Response of an Indicator Species, Dryopteris crassirhizoma, to Temporal and Spatial Variations in Sika Deer Density
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
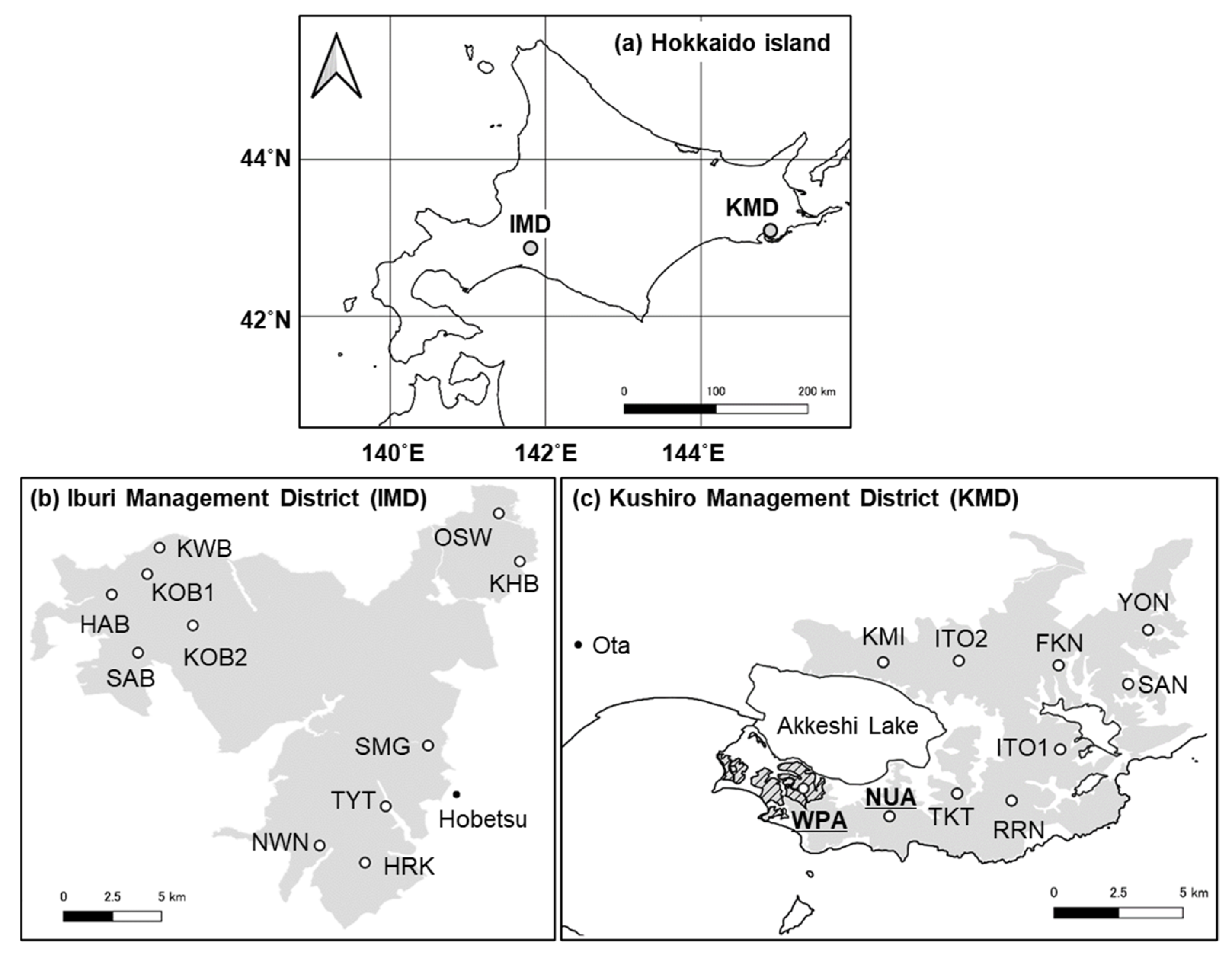
2.1. Study Areas
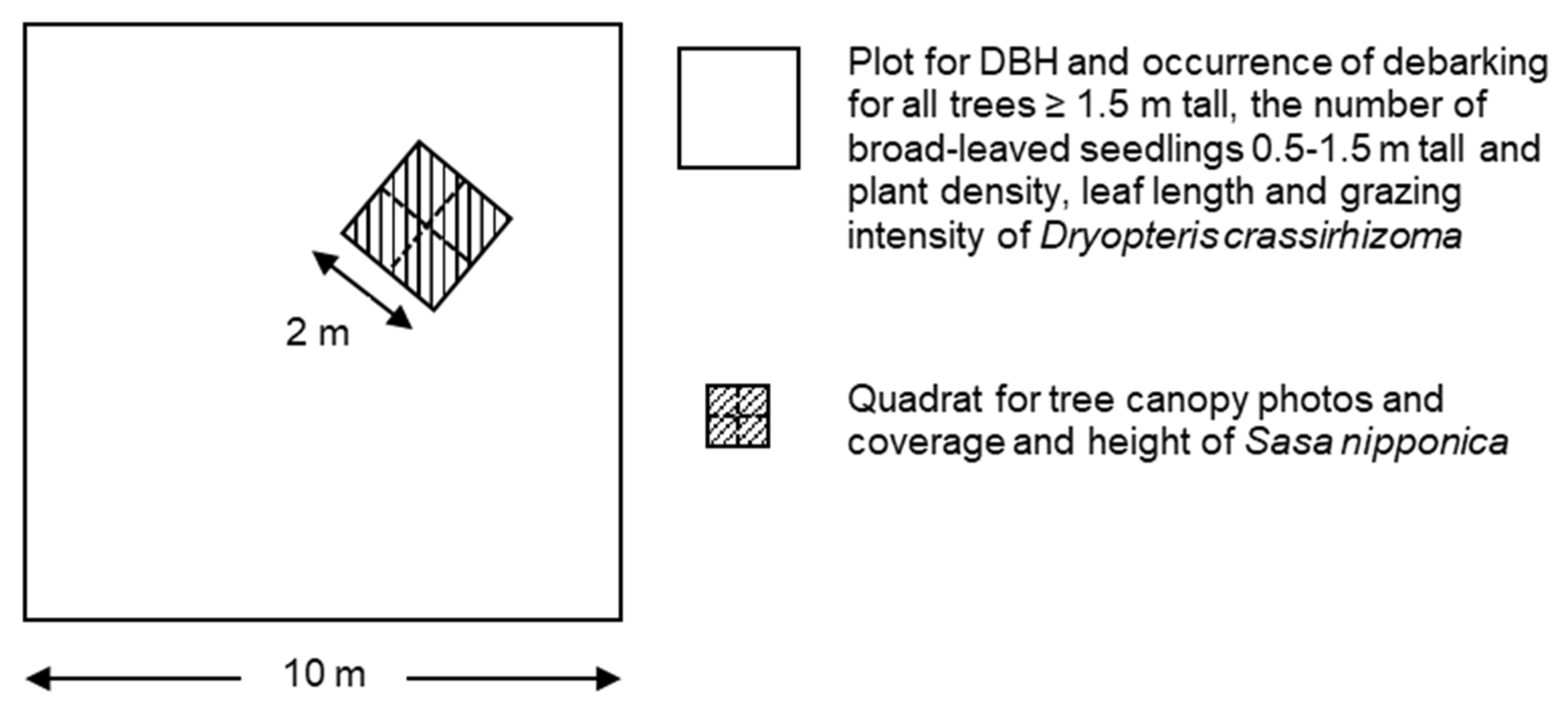
2.2. Temporal Survey of Indicator Species
2.3. Spatial Survey of Indicator Species
2.4. Line-Transect Survey
2.5. Data Analysis
3. Results
3.1. Temporal Survey of Indicator Species
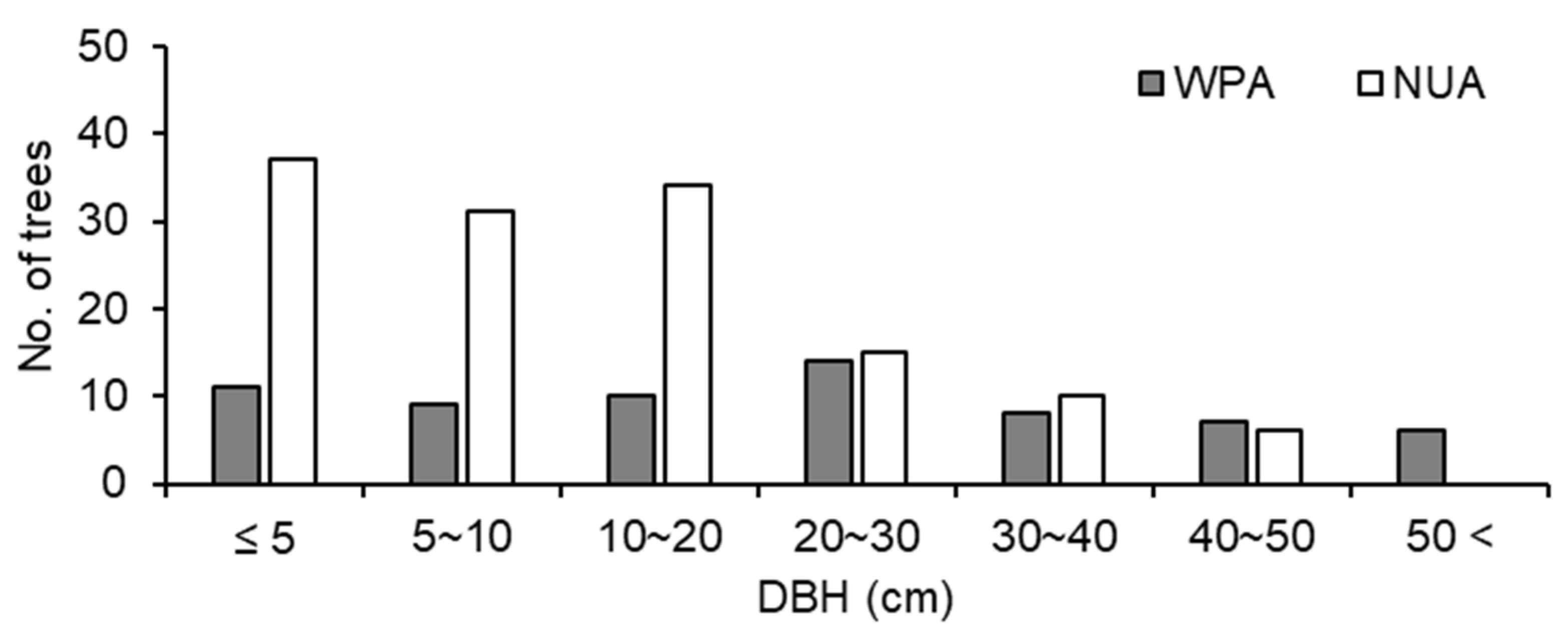
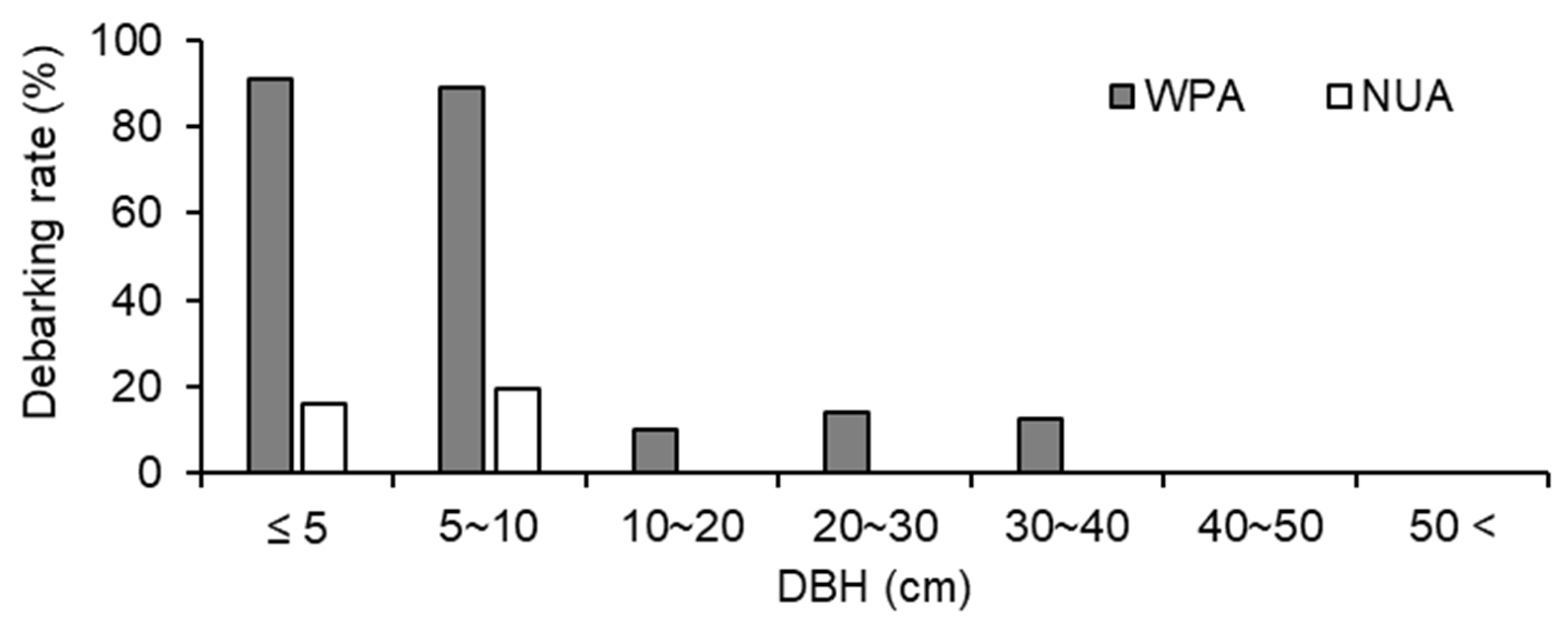
3.2. Spatial Survey of Indicator Species
3.3. Line Transect Survey
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Region | Site | Length (km) | Deer Density (Deer/km2) (95% Confidence Interval) | ||
|---|---|---|---|---|---|
| September 2012 | November 2013 | November 2014 | |||
| KMD | WPA | 4.0 | 82.1 (62.1–108.6) | 36.5 (31.3–42.5) | 22.4 (20.2–24.9) |
| NUA | 4.5 | 19.9 (13.3–29.7) | 26.2 (21.7–31.6) | 13.1 (11.8–14.6) | |
| TKT | 4.5 | 11.4 (8.5–15.4) | 57.0 (48.3–67.4) | 21.1 (19–23.5) | |
| RRN | 4.4 | 5.5 (4.0–7.6) | 35.2 (29.5–42.2) | 19.4 (17.4–21.5) | |
| ITO1 | 4.3 | 9.5 (6.5–14) | 43.1 (35.2–52.7) | 15.5 (14.0–17.3) | |
| FKN | 4.3 | 11.3 (8.1–15.7) | 53.4 (45.5–62.6) | 19.5 (17.5–21.7) | |
| ITO2 | 4.6 | 7.4 (1.2–44.7) | 24.4 (19.8–30.1) | 9.5 (8.5–10.6) | |
| KMI | 4.0 | 17.3 (12.8–23.4) | 32.3 (26.5–39.2) | 10.4 (9.4–11.6) | |
| SAN | 3.7 | 15.6 (11.6–20.9) | 15.5 (12.8–18.7) | 13.3 (12.0–14.8) | |
| YON | 5.4 | 14.1 (9.3–21.6) | 59.0 (51.4–67.8) | 8.3 (7.4–9.2) | |
| IMD | NWN | 3.2 | 42.7 (24.1–75.6) | 11.3 (8.6–14.8) | 8.5 (6.3–11.6) |
| HRK | 4.4 | 4.2 (3.6–5.0) | 6.1 (4.2–8.7) | 1.8 (1.5–2.0) | |
| TYT | 4.4 | 80.8 (47.0–139.0) | 16.8 (13.2–21.4) | 3.6 (2.1–6.0) | |
| SMG | 2.3 | 55.3 (38.7–79) | 15.3 (11.3–20.6) | 9.2 (6.1–13.9) | |
| KHB | 3.3 | 10.6 (6.1–18.6) | 5.4 (2.6–11.1) | 4.8 (3.3–7.1) | |
| OSW | 4.0 | 6.8 (2.9–16.1) | 3.2 (1.6–6.1) | 1.6 (1.2–2.1) | |
| KOB1 | 3.5 | 9.4 (6.3–14.2) | 3.7 (1.8–7.6) | 3.9 (1.1–14.1) | |
| KOB2 | 4.0 | 17.3 (11.8–25.3) | 16.2 (13.5–19.4) | 6.1 (3.1–11.7) | |
| HAB | 5.0 | 9.2 (5.9–14.3) | 4.9 (4.0–6.1) | 2.7 (2.3–3.2) | |
| SAB | 4.7 | 10.0 (6.4–15.7) | 1.5 (0.4–6.0) | 3.3 (1.9–5.7) | |
| KWB | 2.8 | - | 9.7 (6.4–14.7) | 6.9 (5.9–8.0) | |
References
- Rooney, T.P.; Waller, D.M. Direct and indirect effects of white-tailed deer in forest ecosystems. For. Ecol. Manage. 2003, 181, 165–176. [Google Scholar] [CrossRef]
- Yokoyama, S.; Shibata, E. The effects of sika-deer browsing on the biomass and morphology of a dwarf bamboo, Sasa nipponica, in Mt. Ohdaigahara, central Japan. For. Ecol. Manage. 1998, 103, 49–56. [Google Scholar] [CrossRef]
- Takatsuki, S. Effects of sika deer on vegetation in Japan: A review. Biol. Conserv. 2009, 142, 1922–1929. [Google Scholar] [CrossRef]
- Akashi, N.; Nakashizuka, T. Effects of bark-stripping by Sika deer (Cervus nippon) on population dynamics of a mixed forest in Japan. For. Ecol. Manage. 1999, 113, 75–82. [Google Scholar] [CrossRef]
- Iijima, H.; Nagaike, T. Appropriate vegetation indices for measuring the impacts of deer on forest ecosystems. Ecol. Indic. 2015, 48, 457–463. [Google Scholar] [CrossRef]
- Kumar, S.; Takeda, A.; Shibata, E. Effects of 13-year fencing on browsing by sika deer on seedlings on Mt. Ohdaigahara, central Japan. J. For. Res. 2006, 11, 337–342. [Google Scholar] [CrossRef]
- Akashi, N.; Unno, A.; Terazawa, K. Effects of deer abundance on broad-leaf tree seedling establishment in the understory of Abies sachalinensis plantations. J. For. Res. 2011, 16, 500–508. [Google Scholar] [CrossRef]
- Uno, H.; Inatomi, Y.; Ueno, M.; Iijima, H. Effects of sika deer (Cervus nippon) and dwarf bamboo (Sasa senanensis) on tree seedlings in a cool-temperate mixed forest on Hokkaido Island, Japan. Eur. J. For. Res. 2019, 138, 929–938. [Google Scholar] [CrossRef]
- Tamura, A. Potential of soil seed banks in the ecological restoration of overgrazed floor vegetation in a cool-temperate old-growth damp forest in eastern Japan. J. For. Res. 2016, 21, 43–56. [Google Scholar] [CrossRef]
- Beguin, J.; Pothier, D.; Côtá, S.D. Deer browsing and soil disturbance induce cascading effects on plant communities: A multilevel path analysis. Ecol. Appl. 2011, 21, 439–451. [Google Scholar] [CrossRef]
- Anderson, R.C. Height of white-flowered Trillium (Trillium grandiflorum) as an index of deer browsing intensity. Ecol. Appl. 1994, 4, 104–109. [Google Scholar] [CrossRef]
- Augustine, D.J.; Frelich, L.E. Effects of white-tailed deer on populations of an understory forb in fragmented deciduous forests. Conserv. Biol. 1998, 12, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Côté, S.D.; Rooney, T.P.; Tremblay, J.; Dussault, C.; Waller, D.M. Ecological Impacts of Deer Overabundance. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 113–147. [Google Scholar] [CrossRef] [Green Version]
- Koh, S.; Bazely, D.R.; Tanentzap, A.J.; Voigt, D.R.; Da Silva, E. Trillium grandiflorum height is an indicator of white-tailed deer density at local and regional scales. For. Ecol. Manage. 2010, 259, 1472–1479. [Google Scholar] [CrossRef]
- Rooney, T.P. Escaping herbivory: Refuge effects on the morphology and shoot demography of the clonal forest herb Maianthemum canadense. J. Torrey Bot. Soc. 1997, 124, 280–285. [Google Scholar] [CrossRef]
- Kirschbaum, C.D.; Anacker, B.L. The utility of Trillium and Maianthemum as phyto-indicators of deer impact in northwestern Pennsylvania, USA. For. Ecol. Manage. 2005, 217, 54–66. [Google Scholar] [CrossRef]
- Royo, A.A.; Stout, S.L.; DeCalesta, D.S.; Pierson, T.G. Restoring forest herb communities through landscape-level deer herd reductions: Is recovery limited by legacy effects? Biol. Conserv. 2010, 143, 2425–2434. [Google Scholar] [CrossRef]
- Augustine, D.J.; Jordan, P.A. Predictors of white-tailed deer grazing intensity in fragmented deciduous forests. J. Wildl. Manag. 1998, 62, 1076–1085. [Google Scholar] [CrossRef]
- Williams, C.E.; Mosbacher, E.V.; Moriarity, W.J. Use of turtlehead (Chelone glabra L.) and other herbaceous plants to assess intensity of white-tailed deer browsing on Allegheny Plateau riparian forests, USA. Biol. Conserv. 2000, 92, 207–215. [Google Scholar] [CrossRef]
- Augustine, D.J.; DeCalesta, D.S. Defining deer overabundance and threats to forest communities: From individual plants to landscape structure. Ecoscience 2003, 10, 472–486. [Google Scholar] [CrossRef]
- Inatomi, Y.; Uno, H.; Iijima, H. Effects of sika deer (Cervus nippon) and dwarf bamboo (Sasa senanensis) on trillium populations in Akan National Park, Eastern Hokkaido, Japan. Plant. Species Biol. 2017, 32, 423–431. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Fujiki, D. List of food plants and unpalatable plants of sika deer (Cervus nippon) in Japan. Hum. Nat. 2014, 25, 133–160, (In Japanese with English Abstract). [Google Scholar]
- Takahashi, H.; Kaji, K. Fallen leaves and unpalatable plants as alternative foods for sika deer under food limitation. Ecol. Res. 2001, 16, 257–262. [Google Scholar] [CrossRef]
- Miyaki, M.; Kaji, K. Summer forage biomass and the importance of litterfall for a high-density sika deer population. Ecol. Res. 2004, 19, 405–409. [Google Scholar] [CrossRef]
- Murata, G. Poaceae (Gramineae). In Wild Flowers of Japan Woody Plants; Satake, Y., Hara, H., Watari, S., Tominari, T., Eds.; Heibonsha: Tokyo, Japan, 1989; pp. 254–261. (In Japanese) [Google Scholar]
- Takatsuki, S. The importance of Sasa nipponica as a forage for sika deer (Cervus nippon) in Omote-Nikko. Jpn. J. Ecol. 1983, 33, 17–25. [Google Scholar]
- Yokoyama, M.; Kaji, K.; Suzuki, M. Food habits of sika deer and nutritional value of sika deer diets in eastern Hokkaido, Japan. Ecol. Res. 2000, 15, 345–355. [Google Scholar] [CrossRef]
- Iwatsuki, K. Dryopteridaceae. In Ferns and Fern Allies of Japan; Iwatsuki, K., Ed.; Heibonsha: Tokyo, Japan, 1992; pp. 161–206. (In Japanese) [Google Scholar]
- Hirabayashi, H. Dryopteris crassirhizoma Nakai. In Illustrations of Pteridophytes of Japan; Kurata, S., Nakaike, T., Eds.; University of Tokyo Press: Tokyo, Japan, 1985; Volume 4, pp. 344–361. (In Japanese) [Google Scholar]
- Sato, T.; Sakai, A. Phenological study of the leaf of pterophyte in Hokkaido. Jap. J. Ecol. 1980, 30, 369–375, (In Japanese with English Abstract). [Google Scholar]
- Tani, T.; Kudo, G. Storage ability of overwintering leaves and rhizomes in a semi-evergreen fern, Dryopteris crassirhizoma (Dryopteridaceae). Ecol. Res. 2003, 18, 15–24. [Google Scholar] [CrossRef]
- Kawanishi, M.; Kudo, M.; Higa, M.; Sakio, H. Changes in vegetation in Ooyamazawa riparian forest. In Long-Term Ecosystem Changes in Riparian Forests; Sakio, H., Ed.; Springer: Singapore, 2020; pp. 139–161. [Google Scholar]
- Japan Meteorological Agency. Available online: http://www.data.jma.go.jp/obd/stats/etrn/index.php (accessed on 9 December 2021).
- Uno, H.; Ueno, M.; Inatomi, Y.; Osa, Y.; Akashi, N.; Unno, A.; Minamino, K. Estimation of population density for sika deer (Cervus nippon) using distance sampling in the forested habitats of Hokkaido, Japan. Mammal Study 2017, 42, 57–64. [Google Scholar] [CrossRef]
- Akashi, N. Browsing damage by sika deer on trees in young plantations and its relation to relative deer density indices in Hokkaido, Japan. J. Jpn. For. Soc. 2009, 91, 178–183, (In Japanese with English Abstract). [Google Scholar] [CrossRef] [Green Version]
- Akashi, N.; Unno, A.; Terazawa, K. Significance of woody browse preferences in evaluating the impact of sika deer browsing on tree seedlings. J. For. Res. 2015, 20, 396–402. [Google Scholar] [CrossRef]
- Akashi, N.; Unno, A.; Uno, H. The protective effect of dwarf bamboo on broad-leaved seedlings against deer browsing. For. Ecol. Manage. 2021, 494, 119273. [Google Scholar] [CrossRef]
- R_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 4 November 2021).
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiberger, R.M.; Schuetzenmeister, A.; Scheibe, S. Package ‘multcomp’. Simultaneaous Inference in General Parametric Models. R Package Version 1.4-17. 2021. Available online: http://multcomp.R-forge.R-project.org (accessed on 4 November 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Thomas, L.; Buckland, S.T.; Rexstad, E.A.; Laake, J.L.; Strindberg, S.; Hedley, S.L.; Bishop, J.R.B.; Marques, T.A.; Burnham, K.P. Distance software: Design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 2010, 47, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L. Distance Sampling: Estimating Abundance of Biological Populations; Springer Science and Business Media: Berlin, Germany, 1993; p. 446. [Google Scholar]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In 2nd International Symposium on Information Theory; Petrov, B.N., Csaki, F., Eds.; Academiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Gill, R.M.A.; Thomas, M.L.; Stocker, D. The use of portable thermal imaging for estimating deer population density on forest habitats. J. Appl. Ecol. 1997, 34, 1273–1286. [Google Scholar] [CrossRef]
- Akashi, N.; Terazawa, K. Bark stripping damage to conifer plantations in relation to the abundance of sika deer in Hokkaido, Japan. For. Ecol. Manage. 2005, 208, 77–83. [Google Scholar] [CrossRef]
- Maesako, Y.; Takatsuki, S. Efficiency and limitation of deer preventive fence. In The Threat of Deer and Future of Forest—Efficiency and Limitation of Deer Preventive Fence; Maesako, Y., Takatsuki, S., Eds.; Bun-ichi Sogo Shuppan: Tokyo, Japan, 2015; pp. 221–233. (In Japanese) [Google Scholar]

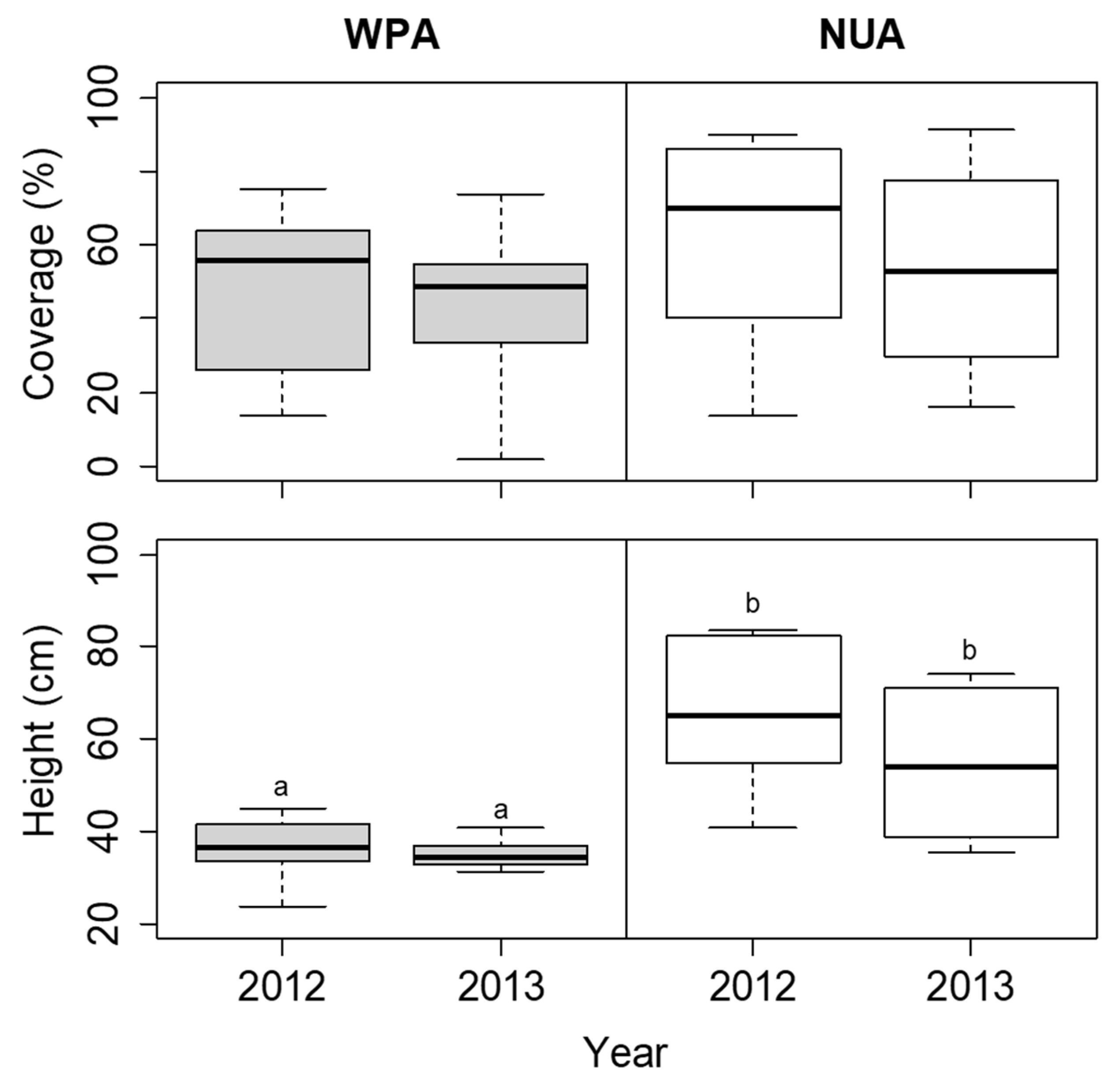
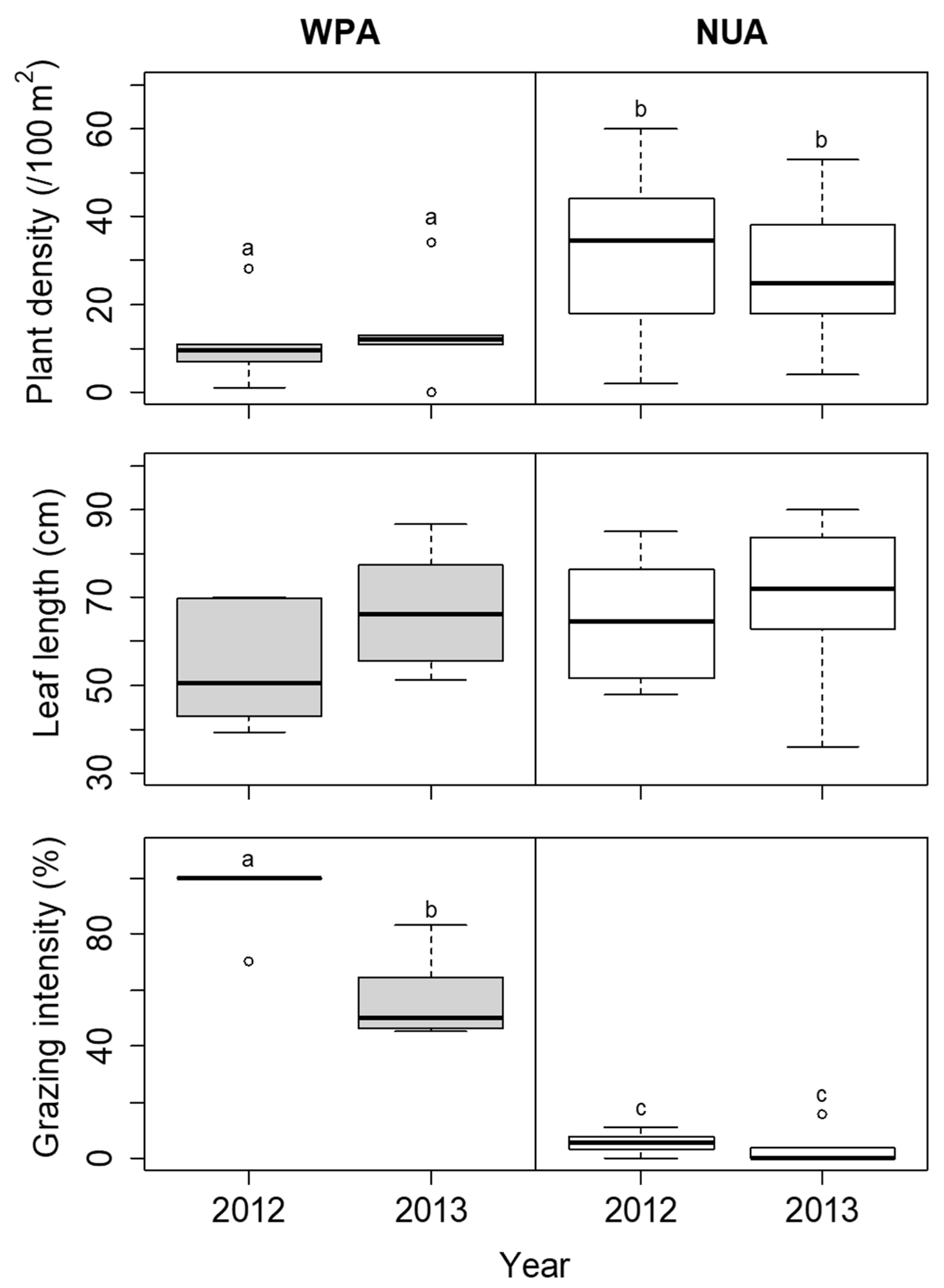
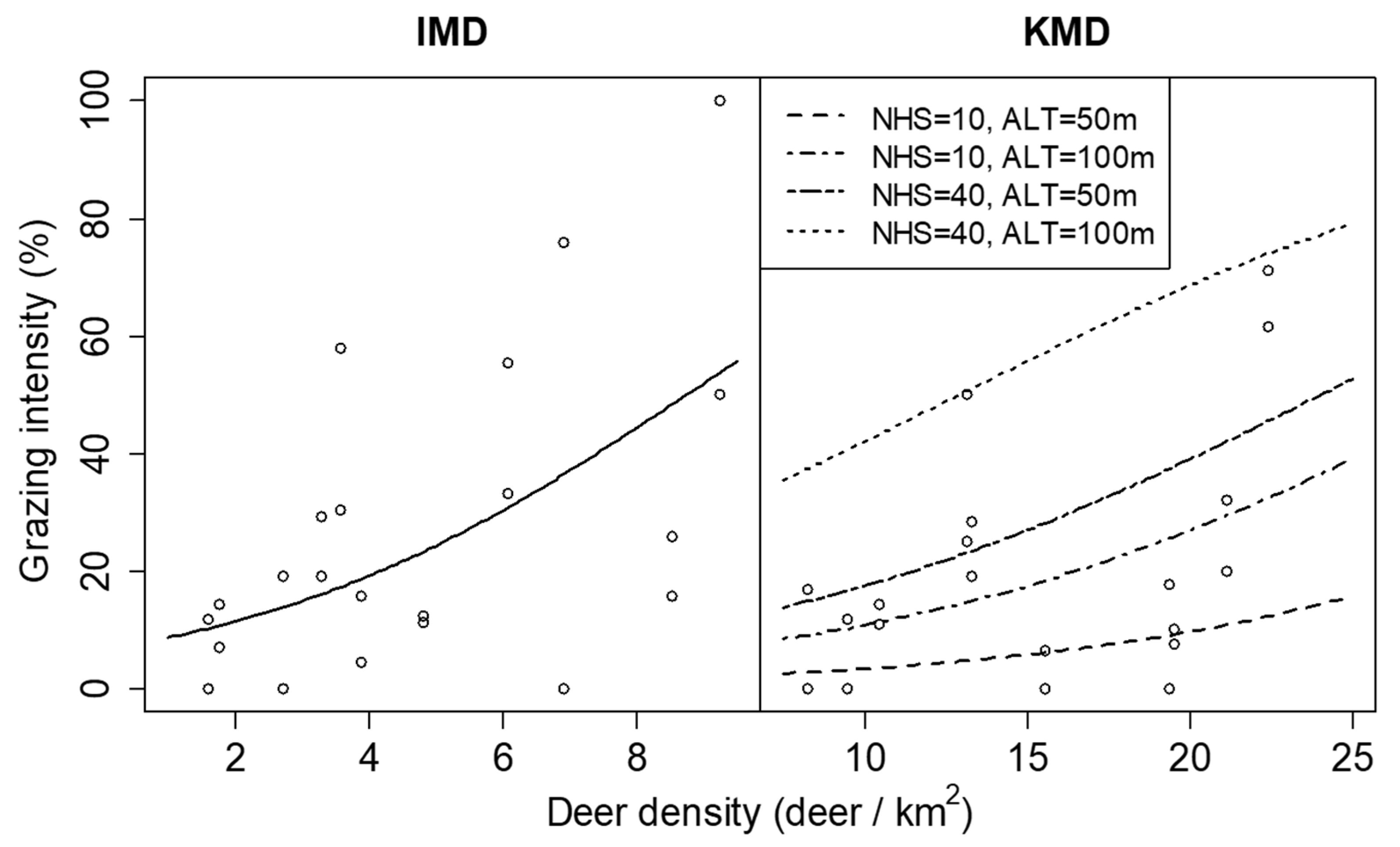
| Site | Density of Seedlings (/100 m2) | Canopy Openness (%) |
|---|---|---|
| WPA | 0.33 ± 0.21 | 8.33 ± 2.13 |
| NUA | 5.00 ± 2.59 | 6.48 ± 1.72 |
| Response Variables | Explanatory Variables | IMD | KMD | ||||
|---|---|---|---|---|---|---|---|
| Estimate ± SE | χ2 | p | Estimate ± SE | χ2 | p | ||
| Plant density | Intercept | 3.000 ± 0.450 | 44.364 | <0.001 | 3.337 ± 0.621 | 28.857 | <0.001 |
| DD | 0.012 ± 0.046 | 0.072 | 0.789 | −0.026 ± 0.033 | 0.640 | 0.424 | |
| NHS | −0.005 ± 0.008 | 0.3445 | 0.557 | 0.009 ± 0.012 | 0.567 | 0.452 | |
| ALT | 2.619 ± 1.697 | 2.380 | 0.123 | −2.177 ± 4.333 | 0.252 | 0.615 | |
| Leaf length | Intercept | 83.309 ± 17.434 | 22.832 | <0.001 | 52.198 ± 20.510 | 6.477 | 0.011 |
| DD | 0.277 ± 0.984 | 0.079 | 0.778 | −0.462 ± 0.788 | 0.344 | 0.557 | |
| NHS | −0.300 ± 0.369 | 0.665 | 0.415 | 0.912 ± 0.486 | 3.525 | 0.060 | |
| ALT | −32.768 ± 61.780 | 0.281 | 0.596 | −93.067 ± 174.068 | 0.286 | 0.593 | |
| Grazing intensity | Intercept | −2.089 ± 1.151 | 3.291 | 0.070 | −6.250 ± 0.733 | 72.724 | <0.001 |
| DD | 0.257 ± 0.103 | 6.197 | 0.013 | 0.110 ± 0.026 | 18.055 | <0.001 | |
| NHS | 0.018 ± 0.021 | 0.747 | 0.387 | 0.059 ± 0.016 | 12.996 | <0.001 | |
| ALT | −5.562 ± 4.812 | 1.336 | 0.248 | 24.539 ± 5.593 | 19.247 | <0.001 | |
| Site | Length (km) | Deer Density (Deer/km2) (95% Confidence Interval) | |
|---|---|---|---|
| September 2012 | November 2013 | ||
| WPA | 4.0 | 82.1 (62.1–108.6) | 36.5 (31.3–42.5) |
| NUA | 4.5 | 19.9 (13.3–29.7) | 26.2 (21.7–31.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inatomi, Y.; Uno, H.; Ueno, M.; Takafumi, H.; Osa, Y. Response of an Indicator Species, Dryopteris crassirhizoma, to Temporal and Spatial Variations in Sika Deer Density. Biology 2022, 11, 302. https://doi.org/10.3390/biology11020302
Inatomi Y, Uno H, Ueno M, Takafumi H, Osa Y. Response of an Indicator Species, Dryopteris crassirhizoma, to Temporal and Spatial Variations in Sika Deer Density. Biology. 2022; 11(2):302. https://doi.org/10.3390/biology11020302
Chicago/Turabian StyleInatomi, Yoshihiro, Hiroyuki Uno, Mayumi Ueno, Hino Takafumi, and Yuichi Osa. 2022. "Response of an Indicator Species, Dryopteris crassirhizoma, to Temporal and Spatial Variations in Sika Deer Density" Biology 11, no. 2: 302. https://doi.org/10.3390/biology11020302
APA StyleInatomi, Y., Uno, H., Ueno, M., Takafumi, H., & Osa, Y. (2022). Response of an Indicator Species, Dryopteris crassirhizoma, to Temporal and Spatial Variations in Sika Deer Density. Biology, 11(2), 302. https://doi.org/10.3390/biology11020302






