Rewilding by Wolf Recolonisation, Consequences for Ungulate Populations and Game Hunting
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Area
2.2. The Wolf in Sweden
2.3. Ungulate Populations in WBR
2.4. Ungulate Density and Distribution
2.5. Prey Selection
2.6. Wolf Kill Rates
2.7. Estimating the Potential for Wolf Density
2.8. Combining Prey Density, Predator Density and Kill Rates
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navarro, L.M.; Pereira, H.M. Rewilding Abandoned Landscapes in Europe. In Rewilding European Landscapes; Pereira, H.M., Navarro, L.M., Eds.; Springer Open: Cham, Switzerland; Heidelberg, Germany; New York, NY, USA, 2015; pp. 3–23. ISBN 978-3-319-12038-6. [Google Scholar]
- Boitani, L.; Linnell, J.D.C. Bringing Large Mammals Back: Large Carnivores in Europe. In Rewilding European Landscapes; Pereira, H.M., Navarro, L.M., Eds.; Springer Open: Cham, Switzerland; Heidelberg, Germany; New York, NY, USA, 2015; pp. 67–84. ISBN 978-3-319-12038-6. [Google Scholar]
- Chapron, G.; Kaczensky, P.; Linnell, J.D.C.; von Arx, M.; Huber, D.; Andrén, H.; López-Bao, J.V.; Adamec, M.; Álvares, F.; Anders, O.; et al. Recovery of Large Carnivores in Europe’s Modern Human-Dominated Landscapes. Science 2014, 346, 1517–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripple, W.J.; Estes, J.A.; Beschta, R.L.; Wilmers, C.C.; Ritchie, E.G.; Hebblewhite, M.; Berger, J.; Elmhagen, B.; Letnic, M.; Nelson, M.P.; et al. Status and Ecological Effects of the World’s Largest Carnivores. Science 2014, 343, 1241484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treves, A.; Karanth, K.U. Human-Carnivore Conflict and Perspectives on Carnivore Management Worldwide. Conserv. Biol. 2003, 17, 1491–1499. [Google Scholar] [CrossRef]
- Llorent-Bedmar, V.; Cobano-Delgado, V.; Navarro-Granados, M. The Rural Exodus of Young People from Empty Spain. Socio-Educational Aspects. J. Rural Stud. 2021, 82, 303–314. [Google Scholar] [CrossRef]
- Recio, M.R.; Sand, H.; Virgós, E. Promoting Grazing or Rewilding Initiatives against Rural Exodus? The Return of the Wolf and Other Large Carnivores Must Be Considered. Environ. Conserv. 2020, 47, 269–276. [Google Scholar] [CrossRef]
- Fernández, N.; Navarro, L.M.; Pereira, H.M. Rewilding: A Call for Boosting Ecological Complexity in Conservation: A Call for Rewilding in Conservation. Conserv. Lett. 2017, 10, 276–278. [Google Scholar] [CrossRef]
- Cerqueira, Y.; Navarro, L.M.; Maes, J.; Marta-Pedroso, C.; Pradinho Honrado, J.; Pereira, H.M. Ecosystem Services: The Opportunities of Rewilding in Europe. In Rewilding European Landscapes; Pereira, H.M., Navarro, L.M., Eds.; Springer Open: Cham, Switzerland; Heidelberg, Germany; New York, NY, USA, 2015; pp. 47–64. ISBN 978-3-319-12038-6. [Google Scholar]
- Svenning, J.-C.; Pedersen, P.B.M.; Donlan, C.J.; Ejrnæs, R.; Faurby, S.; Galetti, M.; Hansen, D.M.; Sandel, B.; Sandom, C.J.; Terborgh, J.W.; et al. Science for a Wilder Anthropocene: Synthesis and Future Directions for Trophic Rewilding Research. Proc. Natl. Acad. Sci. USA 2016, 113, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Bakker, E.S.; Svenning, J.-C. Trophic Rewilding: Impact on Ecosystems under Global Change. Philos. Trans. R. Soc. B 2018, 373, 20170432. [Google Scholar] [CrossRef] [Green Version]
- Lozano, J.; Olszańska, A.; Morales-Reyes, Z.; Castro, A.A.; Malo, A.F.; Moleón, M.; Sánchez-Zapata, J.A.; Cortés-Avizanda, A.; von Wehrden, H.; Dorresteijn, I.; et al. Human-Carnivore Relations: A Systematic Review. Biol. Conserv. 2019, 237, 480–492. [Google Scholar] [CrossRef]
- Ronnenberg, K.; Habbe, B.; Gräber, R.; Strauß, E.; Siebert, U. Coexistence of Wolves and Humans in a Densely Populated Region (Lower Saxony, Germany). Basic Appl. Ecol. 2017, 25, 1–14. [Google Scholar] [CrossRef]
- van Eeden, L.M.; Slagle, K.; Crowther, M.S.; Dickman, C.R.; Newsome, T.M. Linking Social Identity, Risk Perception, and Behavioral Psychology to Understand Predator Management by Livestock Producers. Restor. Ecol. 2020, 28, 902–910. [Google Scholar] [CrossRef]
- Frank, J.; Johansson, M.; Flykt, A. Public Attitude towards the Implementation of Management Actions Aimed at Reducing Human Fear of Brown Bears and Wolves. Wildl. Biol. 2015, 21, 122–130. [Google Scholar] [CrossRef]
- Gross, L. No Place for Predators? PLoS Biol. 2008, 6, e40. [Google Scholar] [CrossRef]
- Gazzola, A.; Capitani, C.; Mattioli, L.; Apollonio, M. Livestock Damage and Wolf Presence. J. Zool. 2008, 274, 261–269. [Google Scholar] [CrossRef]
- Bisi, J.; Liukkonen, T.; Mykrä, S.; Pohja-Mykrä, M.; Kurki, S. The Good Bad Wolf—Wolf Evaluation Reveals the Roots of the Finnish Wolf Conflict. Eur. J. Wildl. Res. 2010, 56, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Fritts, S.H.; Stephenson, R.O.; Hayes, R.D.; Boitani, L. Wolves and Humans. In Wolves. Behaviour, Ecology, and Conservation; Mech, L.D., Boitani, L., Eds.; The University of Chicago Press: Chicago, IL, USA; London, UK, 2003; pp. 289–316. [Google Scholar]
- Mech, L.D.; Boitani, L. Introduction. In Wolves. Behaviour, Ecology, and Conservation; Mech, L.D., Boitani, L., Eds.; The University of Chicago Press: Chicago, IL, USA; London, UK, 2003; pp. XV–XVII. [Google Scholar]
- Janeiro-Otero, A.; Newsome, T.M.; van Eeden, L.M.; Ripple, W.J.; Dormann, C.F. Grey Wolf (Canis Lupus) Predation on Livestock in Relation to Prey Availability. Biol. Conserv. 2020, 243, 108433. [Google Scholar] [CrossRef]
- Fuller, T.K.; Mech, L.D.; Cochrane, J.F. Wolf Population Dynamics. In Wolves: Behavior, Ecology, and Conservation; Mech, L.D., Boitani, L., Eds.; Chicago University Press: Chicago, IL, USA, 2003; p. 33. [Google Scholar]
- Peterson, R.O.; Ciucci, P. The Wolf as a Carnivore. In Wolves: Behavior, Ecology, and Conservation; Mech, L.D., Boitani, L., Eds.; Chicago University Press: Chicago, IL, USA, 2003. [Google Scholar]
- Peterson, R.O.; Vucetich, J.A.; Bump, J.M.; Smith, D.W. Trophic Cascades in a Multicausal World: Isle Royale and Yellowstone. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 325–345. [Google Scholar] [CrossRef]
- Kaczensky, P.; Chapron, G.; von Arx, M.; Huber, D.; Andrén, H.; Linnell, J. Status, Management and Distribution of Large Carnivores—Bear, Lynx, Wolf and Wolverine in Europe. Part 1. IUCN/SSC Large Carnivore Initiative for Europe. 2013. Available online: https://books.google.com.hk/books/about/Status_Management_and_Distribution_of_La.html?id=gqLcjwEACAAJ&redir_esc=yvvvv (accessed on 10 February 2022).
- Recio, M.R.; Zimmermann, B.; Wikenros, C.; Zetterberg, A.; Wabakken, P.; Sand, H. Integrated Spatially-Explicit Models Predict Pervasive Risks to Recolonizing Wolves in Scandinavia from Human-Driven Mortality. Biol. Conserv. 2018, 226, 111–119. [Google Scholar] [CrossRef]
- Kaartinen, S.; Antikainen, H.; Kojola, I. Habitat Model for a Recolonizing Wolf (Canis Lupus) Population in Finland. Ann. Zool. Fenn. 2015, 52, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Mysłajek, R.W.; Tracz, M.; Tracz, M.; Tomczak, P.; Szewczyk, M.; Niedźwiecka, N.; Nowak, S. Spatial Organization in Wolves Canis Lupus Recolonizing North-West Poland: Large Territories at Low Population Density. Mamm. Biol. 2018, 92, 37–44. [Google Scholar] [CrossRef]
- Reinhardt, I.; Kluth, G.; Nowak, C.; Szentiks, C.A.; Krone, O.; Ansorge, H.; Mueller, T. Military Training Areas Facilitate the Recolonization of Wolves in Germany. Conserv. Lett. 2019, 12, e12635. [Google Scholar] [CrossRef]
- Sonne, C.; Alstrup, A.K.O. One Wolf Shot in Denmark Is Too Many. Nature 2018, 558, 519. [Google Scholar] [CrossRef]
- Eggermann, J.; da Costa, G.F.; Guerra, A.M.; Kirchner, W.H.; Petrucci-Fonseca, F. Presence of Iberian Wolf (Canis Lupus Signatus) in Relation to Land Cover, Livestock and Human Influence in Portugal. Mamm. Biol. 2011, 76, 217–221. [Google Scholar] [CrossRef]
- Vilá, C.; Castroviejo, J.; Urios, V. The Iberian Wolf in Spain. In Wolves in Europe: Status and Perspectives; Promberger, C., Schröeder, W., Eds.; Munich Wildlife Society: Ettal, Germany, 1993; pp. 104–109. [Google Scholar]
- Liberg, O.; Suutarinen, J.; Åkesson, M.; Andrén, H.; Wabakken, P.; Wikenros, C.; Sand, H. Poaching-Related Disappearance Rate of Wolves in Sweden Was Positively Related to Population Size and Negatively to Legal Culling. Biol. Conserv. 2020, 243, 108456. [Google Scholar] [CrossRef]
- Wikenros, C.; Sand, H.; Månsson, J.; Maartmann, E.; Eriksen, A.; Wabakken, P.; Zimmermann, B. Impact of a Recolonizing, Cross-Border Carnivore Population on Ungulate Harvest in Scandinavia. Sci. Rep. 2020, 10, 21670. [Google Scholar] [CrossRef]
- Bergerud, A.T. Caribou, Wolves and Man. Trends Ecol. Evol. 1988, 3, 68–72. [Google Scholar] [CrossRef]
- Sand, H.; Wabakken, P.; Zimmermann, B.; Johansson, Ö.; Pedersen, H.C.; Liberg, O. Summer Kill Rates and Predation Pattern in a Wolf–Moose System: Can We Rely on Winter Estimates? Oecologia 2008, 156, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Fuller, T.K. Effect of Snow Depth on Wolf Activity and Prey Selection in North Central Minnesota. Can. J. Zool. 1991, 283–287. [Google Scholar] [CrossRef]
- Jędrzejewski, W.; Schmidt, K.; Theuerkauf, J.; Jędrzejewska, B.; Selva, N.; Zub, K.; Szymura, L. Kill Rates and Predation by Wolves on Ungulate Populations in a Białowieża Primeval Forest (Poland). Ecology 2002, 83, 1341–1356. [Google Scholar] [CrossRef]
- Huggard, D. Prey Selectivity of Wolves in Banff National Park. I. Prey Species. Can. J. Zool. 2011, 71, 130–139. [Google Scholar] [CrossRef]
- Jędrzejewski, W.; Jedrzejewska, B.; Okarma, H.; Schmidt, K.; Zub, K.; Musiani, M. Prey Selection and Predation by Wolves in Bialowieza Primeval Forest, Poland. J. Mammal. 2000, 81, 197–212. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.S.; Garrott, R.A.; White, P.J.; Gower, C.N.; Bergman, E.J.; Jaffe, R. Chapter 16 Wolf Prey Selection in an Elk-Bison System: Choice or Circumstance? In The Ecology of Large Mammals in Central Yellowstone: Sixteen Years of Integrated Field Studies (Terrestrial Ecology); Garrott, R.A., White, P.J., Watson, F.G.R., Eds.; Elsevier: San Diego, CA, USA, 2008; Volume 3, pp. 305–337. [Google Scholar]
- Latham, A.; Latham, M.; Knopff, K.; Hebblewhite, M.; Boutin, S. Wolves, White-Tailed Deer, and Beaver: Implications of Seasonal Prey Switching for Woodland Caribou Declines. Ecography 2013, 36, 1276–1290. [Google Scholar] [CrossRef]
- Garrott, R.A.; Bruggeman, J.E.; Becker, M.S.; Kalinowski, S.T.; White, P.J. Evaluating Prey Switching in Wolf–Ungulate Systems. Ecol. Appl. 2007, 17, 1588–1597. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, B.; Sand, H.; Wabakken, P.; Liberg, O.; Andreassen, H.P. Predator-Dependent Functional Response in Wolves: From Food Limitation to Surplus Killing. J. Anim. Ecol. 2015, 84, 102–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jędrzejewski, W.; Schmidt, K.; Theuerkauf, J.; Jędrzejewska, B.; Kowalczyk, R. Territory Size of Wolves Canis Lupus: Linking Local (Białowieża Primeval Forest, Poland) and Holarctic-Scale Patterns. Ecography 2007, 30, 66–76. [Google Scholar] [CrossRef]
- Jędrzejewski, W.; Niedziałkowska, M.; Hayward, M.W.; Goszczyński, J.; Jędrzejewska, B.; Borowik, T.; Bartoń, K.A.; Nowak, S.; Harmuszkiewicz, J.; Juszczyk, A.; et al. Prey Choice and Diet of Wolves Related to Ungulate Communities and Wolf Subpopulations in Poland. J. Mammal. 2012, 93, 1480–1492. [Google Scholar] [CrossRef]
- Recio, M.R.; Singer, A.; Wabakken, P.; Sand, H. Agent-Based Models Predict Patterns and Identify Constraints of Large Carnivore Recolonizations, a Case Study of Wolves in Scandinavia. Biol. Conserv. 2020, 251, 108752. [Google Scholar] [CrossRef]
- Svensson, L.; Wabakken, P.; Maartmann, E.; Cardoso Palacios, E.; Flagstad, Ø.; Åkesson, M. Inventering Av Varg Vintern 2020-2021. Bestandsovervåking Av Ulv Vinteren 2020-2021. Bestandsstatus for Store Rovdyr i Skandinavia. 1-2020; Rovdata and SLU Viltskadecenter: Trondheim, Norway, 2021; p. 55. [Google Scholar]
- Falcucci, A.; Maiorano, L.; Tempio, G.; Boitani, L.; Ciucci, P. Modeling the Potential Distribution for a Range-Expanding Species: Wolf Recolonization of the Alpine Range. Biol. Conserv. 2013, 158, 63–72. [Google Scholar] [CrossRef]
- Wabakken, P.; Sand, H.; Liberg, O.; Bjärvall, A. The Recovery, Distribution, and Population Dynamics of Wolves on the Scandinavian Peninsula, 1978-1998. Can. J. Zool. 2001, 79, 710–725. [Google Scholar] [CrossRef]
- Karlsson, J.; Brøseth, H.; Sand, H.; Andrén, H. Predicting Occurrence of Wolf Territories in Scandinavia. J. Zool. 2007, 272, 276–283. [Google Scholar] [CrossRef]
- SLU Artdatabanken. Rödlistade Arter i Sverige 2020; SLU: Uppsala, Sweden, 2020. [Google Scholar]
- Åkesson, M.; Liberg, O.; Sand, H.; Wabakken, P.; Bensch, S.; Flagstad, Ø. Genetic Rescue in a Severely Inbred Wolf Population. Mol. Ecol. 2016, 25, 4745–4756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, T.; Dalerum, F. Identifying Potential Areas for an Expanding Wolf Population in Sweden. Biol. Conserv. 2018, 220, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Jensen, W.F. A Review of Circumpolar Moose Populations with Emphasis on Eurasian Moose Distributions and Densities. Alces 2020, 56, 1–16. [Google Scholar]
- Linnell, J.D.C.; Cretois, B.; Nilsen, E.B.; Rolandsen, C.M.; Solberg, E.J.; Veiberg, V.; Kaczensky, P.; Van Moorter, B.; Panzacchi, M.; Rauset, G.R.; et al. The Challenges and Opportunities of Coexisting with Wild Ungulates in the Human-Dominated Landscapes of Europe’s Anthropocene. Biol. Conserv. 2020, 244, 108500. [Google Scholar] [CrossRef]
- Magnusson, M. Population and Management Models for the Swedish Wild Boar (Sus Scrofa). Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2010. [Google Scholar]
- Jansson, G.; Månsson, J.; Magnusson, M. Hur Många Vildsvin Finns Det? Svensk. Jakt. 2010, 4, 86–87. [Google Scholar]
- Jarnemo, A. Seasonal Migration of Male Red Deer (Cervus Elaphus) in Southern Sweden and Consequences for Management. Eur. J. Wildl. Res. 2008, 54, 327–333. [Google Scholar] [CrossRef]
- Swedish Hunter’s Association. Utbredning Och Förekomst Av Kron- Och Dovhjort i Sverige—Analysis of Data from the Swedish Hunters’ Association Wildlife Monitoring 2016. 2017, p. 16. Available online: https://www.viltdata.se/wp-content/uploads/2017/04/Bilaga-kron-och-dov.pdf (accessed on 10 February 2022).
- Menichetti, L.; Touzot, L.; Elofsson, K.; Hyvönen, R.; Kätterer, T.; Kjellander, P. Interactions between a Population of Fallow Deer (Dama Dama), Humans and Crops in a Managed Composite Temperate Landscape in Southern Sweden: Conflict or Opportunity? PLoS ONE 2019, 14, e0215594. [Google Scholar] [CrossRef] [Green Version]
- Ueno, M.; Solberg, E.J.; Iijima, H.; Rolandsen, C.M.; Gangsei, L.E. Performance of Hunting Statistics as Spatiotemporal Density Indices of Moose (Alces Alces) in Norway. Ecosphere 2014, 5, art13. [Google Scholar] [CrossRef]
- Mattisson, J.; Sand, H.; Wabakken, P.; Gervasi, V.; Liberg, O.; Linnell, J.D.C.; Rauset, G.R.; Pedersen, H.C. Home Range Size Variation in a Recovering Wolf Population: Evaluating the Effect of Environmental, Demographic, and Social Factors. Oecologia 2013, 173, 813–825. [Google Scholar] [CrossRef] [Green Version]
- Jonzén, N.; Sand, H.; Wabakken, P.; Swenson, J.E.; Kindberg, J.; Liberg, O.; Chapron, G. Sharing the Bounty—Adjusting Harvest to Predator Return in the Scandinavian Human–Wolf–Bear–Moose System. Ecol. Modell. 2013, 265, 140–148. [Google Scholar] [CrossRef]
- Melis, C.; Nilsen, E.B.; Panzacchi, M.; Linnell, J.D.C.; Odden, J. Roe Deer Face Competing Risks between Predators along a Gradient in Abundance. Ecosphere 2013, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, J.M.; Festa-Bianchet, M.; Yoccoz, N.G.; Loison, A.; Toïgo, C. Temporal Variation in Fitness Components and Population Dynamics of Large Herbivores. Annu. Rev. Ecol. Syst. 2000, 31, 367–393. [Google Scholar] [CrossRef]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild Boar Populations up, Numbers of Hunters down? A Review of Trends and Implications for Europe: Wild Boar and Hunter Trends in Europe. Pest. Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Wikenros, C.; Sand, H.; Ahlqvist, P.; Liberg, O. Biomass Flow and Scavengers Use of Carcasses after Re-Colonization of an Apex Predator. PLoS ONE 2013, 8, e77373. [Google Scholar] [CrossRef] [Green Version]
- Wabakken, P.; Svensson, L.; Maartmann, E.; Åkesson, M.; Flagstad, Ø. Bestandsovervåking Av Ulv Vinteren 2015-2016/Inventering Av Varg Vintern 2015-2016. Bestandsstatus for Store Rovdyr i Skandinavia/Beståndsstatus För Stora Rovdjur i Skandinavien 1-2016; Rovdata and Viltskadecenter, SLU: Trondheim, Norway, 2016; p. 49s. [Google Scholar]
- Sand, H.; Eklund, A.; Zimmermann, B.; Wikenros, C.; Wabakken, P. Prey Selection of Scandinavian Wolves: Single Large or Several Small? PLoS ONE 2016, 11, e0168062. [Google Scholar] [CrossRef]
- Sand, H.; Vucetich, J.A.; Zimmermann, B.; Wabakken, P.; Wikenros, C.; Pedersen, H.C.; Peterson, R.O.; Liberg, O. Assessing the Influence of Prey-Predator Ratio, Prey Age Structure and Packs Size on Wolf Kill Rates. Oikos 2012, 121, 1454–1463. [Google Scholar] [CrossRef]
- Holt, R.D. Predation, Apparent Competition, and the Structure of Prey Communities. Theor. Popul. Biol. 1977, 12, 197–229. [Google Scholar] [CrossRef]
- Miller, J.R.B. Mapping Attack Hotspots to Mitigate Human–Carnivore Conflict: Approaches and Applications of Spatial Predation Risk Modeling. Biodivers. Conserv. 2015, 24, 2887–2911. [Google Scholar] [CrossRef]
- Helmer, W.; Saavedra, D.; Sylvén, M.; Schepers, F. Rewilding Europe: A New Strategy for an Old Continent. In Rewilding european landscapes; Pereira, H.M., Navarro, L.M., Eds.; Springer Open: Cham, Switzerland; Heidelberg, Germany; New York, NY, USA, 2015; ISBN 978-3-319-12038-6. [Google Scholar]
- Soulé, M.E.; Noss, R.F. Rewilding and Biodiversity Conservation as Complementary Goals for Continental Conservation. Wild Earth 1998, 8, 18–28. [Google Scholar]
- Jarvie, S.; Svenning, J.-C. Using Species Distribution Modelling to Determine Opportunities for Trophic Rewilding under Future Scenarios of Climate Change. Philos. Trans. R. Soc. Lond. Ser. B 2018, 373, 20170446. [Google Scholar] [CrossRef] [Green Version]

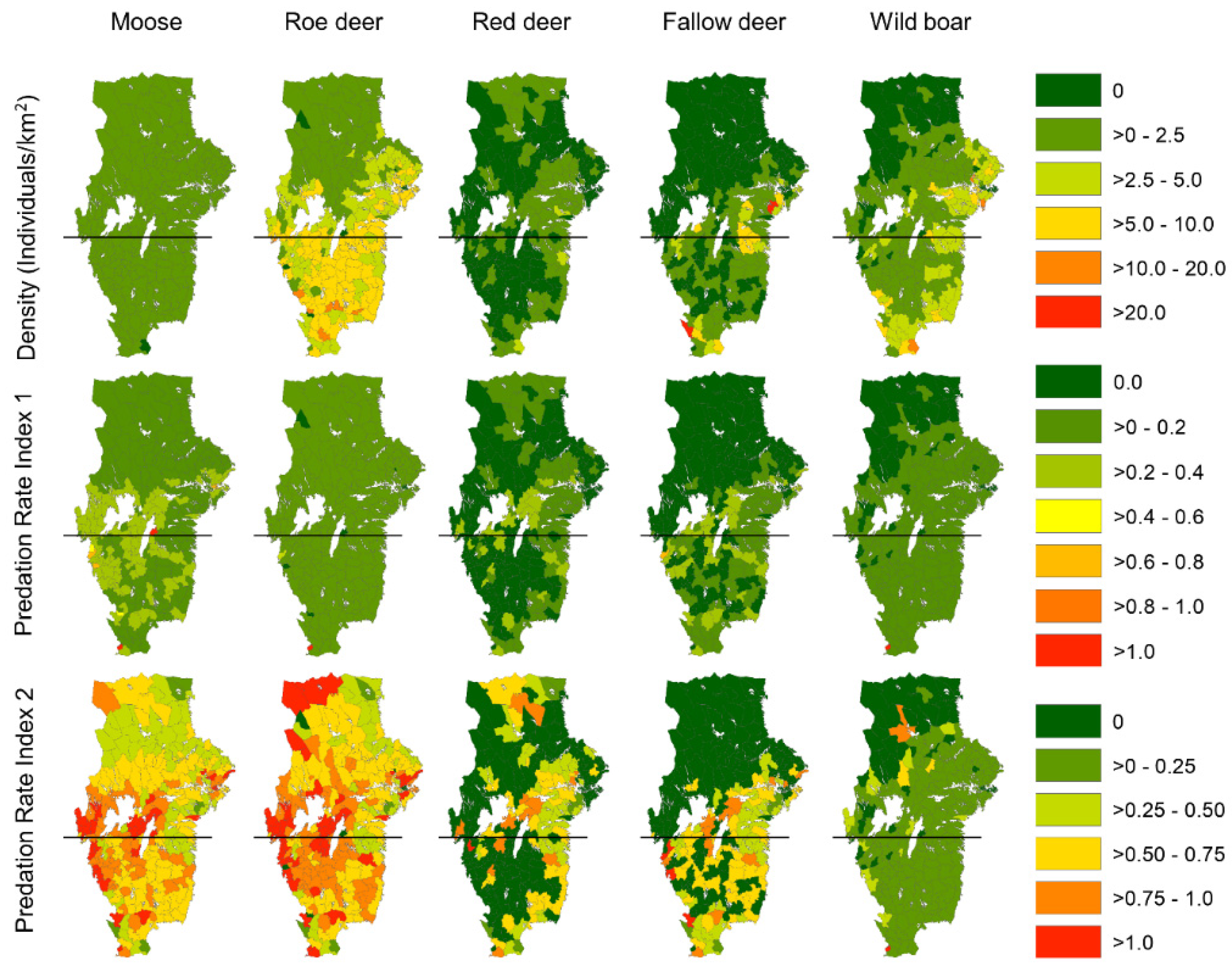

| Pr1 | Pr2 | |||||
|---|---|---|---|---|---|---|
| NWBR | SWBR | Total | NWBR | SWBR | Total | |
| Moose | 0.17 ± 0.04 | 0.20 ± 0.05 | 0.18 ± 0.03 | 0.65 ± 0.14 | 0.74 ± 0.18 | 0.69 ± 0.11 |
| Roe deer | 0.13 ± 0.01 | 0.19 ± 0.01 | 0.15 ± 0.01 | 0.92 ± 0.04 | 1.17 ± 0.04 | 1.02 ± 0.03 |
| Red deer | 0.05 ± 0.01 | 0.04 ± 0.05 | 0.05 ± 0.02 | 0.16 ± 0.12 | 0.13 ± 0.29 | 0.15 ± 0.14 |
| Fallow deer | 0.04 ± 0.01 | 0.09 ± 0.02 | 0.06 ± 0.01 | 0.13 ± 0.03 | 0.28 ± 0.06 | 0.19 ± 0.03 |
| Wild boar | 0.10 ± 0.01 | 0.17 ± 0.05 | 0.13 ± 0.02 | 0.31 ± 0.05 | 0.43 ± 0.11 | 0.36 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Recio, M.; Wikenros, C.; Zimmermann, B.; Sand, H. Rewilding by Wolf Recolonisation, Consequences for Ungulate Populations and Game Hunting. Biology 2022, 11, 317. https://doi.org/10.3390/biology11020317
Rodríguez-Recio M, Wikenros C, Zimmermann B, Sand H. Rewilding by Wolf Recolonisation, Consequences for Ungulate Populations and Game Hunting. Biology. 2022; 11(2):317. https://doi.org/10.3390/biology11020317
Chicago/Turabian StyleRodríguez-Recio, Mariano, Camilla Wikenros, Barbara Zimmermann, and Håkan Sand. 2022. "Rewilding by Wolf Recolonisation, Consequences for Ungulate Populations and Game Hunting" Biology 11, no. 2: 317. https://doi.org/10.3390/biology11020317
APA StyleRodríguez-Recio, M., Wikenros, C., Zimmermann, B., & Sand, H. (2022). Rewilding by Wolf Recolonisation, Consequences for Ungulate Populations and Game Hunting. Biology, 11(2), 317. https://doi.org/10.3390/biology11020317






