Calcined Oyster Shell Powder as a Natural Preservative for Maintaining Quality of White Shrimp (Litopenaeus vannamei)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
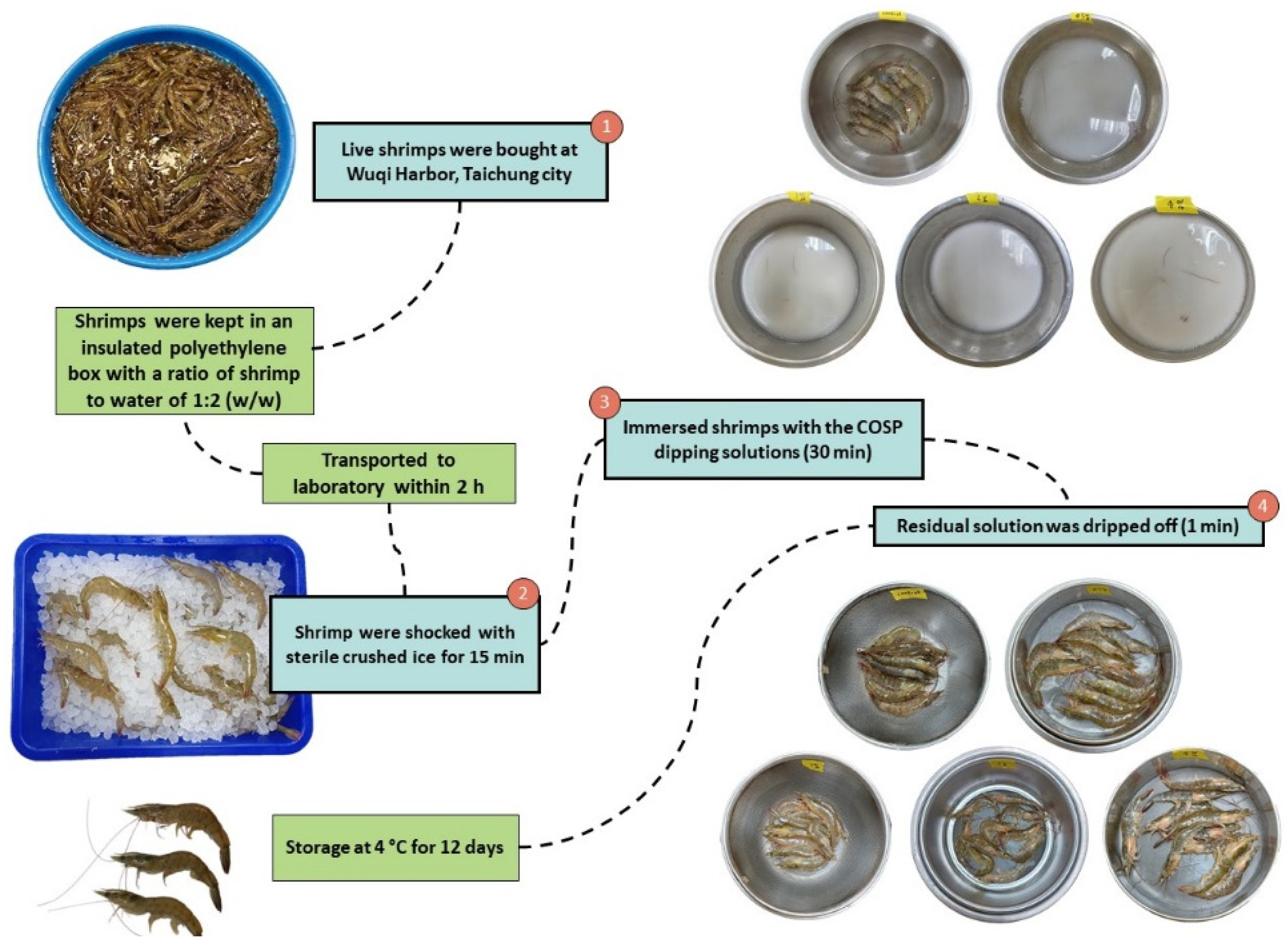
2.1. Materials
2.2. Preparation of COSP
2.3. Dipping Solutions and Refrigerated Storage Conditions for Shrimp
2.4. COSP Characterization
2.5. Biochemistry Quality Evaluation
2.6. Physical Quality Evaluation
2.7. Microbiology Analysis
2.8. Statistical Analyses
3. Results and Discussion
3.1. Characterization of COSP
3.2. Effect of COSP on pH in Shrimp during Refrigerated Storage
3.3. Effect of COSP on TVB-N Content in Shrimp during Refrigerated Storage
3.4. Effect of COSP on PV in Shrimp during Refrigerated Storage
3.5. Effect of COSP on TBARS Content in Shrimp during Refrigerated Storage
3.6. Effect of COSP on the Physical Quality of Shrimp during Refrigerated Storage
3.7. Microbiological Analysis of Refrigerated Shrimp
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cakli, S.; Kilinc, B.; Cadun, A.; Dincer, T.; Tolasa, S. Quality differences of whole ungutted sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) while stored in ice. Food Control 2007, 18, 391–397. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Varble, S.; Secchi, S. Fish Consumers: Environmental Attitudes and Purchasing Behavior. J. Food Prod. Market. 2017, 23, 267–282. [Google Scholar] [CrossRef]
- Silva, T.H.; Mesquita-Guimarães, I.; Henriques, B.; Silva, F.S.; Fredel, M.C. The potential use of oyster shell waste in new value-added by product. Resources 2019, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Choi, Y.; Noh, D.; Cho, S.; Suh, H.J. The effect of oyster shell powder on the extension of the shelf life of tofu. Food Chem. 2007, 103, 155–160. [Google Scholar] [CrossRef]
- Choi, Y.; Whang, J.; Kim, J.; Suh, H.J. The effect of oyster shell powder on the extension of the shelf-life of Kimchi. Food Control 2006, 17, 695–699. [Google Scholar] [CrossRef]
- Oikawa, K.; Asada, T.; Yamamoto, K.; Wakabayashi, H.; Sasaki, M.; Sato, M.; Matsuda, J. Antibacterial Activity of Calcined Shell Calcium Prepared from Wild Surf Clam. J. Health Sci. 2000, 46, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, K.; Park, K.; Seo, J. Oyster Shell Disposal: Potential as a Novel Ecofriendly Antimicrobial Agent for Packaging: A Mini Review. Korean. J. Packag. Sci. Technol. 2019, 25, 57–62. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.Z.; Yang, R.; Antenucci, R.; Mills, B.; Cassidy, J.; Scullen, O.J.; Sites, J.E.; Rajkowski, K.T.; Sommers, C.H. Inactivation of natural microflora and inoculated Listeria innocua on whole raw shrimp by ozonated water, antimicrobial coatings, and cryogenic freezing. Food Control 2013, 34, 24–30. [Google Scholar] [CrossRef]
- Okpala, C.O.R. Investigation of quality attributes of ice-stored pacific white shrimp (Litopenaeus vannamei) as affected by sequential minimal ozone treatment. LWT-Food Sci. Technol. 2014, 57, 538–549. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists International: Rockville, MD, USA, 2000; Volume II, Chapter 39; pp. 1–27. [Google Scholar]
- Wang, H.B. Effect of dandelion polysacchardies on the retardation of the quality changes of white shrimp. Int. J. Biol. Macromol. 2014, 68, 205–208. [Google Scholar] [CrossRef] [PubMed]
- AOCS. Official Methods and Recommended Practices of the American and Chemists Society, 4th ed.; AOCS: Champaign, II, USA, 1990. [Google Scholar]
- Tongwanichniyom, S.; Pattamapitoon, T.; Sangvichien, N.; Phornphisutthimas, S. Production of calcium oxide from waste oyster shells for a value-added application of antibacteria. Ecol. Enviroment Conserv. 2021, 27, 539–549. [Google Scholar]
- Nirmal, N.P.; Benjakul, S. Retardation of quality changes of Pacific white shrimp by green tea extract treatment and modified atmosphere packaging during retrigerated storage. Int. J. Biol. Macromol. 2011, 149, 247–253. [Google Scholar]
- Arancibia, M.Y.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Chitosan coatings enriched with active shrimp waste for shrimp preservation. Food Control 2015, 54, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Farajzadeh, F.; Motamedzadegan, A.; Shahidi, S.; Hamzeh, S. The effect of chitosan-gelatin coating on the quality of shrimp (Litopenaeus vannamei) under refrigerated condition. Food Control 2016, 67, 163–170. [Google Scholar] [CrossRef]
- Nowzari, F.; Shábanpoun, B.; Ojagh, S.M. Comparison of chitosan-gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef]
- Klinmalai, P.; Fong-in, S.; Phongthai, S.; Klunklin, W. Improving the quality of frozen fillets of semi-dried Gourami Fish (Trichogaster pectoralis) by using sorbitol and citric Acid. Foods 2021, 10, 2763. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kamil, J.Y.; Shahidi, F. Chitosan as an edible invisible film for quality preservation of herring and Atlantic cod. J. Agric. Food Chem. 2002, 20, 5167–5178. [Google Scholar] [CrossRef]
- Sathiel, S.; Liu, Q.; Huang, J.; Prinyawiwatkul, W. The influence of chitosan glazing on the quality of skinless pink salmon (Oncorhynchus garbuscha) fillets during frozen sotrage. J. Food Eng. 2007, 83, 366–373. [Google Scholar] [CrossRef]
- Dirapan, P.; Boonyakiat, D.; Poonlarp, P. Improving shelf life, maintaining quality, and delaying microbial growth of broccoli in supply chain using commercial vacuum cooling and package icing. Horticulturae 2021, 7, 506. [Google Scholar] [CrossRef]
- Aziman, N.; Jawaid, M.; Mutalib, N.A.A.; Yusof, N.L.; Nadrah, A.H.; Nazatul, U.K.; Tverezovskiy, V.V.; Tverezovskaya, O.A.; Fouad, H.; Braganca, R.M.; et al. Antimicrobial potential of plastic films incorporated with sage extract on chicken meat. Foods 2021, 10, 2812. [Google Scholar] [CrossRef] [PubMed]
- Bindu, J.; Ginson, J.; Kamalakanth, C.K.; Asha, K.K.; Srinivasa Gopal, T.K. Physicochemical changes in high pressure coated Indian white prawn (Fenneropenacus indicus) during chill storage. Innov. Food Sci. Emerg. 2013, 17, 37–42. [Google Scholar] [CrossRef]
- Speranza, B.; Racioppo, A.; Bevilacqua, A.; Buzzo, V.; Marigliano, P.; Mocerino, E.; Scognamiglio, R.; Corbo, M.R.; Scognamiglio, G.; Sinigaglia, M. Innovative preservation methods improving the quality and safety of fish products: Beneficial effects and limits. Foods 2021, 10, 2854. [Google Scholar] [CrossRef] [PubMed]
- Firdous, A.; Ringø, E.; Elumalai, P. Effects of green tea- and amla extracts on quality and melanosis of Indian white prawn (Fenneropenaeus indicus, Milne Edwards, 1837) during chilled storage. Aquac. Fish. 2021, 6, 617–627. [Google Scholar] [CrossRef]
- Nagarajan, M.; Rajasekaran, B.; Benjakul, S.; Venkatachalam, K. Influence of chitosan-gelatin edible coating incorporated with longkong pericarp extract on refrigerated black tiger Shrimp (Penaeus monodon). Cur. Res. Food Sci. 2021, 4, 345–353. [Google Scholar] [CrossRef]
- Do, D.T.B.; Bui, T.H.; Phan, D.T.A. Persea Americana Mill seed extracts: Understanding insights into the antioxidant and antityrosinase activities and effects on preserving qualities of whiteleg shrimp (Litopenaus vannamei) during refrigerated storage. Food Chem. 2022, 373, 131469. [Google Scholar] [CrossRef]
- Kontominas, M.G.; Badeka, A.V.; Kosma, I.S.; Nathanailides, C.I. Innovative seafood preservation technologies: Recent developments. Animals 2021, 11, 92. [Google Scholar] [CrossRef]
- Mace, S.; Cardinal, M.; Jaffres, E.; Cornet, J.; Lalanne, V.; Chevalier, F.; Sérot, T.; Pilet, M.; Dousset, X.; Joffraud, J. Evaluation of the spoilage potential of bacterial isolated from spoiled cooked whole tropical shrimp (Penaeus vannamei) stored under modified atmosphere packaging. Food Microbiol. 2014, 40, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jia, Y.; Hu, Y.; Xia, X.; Li, Y.; Zhou, J.; Liu, Y. Effect of Citrus wilsonii Tanaka extract combined with alginate-calcium coating on quality maintenance of white shrimps (Litopenaeus vannamei Boone). Food Control 2016. [Google Scholar] [CrossRef]








| Attributes | COSP (%) | Storage Days | ||||
|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | ||
| pH | Control | 6.58 ± 0.06 a | 6.67 ± 0.03 a | 7.01 ± 0.06 a | 7.48 ± 0.07 a | 7.68 ± 0.09 a |
| 0.5% | 6.62 ± 0.11 a | 6.64 ± 0.02 a | 6.90 ± 0.07 b | 7.37 ± 0.05 a | 7.61 ± 0.02 a | |
| 1.0% | 6.56 ± 0.09 a | 6.60 ± 0.04 a | 6.83 ± 0.03 b | 6.88 ± 0.04 b | 6.91 ± 0.04 b | |
| 2.0% | 6.61 ± 0.04 a | 6.62 ± 0.06 a | 6.79 ± 0.09 bc | 6.82 ± 0.05 b | 6.84 ± 0.06 b | |
| 4.0% | 6.67 ± 0.13 a | 6.63 ± 0.03 a | 6.72 ± 0.05 c | 6.78 ± 0.09 b | 6.80 ± 0.05 b | |
| COSP (%) | Storage Days | |||||
|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | ||
| TVC | Control | 1.47 ± 0.06 a × 102 | 1.13 ± 0.22 a × 103 | 2.34 ± 0.21 a × 104 | 4.66 ± 0.22 a × 106 | 1.58 ± 0.32 a × 108 |
| 0.5% | 1.21 ± 0.11 b × 102 | 6.34 ± 0.23 b × 102 | 9.17 ± 1.50 b × 103 | 2.82 ± 0.14 b × 105 | 8.22 ± 0.66 b × 107 | |
| 1.0% | 1.07 ± 0.15 b × 102 | 3.77 ± 0.12 c × 102 | 4.67 ± 0.35 c × 103 | 6.15 ± 0.18 c × 104 | 5.21 ± 0.23 c × 106 | |
| 2.0% | 7.23 ± 0.56 c × 101 | 3.14 ± 0.06 d × 102 | 1.03 ± 0.15 d × 103 | 3.25 ± 0.18 d × 104 | 3.16 ± 0.54 d × 105 | |
| 4.0% | 4.57 ± 0.23 d × 101 | 6.25 ± 0.12 e × 101 | 3.67 ± 0.22 e × 102 | 5.25 ± 0.18 e × 103 | 6.22 ± 0.15 e × 104 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.-C.; Chiu, C.-S.; Hsieh, C.-W.; Chan, Y.-J.; Liang, Z.-C.; Wang, C.-C.R.; Mulio, A.T.; Le, D.H.T.; Li, P.-H. Calcined Oyster Shell Powder as a Natural Preservative for Maintaining Quality of White Shrimp (Litopenaeus vannamei). Biology 2022, 11, 334. https://doi.org/10.3390/biology11020334
Lu W-C, Chiu C-S, Hsieh C-W, Chan Y-J, Liang Z-C, Wang C-CR, Mulio AT, Le DHT, Li P-H. Calcined Oyster Shell Powder as a Natural Preservative for Maintaining Quality of White Shrimp (Litopenaeus vannamei). Biology. 2022; 11(2):334. https://doi.org/10.3390/biology11020334
Chicago/Turabian StyleLu, Wen-Chien, Chien-Shan Chiu, Chang-Wei Hsieh, Yung-Jia Chan, Zeng-Chin Liang, Chiun-C. Roger Wang, Amanda Tresiliana Mulio, Dung Huynh Thi Le, and Po-Hsien Li. 2022. "Calcined Oyster Shell Powder as a Natural Preservative for Maintaining Quality of White Shrimp (Litopenaeus vannamei)" Biology 11, no. 2: 334. https://doi.org/10.3390/biology11020334
APA StyleLu, W. -C., Chiu, C. -S., Hsieh, C. -W., Chan, Y. -J., Liang, Z. -C., Wang, C. -C. R., Mulio, A. T., Le, D. H. T., & Li, P. -H. (2022). Calcined Oyster Shell Powder as a Natural Preservative for Maintaining Quality of White Shrimp (Litopenaeus vannamei). Biology, 11(2), 334. https://doi.org/10.3390/biology11020334









