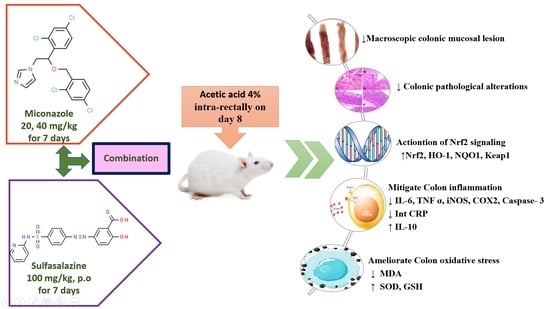
Miconazole Mitigates Acetic Acid-Induced Experimental Colitis in Rats: Insight into Inflammation, Oxidative Stress and Keap1/Nrf-2 Signaling Crosstalk
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and Chemicals
2.3. Induction of Colitis
2.4. Experimental Design
2.4.1. Collection of Samples
2.4.2. Colonic Wet Weight Assay
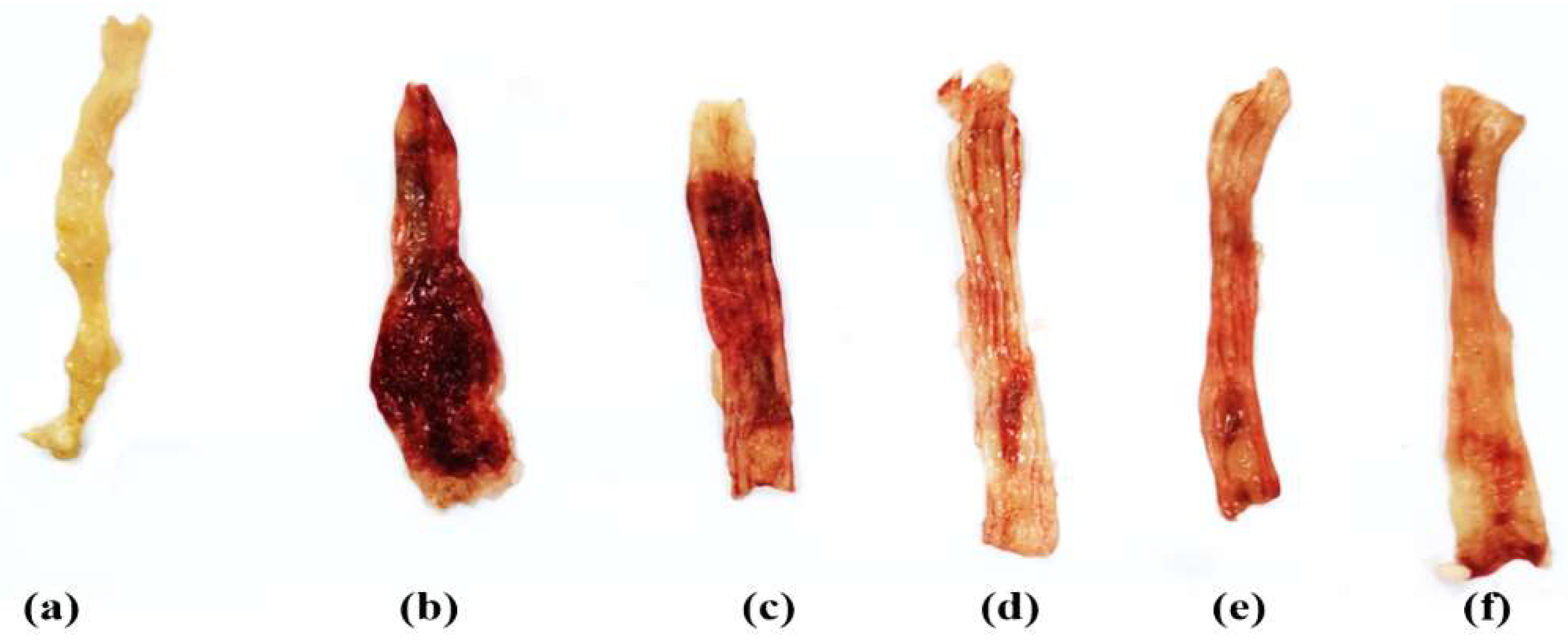
2.4.3. Macroscopic Colonic Damage Scoring
- (0)
- There have been no modifications in the macroscopic findings.
- (1)
- Mucosal erythema is the only symptom.
- (2)
- The presence of mild mucosal edema, minor bleeding or minor erosions.
- (3)
- Bleeding ulcers or erosions, as well as moderate edema.
- (4)
- Necrosis, edema and severe ulceration/erosion of the tissue.
2.5. Assessment of Colonic Inflammatory Mediators
2.6. Assessment of Colonic Oxidative Markers
2.7. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
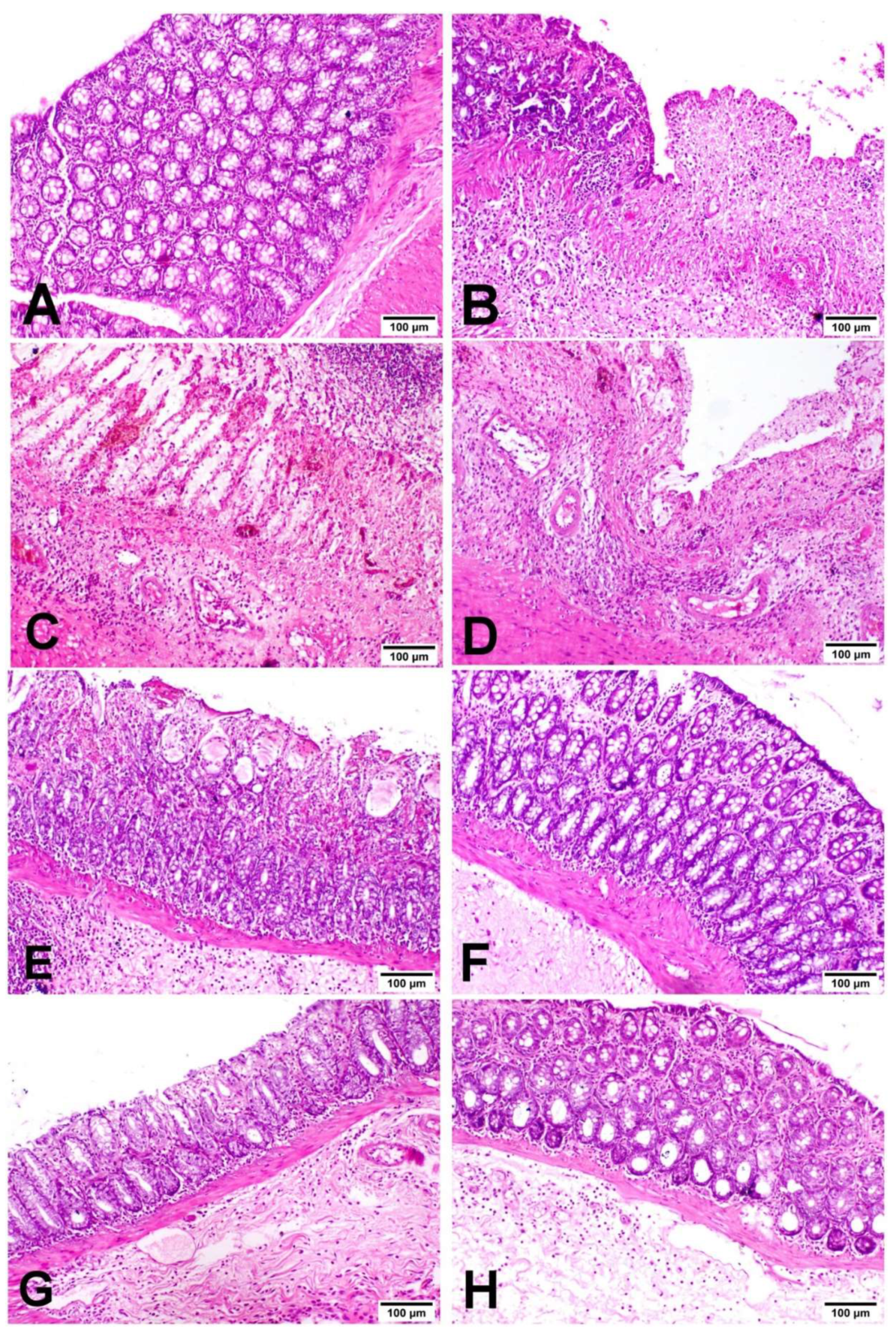
2.8. Histopathological Examination of Colon Tissue
2.9. Statistical Analysis
3. Results
3.1. Miconazole Reduced Colon Weight in AA-Induced Colitis
3.2. Miconazole Attenuated Macroscopic Alterations in AA-Induced Colitis
3.3. Miconazole Alleviated Oxidative Stress in AA-Induced Colitis
3.4. Miconazole Downregulated the Pro-Inflammatory and Upregulated the Anti-Inflammatory Mediators in AA-Induced Colitis
3.5. Miconazole Upregulated Nrf2 and Heme Oxygenase-1 (HO-1) and Downregulated CRP in AA-Induced Colitis
3.6. Miconazole Amended Histopathological Alterations in AA-Induced Colitis
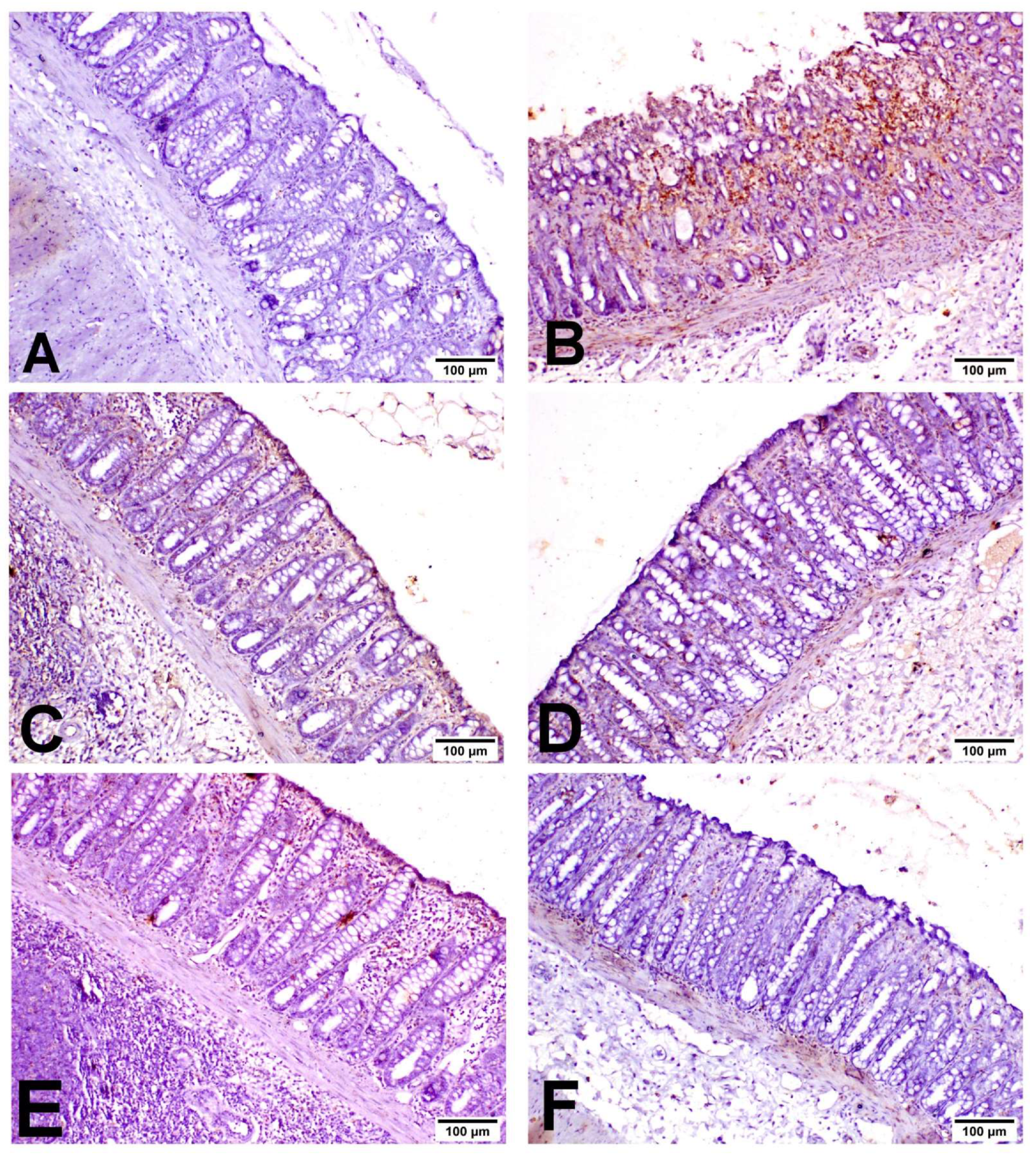
3.7. Miconazole Downregulates INOS, COX2 and Caspase-3 Protein Expression in AA-Induced Colitis
3.8. Miconazole Activates Nrf2/HO-1 Signaling in AA-Induced Colitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malago, J.J.; Sangu, C.L. Intraperitoneal administration of butyrate prevents the severity of acetic acid colitis in rats. J. Zhejiang Univ. Sci. B 2015, 16, 224–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Baz, A.M.; Khodir, A.E.; Adel El-Sokkary, M.M.; Shata, A. The protective effect of Lactobacillus versus 5-aminosalicylic acid in ulcerative colitis model by modulation of gut microbiota and Nrf2/Ho-1 pathway. Life Sci. 2020, 256, 117927. [Google Scholar] [CrossRef] [PubMed]
- Tanida, S.; Mizoshita, T.; Mizushima, T.; Sasaki, M.; Shimura, T.; Kamiya, T.; Kataoka, H.; Joh, T. Involvement of oxidative stress and mucosal address in cell adhesion molecule-1 (MAdCAM-1) in inflammatory bowel disease. J. Clin. Biochem. Nutr. 2011, 48, 112–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, C.; Grácio, D.; Teixeira, J.P.; Magro, F. Oxidative Stress and DNA Damage: Implications in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Zhang, J. Recent Advances: The Imbalance of Cytokines in the Pathogenesis of Inflammatory Bowel Disease. Mediat. Inflamm. 2017, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nikkhah-Bodaghi, M.; Maleki, I.; Agah, S.; Hekmatdoost, A. Zingiber officinale and oxidative stress in patients with ulcerative colitis: A randomized, placebo-controlled, clinical trial. Complement. Ther. Med. 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Tahan, G.; Aytac, E.; Aytekin, H.; Gunduz, F.; Dogusoy, G.; Aydin, S.; Tahan, V.; Uzun, H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can. J. Surg. 2011, 54, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Al-Rejaie, S.S.; Abuohashish, H.M.; Al-Enazi, M.M.; Al-Assaf, A.H.; Parmar, M.Y.; Ahmed, M.M. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J. Gastroenterol. 2013, 19, 5633–5644. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell Longev. 2017, 3, 1–18. [Google Scholar] [CrossRef]
- Lu, M.-C.; Ji, J.-A.; Jiang, Y.-L.; Chen, Z.-Y.; Yuan, Z.-W.; You, Q.-D.; Jiang, Z.-Y. An inhibitor of the Keap1-Nrf2 protein-protein interaction protects NCM460 colonic cells and alleviates experimental colitis. Sci. Rep. 2016, 6, 26585. [Google Scholar] [CrossRef] [PubMed]
- Song, C.H.; Kim, N.; Lee, S.M.; Nam, R.H.; Choi, S.I.; Kang, S.R.; Shin, E.; Lee, D.H.; Lee, H.N.; Surh, Y.J. Effects of 17β-estradiol on colorectal cancer development after azoxymethane/dextran sulfate sodium treatment of ovariectomized mice. Biochem. Pharmacol. 2019, 164, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brechmann, T.; Günther, K.; Neid, M.; Schmiegel, W.; Tannapfel, A. Triggers of histologically suspected drug-induced colitis. World J Gastroenterol. 2019, 25, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Peluso, R.; Manguso, F.; Vitiello, M.; Iervolino, S.; Di Minno, M.N.D. Management of arthropathy in inflammatory bowel diseases. Ther. Adv. Chronic Dis. 2015, 6, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Luo, L.; Sun, S.; Jiang, Z.; Guo, X. Enantioselective separation and determination of miconazole in rat plasma by chiral LC-MS/MS: Application in a stereoselective pharmacokinetic study. Anal. Bioanal. Chem. 2017, 409, 6315–6323. [Google Scholar] [CrossRef]
- Piérard, G.E.; Hermanns-Lê, T.; Delvenne, P.; Piérard-Franchimont, C. Miconazole, a pharmacological barrier to skin fungal infections. Expert Opin. Pharmacother. 2012, 13, 1187–1194. [Google Scholar] [CrossRef]
- Gøtzsche, P.C.; Johansen, H.K. Routine versus selective antifungal administration for control of fungal infections in patients with cancer. Cochrane Database Syst. Rev. 2014, 2, Cd000026. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.-F.; Chen, P.-C.; Lin, Y.-C.; Chou, K.-Y.; Chen, H.-E.; Ho, C.-Y.; Lin, J.-F.; Hwang, T.I.S. Miconazole Contributes to NRF2 Activation by Noncanonical P62-KEAP1 Pathway in Bladder Cancer Cells. Drug Des. Devel. Ther. 2020, 14, 1209–1218. [Google Scholar] [CrossRef] [Green Version]
- Barbosa Bezerra, G.; de Menezes de Souza, L.; Dos Santos, A.S.; de Almeida, G.K.; Souza, M.T.; Santos, S.L.; Aparecido Camargo, E.; Dos Santos Lima, B.; de Souza Araújo, A.A.; Cardoso, J.C.; et al. Hydroalcoholic extract of Brazilian red propolis exerts protective effects on acetic acid-induced ulcerative colitis in a rodent model. Biomed. Pharmacother. 2017, 85, 687–696. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Mei, Q.B.; Liu, L.; Guo, X.; Qiu, B.S.; Zhao, D.H.; Cho, C.H. Delivery of glucocorticoid conjugate in rat gastrointestinal tract and its treatment for ulcerative colitis. Acta Pharmacol. Sin. 2001, 22, 761–764. [Google Scholar] [PubMed]
- El-Alfy, T.S.; Hetta, M.; Yassin, N.; Abdel-Rahman, R.; Kadry, E.M. Estrogenic activity of Citrus medica L. leaves growing in Egypt. J. App. Pharm. Sci. 2012, 2, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Millar, A.D.; Rampton, D.S.; Chander, C.L.; Claxson, A.W.; Blades, S.; Coumbe, A.; Panetta, J.; Morris, C.J.; Blake, D.R. Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut 1996, 39, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogaly, H.A.; Alsherbiny, M.A.; El Badawy, S.A.; Abd-Elsalam, R.M.; Guang Li, C.; Azouz, A.A. Gastroprotective effects and metabolomic profiling of Chasteberry fruits against indomethacin-induced gastric injury in rats. J. Funct. Foods 2021, 86, 104732. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Churchill Livingstone: London, UK; Elsevier: Amsterdam, The Netherlands, 2008; pp. 126–127. [Google Scholar]
- Stillie, R.; Stadnyk, A.W. Role of TNF receptors, TNFR1 and TNFR2, in dextran sodium sulfate-induced colitis. Inflamm. Bowel Dis. 2009, 15, 1515–1525. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.F.; Alqasoumi, S.I.; Ogaly, H.A.; Abd-Elsalam, R.M.; El-Banna, H.A.; Soliman, G.A. Propolis ameliorates cerebral injury in focal cerebral ischemia/reperfusion (I/R) rat model via upregulation of TGF-β1. Saudi Pharm. J. 2020, 28, 116–126. [Google Scholar] [CrossRef]
- Khamis, G.; Hassan, M.; Morsy, M.; Ibrahim, M.A. Innovative application of helium-neon laser: Enhancing the germination of Adansonia digitata and evaluating the hepatoprotective activities in mice. Environ. Sci. Pollut. Res. Int. 2020, 27, 26520–26531. [Google Scholar] [CrossRef]
- Abu-Elala, N.M.; Mohamed, S.H.; Zaki, M.M.; Eissa, A.E. Assessment of the immune-modulatory and antimicrobial effects of dietary chitosan on Nile tilapia (Oreochrmis niloticus) with special emphasis to its bio-remediating impacts. Fish Shellfish Immunol. 2015, 46, 678–685. [Google Scholar] [CrossRef]
- Abd-Elsalam, R.M.; El Badawy, S.A.; Ogaly, H.A.; Ibrahim, F.M.; Farag, O.M.; Ahmed, K.A. Eruca sativa seed extract modulates oxidative stress and apoptosis and up-regulates the expression of Bcl-2 and Bax genes in acrylamide-induced testicular dysfunction in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 53249–53266. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, Y.; Zhang, Y.; Su, X.; Luo, C.; Alarifi, S.; Yang, H. Dieckol alleviates dextran sulfate sodium-induced colitis via inhibition of inflammatory pathway and activation of Nrf2/HO-1 signaling pathway. Environ. Toxicol. 2021, 36, 782–788. [Google Scholar] [CrossRef]
- Khodir, A.E.; Said, E.; Atif, H.; ElKashef, H.A.; Salem, H.A. Targeting Nrf2/HO-1 signaling by crocin: Role in attenuation of AA-induced ulcerative colitis in rats. Biomed. Pharmacother. 2019, 110, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, H.N.; Ciorba, M.A. Biomarkers in inflammatory bowel disease: Current practices and recent advances. Transl. Res. 2012, 159, 313–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sales-Campos, H.; Basso, P.J.; Alves, V.B.; Fonseca, M.T.; Bonfá, G.; Nardini, V.; Cardoso, C.R. Classical and recent advances in the treatment of inflammatory bowel diseases. Braz. J. Med. Biol. Res. 2015, 48, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Heng, X.; Jiang, Y.; Chu, W. Influence of Fluconazole Administration on Gut Microbiome, Intestinal Barrier, and Immune Response in Mice. Antimicrob. Agents Chemother. 2021, 65, e02552-20. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, D.; Ziaei, M.; Hosseini, S.V.; Ghahramani, L.; Bananzadeh, A.M.; Ashraf, M.J.; Amini, A.; Amini, M.; Tanideh, N. The effect of calendula officinalis in therapy of acetic Acid induced ulcerative colitis in dog as an animal model. Iran Red Crescent Med. J. 2011, 13, 884–890. [Google Scholar] [PubMed]
- Mehrabani, D.; Bahrami, F.; Hosseini, S.V.; Ashraf, M.J.; Tanideh, N.; Rezaianzadeh, A.; Amini, M.; Amini, A. The Healing Effect of Teucrium polium in Acetic Acid-Induced Ulcerative Colitis in the Dog as an Animal Model. Middle East J. Dig. Dis. 2012, 4, 40–47. [Google Scholar]
- El-Abhar, H.S.; Hammad, L.N.; Gawad, H.S. Modulating effect of ginger extract on rats with ulcerative colitis. J. Ethnopharmacol. 2008, 118, 367–372. [Google Scholar] [CrossRef]
- Harputluoglu, M.M.; Demirel, U.; Yücel, N.; Karadağ, N.; Temel, I.; Firat, S.; Ara, C.; Aladağ, M.; Karincaoğlu, M.; Hilmioğlu, F. The effects of Gingko biloba extract on acetic acid-induced colitis in rats. Turk. J. Gastroenterol. 2006, 17, 177–182. [Google Scholar]
- Patel, M.A.; Patel, P.K.; Patel, M.B. Effects of ethanol extract of Ficus bengalensis (bark) on inflammatory bowel disease. Indian J. Pharmacol. 2010, 42, 214–218. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Franco, R.; Cidlowski, J.A. Glutathione efflux and cell death. Antioxid. Redox Signal. 2012, 17, 1694–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, S.V.; Sharma, S.; Prasad, K.K.; Sinha, S.K.; Singh, K. Role of oxidative stress & antioxidant defence in ulcerative colitis patients from north India. Indian J. Med. Res. 2014, 139, 568–571. [Google Scholar] [PubMed]
- Tanimoto, A.; Witaicenis, A.; Caruso, Í.P.; Piva, H.M.R.; Araujo, G.C.; Moraes, F.R.; Fossey, M.C.; Cornélio, M.L.; Souza, F.P.; Di Stasi, L.C. 4-Methylesculetin, a natural coumarin with intestinal anti-inflammatory activity, elicits a glutathione antioxidant response by different mechanisms. Chem. Biol. Interact. 2020, 315, 108876. [Google Scholar] [CrossRef] [PubMed]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [Green Version]
- Aleisa, A.M.; Al-Rejaie, S.S.; Abuohashish, H.M.; Ola, M.S.; Parmar, M.Y.; Ahmed, M.M. Pretreatment of Gymnema sylvestre revealed the protection against acetic acid-induced ulcerative colitis in rats. BMC Complement. Altern. Med. 2014, 14, 49. [Google Scholar] [CrossRef] [Green Version]
- Ngo, B.; Farrell, C.P.; Barr, M.; Wolov, K.; Bailey, R.; Mullin, J.M.; Thornton, J.J. Tumor necrosis factor blockade for treatment of inflammatory bowel disease: Efficacy and safety. Curr. Mol. Pharmacol. 2010, 3, 145–152. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, X.; Liu, X.; Liu, Y.; Fan, H. Rho kinase Blockade Ameliorates DSS-Induced Ulcerative Colitis in Mice Through Dual Inhibition of the NF-κB and IL-6/STAT3 Pathways. Inflammation 2020, 43, 857–867. [Google Scholar] [CrossRef]
- Sacerdote, P.; Franchi, S.; Moretti, S.; Castelli, M.; Procacci, P.; Magnaghi, V.; Panerai, A.E. Cytokine modulation is necessary for efficacious treatment of experimental neuropathic pain. J. Neuroimmune Pharmacol. 2013, 8, 202–211. [Google Scholar] [CrossRef]
- Tatiya-aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 1–10. [Google Scholar] [CrossRef]
- Majagi, S.I.; Khan, M.D.M.A.; Patil, P.A. Anti-inflammatory and analgesic activity of ketoconazole and miconazole in wistar rats. J. Pharm. Res. 2011, 4, 714–716. [Google Scholar]
- Zhang, L.; Chen, X.; Liu, Z.; Han, Q.; Tang, L.; Tian, Z.; Ren, Z.; Rong, C.; Xu, H. Miconazole alleviates peripheral nerve crush injury by mediating a macrophage phenotype change through the NF-κB pathway. Brain Behav. 2019, 9, e01400. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.J.; Yun, J.; Son, D.J.; Han, S.-B.; Hong, J.T. Antifungal drug miconazole ameliorated memory deficits in a mouse model of LPS-induced memory loss through targeting iNOS. Cell Death Dis. 2020, 11, 623. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Su, Z.-y.; Kong, A.-N.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef]
- Iranshahy, M.; Iranshahi, M.; Abtahi, S.R.; Karimi, G. The role of nuclear factor erythroid 2-related factor 2 in hepatoprotective activity of natural products: A review. Food Chem. Toxicol. 2018, 120, 261–276. [Google Scholar] [CrossRef]
- Yamamoto, T.; Suzuki, T.; Kobayashi, A.; Wakabayashi, J.; Maher, J.; Motohashi, H.; Yamamoto, M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell Biol. 2008, 28, 2758–2770. [Google Scholar] [CrossRef] [Green Version]
- Zeng, T.; Zhang, C.L.; Song, F.Y.; Zhao, X.L.; Yu, L.H.; Zhu, Z.P.; Xie, K.Q. The activation of HO-1/Nrf-2 contributes to the protective effects of diallyl disulfide (DADS) against ethanol-induced oxidative stress. Biochim. Biophys. Acta 2013, 1830, 4848–4859. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, Y.M.; Park, S.W.; Kim, H.J.; Lee, J.H.; Lee, D.U.; Chang, K.C. Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula helenium L. reduces inflammation in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Food Chem. Toxicol. 2013, 55, 386–395. [Google Scholar] [CrossRef]
- Paul, G.; Bataille, F.; Obermeier, F.; Bock, J.; Klebl, F.; Strauch, U.; Lochbaum, D.; Rümmele, P.; Farkas, S.; Schölmerich, J.; et al. Analysis of intestinal haem-oxygenase-1 (HO-1) in clinical and experimental colitis. Clin. Exp. Immunol. 2005, 140, 547–555. [Google Scholar] [CrossRef]
- Saber, S.; Khalil, R.M.; Abdo, W.S.; Nassif, D.; El-Ahwany, E. Olmesartan ameliorates chemically-induced ulcerative colitis in rats via modulating NFκB and Nrf-2/HO-1 signaling crosstalk. Toxicol. Appl. Pharmacol. 2019, 364, 120–132. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hariharan, A.; Hakeem, A.R.; Radhakrishnan, S.; Reddy, M.S.; Rela, M. The Role and Therapeutic Potential of NF-kappa-B Pathway in Severe COVID-19 Patients. Inflammopharmacology 2021, 29, 91–100. [Google Scholar] [CrossRef] [PubMed]
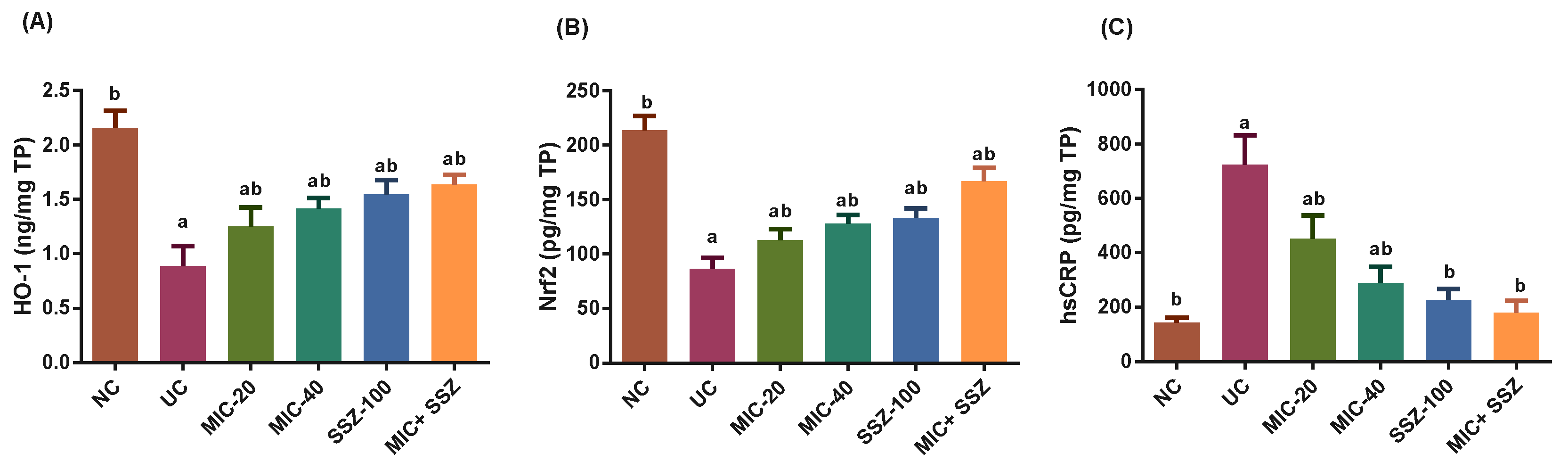




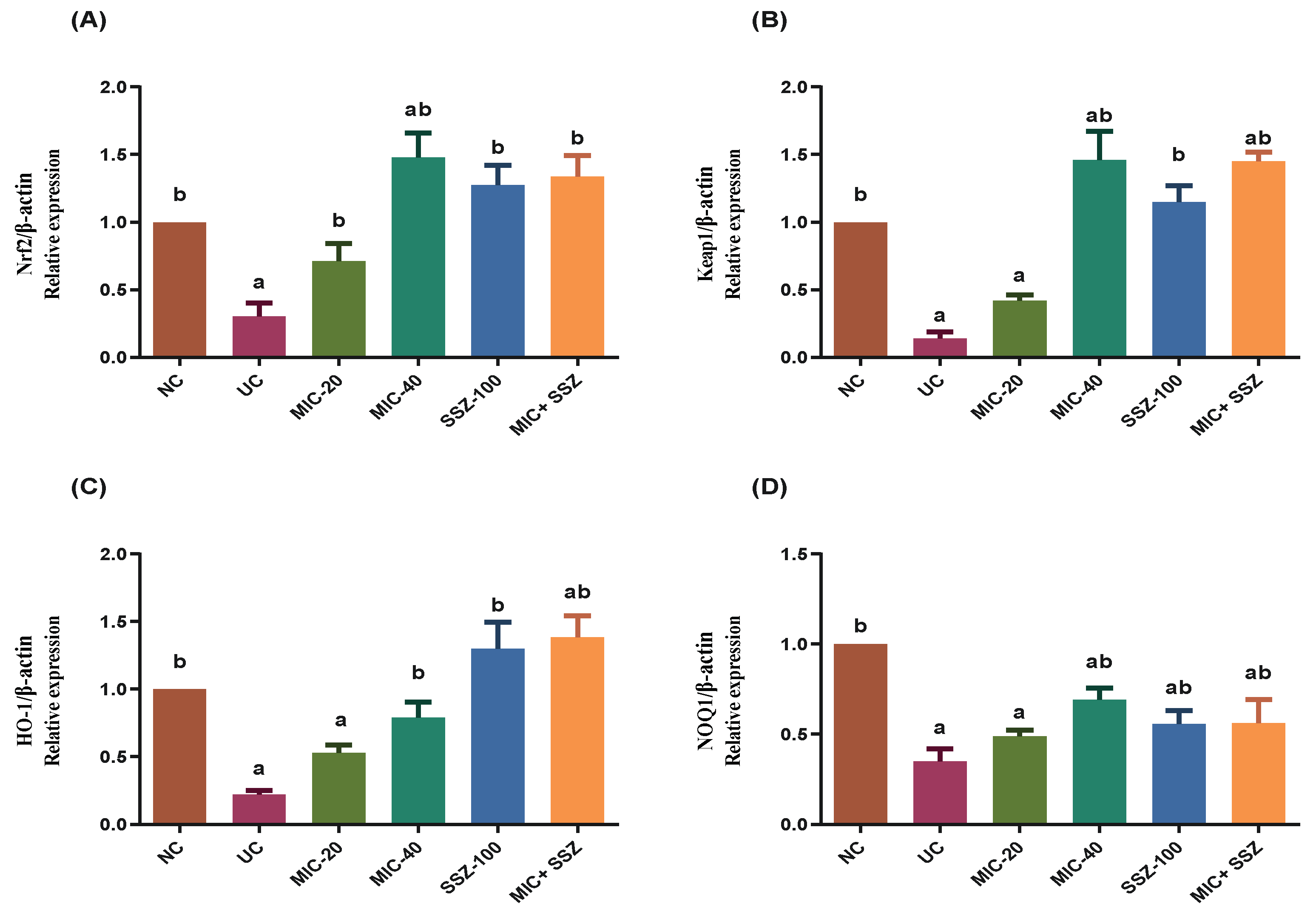
| Gene | Accession No. | Product Size | Sequence (5′–3′) | |
|---|---|---|---|---|
| Nrf2 | XM_006234398.3 | 121 | F | CACATCCAGACAGACACCAGT |
| R | CTACAAATGGGAATGTCTCTGC | |||
| NQO1 | NM_017000.3 | 162 | F | CTGTGAGGGACTCTGGTCTTTG |
| R | CTGAAAGCAAGCCAGGCAAAC | |||
| Keap-1 | NM_017000.3 | 190 | F | AACTCGGCAGAATGTTACTACCC |
| R | CTACGAAAGTCCAGGTCTCTGTCTC | |||
| HO-1 | NM_012580.2 | 107 | F | ACAGGGTGACAGAAGAGGCTAA |
| R | TCAAGAGGAGCAGAAAAAGAACAAG | |||
| β-actin | NM_031144.3 | 97 bp | F | GGTGGGTATGGGTCAG |
| R | ATGCCGTGTTCAATGG | |||
| Inflammatory Cell Infiltration | |
| 0. None | |
| 1. Occasional, limited to submucosa | |
| 2. Significant focal areas in submucosa | |
| 3. Significant focal areas in submucosa and lamina propria | |
| 4. Diffuse large areas in submucosa, around blood vessels and lamina propria | |
| 5. Transmural infiltration from mucosa to muscularis | |
| Crypt Damage | |
| 0. No change | |
| 1. Some crypt damage, spaces between crypts | |
| 2. Goblet cell loss, some shortening of crypts with larger spaces between them | |
| 3. Large areas without crypts | |
| 4. No crypt | |
| Ulceration | |
| 0. None | |
| 1. Small focal ulcers | |
| 2. Frequent small ulcers | |
| 3. Large areas without surface epithelium | |
| Edema | |
| 0. Absent | |
| 1. Present | |
| Total lesion score | 0–13 |
| Group | Relative Colon Weight (g) | W/L Ratio (g/cm) | Lesion Score |
|---|---|---|---|
| NC | 0.34 b ± 0.04 | 0.071 b ± 0.008 | 0 ± 0 |
| UC | 0.65 a ± 0.06 | 0.133 a ± 0.01 | 3.52 ± 0.19 |
| MIC-20 | 0.62 a ± 0.03 | 0.129 a ± 0.007 | 2.57 ± 0.20 |
| MIC-40 | 0.53 a ± 0.01 | 0.102 ab ± 0.004 | 1.71 ± 0.29 |
| SSZ-100 | 0.53 a ± 0.02 | 0.102 ab ± 0.005 | 1.86 ± 0.26 |
| MIC + SSZ | 0.53 a ± 0.02 | 0.106 ab ± 0.002 | 1.43 ± 0.20 |
| Group | MDA (nMol/mL) | GSH (nMol/dL) | SOD (U/mL) |
|---|---|---|---|
| NC | 8.5 b ± 0.31 | 0.9 b ± 0.03 | 17.6 b ± 0.37 |
| UC | 13.8 a ± 0.43 | 0.6 a ± 0.02 | 11.7 a ± 0.23 |
| MIC-20 | 11.7 ab ± 0.4 | 0.7 ab ± 0.02 | 13.0 ab ± 0.23 |
| MIC-40 | 11.4 ab ± 0.23 | 0.7 ab ± 0.02 | 13.9 ab ± 0.38 |
| SSZ-100 | 10.7 ab ± 0.26 | 0.8 ab ± 0.01 | 15.0 ab ± 0.18 |
| MIC + SSZ | 10.0 ab ± 0.23 | 0.8 ab ± 0.01 | 16.4 b ± 0.39 |
| Group | IL-10 (pg/mg TP) | IL-6 (pg/mg TP) | TNF-α (pg/mg TP) |
|---|---|---|---|
| NC | 262.7 b ± 16.34 | 154.6 b ± 5.84 | 198.1 b ± 3.87 |
| UC | 145.2 a ± 3.74 | 309.6 a ± 13.75 | 342.7 a ± 17.07 |
| MIC-20 | 176.9 a ± 6.52 | 261.0 ab ± 6.76 | 286.5 ab ± 15.63 |
| MIC-40 | 193.7 ab ± 8.14 | 249.2 ab ± 14.27 | 254.4 ab ± 12.96 |
| SSZ-100 | 224.6 ab ± 6.65 | 208.4 ab ± 13.08 | 241.8 b ± 6.16 |
| MIC + SSZ | 238.8 b ± 2.46 | 166.2 b ± 3.31 | 207.6 b ± 7.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsharif, I.A.; Fayed, H.M.; Abdel-Rahman, R.F.; Abd-Elsalam, R.M.; Ogaly, H.A. Miconazole Mitigates Acetic Acid-Induced Experimental Colitis in Rats: Insight into Inflammation, Oxidative Stress and Keap1/Nrf-2 Signaling Crosstalk. Biology 2022, 11, 303. https://doi.org/10.3390/biology11020303
Alsharif IA, Fayed HM, Abdel-Rahman RF, Abd-Elsalam RM, Ogaly HA. Miconazole Mitigates Acetic Acid-Induced Experimental Colitis in Rats: Insight into Inflammation, Oxidative Stress and Keap1/Nrf-2 Signaling Crosstalk. Biology. 2022; 11(2):303. https://doi.org/10.3390/biology11020303
Chicago/Turabian StyleAlsharif, Ifat A., Hany M. Fayed, Rehab F. Abdel-Rahman, Reham M. Abd-Elsalam, and Hanan A. Ogaly. 2022. "Miconazole Mitigates Acetic Acid-Induced Experimental Colitis in Rats: Insight into Inflammation, Oxidative Stress and Keap1/Nrf-2 Signaling Crosstalk" Biology 11, no. 2: 303. https://doi.org/10.3390/biology11020303
APA StyleAlsharif, I. A., Fayed, H. M., Abdel-Rahman, R. F., Abd-Elsalam, R. M., & Ogaly, H. A. (2022). Miconazole Mitigates Acetic Acid-Induced Experimental Colitis in Rats: Insight into Inflammation, Oxidative Stress and Keap1/Nrf-2 Signaling Crosstalk. Biology, 11(2), 303. https://doi.org/10.3390/biology11020303