Different Arbuscular Mycorrhizal Fungi Established by Two Inoculation Methods Improve Growth and Drought Resistance of Cinnamomum Migao Seedlings Differently
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Seeds, AMF, and Soil
2.2. Study Site
2.3. Experimental Design
2.4. Parameters Measured
2.4.1. Mycorrhizal Colonization
2.4.2. AMF Spore Density Per 10 g Soil
2.4.3. Root Vigor
2.4.4. RWC
2.4.5. Soil Moisture
2.4.6. Plant Growth and Mycorrhizal Dependency Index
(NM plants are the plants without AMF inoculation)
2.4.7. Antioxidant Enzyme Activity
2.4.8. Osmotic Adjustment Substances
2.5. Statistical Analysis
3. Results
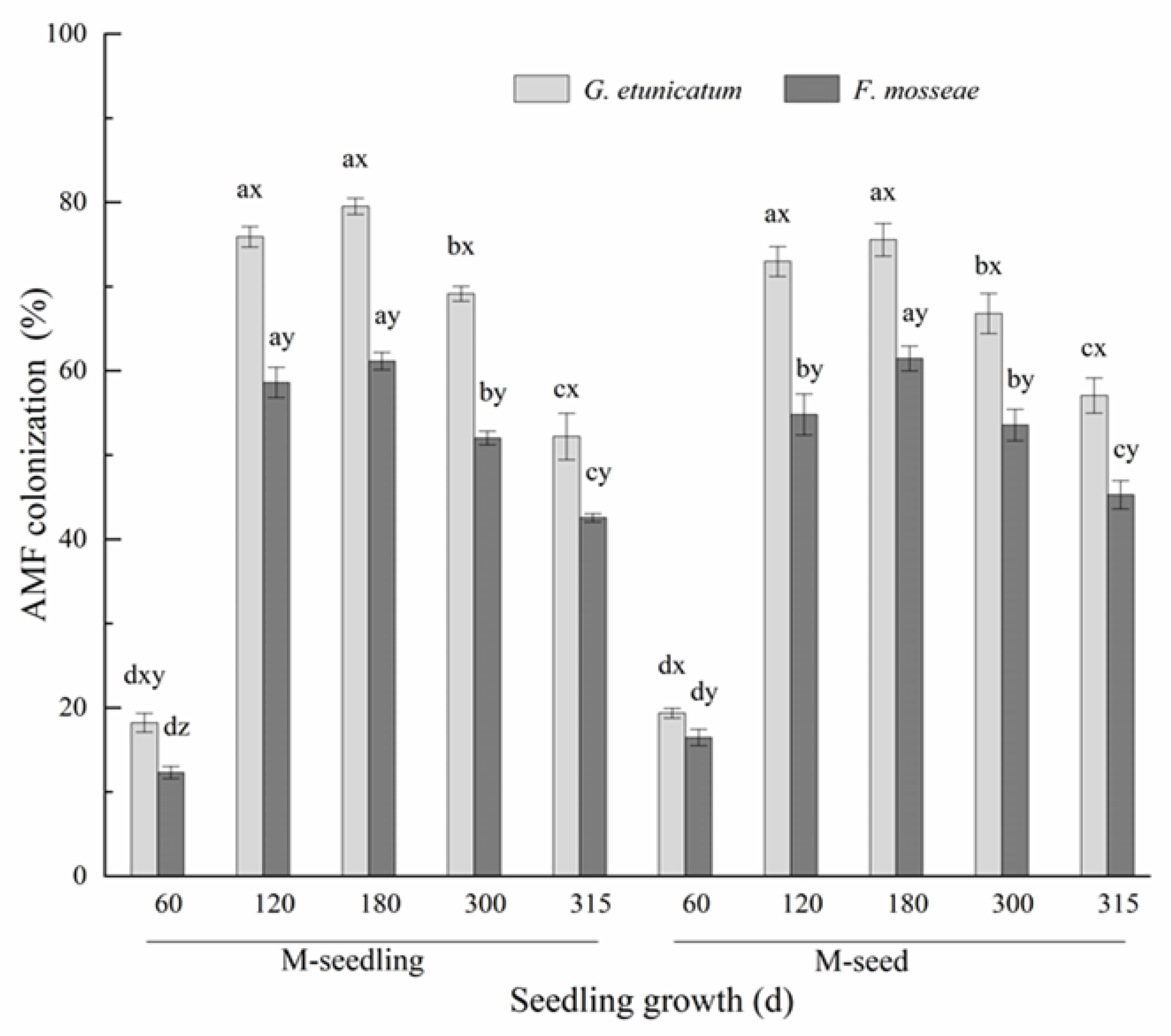
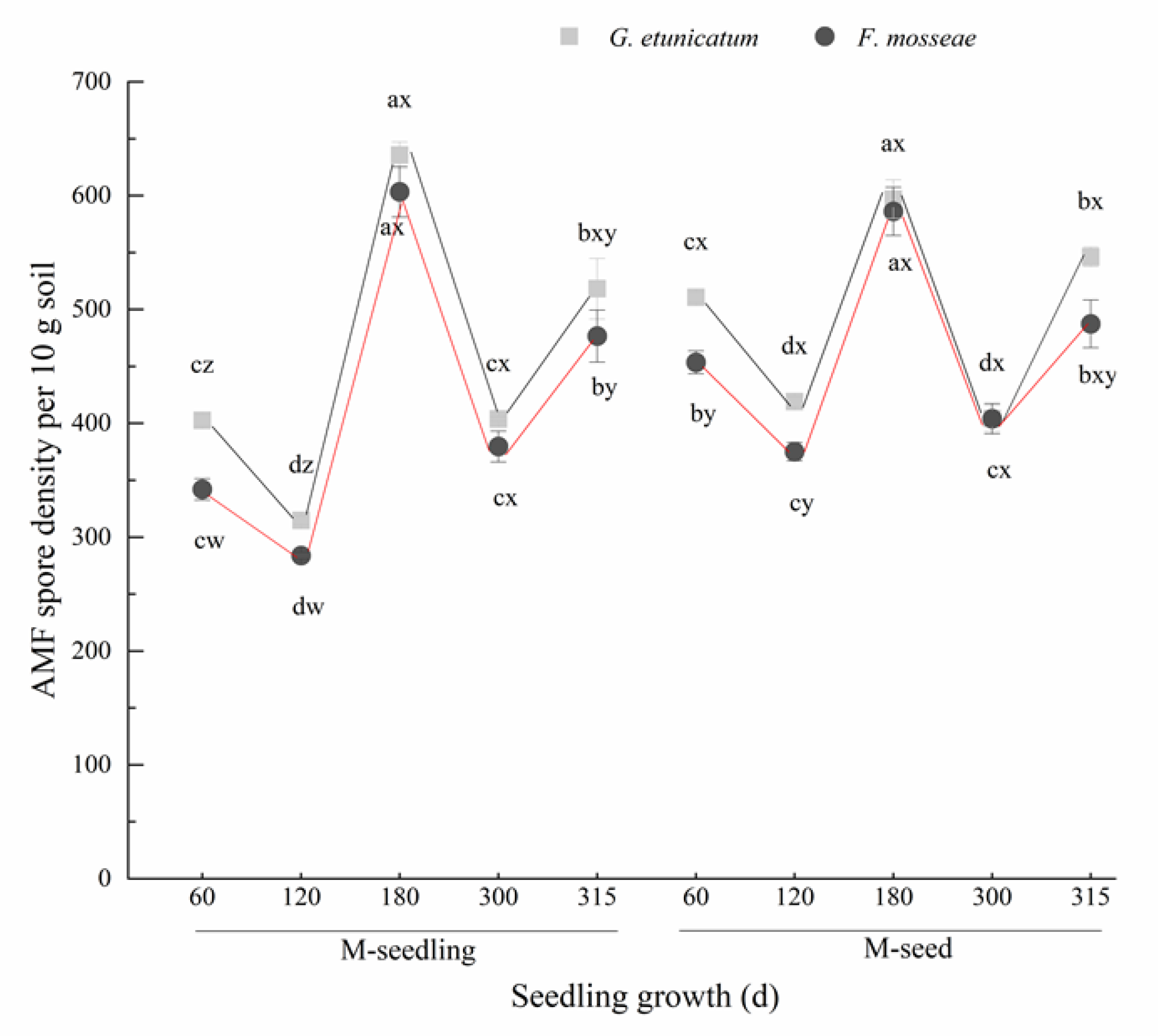
3.1. AMF Colonization and Spore Density
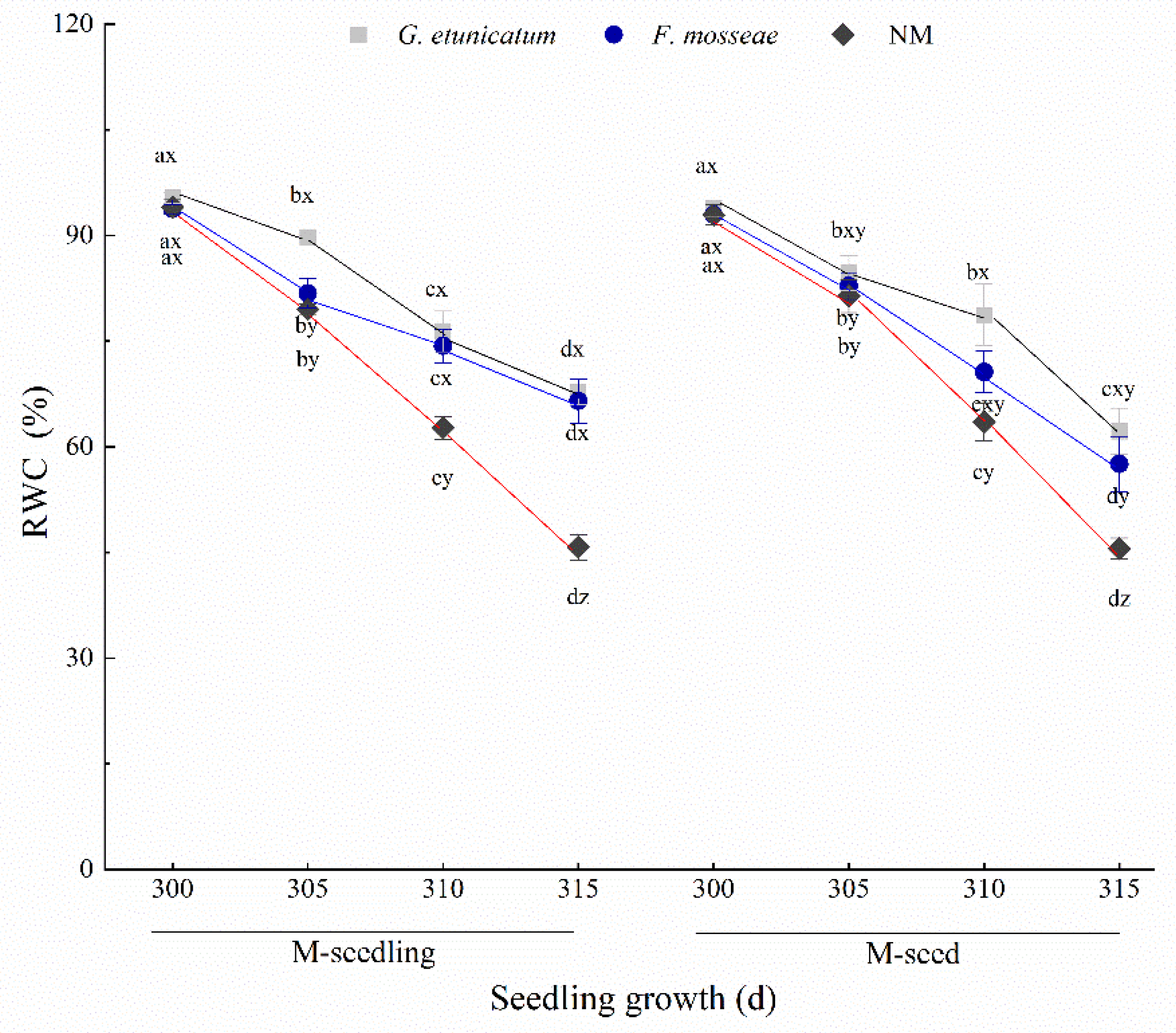
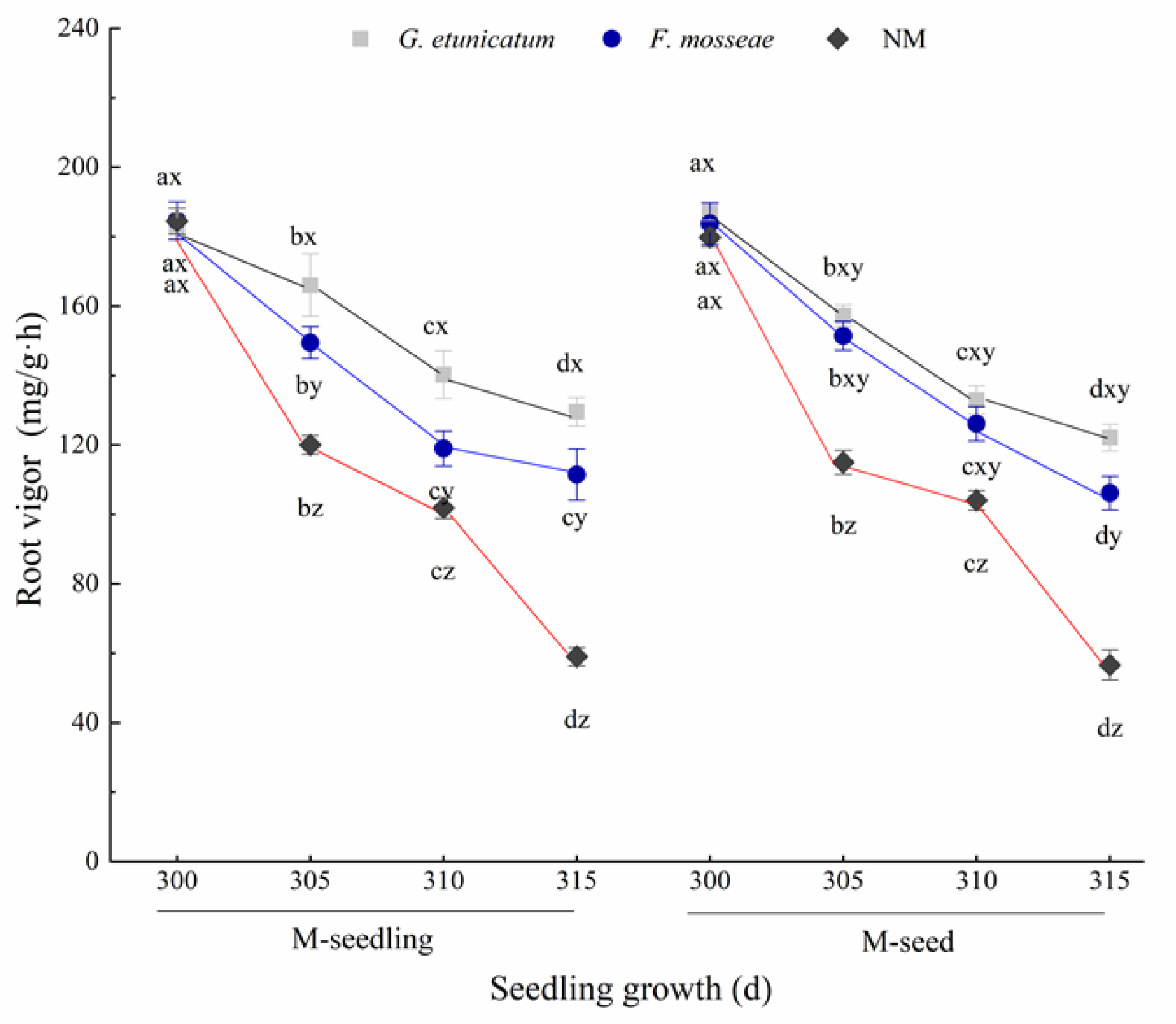
3.2. Root Vigor and RWC
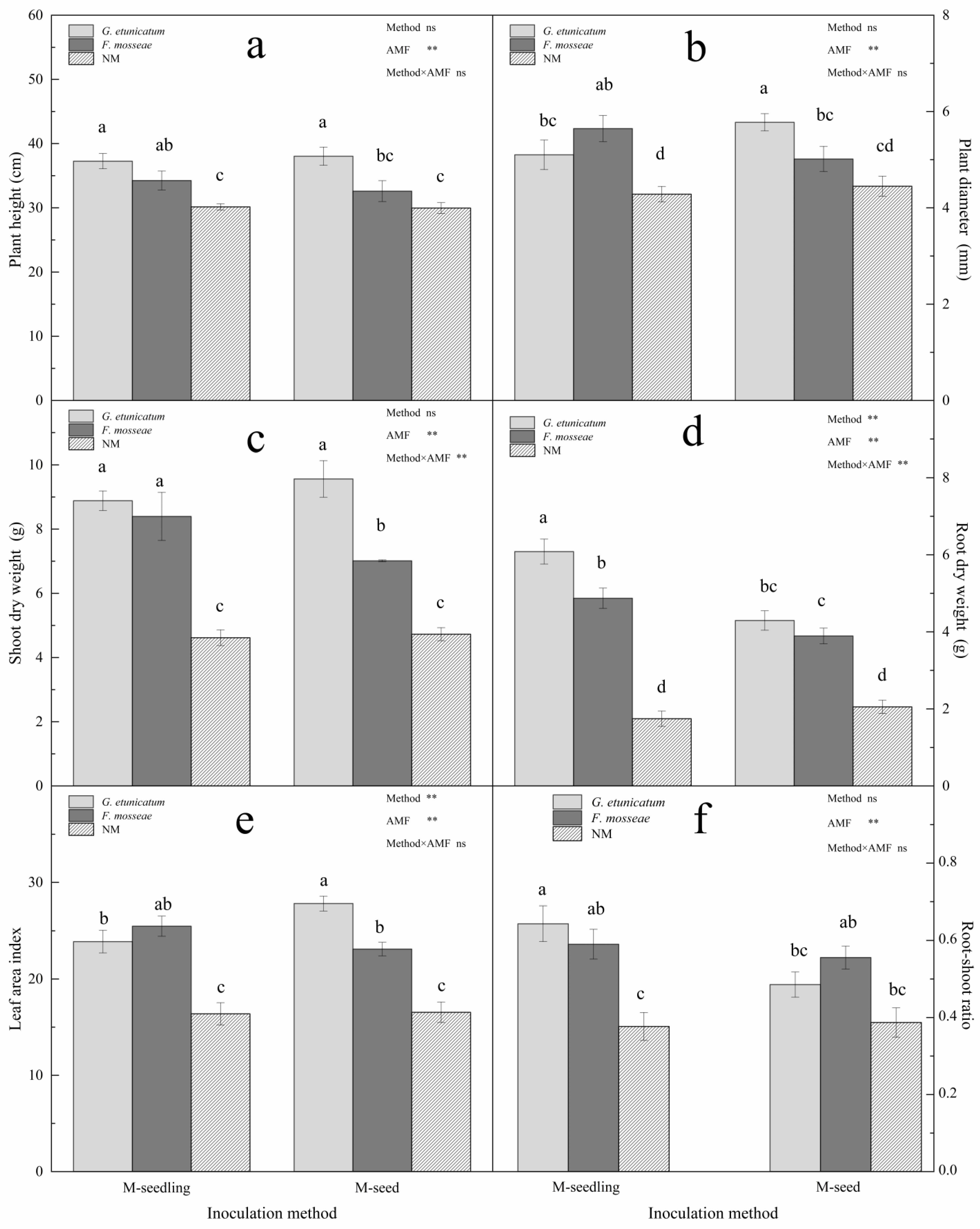
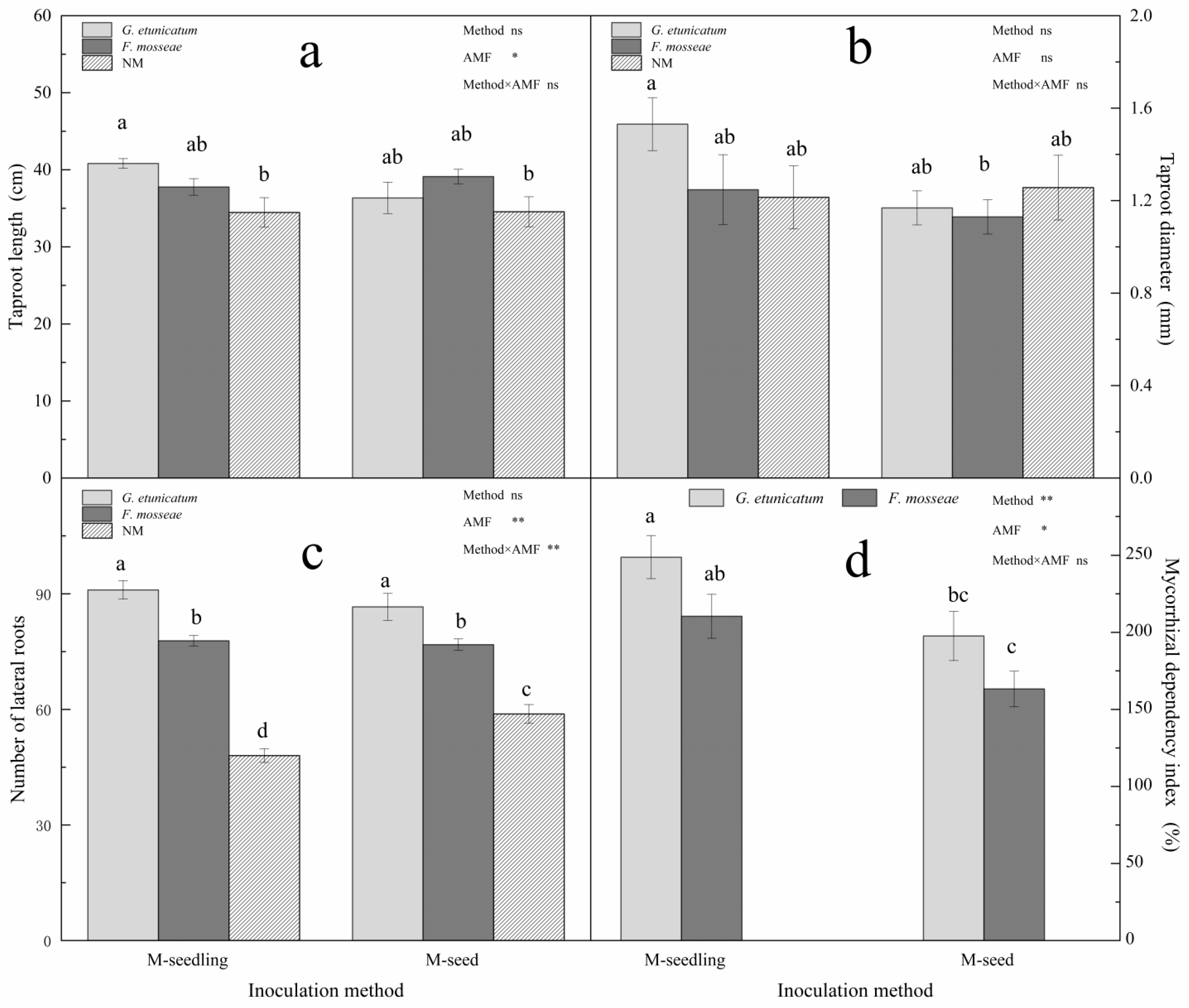
3.3. Plant Growth and MDI
3.4. Antioxidant Enzyme Activity
3.5. Osmotic Adjustment Substances
4. Discussion
4.1. AMF Colonization and Spore Density
4.2. Root Vigor and RWC
4.3. Plant Growth
4.4. Antioxidant Enzyme Activity
4.5. Osmotic Adjustment Substances
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelley, C.P.; Mohtadi, S.; Cane, M.A.; Seager, R.; Kushnir, Y. Climate change in the fertile crescent and implications of the recent Syrian drought. Proc. Natl. Acad. Sci. USA 2015, 112, 3241–3246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, N.; Hadi, F.; Ditta, A.; Suleman, M.; Ullah, M. Zinc-induced anti-oxidative defense and osmotic adjustments to enhance drought stress tolerance in sunflower (Helianthus annuus L.). Environ. Exp. Bot. 2022, 193, 104682. [Google Scholar] [CrossRef]
- Ullah, N.; Ditta, A.; Imtiaz, M.; Li, X.M.; Jan, A.U.; Mehmood, S.; Rizwan, M.S.; Rizwan, M. Appraisal for organic amendments and plant growth-promoting rhizobacteria to enhance crop productivity under drought stress: A review. J. Agro. Crop. Sci. 2021, 207, 783–802. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, C.; Anderegg, W.; Michalak, A.; Fisher, J.; Biondi, F.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.; Wolf, A.; et al. Global patterns of drought recovery. Nature 2017, 548, 202–205. [Google Scholar] [CrossRef]
- Dao, X. On the Karst Ecosystem. Acta Geol. Sin.-Engl. Ed. 2001, 75, 336–338. [Google Scholar] [CrossRef]
- Shi, P.; Duan, J.; Zhang, Y.; Li, P.; Wang, X.; Li, Z.; Xiao, L.; Xu, G.; Lu, K.; Cheng, S.; et al. The effects of ecological construction and topography on soil organic carbon and total nitrogen in the Loess Plateau of China. Environ. Earth Sci. 2019, 78, 5. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, G.; Yuan, D.; Polk, J. Evolution of major environmental geological problems in karst areas of Southwestern China. Environ. Earth Sci. 2013, 69, 2427–2435. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Deng, Y.; Peng, P.; Fan, J. A discussion on concept definition and academic value of ecological defense. J. Mt. Sci. 2005, 23, 431–436. [Google Scholar]
- Li, D.; Liu, J.; Chen, H.; Zheng, L.; Wang, K. Soil microbial community responses to forage grass cultivation in degraded karst soils, Southwest China. Land Degrad. Dev. 2018, 29, 4262–4270. [Google Scholar] [CrossRef]
- Dai, Q.; Peng, X.; Wang, P.; Li, C.; Shao, H. Surface erosion and underground leakage of yellow soil on slopes in karst regions of southwest China. Land Degrad. Dev. 2018, 29, 2438–2448. [Google Scholar] [CrossRef]
- Qi, D.; Wieneke, X.; Zhou, X.; Jiang, X.; Xue, P. Succession of plant community composition and leaf functional traits in responding to karst rocky desertification in the Wushan County in Chongqing, China. Community Ecol. 2017, 18, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, R.; Parise, M. Karst environments: Problems, management, human impacts, and sustainability: An introduction to the special issue. J. Cave Karst Stud. 2012, 74, 135–136. [Google Scholar] [CrossRef]
- Chen, H.; Nie, Y.; Wang, K. Spatio-temporal heterogeneity of water and plant adaptation mechanisms in karst regions: A review. Acta Ecol. Sin. 2013, 33, 317–326. (In Chinese) [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Chen, J.; Liu, J.; Li, J.; Wu, M.; Tong, B. Autotoxicity Hinders the Natural Regeneration of Cinnamomum migao H. W. Li in Southwest China. Forests 2019, 10, 919. [Google Scholar] [CrossRef] [Green Version]
- Staddon, P.L.; Gregersen, R.; Jakobsen, I. The response of two Glomus mycorrhizal fungi and a fine endophyte to elevated atmospheric CO2, soil warming and drought. Glob. Chang. Biol. 2004, 10, 1909–1921. [Google Scholar] [CrossRef]
- Vandana, U.K.; Rajkumari, J.; Singha, L.P.; Satish, L.; Alavilli, H.; Sudheer, P.D.V.N.; Chauhan, S.; Ratnala, R.; Satturu, V.; Mazumder, P.B.; et al. The Endophytic Microbiome as a Hotspot of Synergistic Interactions, with Prospects of Plant Growth Promotion. Biology 2021, 10, 101. [Google Scholar] [CrossRef]
- Hodge, A.; Campbell, C.; Fitter, A. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 297–299. [Google Scholar] [CrossRef] [Green Version]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bárzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol. Plant-Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef] [Green Version]
- Pozo, M.J.; López-Ráez, J.A.; Azcón-Aguilar, C.; García-Garrido, J.M. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 2015, 205, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Sánchez, M.; Aroca, R.; Muñoz, Y.; Polon, R.; Ruiz-Lozano, J.M. The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J. Plant Physiol. 2010, 167, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, S.; Zhang, J.; Zhou, C.; Chen, L. Effects of mycorrhizal fungus inoculation on the root of Cupressus duclouxiana and Catalpa bungei seedings under drought stress. J. Nanjing For. Univ. Nat. Sci. Edn. 2012, 36, 23–27. (In Chinese) [Google Scholar]
- Wei, Y.; Wang, S.; Liu, X.; Huang, T. Genetic diversity of arbuscular mycorrhizal fungi in karst microhabitats of Guizhou Province, China. Chin. J. Plant Ecol. 2012, 35, 1083–1090. (In Chinese) [Google Scholar]
- Tong, B.L.; Liu, J.M.; Chen, J.Z.; Wu, M.Y.; Guan, R.T. Correlation between fungal diversity in rhizosphere soil and medicinal active components in fruits of Cinnamomum migao. Mycosystema 2019, 38, 1058–1070. (In Chinese) [Google Scholar]
- Chen, J.; Huang, X.; Tong, B.; Wang, D.; Liu, J.; Liao, X.; Sun, Q. Effects of rhizosphere fungi on the chemical composition of fruits of the medicinal plant Cinnamomum migao endemic to southwestern China. BMC Microbiol. 2021, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Chen, J.; Guan, R.; Liu, J.; Sun, Q. Two arbuscular mycorrhizal fungi alleviates drought stress and improves plant growth in Cinnamomum migao seedlings. Mycobiology 2021, 49, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Sanam, T.; Triveni, S.; Nerella, S.G.; Ningoji, S.N.; Desai, S. Correlation and regression models of tomato yield in response to plant growth by different bacterial inoculants and inoculation methods. Agron. J. 2021, 1–9. [Google Scholar] [CrossRef]
- Morton, J.B.; Bentivenga, S.P.; Wheeler, W.W. Germ plasm in the international collection of Arbuscular Mycorrhizal Fungi (INVAM) and procedures for culture development, documentation and storage. Mycotaxon 1993, 48, 491–528. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- He, J.; Dong, T.; Wu, H.; Zou, Y.; Wu, Q.; Kuca, K. Mycorrhizas induce diverse responses of root TIP aquaporin gene expression to drought stress in trifoliate orange. Sci. Hortic. 2019, 243, 64–69. [Google Scholar] [CrossRef]
- Xu, F.; Song, S.; Ma, C.; Zhang, W.; Dai, C. Endophytic fungus improves peanut drought resistance by reassembling the root-dwelling community of arbuscular mycorrhizal fungi. Fungal Ecol. 2020, 48, 100993. [Google Scholar] [CrossRef]
- Ferguson, J.J.; Menge, J.A. The influence of light intensity and artificially extended photoperiod upon infection and sporulation of Glomus fasciculatus on Sudan grass and on root exudation by Sudan grass. New Phytol 1982, 92, 183–186. [Google Scholar] [CrossRef]
- Li, H. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Zhang, X.; Wang, Z.; Han, Q.; Wang, Z.; Nie, J. Effects of water stress on the root structure and physiological characteristics of early-stage maize. Acta Ecol. Sin. 2016, 36, 2969–2977. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Mishra, S. Influence of Arbuscular Mycorrhizal (AM) Fungi and Salinity on Seedling Growth, Solute Accumulation, and Mycorrhizal Dependency of Jatropha curcas L. J. Plant Growth Regul. 2010, 29, 297–306. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zegaoui, Z.; Planchais, S.; Cabassa, C.; Djebbar, R.; Belbachir, O.; Carol, P. Variation in relative water content, proline accumulation and stress gene expression in two cowpea land races under drought. J. Plant Physiol. 2017, 218, 26–34. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Homaee, M.; Bannayan, M. Quantitative trend, sensitivity and contribution analyses of reference evapotranspiration in somearid environments under climate change. Water Resour Manag. 2017, 31, 2207–2224. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef] [Green Version]
- Pedranzani, H.; Rodríguez-Rivera, M.; Gutiérrez, M.; Porcel, R.; Hause, B.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza 2016, 26, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Zou, Z.; He, C.; He, Z.; Zhang, Z.; Li, J. Physiological and photosynthetic responses of melon (Cucumis melo L.) seedlings to three Glomus species under water deficit. Plant Soil. 2011, 339, 391–399. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, Y.; Wu, Q. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, Y.; Wu, Q. Effects of Funneliformis mosseae on the expression of antioxidant enzyme genes in trifoliate orange exposed to drought stress. Mycosystema 2019, 38, 2043–2050. (In Chinese) [Google Scholar]
- He, F.; Sheng, M.; Tang, M. Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought stress. Front. Plant Sci. 2017, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Essahibi, A.; Benhiba, L.; Babram, M.A.; Ghoulam, C.; Qaddoury, A. Influence of arbuscular mycorrhizal fungi on the functional mechanisms associated with drought tolerance in carob (Ceratonia siliqua L.). Trees 2018, 32, 87–97. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Etemadi, N.; Sabzalian, M.R. Nutritional status, essential oil changes and water-use efficiency of rose geranium in response to arbuscular mycorrhizal fungi and water deficiency stress. Symbiosis 2017, 73, 15–25. [Google Scholar] [CrossRef]
- Wu, Q.; He, J.; Srivastava, A.K.; Zou, Y.; Kuca, K. Mycorrhiza enhances drought tolerance of citrus by altering root fatty acid compositions and their saturation levels. Tree Physiol. 2019, 39, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Huang, Y. Effects of arbuscular mycorrhizal fungi on the drought tolerance of Cyclobalanopsis glauca seedings under greenhouse conditions. New For. 2014, 45, 545–556. [Google Scholar] [CrossRef]
- Bainard, L.D.; Klironomos, J.N.; Gordon, A.M. Arbuscular mycorrhizal fungi in tree-based intercropping systems: A review of their abundance and diversity. Pedobiologia 2011, 54, 57–61. [Google Scholar] [CrossRef]
- Li, X.J. Microbiology; Higher Education Press: Beijing, China, 2013. (In Chinese) [Google Scholar]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2010, 154, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.T.; Zhu, Y.; Chen, Y.; Ren, H.; Li, J.; Kay, A. Xiong, Y. Arbuscular mycorrhizal fungus alters root-sourced signal (abscisic acid) for better drought acclimation in Zea mays L. seedings. Environ. Exp. Bot. 2019, 167, 103824. [Google Scholar] [CrossRef]
- Wu, Q.; Srivastava, A.K.; Zou, Y. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hortic. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.J.; Hao, Z.P.; Li, H.; Wang, Y.; Chen, B. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Ruth, B.; Khalvati, M.; Schmidhalter, U. Quantification of mycorrhizal water uptake via high-resolution on-line water content sensors. Plant Soil 2011, 342, 459–468. [Google Scholar] [CrossRef]
- Li, T.; Lin, G.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, B. Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza 2014, 24, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Tang, M.; Chen, H.; Zhang, Q.; Feng, X. Effects of two Glomus species on the growth and physiological performance of Sophora davidii seedlings under water stress. New For. 2013, 44, 399–408. [Google Scholar] [CrossRef]
- Patanita, M.; Campos, M.D.; Félix, M.d.R.; Carvalho, M.; Brito, I. Effect of Tillage System and Cover Crop on Maize Mycorrhization and Presence of Magnaporthiopsis maydis. Biology 2020, 9, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bargali, K.; Tewari, A. Growth and water relation parameters in drought stressed Coriaria nepalensis seedings. J. Arid Environ. 2004, 58, 505–512. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Ding, G.; Li, Z. Effect of water stress on physiology and photosynthesis properties of ectomycorrhizal Pinus massoniana seedings. J. Zhejiang For. Sci. Technol. 2019, 39, 1–8. (In Chinese) [Google Scholar]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, B.; Singh, G.; Farooq, M.; Abd-Allah, E. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Staddon, P.L.; Thompson, K.; Jakobsen, I.; Grime, J.P.; Fitter, A.H. Mycorrhizal fungal abundance is affected by long-term climatic manipulations in the field. Glob. Chang. Biol. 2003, 9, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Gholamhoseini, M.; Ghalavand, A.; Dolatabadian, A.; Jamshidi, E.; Khodaei-Joghan, A. Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric. Water Manag. 2013, 117, 106–114. [Google Scholar] [CrossRef]
- Bowles, T.M.; Jackson, L.E.; Cavagnaro, T.R. Mycorrhizal fungi enhance plant nutrient acquisition and modulate nitrogen loss with variable water regimes. Glob. Chang. Biol. 2018, 24, e171–e182. [Google Scholar] [CrossRef]
- Campos, C.; Nobre, T.; Goss, M.J.; Faria, J.; Barrulas, P.; Carvalho, M. Transcriptome Analysis of Wheat Roots Reveals a Differential Regulation of Stress Responses Related to Arbuscular Mycorrhizal Fungi and Soil Disturbance. Biology 2019, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hoffland, E.; Feng, G.; Kuyper, T.W. Phosphate uptake from phytate due to hyphae-mediated phytase activity by arbuscular mycorrhizal maize. Front. Plant Sci. 2017, 8, 684. [Google Scholar] [CrossRef] [Green Version]
- Ferrol, N.; Azcón-Aguilar, C.; Pérez-Tienda, J. Review: Arbuscular mycorrhizas as key players in sustainable plant phosphorus acquisition: An overview on the mechanisms involved. Plant Sci. 2019, 280, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Paymaneh, Z.; Sarcheshmehpour, M.; Petra, B.; Jansa, J. Could indigenous arbuscular mycorrhizal communities be used to improve tolerance of pistachio to salinity and/or drought? Symbiosis 2019, 79, 269–283. [Google Scholar] [CrossRef]
- Abbaspour, H.; Saeidi-Sar, S.; Afshari, H.; Abdel-Wahhab, M.A. Tolerance of mycorrhiza infected pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J. Plant Physiol. 2012, 169, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tang, M.; Sulpice, R.; Chen, H.; Tian, S.; Ban, Y. Arbuscular mycorrhizal fungi alter fractal dimension characteristics of Robinia pseudoacacia L. seedings through regulating plant growth, leaf water status, photosynthesis, and nutrient concentration under drought stress. J. Plant Growth Regul. 2014, 33, 612–625. [Google Scholar] [CrossRef]
- Xu, P.; Wang, F.; Qi, Y.; Zhang, F.; Yang, Q.; Xiao, B. Effect of arbuscular mycorrhiza fungi on drought resistance in tea plant (Camellia sinensis). Acta Agric. Boreali-Occident. Sin. 2017, 26, 1033–1040. (In Chinese) [Google Scholar] [CrossRef]
- Mathimaran, N.; Sharma, M.P.; Mohan, R.B.; Bagyaraj, D.J. Arbuscular mycorrhizal symbiosis and drought tolerance in crop plants. Mycosphere 2017, 8, 361–376. [Google Scholar] [CrossRef]
- Arsl, A.; Oao, B.; Er, C.; Rm, C. To what extent arbuscular mycorrhiza can protect chicory (Cichorium intybus L.) against drought stress. Sci. Hortic. 2020, 263, 109109. [Google Scholar] [CrossRef]
- Wu, H.; Zou, Y.; Rahman, M.; Ni, Q.; Wu, Q. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci. Rep. 2017, 7, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; He, X. Effect of AM fungal on the protective system in leaves of Artemisia ordosica under drought stress. Biotechnol Bull. 2007, 3, 129–133. [Google Scholar]
- Azmat, R.; Moin, S. The remediation of drought stress under VAM inoculation through proline chemical transformation action. J. Photochem. Photobiol. B Biol. 2019, 193, 155–161. [Google Scholar] [CrossRef]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 2018, 9, 2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahadur, A.; Batool, A.; Nasir, F. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Inoculation Method | AMF Status | MDA (nmol·g−1) | SOD (U·g−1) | POD (U·g−1) | CAT (U·g−1) |
|---|---|---|---|---|---|
| M-seedling | G. etunicatum | 35.88 ± 1.70 b | 287.55 ± 3.96 a | 411.52 ± 5.86 a | 234.32 ± 6.86 a |
| F. mosseae | 41.82 ± 2.42 b | 232.91 ± 12.84 c | 259.08 ± 12.26 b | 201.82 ± 5.56 b | |
| NM | 80.19 ± 3.14 a | 122.92 ± 3.83 e | 116.94 ± 4.81 d | 60.31 ± 5.68 c | |
| M-seed | G. etunicatum | 38.29 ± 2.35 b | 258.57 ± 4.46 b | 402.39 ± 3.48 a | 223.60 ± 8.36 a |
| F. mosseae | 42.32 ± 1.18 b | 203.19 ± 2.20 d | 206.25 ± 4.17 c | 192.97 ± 7.50 b | |
| NM | 76.15 ± 3.34 a | 125.96 ± 3.22 e | 118.17 ± 7.25 d | 64.80 ± 6.00 c | |
| Two-way ANOVA (significance level) | |||||
| AMF | ** | ** | ** | ** | |
| Method | ns | ** | ** | ns | |
| AMF × Method | ns | * | ** | ns | |
| Inoculation Method | AMF Status | Pro (U·g−1) | SP (mg·g−1) | SS (mg·g−1) |
|---|---|---|---|---|
| M-seedling | G. etunicatum | 0.75 ± 0.07 b | 10.73 ± 0.31 a | 3.42 ± 0.20 a |
| F. mosseae | 0.61 ± 0.03 b | 10.31 ± 0.31 a | 3.11 ± 0.34 ab | |
| NM | 1.11 ± 0.08 a | 10.17 ± 0.67 a | 2.49 ± 0.23 bc | |
| M-seed | G. etunicatum | 0.70 ± 0.08 b | 11.77 ± 0.92 a | 3.00 ± 0.21 ab |
| F. mosseae | 0.65 ± 0.08 b | 10.23 ± 0.36 a | 3.13 ± 0.05 ab | |
| NM | 1.16 ± 0.06 a | 10.37 ± 0.34 a | 2.28 ± 0.36 c | |
| Two-way ANOVA (significance level) | ||||
| AMF | ** | ns | * | |
| Method | ns | ns | ns | |
| AMF × Method | ns | ns | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Chen, J.; Liao, X.; Yan, Q.; Liang, G.; Liu, J.; Wang, D.; Guan, R. Different Arbuscular Mycorrhizal Fungi Established by Two Inoculation Methods Improve Growth and Drought Resistance of Cinnamomum Migao Seedlings Differently. Biology 2022, 11, 220. https://doi.org/10.3390/biology11020220
Xiao X, Chen J, Liao X, Yan Q, Liang G, Liu J, Wang D, Guan R. Different Arbuscular Mycorrhizal Fungi Established by Two Inoculation Methods Improve Growth and Drought Resistance of Cinnamomum Migao Seedlings Differently. Biology. 2022; 11(2):220. https://doi.org/10.3390/biology11020220
Chicago/Turabian StyleXiao, Xuefeng, Jingzhong Chen, Xiaofeng Liao, Qiuxiao Yan, Gelin Liang, Jiming Liu, Deng Wang, and Ruiting Guan. 2022. "Different Arbuscular Mycorrhizal Fungi Established by Two Inoculation Methods Improve Growth and Drought Resistance of Cinnamomum Migao Seedlings Differently" Biology 11, no. 2: 220. https://doi.org/10.3390/biology11020220





