UV Protection in the Cornea: Failure and Rescue
Abstract
:Simple Summary
Abstract
1. Introduction
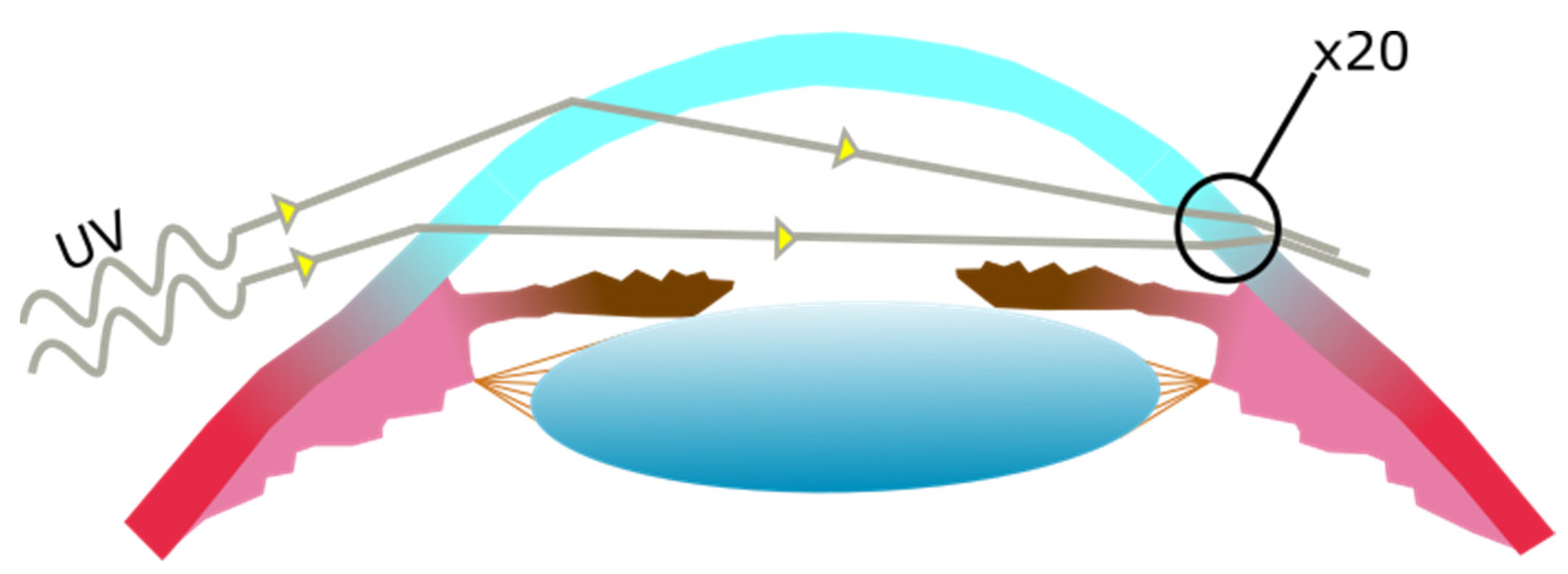
The Cornea
2. UV Effects on the Cornea
2.1. UV Damage and Repair Mechanisms
2.2. Pterygium Aetiology and Pathogenesis
2.3. UV-Induced DNA Lesion Formation
2.4. The Role of Genotoxic Stress
2.5. Reactive Oxygen Species
2.6. Disruption of Autophagy Mechanisms
2.7. Apoptosis
3. UV Pathogenesis and Rescue
4. Perspective: Therapeutic Opportunities for UV-Induced Conditions including Pterygium
4.1. T4 Endonuclease V and Photolyases as Photolesion-Repairing Treatment Strategies
4.2. Autophagy-Induction as a Treatment Strategy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.M. The structure and transparency of the cornea. J. Physiol. 1957, 136, 263–286. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, W.; Keyserlingk, D.G. On the fine structure of the human cornea with special reference to the problem of transparency. Z. Zellforsch Mikrosk. Anat. 1966, 73, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Osawa, T.; Tohyama, K. Comparative observations on corneas, with special reference to Bowman’s layer and Descemet’s membrane in mammals and amphibians. J. Morphol. 2002, 254, 247–258. [Google Scholar] [CrossRef]
- Dubbelman, M.; Sicam, V.A.; Van der Heijde, G.L. The shape of the anterior and posterior surface of the aging human cornea. Vision Res. 2006, 46, 993–1001. [Google Scholar] [CrossRef] [Green Version]
- Tong, L.; Corrales, R.M.; Chen, Z.; Villarreal, A.L.; De Paiva, C.S.; Beuerman, R.; Li, D.Q.; Pflugfelder, S.C. Expression and regulation of cornified envelope proteins in human corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1938–1946. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.E.; Mohan, R.R.; Mohan, R.R.; Ambrosio, R., Jr.; Hong, J.; Lee, J. The corneal wound healing response: Cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin. Eye Res. 2001, 20, 625–637. [Google Scholar] [CrossRef]
- Huang, M.; Wang, B.; Wan, P.; Liang, X.; Wang, X.; Liu, Y.; Zhou, Q.; Wang, Z. Roles of limbal microvascular net and limbal stroma in regulating maintenance of limbal epithelial stem cells. Cell Tissue Res. 2015, 359, 547–563. [Google Scholar] [CrossRef]
- Papas, E.B. The limbal vasculature. Cont. Lens. Anterior Eye 2003, 26, 71–76. [Google Scholar] [CrossRef]
- Kruse, F.E. Stem cells and corneal epithelial regeneration. Eye 1994, 8, 170–183. [Google Scholar] [CrossRef]
- Zieske, J.D. Perpetuation of stem cells in the eye. Eye 1994, 8, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.J.; Ismail, S.; Sherwin, T. Limbal stem cells: Central concepts of corneal epithelial homeostasis. World J. Stem Cells 2014, 6, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Notara, M.; Behoudifard, S.; S. Kluth, M.A.; Masslo, C.; Ganss, C.; Frank, M.H.; Cursiefen, C. UV light-blocking contact lenses prevent UVB-induced DNA and oxidative damage of the limbal stem cell niche, protect against inflammation and maintain putative stem cell phenotype. Investig. Ophthalmol. Vis. Sci. 2019, 60, 920. [Google Scholar]
- Notara, M.; Refaian, N.; Brown, G.; Steven, P.; Bock, F.; Cursiefen, C. Effects of UVB irradiation on limbal stem cell niche and its role in cornea lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5622. [Google Scholar]
- Notara, M.; Refaian, N.; Braun, G.; Steven, P.; Bock, F.; Cursiefen, C. Short-Term Ultraviolet A Irradiation Leads to Dysfunction of the Limbal Niche Cells and an Antilymphangiogenic and Anti-inflammatory Micromilieu. Investig. Ophthalmol. Vis. Sci. 2016, 57, 928–939. [Google Scholar] [CrossRef] [Green Version]
- Notara, M.; Braun, G.; Dreisow, M.L.; Bock, F.; Cursiefen, C. Avastin effects on human limbal epithelial cell function and phenotype in vitro. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4343. [Google Scholar]
- Gao, X.; Guo, K.; Santosa, S.M.; Montana, M.; Yamakawa, M.; Hallak, J.A.; Han, K.Y.; Doh, S.J.; Rosenblatt, M.I.; Chang, J.H.; et al. Application of corneal injury models in dual fluorescent reporter transgenic mice to understand the roles of the cornea and limbus in angiogenic and lymphangiogenic privilege. Sci. Rep. 2019, 9, 12331. [Google Scholar] [CrossRef] [Green Version]
- Azar, D.T. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 264–302. [Google Scholar]
- Klyce, S.D. 12 Endothelial pump and barrier function. Exp. Eye Res. 2020, 198, 108068. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [Green Version]
- Delic, N.C.; Lyons, J.G.; Di Girolamo, N.; Halliday, G.M. Damaging Effects of Ultraviolet Radiation on the Cornea. Photochem. Photobiol. 2017, 93, 920–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, A.R.; Claveau, J.; Rossi, A.B. Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J. Am. Acad. Dermatol. 2017, 76, S100–S109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siiskonen, H.; Smorodchenko, A.; Krause, K.; Maurer, M. Ultraviolet radiation and skin mast cells: Effects, mechanisms and relevance for skin diseases. Exp. Dermatol. 2018, 27, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. Adv. Exp. Med. Biol. 2017, 996, 71–87. [Google Scholar] [CrossRef]
- Christensen, L.; Suggs, A.; Baron, E. Ultraviolet Photobiology in Dermatology. Adv. Exp. Med. Biol. 2017, 996, 89–104. [Google Scholar] [CrossRef]
- Kronschlager, M.; Talebizadeh, N.; Yu, Z.; Meyer, L.M.; Lofgren, S. Apoptosis in Rat Cornea After In Vivo Exposure to Ultraviolet Radiation at 300 nm. Cornea 2015, 34, 945–949. [Google Scholar] [CrossRef]
- Miller, S. A focus on ultraviolet keratitis. Nursing 2009, 4, 12–16. [Google Scholar] [CrossRef]
- Najjar, D.M.; Awwad, S.T.; Zein, W.M.; Haddad, W.F. Assessment of the corneal endothelium in acute ultraviolet keratitis. Med. Sci. Monit. 2006, 12, MT23–MT25. [Google Scholar]
- Willmann, G. Ultraviolet Keratitis: From the Pathophysiological Basis to Prevention and Clinical Management. High Alt. Med. Biol. 2015, 16, 277–282. [Google Scholar] [CrossRef]
- Coroi, M.C.; Rosca, E.; Mutiu, G.; Coroi, T. Squamous carcinoma of the conjunctiva. Rom. J. Morphol. Embryol. 2011, 52 (Suppl. 1), 513–515. [Google Scholar]
- Olah, Z. Malignant tumor of the cornea and exroderma pigmentosum. Ceskoslovenska Oftalmol. 1968, 24, 119–122. [Google Scholar]
- Toshida, H.; Nakayasu, K.; Okisaka, S.; Kanai, A. Incidence of tumors and tumor-like lesions in the conjunctiva and the cornea. Nippon Ganka Gakkai Zasshi 1995, 99, 186–189. [Google Scholar] [PubMed]
- Newton, R.; Ferlay, J.; Reeves, G.; Beral, V.; Parkin, D.M. Effect of ambient solar ultraviolet radiation on incidence of squamous-cell carcinoma of the eye. Lancet 1996, 347, 1450–1451. [Google Scholar] [CrossRef]
- Hatsusaka, N.; Yamamoto, N.; Miyashita, H.; Shibuya, E.; Mita, N.; Yamazaki, M.; Shibata, T.; Ishida, H.; Ukai, Y.; Kubo, E.; et al. Association among pterygium, cataracts, and cumulative ocular ultraviolet exposure: A cross-sectional study in Han people in China and Taiwan. PLoS ONE 2021, 16, e0253093. [Google Scholar] [CrossRef] [PubMed]
- Sekelj, S.; Dekaris, I.; Kondza-Krstonijevic, E.; Gabric, N.; Predovic, J.; Mitrovic, S. Ultraviolet light and pterygium. Coll. Antropol. 2007, 31 (Suppl. 1), 45–47. [Google Scholar] [PubMed]
- Coroneo, M.T.; Muller-Stolzenburg, N.W.; Ho, A. Peripheral light focusing by the anterior eye and the ophthalmohelioses. Ophthalmic. Surg. 1991, 22, 705–711. [Google Scholar] [CrossRef]
- Dushku, N.; Reid, T.W. Immunohistochemical evidence that human pterygia originate from an invasion of vimentin-expressing altered limbal epithelial basal cells. Curr. Eye Res. 1994, 13, 473–481. [Google Scholar] [CrossRef]
- Willis, I.; Cylus, L. UVA erythema in skin: Is it a sunburn? J. Investig. Dermatol. 1977, 68, 128–129. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Gaddameedhi, S. UV-B-Induced Erythema in Human Skin: The Circadian Clock Is Ticking. J. Investig. Dermatol. 2018, 138, 248–251. [Google Scholar] [CrossRef] [Green Version]
- Sample, A.; He, Y.Y. Autophagy in UV Damage Response. Photochem. Photobiol. 2017, 93, 943–955. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem. Photobiol. Sci. 2018, 17, 1816–1841. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P. Mechanisms of UV-induced mutations and skin cancer. Genome Instab. Dis. 2020, 1, 99–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishigori, C.; Hattori, Y.; Toyokuni, S. Role of reactive oxygen species in skin carcinogenesis. Antioxid. Redox Signal. 2004, 6, 561–570. [Google Scholar] [CrossRef]
- Kao, A.A.; Galor, A.; Karp, C.L.; Abdelaziz, A.; Feuer, W.J.; Dubovy, S.R. Clinicopathologic correlation of ocular surface squamous neoplasms at Bascom Palmer Eye Institute: 2001 to 2010. Ophthalmology 2012, 119, 1773–1776. [Google Scholar] [CrossRef]
- Whittaker, K.W.; Trivedi, D.; Bridger, J.; Sandramouli, S. Ocular surface squamous neoplasia: Report of an unusual case and review of the literature. Orbit 2002, 21, 209–215. [Google Scholar] [CrossRef]
- Basti, S.; Macsai, M.S. Ocular surface squamous neoplasia: A review. Cornea 2003, 22, 687–704. [Google Scholar] [CrossRef]
- Godic, A.; Poljsak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell Longev. 2014, 2014, 860479, Erratum in Oxid. Med. Cell Longev. 2020, 2020, 1969760. [Google Scholar] [CrossRef]
- Rivas, J.M.; Ullrich, S.E. The role of IL-4, IL-10, and TNF-alpha in the immune suppression induced by ultraviolet radiation. J. Leukoc. Biol. 1994, 56, 769–775. [Google Scholar] [CrossRef]
- Hart, P.H.; Norval, M. Ultraviolet radiation-induced immunosuppression and its relevance for skin carcinogenesis. Photochem. Photobiol. Sci. 2018, 17, 1872–1884. [Google Scholar] [CrossRef]
- Yagura, T.; Makita, K.; Yamamoto, H.; Menck, C.F.; Schuch, A.P. Biological sensors for solar ultraviolet radiation. Sensors 2011, 11, 4277–4294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Ayala, M.N.; Michael, R.; Soderberg, P.G. Influence of exposure time for UV radiation-induced cataract. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3539–3543. [Google Scholar]
- Roberts, J.E. Ultraviolet radiation as a risk factor for cataract and macular degeneration. Eye Contact Lens. 2011, 37, 246–249. [Google Scholar] [CrossRef]
- Bashir, H.; Seykora, J.T.; Lee, V. Invisible shield: Review of the corneal epithelium as a barrier to UV radiation, pathogens, and other environmental stimuli. J. Ophthalmic Vis. Res. 2017, 12, 305. [Google Scholar]
- Morishita, H.; Mizushima, N. Autophagy in the lens. Exp. Eye Res. 2016, 144, 22–28. [Google Scholar] [CrossRef]
- Li, J.; Ye, W.; Xu, W.; Chang, T.; Zhang, L.; Ma, J.; Pei, R.; He, M.; Zhou, J. Activation of autophagy inhibits epithelial to mesenchymal transition process of human lens epithelial cells induced by high glucose conditions. Cell Signal. 2020, 75, 109768. [Google Scholar] [CrossRef]
- Costello, M.J.; Brennan, L.A.; Basu, S.; Chauss, D.; Mohamed, A.; Gilliland, K.O.; Johnsen, S.; Menko, S.; Kantorow, M. Autophagy and mitophagy participate in ocular lens organelle degradation. Exp. Eye Res. 2013, 116, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Z.K.; Lim, L.L.; Rogers, S.L.; Hall, A.J. Patterns of Vitamin D Levels and Exposures in Active and Inactive Noninfectious Uveitis Patients. Ophthalmology 2020, 127, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.C.; Liou, J.C.; Kuo, C.Y.; Tsai, Y.S.; Lin, E.C.; Hsieh, C.J.; Lin, S.P.; Chen, B.Y. UVB promotes the initiation of uveitic inflammatory injury in vivo and is attenuated by UV-blocking protection. Mol. Vis. 2017, 23, 219–227. [Google Scholar] [PubMed]
- Santeford, A.; Wiley, L.A.; Park, S.; Bamba, S.; Nakamura, R.; Gdoura, A.; Ferguson, T.A.; Rao, P.K.; Guan, J.L.; Saitoh, T.; et al. Impaired autophagy in macrophages promotes inflammatory eye disease. Autophagy 2016, 12, 1876–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.H.; Moon, J.D.; Park, W.J.; Kang, W.Y.; Kim, S.H.; Lim, H.M.; Ahn, J.S.; Chae, H.J. Case series of keratitis in poultry abattoir workers induced by exposure to the ultraviolet disinfection lamp. Ann. Occup. Environ. Med. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Xue, Y.; Wang, Y.; Dong, F.; Shen, M.; Zong, R.; Liu, Z.; Li, C. The role of autophagy in the pathogenesis of exposure keratitis. J. Cell Mol. Med. 2019, 23, 4217–4228. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.C.; Maske, R. Pathogenesis of pterygium. Eye 1989, 3, 218–226. [Google Scholar] [CrossRef]
- Fonseca, E.C.; Rocha, E.M.; Arruda, G.V. Comparison among adjuvant treatments for primary pterygium: A network meta-analysis. Br. J. Ophthalmol. 2018, 102, 748–756. [Google Scholar] [CrossRef]
- Lu, P.; Chen, X.; Kang, Y.; Ke, L.; Wei, X.; Zhang, W. Pterygium in Tibetans: A population-based study in China. Clin. Exp. Ophthalmol. 2007, 35, 828–833. [Google Scholar] [CrossRef]
- Stevenson, L.J.; Mackey, D.A.; Lingham, G.; Burton, A.; Brown, H.; Huynh, E.; Tan, I.J.; Franchina, M.; Sanfilippo, P.G.; Yazar, S. Has the Sun Protection Campaign in Australia Reduced the Need for Pterygium Surgery Nationally? Ophthalmic. Epidemiol. 2021, 28, 105–113. [Google Scholar] [CrossRef]
- Hirst, L.W.; Smith, J. Accuracy of diagnosis of pterygium by optometrists and general practitioners in Australia. Clin. Exp. Optom. 2020, 103, 197–200. [Google Scholar] [CrossRef]
- Khanna, R.C.; Marmamula, S.; Cicinelli, M.V.; Mettla, A.L.; Giridhar, P.; Banerjee, S.; Shekhar, K.; Chakrabarti, S.; Murthy, G.V.S.; Gilbert, C.E.; et al. Fifteen-year incidence rate and risk factors of pterygium in the Southern Indian state of Andhra Pradesh. Br. J. Ophthalmol. 2021, 105, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.L.; Chong, C.C.Y.; Thakur, S.; Da Soh, Z.; Teo, Z.L.; Majithia, S.; Lim, Z.W.; Rim, T.H.; Sabanayagam, C.; Wong, T.Y.; et al. Ethnic differences in the incidence of pterygium in a multi-ethnic Asian population: The Singapore Epidemiology of Eye Diseases Study. Sci. Rep. 2021, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Rim, T.H.; Kang, M.J.; Choi, M.; Seo, K.Y.; Kim, S.S. The incidence and prevalence of pterygium in South Korea: A 10-year population-based Korean cohort study. PLoS ONE 2017, 12, e0171954. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, J.Y.; Geng, J.; Yuan, Z.; Huang, D.S. Geographical prevalence and risk factors for pterygium: A systematic review and meta-analysis. BMJ Open 2013, 3, e003787. [Google Scholar] [CrossRef] [PubMed]
- Song, P.G.; Chang, X.L.; Wang, M.L.; An, L. Variations of pterygium prevalence by age, gender and geographic characteristics in China: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0174587. [Google Scholar] [CrossRef] [Green Version]
- Kwok, L.S.; Kuznetsov, V.A.; Ho, A.; Coroneo, M.T. Prevention of the adverse photic effects of peripheral light-focusing using UV-blocking contact lenses. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1501–1507. [Google Scholar] [CrossRef]
- Sage, E. Distribution and repair of photolesions in DNA: Genetic consequences and the role of sequence context. Photochem. Photobiol. 1993, 57, 163–174. [Google Scholar] [CrossRef]
- Rochette, P.J.; Bastien, N.; Todo, T.; Drouin, R. Pyrimidine (6-4) pyrimidone photoproduct mapping after sublethal UVC doses: Nucleotide resolution using terminal transferase-dependent PCR. Photochem. Photobiol. 2006, 82, 1370–1376. [Google Scholar] [CrossRef]
- Liu, Z.; Tan, C.; Guo, X.; Kao, Y.T.; Li, J.; Wang, L.; Sancar, A.; Zhong, D. Dynamics and mechanism of cyclobutane pyrimidine dimer repair by DNA photolyase. Proc. Natl. Acad. Sci. USA 2011, 108, 14831–14836. [Google Scholar] [CrossRef] [Green Version]
- Torizawa, T.; Ueda, T.; Kuramitsu, S.; Hitomi, K.; Todo, T.; Iwai, S.; Morikawa, K.; Shimada, I. Investigation of the cyclobutane pyrimidine dimer (CPD) photolyase DNA recognition mechanism by NMR analyses. J. Biol. Chem. 2004, 279, 32950–32956. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, F. Induction of tandem-base change mutations. Mutat. Res. 1994, 309, 11–15. [Google Scholar] [CrossRef]
- Yamada, D.; Dokainish, H.M.; Iwata, T.; Yamamoto, J.; Ishikawa, T.; Todo, T.; Iwai, S.; Getzoff, E.D.; Kitao, A.; Kandori, H. Functional Conversion of CPD and (6-4) Photolyases by Mutation. Biochemistry 2016, 55, 4173–4183. [Google Scholar] [CrossRef]
- Mees, A.; Klar, T.; Gnau, P.; Hennecke, U.; Eker, A.P.; Carell, T.; Essen, L.O. Crystal structure of a photolyase bound to a CPD-like DNA lesion after in situ repair. Science 2004, 306, 1789–1793. [Google Scholar] [CrossRef]
- McGregor, W.G.; Chen, R.H.; Lukash, L.; Maher, V.M.; McCormick, J.J. Cell cycle-dependent strand bias for UV-induced mutations in the transcribed strand of excision repair-proficient human fibroblasts but not in repair-deficient cells. Mol. Cell Biol. 1991, 11, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Steurer, B.; Turkyilmaz, Y.; van Toorn, M.; van Leeuwen, W.; Escudero-Ferruz, P.; Marteijn, J.A. Fluorescently-labelled CPD and 6-4PP photolyases: New tools for live-cell DNA damage quantification and laser-assisted repair. Nucleic Acids Res. 2019, 47, 3536–3549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.L.; Nairn, R.S. The biology of the (6-4) photoproduct. Photochem. Photobiol. 1989, 49, 805–819. [Google Scholar] [CrossRef]
- Boros, G.; Miko, E.; Muramatsu, H.; Weissman, D.; Emri, E.; van der Horst, G.T.; Szegedi, A.; Horkay, I.; Emri, G.; Kariko, K.; et al. Identification of Cyclobutane Pyrimidine Dimer-Responsive Genes Using UVB-Irradiated Human Keratinocytes Transfected with In Vitro-Synthesized Photolyase mRNA. PLoS ONE 2015, 10, e0131141. [Google Scholar] [CrossRef] [Green Version]
- Boros, G.; Kariko, K.; Muramatsu, H.; Miko, E.; Emri, E.; Hegedus, C.; Emri, G.; Remenyik, E. Transfection of Human Keratinocytes with Nucleoside-Modified mRNA Encoding CPD-Photolyase to Repair DNA Damage. Methods Mol. Biol. 2016, 1428, 219–228. [Google Scholar] [CrossRef]
- Todo, T.; Tsuji, H.; Otoshi, E.; Hitomi, K.; Kim, S.T.; Ikenaga, M. Characterization of a human homolog of (6-4) photolyase. Mutat. Res. 1997, 384, 195–204. [Google Scholar] [CrossRef]
- Marizcurrena, J.J.; Lamparter, T.; Castro-Sowinski, S. A (6-4)-photolyase from the Antarctic bacterium Sphingomonas sp. UV9: Recombinant production and in silico features. Extremophiles 2020, 24, 887–896. [Google Scholar] [CrossRef]
- Marizcurrena, J.J.; Acosta, S.; Canclini, L.; Hernandez, P.; Valles, D.; Lamparter, T.; Castro-Sowinski, S. A natural occurring bifunctional CPD/(6-4)-photolyase from the Antarctic bacterium Sphingomonas sp. UV9. Appl. Microbiol. Biotechnol. 2020, 104, 7037–7050. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Kim, S.T.; Sancar, A. Evidence for lack of DNA photoreactivating enzyme in humans. Proc. Natl. Acad. Sci. USA 1993, 90, 4389–4393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Spek, P.J.; Kobayashi, K.; Bootsma, D.; Takao, M.; Eker, A.P.; Yasui, A. Cloning, tissue expression, and mapping of a human photolyase homolog with similarity to plant blue-light receptors. Genomics 1996, 37, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz, M.J.; Nieto Moreno, N.; Giono, L.E.; Cambindo Botto, A.E.; Dujardin, G.; Bastianello, G.; Lavore, S.; Torres-Mendez, A.; Menck, C.F.M.; Blencowe, B.J.; et al. Major Roles for Pyrimidine Dimers, Nucleotide Excision Repair, and ATR in the Alternative Splicing Response to UV Irradiation. Cell Rep. 2017, 18, 2868–2879. [Google Scholar] [CrossRef] [Green Version]
- Hsu, P.H.; Hanawalt, P.C.; Nouspikel, T. Nucleotide excision repair phenotype of human acute myeloid leukemia cell lines at various stages of differentiation. Mutat. Res.-Fund. Mol. Mech. Mutagenesis 2007, 614, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Marteijn, J.A.; Lans, H.; Vermeulen, W.; Hoeijmakers, J.H. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014, 15, 465–481. [Google Scholar] [CrossRef]
- Langie, S.A.; Wilms, L.C.; Hamalainen, S.; Kleinjans, J.C.; Godschalk, R.W.; van Schooten, F.J. Modulation of nucleotide excision repair in human lymphocytes by genetic and dietary factors. Br. J. Nutr. 2010, 103, 490–501. [Google Scholar] [CrossRef]
- de Boer, J.; Hoeijmakers, J.H. Nucleotide excision repair and human syndromes. Carcinogenesis 2000, 21, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, W.; de Boer, J.; Citterio, E.; van Gool, A.J.; van der Horst, G.T.; Jaspers, N.G.; de Laat, W.L.; Sijbers, A.M.; van der Spek, P.J.; Sugasawa, K.; et al. Mammalian nucleotide excision repair and syndromes. Biochem. Soc. Trans. 1997, 25, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Rapin, I. Disorders of nucleotide excision repair. Handb. Clin. Neurol. 2013, 113, 1637–1650. [Google Scholar] [CrossRef]
- Cohen, V.M.L.; O’Day, R.F. Management Issues in Conjunctival Tumours: Ocular Surface Squamous Neoplasia. Ophthalmol. Ther. 2020, 9, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwich, R.; Ghazawi, F.M.; Le, M.; Rahme, E.; Alghazawi, N.; Zubarev, A.; Moreau, L.; Sasseville, D.; Burnier, M.N., Jr.; Litvinov, I.V. Epidemiology of invasive ocular surface squamous neoplasia in Canada during 1992–2010. Br. J. Ophthalmol. 2020, 104, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Gupta, N.; Singh, R.; Patil, M.; Meel, R.; Vanathi, M.; Kashyap, S.; Tandon, R. Role of Conjunctival Ultraviolet Autofluorescence in Ocular Surface Squamous Neoplasia. Ocul. Oncol. Pathol. 2020, 6, 422–429. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, J.M.; Mayr, A.J.; Martin, W.J. DNA of human papillomavirus type 16 in dysplastic and malignant lesions of the conjunctiva and cornea. N. Engl. J. Med. 1989, 320, 1442–1446. [Google Scholar] [CrossRef]
- Vempuluru, V.S.; Pattnaik, M.; Ghose, N.; Kaliki, S. Bilateral ocular surface squamous neoplasia: A study of 25 patients and review of literature. Eur. J. Ophthalmol. 2021, 11206721211007109. [Google Scholar] [CrossRef]
- Walsh, J.E.; Bergmanson, J.P.; Wallace, D.; Saldana, G.; Dempsey, H.; McEvoy, H.; Collum, L.M. Quantification of the ultraviolet radiation (UVR) field in the human eye in vivo using novel instrumentation and the potential benefits of UVR blocking hydrogel contact lens. Br. J. Ophthalmol. 2001, 85, 1080–1085. [Google Scholar] [CrossRef] [Green Version]
- Cardenas-Cantu, E.; Zavala, J.; Valenzuela, J.; Valdez-Garcia, J.E. Molecular Basis of Pterygium Development. Semin. Ophthalmol. 2016, 31, 567–583. [Google Scholar] [CrossRef]
- Mallet, J.D.; Dorr, M.M.; Drigeard Desgarnier, M.C.; Bastien, N.; Gendron, S.P.; Rochette, P.J. Faster DNA Repair of Ultraviolet-Induced Cyclobutane Pyrimidine Dimers and Lower Sensitivity to Apoptosis in Human Corneal Epithelial Cells than in Epidermal Keratinocytes. PLoS ONE 2016, 11, e0162212. [Google Scholar] [CrossRef]
- Mann, A.; Tighe, B. Contact lens interactions with the tear film. Exp. Eye Res. 2013, 117, 88–98. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, B.; Gu, Y. Changes of tear film function after pterygium operation. Ophthalmic. Res. 2011, 45, 210–215. [Google Scholar] [CrossRef]
- Bergstresser, P.R.; Pariser, R.J.; Taylor, J.R. Counting and sizing of epidermal cells in normal human skin. J. Investig. Dermatol. 1978, 70, 280–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimmura, S.; Kawakita, T. Accessory cell populations in the cornea. Ocul. Surf. 2006, 4, 74–80. [Google Scholar] [CrossRef]
- Hadley, M.E.; Quevedo, W.C., Jr. Vertebrate epidermal melanin unit. Nature 1966, 209, 1334–1335. [Google Scholar] [CrossRef] [PubMed]
- Higa, K.; Shimmura, S.; Miyashita, H.; Shimazaki, J.; Tsubota, K. Melanocytes in the corneal limbus interact with K19-positive basal epithelial cells. Exp. Eye Res. 2005, 81, 218–223. [Google Scholar] [CrossRef]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.; Halaban, R.; Douki, T.; Brash, D.E. Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science 2015, 347, 842–847. [Google Scholar] [CrossRef]
- Cai, C.X.; Birk, D.E.; Linsenmayer, T.F. Nuclear ferritin protects DNA from UV damage in corneal epithelial cells. Mol. Biol. Cell 1998, 9, 1037–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thilly, W.G. Analysis of chemically induced mutation in single cell populations. Basic Life Sci. 1983, 23, 337–378. [Google Scholar] [CrossRef]
- Poljsak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [Green Version]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxidants—Quo vadis? Trends Pharmacol. Sci. 2011, 32, 125–130. [Google Scholar] [CrossRef]
- Cheeseman, K.H.; Slater, T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Suput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.L.; Izumi, T.; Mitra, S. Oxidized base damage and single-strand break repair in mammalian genomes: Role of disordered regions and posttranslational modifications in early enzymes. Prog. Mol. Biol. Transl. Sci. 2012, 110, 123–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakabeppu, Y. Cellular levels of 8-oxoguanine in either DNA or the nucleotide pool play pivotal roles in carcinogenesis and survival of cancer cells. Int. J. Mol. Sci. 2014, 15, 12543–12557. [Google Scholar] [CrossRef] [Green Version]
- Popov, A.V.; Endutkin, A.V.; Yatsenko, D.D.; Yudkina, A.V.; Barmatov, A.E.; Makasheva, K.A.; Raspopova, D.Y.; Diatlova, E.A.; Zharkov, D.O. Molecular dynamics approach to identification of new OGG1 cancer-associated somatic variants with impaired activity. J. Biol. Chem. 2021, 296, 100229. [Google Scholar] [CrossRef]
- Hegde, M.L.; Hegde, P.M.; Holthauzen, L.M.; Hazra, T.K.; Rao, K.S.; Mitra, S. Specific Inhibition of NEIL-initiated repair of oxidized base damage in human genome by copper and iron: Potential etiological linkage to neurodegenerative diseases. J. Biol. Chem. 2010, 285, 28812–28825. [Google Scholar] [CrossRef] [Green Version]
- Scott, T.L.; Rangaswamy, S.; Wicker, C.A.; Izumi, T. Repair of oxidative DNA damage and cancer: Recent progress in DNA base excision repair. Antioxid. Redox Signal. 2014, 20, 708–726. [Google Scholar] [CrossRef] [Green Version]
- Terman, A. Garbage catastrophe theory of aging: Imperfect removal of oxidative damage? Redox Rep. 2001, 6, 15–26. [Google Scholar] [CrossRef]
- Notara, M.; Lentzsch, A.; Coroneo, M.; Cursiefen, C. The Role of Limbal Epithelial Stem Cells in Regulating Corneal (Lymph)angiogenic Privilege and the Micromilieu of the Limbal Niche following UV Exposure. Stem Cells Int. 2018, 2018, 8620172. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Whiteman, M.W.; Lian, H.; Wang, G.; Singh, A.; Huang, D.; Denmark, T. A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating Beclin 1. J. Biol. Chem. 2009, 284, 21412–21424. [Google Scholar] [CrossRef] [Green Version]
- Alexander, A.; Cai, S.L.; Kim, J.; Nanez, A.; Sahin, M.; MacLean, K.H.; Inoki, K.; Guan, K.L.; Shen, J.; Person, M.D.; et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc. Natl. Acad. Sci. USA 2010, 107, 4153–4158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Z.; Zhang, H.; Levine, A.J.; Jin, S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc. Natl. Acad. Sci. USA 2005, 102, 8204–8209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimprich, K.A.; Cortez, D. ATR: An essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 2008, 9, 616–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.; Tho, L.M.; Xu, N.; Gillespie, D.A. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv. Cancer Res. 2010, 108, 73–112. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, T.; Zhang, X.; Liu, C.; Wu, Z.; Yao, L.; Xie, C.; Xia, H.; Lin, Q.; Xie, L.; et al. ATR/Chk1 signaling induces autophagy through sumoylated RhoB-mediated lysosomal translocation of TSC2 after DNA damage. Nat. Commun. 2018, 9, 4139. [Google Scholar] [CrossRef] [Green Version]
- Maltzman, W.; Czyzyk, L. UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol. Cell Biol. 1984, 4, 1689–1694. [Google Scholar] [CrossRef]
- Tasdemir, E.; Chiara Maiuri, M.; Morselli, E.; Criollo, A.; D’Amelio, M.; Djavaheri-Mergny, M.; Cecconi, F.; Tavernarakis, N.; Kroemer, G. A dual role of p53 in the control of autophagy. Autophagy 2008, 4, 810–814. [Google Scholar] [CrossRef] [Green Version]
- Maclean, K.H.; Dorsey, F.C.; Cleveland, J.L.; Kastan, M.B. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. J. Clin. Investig. 2008, 118, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Yan, T.; Schupp, J.E.; Seo, Y.; Kinsella, T.J. DNA mismatch repair initiates 6-thioguanine--induced autophagy through p53 activation in human tumor cells. Clin. Cancer Res. 2007, 13, 1315–1321. [Google Scholar] [CrossRef] [Green Version]
- Amaravadi, R.K.; Yu, D.; Lum, J.J.; Bui, T.; Christophorou, M.A.; Evan, G.I.; Thomas-Tikhonenko, A.; Thompson, C.B. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Investig. 2007, 117, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Tasdemir, E.; Maiuri, M.C.; Galluzzi, L.; Vitale, I.; Djavaheri-Mergny, M.; D’Amelio, M.; Criollo, A.; Morselli, E.; Zhu, C.; Harper, F.; et al. Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 2008, 10, 676–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tendler, Y.; Pokroy, R.; Panshin, A.; Weisinger, G. p53 protein subcellular localization and apoptosis in rodent corneal epithelium cell culture following ultraviolet irradiation. Int. J. Mol. Med. 2013, 31, 540–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, B.; Abrams, J. p53: The Janus of autophagy? Nat. Cell Biol. 2008, 10, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Rosenbluth, J.M.; Pietenpol, J.A. mTOR regulates autophagy-associated genes downstream of p73. Autophagy 2009, 5, 114–116. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Feng, P.; Ku, B.; Dotan, I.; Canaani, D.; Oh, B.H.; Jung, J.U. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat. Cell Biol. 2006, 8, 688–699. [Google Scholar] [CrossRef]
- Fujita, N.; Itoh, T.; Omori, H.; Fukuda, M.; Noda, T.; Yoshimori, T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell 2008, 19, 2092–2100. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.; Chen, Y.; Zhao, S.; Cui, G.H. Autophagy counteracts apoptosis in human multiple myeloma cells exposed to oridonin in vitro via regulating intracellular ROS and SIRT1. Acta Pharmacol. Sin. 2012, 33, 91–100. [Google Scholar] [CrossRef]
- Garva, R.; Thepmalee, C.; Yasamut, U.; Sudsaward, S.; Guazzelli, A.; Rajendran, R.; Tongmuang, N.; Khunchai, S.; Meysami, P.; Limjindaporn, T.; et al. Sirtuin Family Members Selectively Regulate Autophagy in Osteosarcoma and Mesothelioma Cells in Response to Cellular Stress. Front. Oncol. 2019, 9, 949. [Google Scholar] [CrossRef]
- Timmers, S.; Auwerx, J.; Schrauwen, P. The journey of resveratrol from yeast to human. Aging 2012, 4, 146–158. [Google Scholar] [CrossRef]
- Mohar, D.S.; Malik, S. The Sirtuin System: The Holy Grail of Resveratrol? J. Clin. Exp. Cardiolog. 2012, 3, 216. [Google Scholar] [CrossRef] [Green Version]
- Morselli, E.; Marino, G.; Bennetzen, M.V.; Eisenberg, T.; Megalou, E.; Schroeder, S.; Cabrera, S.; Benit, P.; Rustin, P.; Criollo, A.; et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J. Cell Biol. 2011, 192, 615–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, W.; Hudson, L.G.; Sun, X.; Feng, C.; Liu, K.J. As(III) inhibits ultraviolet radiation-induced cyclobutane pyrimidine dimer repair via generation of nitric oxide in human keratinocytes. Free Radic. Biol. Med. 2008, 45, 1065–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Yin, N.; Xuan, L.L.; Yao, C.S.; Meng, A.M.; Hou, Q. Vam3, a derivative of resveratrol, attenuates cigarette smoke-induced autophagy. Acta Pharmacol. Sin. 2012, 33, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Arakawa, S.; Fujitani, K.; Yamaguchi, H.; Mizuta, T.; Kanaseki, T.; Komatsu, M.; Otsu, K.; Tsujimoto, Y.; Shimizu, S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 2009, 461, 654–658, Erratum in Nature 2016, 533, 130. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Lu, S.; Kivlin, R.; Wallin, B.; Card, E.; Bagdasarian, A.; Tamakloe, T.; Wang, W.J.; Song, X.; Chu, W.M.; et al. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J. Cell Mol. Med. 2009, 13, 3632–3643. [Google Scholar] [CrossRef] [Green Version]
- Ming, M.; Soltani, K.; Shea, C.R.; Li, X.; He, Y.Y. Dual role of SIRT1 in UVB-induced skin tumorigenesis. Oncogene 2015, 34, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Shamsher, E.; Guo, L.; Davis, B.M.; Luong, V.; Ravindran, N.; Somavarapu, S.; Cordeiro, M.F. Resveratrol nanoparticles are neuroprotective in a rat model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2021, 62, 2423. [Google Scholar]
- Abu-Amero, K.K.; Kondkar, A.A.; Chalam, K.V. Resveratrol and Ophthalmic Diseases. Nutrients 2016, 8, 200. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.Y.; Chen, T.C.; Wang, I.J.; Yeh, C.Y.; Su, M.J.; Chen, R.H.; Tsai, T.H.; Hu, F.R. The Effect of Resveratrol on Protecting Corneal Epithelial Cells from Cytotoxicity Caused by Moxifloxacin and Benzalkonium Chloride. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.S.; Yu, Z.; Feng, S.F.; Chen, H.J.; Chen, H.Y.; Lu, X.H. Corneal autophagy and ocular surface inflammation: A new perspective in dry eye. Exp. Eye Res. 2019, 184, 126–134. [Google Scholar] [CrossRef]
- Van Acker, S.I.; van den Bogerd, B.; Haagdorens, M.; Siozopoulou, V.; Dhubhghaill, S.N.; Pintelon, I.; Koppen, C. Pterygium-The Good, the Bad, and the Ugly. Cells 2021, 10, 1567. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.M.; Jeyabalan, N.; Tripathi, R.; Panigrahi, T.; Johnson, P.J.; Ghosh, A.; Mohan, R.R. Autophagy in corneal health and disease: A concise review. Ocul. Surf. 2019, 17, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Danno, K.; Horio, T. Sunburn cell: Factors involved in its formation. Photochem. Photobiol. 1987, 45, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Kulms, D.; Schwarz, T. Molecular mechanisms of UV-induced apoptosis. Photodermatol. Photoimmunol. Photomed. 2000, 16, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Teraki, Y.; Shiohara, T. Apoptosis and the skin. Eur. J. Dermatol. 1999, 9, 413–425, quiz 426. [Google Scholar] [PubMed]
- Cao, C.; Healey, S.; Amaral, A.; Lee-Couture, A.; Wan, S.; Kouttab, N.; Chu, W.; Wan, Y. ATP-sensitive potassium channel: A novel target for protection against UV-induced human skin cell damage. J. Cell Physiol. 2007, 212, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Papucci, L.; Schiavone, N.; Witort, E.; Donnini, M.; Lapucci, A.; Tempestini, A.; Formigli, L.; Zecchi-Orlandini, S.; Orlandini, G.; Carella, G.; et al. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J. Biol. Chem. 2003, 278, 28220–28228. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.Y.; Hwang, B.J.; Ford, J.M.; Hanawalt, P.C.; Chu, G. Xeroderma pigmentosum p48 gene enhances global genomic repair and suppresses UV-induced mutagenesis. Mol. Cell 2000, 5, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Hughes, F.M., Jr.; Bortner, C.D.; Purdy, G.D.; Cidlowski, J.A. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J. Biol. Chem. 1997, 272, 30567–30576. [Google Scholar] [CrossRef] [Green Version]
- Singleton, K.R.; Will, D.S.; Schotanus, M.P.; Haarsma, L.D.; Koetje, L.R.; Bardolph, S.L.; Ubels, J.L. Elevated extracellular K+ inhibits apoptosis of corneal epithelial cells exposed to UV-B radiation. Exp. Eye Res. 2009, 89, 140–151. [Google Scholar] [CrossRef]
- Leerar, J.R.; Glupker, C.D.; Schotanus, M.P.; Ubels, J.L. The effect of K(+) on caspase activity of corneal epithelial cells exposed to UVB. Exp. Eye Res. 2016, 151, 23–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleaver, J.E.; Lam, E.T.; Revet, I. Disorders of nucleotide excision repair: The genetic and molecular basis of heterogeneity. Nat. Rev. Genet. 2009, 10, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Masutani, C.; Kusumoto, R.; Yamada, A.; Dohmae, N.; Yokoi, M.; Yuasa, M.; Araki, M.; Iwai, S.; Takio, K.; Hanaoka, F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature 1999, 399, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Masutani, C.; Araki, M.; Yamada, A.; Kusumoto, R.; Nogimori, T.; Maekawa, T.; Iwai, S.; Hanaoka, F. Xeroderma pigmentosum variant (XP-V) correcting protein from HeLa cells has a thymine dimer bypass DNA polymerase activity. EMBO J. 1999, 18, 3491–3501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleaver, J.E.; Revet, I. Clinical implications of the basic defects in Cockayne syndrome and xeroderma pigmentosum and the DNA lesions responsible for cancer, neurodegeneration and aging. Mech. Ageing Dev. 2008, 129, 492–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zheng, Y.; Yan, X.; Huang, Y.; Jiang, Y.; Li, H. Ocular findings in a patient with Cockayne syndrome with two mutations in the ERCC6 gene. Ophthalmic. Genet. 2017, 38, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Traboulsi, E.I.; De Becker, I.; Maumenee, I.H. Ocular findings in Cockayne syndrome. Am. J. Ophthalmol. 1992, 114, 579–583. [Google Scholar] [CrossRef]
- McElvanney, A.M.; Wooldridge, W.J.; Khan, A.A.; Ansons, A.M. Ophthalmic management of Cockayne’s syndrome. Eye 1996, 10, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Nance, M.A.; Berry, S.A. Cockayne syndrome: Review of 140 cases. Am. J. Med. Genet. 1992, 42, 68–84. [Google Scholar] [CrossRef]
- Gorgels, T.G.; van der Pluijm, I.; Brandt, R.M.; Garinis, G.A.; van Steeg, H.; van den Aardweg, G.; Jansen, G.H.; Ruijter, J.M.; Bergen, A.A.; van Norren, D.; et al. Retinal degeneration and ionizing radiation hypersensitivity in a mouse model for Cockayne syndrome. Mol. Cell Biol. 2007, 27, 1433–1441. [Google Scholar] [CrossRef] [Green Version]
- Brooks, B.P.; Thompson, A.H.; Bishop, R.J.; Clayton, J.A.; Chan, C.C.; Tsilou, E.T.; Zein, W.M.; Tamura, D.; Khan, S.G.; Ueda, T.; et al. Ocular manifestations of xeroderma pigmentosum: Long-term follow-up highlights the role of DNA repair in protection from sun damage. Ophthalmology 2013, 120, 1324–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, B.J.; Ford, J.M.; Hanawalt, P.C.; Chu, G. Expression of the p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair. Proc. Natl. Acad. Sci. USA 1999, 96, 424–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, B.J.; Toering, S.; Francke, U.; Chu, G. p48 Activates a UV-damaged-DNA binding factor and is defective in xeroderma pigmentosum group E cells that lack binding activity. Mol. Cell Biol. 1998, 18, 4391–4399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paridaens, A.D.; McCartney, A.C.; Hungerford, J.L. Premalignant melanosis of the conjunctiva and the cornea in xeroderma pigmentosum. Br. J. Ophthalmol. 1992, 76, 120–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landry, L.G.; Stapleton, A.E.; Lim, J.; Hoffman, P.; Hays, J.B.; Walbot, V.; Last, R.L. An Arabidopsis photolyase mutant is hypersensitive to ultraviolet-B radiation. Proc. Natl. Acad. Sci. USA 1997, 94, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Vechtomova, Y.L.; Telegina, T.A.; Kritsky, M.S. Evolution of Proteins of the DNA Photolyase/Cryptochrome Family. Biochemistry 2020, 85, S131–S153. [Google Scholar] [CrossRef]
- Roh, D.S.; Du, Y.; Gabriele, M.L.; Robinson, A.R.; Niedernhofer, L.J.; Funderburgh, J.L. Age-related dystrophic changes in corneal endothelium from DNA repair-deficient mice. Aging Cell 2013, 12, 1122–1131. [Google Scholar] [CrossRef] [Green Version]
- Storchova, Z.; Pellman, D. From polyploidy to aneuploidy, genome instability and cancer. Nat. Rev. Mol. Cell Biol. 2004, 5, 45–54. [Google Scholar] [CrossRef]
- Roh, D.; Du, Y.; Robinson, A.; Gabriele, M.; Niedernhofer, L.; Funderburgh, J. Corneal Endothelial Changes in DNA Repair-Deficient Mice. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4290. [Google Scholar]
- Deshpande, N.; Melangath, G.; Vasanth, S.; Price, M.; Price, F.; Jurkunas, U.V. Defective DNA Repair in Fuchs endothelial corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2021, 62, 838. [Google Scholar]
- Deshpande, P.; Notara, M.; Bullett, N.; Daniels, J.T.; Haddow, D.B.; MacNeil, S. Development of a Surface-Modified Contact Lens for the Transfer of Cultured Limbal Epithelial Cells to the Cornea for Ocular Surface Diseases. Tissue Eng. Part A 2009, 15, 2889–2902. [Google Scholar] [CrossRef] [PubMed]
- Asahina, H.; Han, Z.; Kawanishi, M.; Kato, T., Jr.; Ayaki, H.; Todo, T.; Yagi, T.; Takebe, H.; Ikenaga, M.; Kimura, S.H. Expression of a mammalian DNA photolyase confers light-dependent repair activity and reduces mutations of UV-irradiated shuttle vectors in xeroderma pigmentosum cells. Mutat. Res. 1999, 435, 255–262. [Google Scholar] [CrossRef]
- Zwetsloot, J.C.; Vermeulen, W.; Hoeijmakers, J.H.; Yasui, A.; Eker, A.P.; Bootsma, D. Microinjected photoreactivating enzymes from Anacystis and Saccharomyces monomerize dimers in chromatin of human cells. Mutat. Res. 1985, 146, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Jans, J.; Schul, W.; Sert, Y.G.; Rijksen, Y.; Rebel, H.; Eker, A.P.; Nakajima, S.; van Steeg, H.; de Gruijl, F.R.; Yasui, A.; et al. Powerful skin cancer protection by a CPD-photolyase transgene. Curr. Biol. 2005, 15, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Schul, W.; Jans, J.; Rijksen, Y.M.; Klemann, K.H.; Eker, A.P.; de Wit, J.; Nikaido, O.; Nakajima, S.; Yasui, A.; Hoeijmakers, J.H.; et al. Enhanced repair of cyclobutane pyrimidine dimers and improved UV resistance in photolyase transgenic mice. EMBO J. 2002, 21, 4719–4729. [Google Scholar] [CrossRef] [Green Version]
- Zelle, B.; Reynolds, R.J.; Kottenhagen, M.J.; Schuite, A.; Lohman, P.H. The influence of the wavelength of ultraviolet radiation on survival, mutation induction and DNA repair in irradiated Chinese hamster cells. Mutat. Res. 1980, 72, 491–509. [Google Scholar] [CrossRef]
- van Zeeland, A.A.; Smith, C.A.; Hanawalt, P.C. Sensitive determination of pyrimidine dimers in DNA of UV-irradiated mammalian cells. Introduction of T4 endonuclease V into frozen and thawed cells. Mutat. Res. 1981, 82, 173–189. [Google Scholar] [CrossRef]
- Bohr, V.A.; Smith, C.A.; Okumoto, D.S.; Hanawalt, P.C. DNA repair in an active gene: Removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell 1985, 40, 359–369. [Google Scholar] [CrossRef]
- Collins, A.R.; Mitchell, D.L.; Zunino, A.; de Wit, J.; Busch, D. UV-sensitive rodent mutant cell lines of complementation groups 6 and 8 differ phenotypically from their human counterparts. Environ. Mol. Mutagen. 1997, 29, 152–160. [Google Scholar] [CrossRef]
- Boros, G.; Miko, E.; Muramatsu, H.; Weissman, D.; Emri, E.; Rozsa, D.; Nagy, G.; Juhasz, A.; Juhasz, I.; van der Horst, G.; et al. Transfection of pseudouridine-modified mRNA encoding CPD-photolyase leads to repair of DNA damage in human keratinocytes: A new approach with future therapeutic potential. J. Photochem. Photobiol. B 2013, 129, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Camarillo, C.; Ocampo, E.A.; Casamichana, M.L.; Perez-Plasencia, C.; Alvarez-Sanchez, E.; Marchat, L.A. Protein Kinases and Transcription Factors Activation in Response to UV-Radiation of Skin: Implications for Carcinogenesis. Int. J. Mol. Sci. 2012, 13, 142–172. [Google Scholar] [CrossRef] [PubMed]
- Marizcurrena, J.J.; Martinez-Lopez, W.; Ma, H.; Lamparter, T.; Castro-Sowinski, S. A highly efficient and cost-effective recombinant production of a bacterial photolyase from the Antarctic isolate Hymenobacter sp. UV11. Extremophiles 2019, 23, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Sekiguchi, M. T4 endonuclease involved in repair of DNA. Proc. Natl. Acad. Sci. USA 1970, 67, 1839–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarosh, D.B. Enhanced DNA repair of cyclobutane pyrimidine dimers changes the biological response to UV-B radiation. Mutat. Res. 2002, 509, 221–226. [Google Scholar] [CrossRef]
- Yarosh, D.; Bucana, C.; Cox, P.; Alas, L.; Kibitel, J.; Kripke, M. Localization of liposomes containing a DNA repair enzyme in murine skin. J. Investig. Dermatol. 1994, 103, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Yarosh, D.; Klein, J.; O’Connor, A.; Hawk, J.; Rafal, E.; Wolf, P. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: A randomised study. Xeroderma Pigmentosum Study Group. Lancet 2001, 357, 926–929. [Google Scholar] [CrossRef]
- Cafardi, J.A.; Elmets, C.A. T4 endonuclease V: Review and application to dermatology. Expert. Opin. Biol. Ther. 2008, 8, 829–838. [Google Scholar] [CrossRef]
- Zahid, S.; Brownell, I. Repairing DNA damage in xeroderma pigmentosum: T4N5 lotion and gene therapy. J. Drugs Dermatol. 2008, 7, 405–408. [Google Scholar]
- Piquero-Casals, J.; Morgado-Carrasco, D.; Gilaberte, Y.; Del Rio, R.; Macaya-Pascual, A.; Granger, C.; Lopez-Estebaranz, J.L. Management Pearls on the Treatment of Actinic Keratoses and Field Cancerization. Dermatol. Ther. 2020, 10, 903–915. [Google Scholar] [CrossRef]
- Gomes, C.; Silva, A.C.; Marques, A.C.; Lobo, J.S.; Amaral, M.H. Biotechnology Applied to Cosmetics and Aesthetic Medicines. Cosmetics 2020, 7, 33. [Google Scholar] [CrossRef]
- Moscarella, E.; Argenziano, G.; Longo, C.; Aladren, S. Management of cancerization field with a medical device containing photolyase: A randomized, double-blind, parallel-group pilot study. J. Eur. Acad. Dermatol. 2017, 31, E410–E413. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Granger, C.; Garre, A.; Trullas, C.; Sanmartin, O.; Argenziano, G. Review of Clinical Evidence over 10 Years on Prevention and Treatment of a Film-Forming Medical Device Containing Photolyase in the Management of Field Cancerization in Actinic Keratosis. Dermatol. Ther. 2019, 9, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eibenschutz, L.; Silipo, V.; De Simone, P.; Buccini, P.L.; Ferrari, A.; Carbone, A.; Catricala, C. A 9-month, randomized, assessor-blinded, parallel-group study to evaluate clinical effects of film-forming medical devices containing photolyase and sun filters in the treatment of field cancerization compared with sunscreen in patients after successful photodynamic therapy for actinic keratosis. Br. J. Dermatol. 2016, 175, 1391–1393. [Google Scholar] [CrossRef] [PubMed]
- Stege, H. Effect of xenogenic repair enzymes on photoimmunology and photocarcinogenesis. J. Photochem. Photobiol. B 2001, 65, 105–108. [Google Scholar] [CrossRef]
- Stege, H.; Roza, L.; Vink, A.A.; Grewe, M.; Ruzicka, T.; Grether-Beck, S.; Krutmann, J. Enzyme plus light therapy to repair DNA damage in ultraviolet-B-irradiated human skin. Proc. Natl. Acad. Sci. USA 2000, 97, 1790–1795. [Google Scholar] [CrossRef] [Green Version]
- Yarosh, D.; Alas, L.G.; Yee, V.; Oberyszyn, A.; Kibitel, J.T.; Mitchell, D.; Rosenstein, R.; Spinowitz, A.; Citron, M. Pyrimidine dimer removal enhanced by DNA repair liposomes reduces the incidence of UV skin cancer in mice. Cancer Res. 1992, 52, 4227–4231. [Google Scholar]
- Yarosh, D.; Klein, J.; Kibitel, J.; Alas, L.; O’Connor, A.; Cummings, B.; Grob, D.; Gerstein, D.; Gilchrest, B.A.; Ichihashi, M.; et al. Enzyme therapy of xeroderma pigmentosum: Safety and efficacy testing of T4N5 liposome lotion containing a prokaryotic DNA repair enzyme. Photodermatol. Photoimmunol. Photomed. 1996, 12, 122–130. [Google Scholar] [CrossRef]
- Tanaka, K.; Hayakawa, H.; Sekiguchi, M.; Okada, Y. Specific action of T4 endonuclease V on damaged DNA in xeroderma pigmentosum cells in vivo. Proc. Natl. Acad. Sci. USA 1977, 74, 2958–2962. [Google Scholar] [CrossRef] [Green Version]
- Matthaei, M.; Hu, J.F.; Meng, H.; Lackner, E.M.; Eberhart, C.G.; Qian, J.; Hao, H.P.; Jun, A.S. Endothelial Cell Whole Genome Expression Analysis in a Mouse Model of Early-Onset Fuchs’ Endothelial Corneal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1931–1940. [Google Scholar] [CrossRef] [Green Version]
- Shetty, R.; Sharma, A.; Pahuja, N.; Chevour, P.; Padmajan, N.; Dhamodaran, K.; Jayadev, C.; Nuijts, R.M.M.A.; Ghosh, A.; Nallathambi, J. Oxidative stress induces dysregulated autophagy in corneal epithelium of keratoconus patients. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One Drug, Many Effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gidfar, S.; Milani, F.Y.; Milani, B.Y.; Shen, X.; Eslani, M.; Putra, I.; Huvard, M.J.; Sagha, H.; Djalilian, A.R. Rapamycin Prolongs the Survival of Corneal Epithelial Cells in Culture. Sci. Rep. 2017, 7, 40308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cejkova, J.; Cejka, C.; Ardan, T.; Sirc, J.; Michalek, J.; Luyckx, J. Reduced UVB-induced corneal damage caused by reactive oxygen and nitrogen species and decreased changes in corneal optics after trehalose treatment. Histol. Histopathol. 2010, 25, 1403–1416. [Google Scholar] [PubMed]
- Cejkova, J.; Cejka, C.; Luyckx, J. Trehalose treatment accelerates the healing of UVB-irradiated corneas. Comparative immunohistochemical studies on corneal cryostat sections and corneal impression cytology. Histol. Histopathol. 2012, 27, 1029–1040. [Google Scholar] [PubMed]
- Park, J.W.; Ko, J.H.; Kim, B.H.; Ryu, J.S.; Kim, H.J.; Kim, M.K.; Oh, J.Y. Inhibition of mTOR by Rapamycin Aggravates Corneal Epithelial Stem Cell Deficiency by Upregulating Inflammatory Response. Stem Cells 2019, 37, 1212–1222. [Google Scholar] [CrossRef] [PubMed]




| Damage | Skin | Cornea | Repair and Prevention |
|---|---|---|---|
| Erythema/Sunburn | ✓ [38,39] | Wound Healing, Autophagy [40,41] | |
| DNA lesion | ✓ [42,43,44] | ✓ [45,46,47] | NER, Apoptosis, Antioxidants [48,49] |
| Immunodeficiency | ✓ [49,50] | None | |
| Premature aging | ✓ [51,52] | NER, Apoptosis, Antioxidants [53,54] | |
| Cataracts | ✓ [55,56] | Wound Healing, Autophagy [57,58,59,60] | |
| Uveitis | ✓ [61,62] | Wound Healing, Autophagy [57,63] | |
| Keratitis | ✓ [64,65,66] | Wound Healing, Autophagy [57,67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volatier, T.; Schumacher, B.; Cursiefen, C.; Notara, M. UV Protection in the Cornea: Failure and Rescue. Biology 2022, 11, 278. https://doi.org/10.3390/biology11020278
Volatier T, Schumacher B, Cursiefen C, Notara M. UV Protection in the Cornea: Failure and Rescue. Biology. 2022; 11(2):278. https://doi.org/10.3390/biology11020278
Chicago/Turabian StyleVolatier, Thomas, Björn Schumacher, Claus Cursiefen, and Maria Notara. 2022. "UV Protection in the Cornea: Failure and Rescue" Biology 11, no. 2: 278. https://doi.org/10.3390/biology11020278
APA StyleVolatier, T., Schumacher, B., Cursiefen, C., & Notara, M. (2022). UV Protection in the Cornea: Failure and Rescue. Biology, 11(2), 278. https://doi.org/10.3390/biology11020278







