Kudineer (KSK), a poly-herbal Siddha medicine, reduced SARS-CoV-2 viral load in asymptomatic COVID-19 individuals as compared to vitamin C and zinc supplementation: Findings froma prospective, exploratory, open-labeled, comparative, randomized controlled trial, Tamil Nadu, India. Trials 2021, 22, 623. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, S.; Soliman, S.; Esmail, E.S.; Khalaf, M.; Mostafa, E.F.; Medhat, M.A.; Ahmed, O.A.; Abd El Ghafar, M.S.; Alboraire, M.; Hassany, S.M. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine?: A randomized, multicenter trial. Biol. Trace Elem. Res. 2021, 199, 3642–3646. [Google Scholar] [CrossRef]
- Xue, J.; Moyer, A.; Peng, B.; Wu, J.; Hannafon, B.N.; Ding, W.Q. Chloroquine is a zinc ionophore. PLoS ONE 2014, 9, e109180. [Google Scholar] [CrossRef] [Green Version]
 : increased activity;
: increased activity;  : inhibited activity;
: inhibited activity;  : trend toward an increase; ↓: inhibited secretion. *: in vitro studies.
: trend toward an increase; ↓: inhibited secretion. *: in vitro studies.
 : increased activity;
: increased activity;  : inhibited activity;
: inhibited activity;  : trend toward an increase; ↓: inhibited secretion. *: in vitro studies.
: trend toward an increase; ↓: inhibited secretion. *: in vitro studies.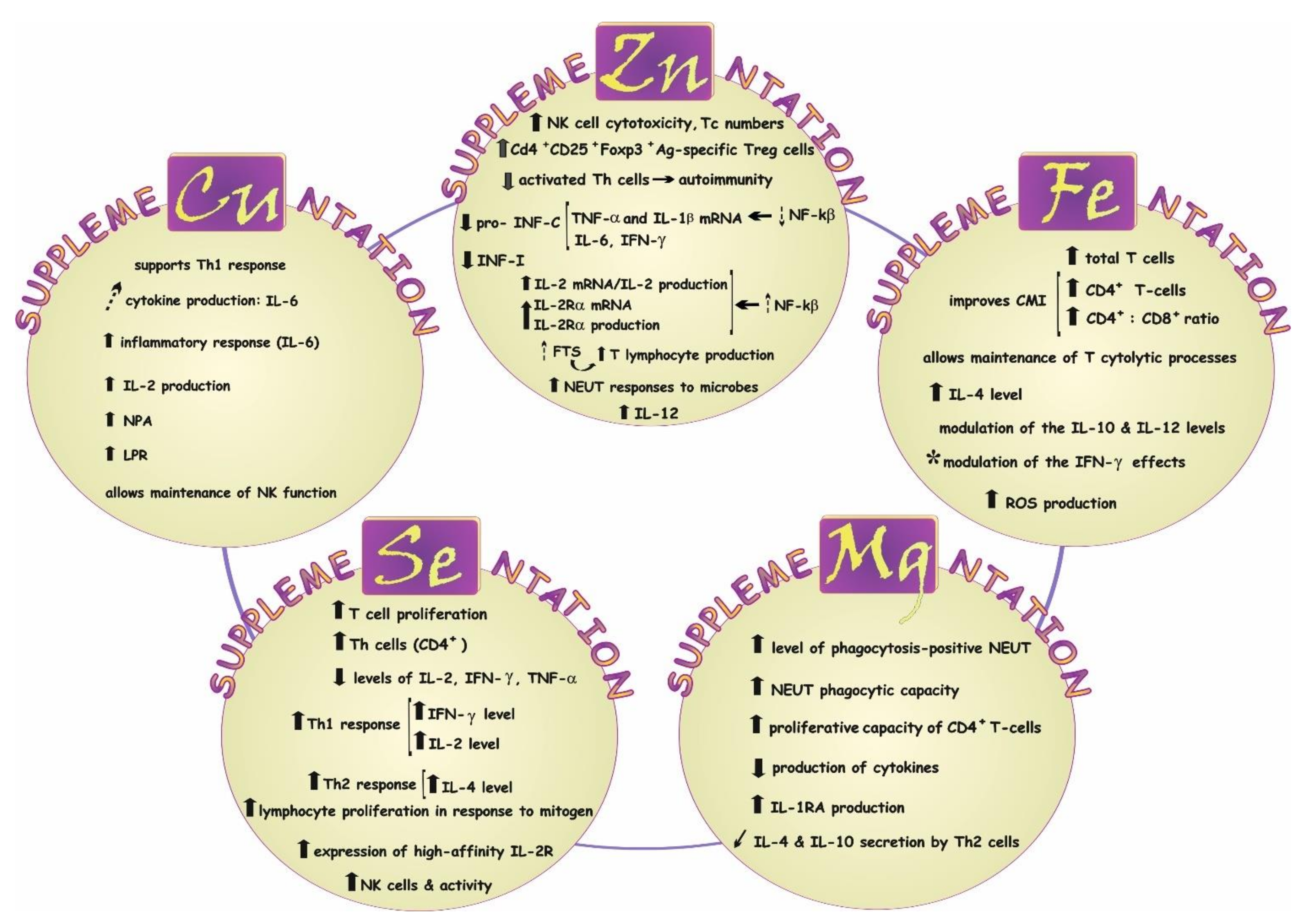
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ścibior, A.; Wnuk, E. Elements and COVID-19: A Comprehensive Overview of Studies on Their Blood/Urinary Levels and Supplementation with an Update on Clinical Trials. Biology 2022, 11, 215. https://doi.org/10.3390/biology11020215
Ścibior A, Wnuk E. Elements and COVID-19: A Comprehensive Overview of Studies on Their Blood/Urinary Levels and Supplementation with an Update on Clinical Trials. Biology. 2022; 11(2):215. https://doi.org/10.3390/biology11020215
Chicago/Turabian StyleŚcibior, Agnieszka, and Ewa Wnuk. 2022. "Elements and COVID-19: A Comprehensive Overview of Studies on Their Blood/Urinary Levels and Supplementation with an Update on Clinical Trials" Biology 11, no. 2: 215. https://doi.org/10.3390/biology11020215
APA StyleŚcibior, A., & Wnuk, E. (2022). Elements and COVID-19: A Comprehensive Overview of Studies on Their Blood/Urinary Levels and Supplementation with an Update on Clinical Trials. Biology, 11(2), 215. https://doi.org/10.3390/biology11020215