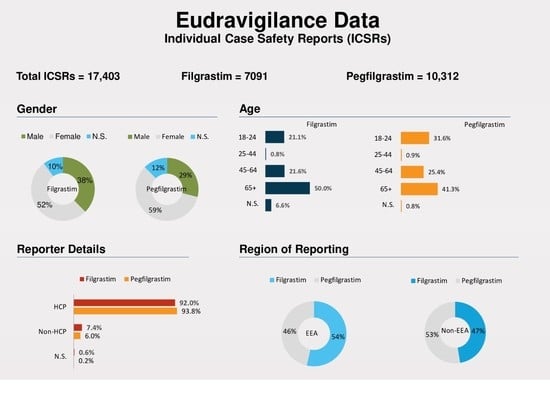
Comparative Study of Adverse Drug Reactions Associated with Filgrastim and Pegfilgrastim Using the EudraVigilance Database
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methodology
2.1. Data Source
2.2. Data Selection
2.3. Data Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aapro, M.; Bohlius, J.; Cameron, D.; Lago, L.D.; Donnelly, J.P.; Kearney, N.; Lyman, G.; Pettengell, R.; Tjan-Heijnen, V.; Walewski, J.; et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur. J. Cancer 2011, 47, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Ajithkumar, T.V.; Hatcher, H. Specialist Training in Oncology E-Book; Elsevier Health Sciences: St Louis, MO, USA, 2011. [Google Scholar]
- Klastersky, J.; de Naurois, J.; Rolston, K.; Rapoport, B.; Maschmeyer, G.; Aapro, M.; Herrstedt, J. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 2016, 27, v111–v118. [Google Scholar] [CrossRef] [PubMed]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.; et al. Outpatient Management of Fever and Neutropenia in Adults Treated for Malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1443–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Baser, O.; Kutikova, L.; Page, J.H.; Barron, R. The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: A systematic review and meta-analysis of randomized controlled trials. Support. Care Cancer 2015, 23, 3131–3140. [Google Scholar] [CrossRef] [Green Version]
- Caggiano, V.; Weiss, R.V.; Rickert, T.S.; Linde-Zwirble, W.T. Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 2005, 103, 1916–1924. [Google Scholar] [CrossRef]
- Hirsch, B.R.; Lyman, G.H. Pharmacoeconomics of the Myeloid Growth Factors. PharmacoEconomics 2012, 30, 497–511. [Google Scholar] [CrossRef]
- Bennett, C.L.; Djulbegovic, B.; Norris, L.B.; Armitage, J.O. Colony-stimulating factors for febrile neutropenia during cancer therapy. N. Engl. J. Med. 2013, 368, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Baden, L.R.; Swaminathan, S.; Angarone, M.; Blouin, G.; Camins, B.C.; Casper, C.; Cooper, B.; Dubberke, E.R.; Engemann, A.M.; Freifeld, A.G.; et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 882–913. [Google Scholar] [CrossRef]
- Carmona-Bayonas, A.; Jimenez-Fonseca, P.; De Castro, E.M.; Mata, E.; Biosca, M.; Custodio, A.; Espinosa, J.; Vázquez, E.G.; Henao, F.; De La Peña, F.A. SEOM clinical practice guideline: Management and prevention of febrile neutropenia in adults with solid tumors (2018). Clin. Transl. Oncol. 2018, 21, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Welte, K.; Gabrilove, J.; Bronchud, M.H.; Platzer, E.; Morstyn, G. Filgrastim (r-metHuG-CSF): The first 10 years. Blood 1996, 88, 1907–1929. [Google Scholar] [CrossRef] [Green Version]
- Mehta, H.M.; Malandra, M.; Corey, S.J. G-CSF and GM-CSF in Neutropenia. J. Immunol. 2015, 195, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Molineux, G. Pegfilgrastim: Using pegylation technology to improve neutropenia support in cancer patients. Anti-Cancer Drugs 2003, 14, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Molineux, G.; Kinstler, O.; Briddell, B.; Hartley, C.; McElroy, P.; Kerzic, P.; Sutherland, W.; Stoney, G.; Kern, B.; Fletcher, F.A.; et al. A new form of Filgrastim with sustained duration in vivo and enhanced ability to mobilize PBPC in both mice and humans. Exp. Hematol. 1999, 27, 1724–1734. [Google Scholar] [CrossRef]
- Johnston, E.; Crawford, J.; Blackwell, S.; Bjurstrom, T.; Lockbaum, P.; Roskos, L.; Yang, B.-B.; Gardner, S.; Miller-Messana, M.A.; Shoemaker, D.; et al. Randomized, dose-escalation study of SD/01 compared with daily filgrastim in patients receiving chemotherapy. J. Clin. Oncol. 2000, 18, 2522–2528. [Google Scholar] [CrossRef]
- Holmes, F.; O’shaughnessy, J.; Vukelja, S.; Jones, S.; Shogan, J.; Savin, M.; Richards, D.; Glaspy, J.; Meza, L.; Cohen, G.; et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J. Clin. Oncol. 2002, 20, 727–731. [Google Scholar] [CrossRef]
- Holmes, F.A.; Jones, S.E.; O’Shaughnessy, J.; Vukelja, S.; George, T.; Savin, M.; Richards, D.; Glaspy, J.; Meza, L.; Cohen, G.; et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim inchemotherapy-induced neutropenia: Amulticenterdose-finding study in women with breast cancer. Ann. Oncol. 2002, 13, 903–909. [Google Scholar] [CrossRef]
- Vose, J.M.; Crump, M.; Lazarus, H.; Emmanouilides, C.; Schenkein, D.; Moore, J.; Frankel, S.; Flinn, I.; Lovelace, W.; Hackett, J.; et al. Randomized, multicenter, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma. J. Clin. Oncol. 2003, 21, 514–519. [Google Scholar] [CrossRef]
- Agency, E.M. European Public Assessment Report for Lonquex. 2013. Available online: http://www.ema.europa.eu/docs/en-GB/document-library/EPAR-Summaryforthepublic/human/002556/WC500148383.pdf (accessed on 30 October 2021).
- Welte, K.; Reiter, A.; Mempel, K.; Pfetsch, M.; Schwab, G.; Schrappe, M.; Riehm, H. A randomized phase-III study of the efficacy of granulocyte colony-stimulating factor in children with high-risk acute lymphoblastic leukemia. Berlin-Frankfurt-Münster Study Group. Blood 1996, 87, 3143–3150. [Google Scholar] [CrossRef] [Green Version]
- Murata, M.; Harada, M.; Kato, S.; Takahashi, S.; Ogawa, H.; Okamoto, S.; Tsuchiya, S.; Sakamaki, H.; Akiyama, Y.; Kodera, Y. Peripheral blood stem cell mobilization and apheresis: Analysis of adverse events in 94 normal donors. Bone Marrow Transplant. 1999, 24, 1065–1071. [Google Scholar] [CrossRef] [Green Version]
- Kubo, K.; Miyazaki, Y.; Murayama, T.; Shimazaki, R.; Usui, N.; Urabe, A.; Hotta, T.; Tamura, K. A randomized, double-blind trial of pegfilgrastim versus filgrastim for the management of neutropenia during CHASE (R) chemotherapy for malignant lymphoma. Br. J. Haematol. 2016, 174, 563–570. [Google Scholar] [CrossRef]
- Rastogi, S.; Shukla, S.; Sharma, A.K.; Sarwat, M.; Srivastava, P.; Katiyar, T.; Kalaiselvan, V.; Singh, G.N. Towards a comprehensive safety understanding of granulocyte-colony stimulating factor biosimilars in treating chemotherapy associated febrile neutropenia: Trends from decades of data. Toxicol. Appl. Pharmacol. 2020, 395, 114976. [Google Scholar] [CrossRef] [PubMed]
- Postigo, R.; Brosch, S.; Slattery, J.; van Haren, A.; Dogné, J.M.; Kurz, X.; Candore, G.; Domergue, F.; Arlett, P. EudraVigilance medicines safety database: Publicly accessible data for research and public health protection. Drug Saf. 2018, 41, 665–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozzicato, P. MedDRA. Pharm. Med. 2009, 23, 65–75. [Google Scholar] [CrossRef]
- di Mauro, G.; Zinzi, A.; Scavone, C.; Mascolo, A.; Gaio, M.; Sportiello, L.; Ferrajolo, C.; Rafaniello, C.; Rossi, F.; Capuano, A. PCSK9 Inhibitors and Neurocognitive Adverse Drug Reactions: Analysis of Individual Case Safety Reports from the Eudravigilance Database. Drug Saf. 2020, 44, 337–349. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.E.; Hayley Bee, B.; Darby Tozer, B.; Train, J.E. Impact on Patients and Families. Oncology Nurse Advisor. 2012, pp. 29–32. Available online: https://www.oncologynurseadvisor.com/wp-content/uploads/sites/13/2019/01/ona_filgrastim0612_8956.pdf (accessed on 24 September 2021).
- Leonard, R.C.F.; Mansi, J.L.; Keerie, C.; Yellowlees, A.; Crawford, S.; Benstead, K.; Matthew, R.; Adamson, D.; Chan, S.; Grieve, R. A randomised trial of secondary prophylaxis using granulocyte colony-stimulating factor (‘SPROG’ trial) for maintaining dose intensity of standard adjuvant chemotherapy for breast cancer by the Anglo-Celtic Cooperative Group and NCRN. Ann. Oncol. 2015, 26, 2437–2441. [Google Scholar] [CrossRef]
- Moore, D.C.; Pellegrino, A.E. Pegfilgrastim-Induced Bone Pain: A Review on Incidence, Risk Factors, and Evidence-Based Management. Ann. Pharmacother. 2017, 51, 797–803. [Google Scholar] [CrossRef]
- Lyman, G.H.; Lalla, A.; Barron, R.L.; Dubois, R.W. Cost-effectiveness of pegfilgrastim versus filgrastim primary prophylaxis in women with early-stage breast cancer receiving chemotherapy in the united states. Clin. Ther. 2009, 31, 1092–1104. [Google Scholar] [CrossRef]
- Green, M.D.; Koelbl, H.; Baselga, J.; Galid, A.; Guillem, V.; Gascon, P.; Siena, S.; Lalisang, R.I.; Samonigg, H.; Clemens, M.R.; et al. A randomized double-blind multicenter phase III study offixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann. Oncol. 2003, 14, 29–35. [Google Scholar] [CrossRef]
- Rastogi, S.; Kalaiselvan, V.; Ali, S.; Ahmad, A.; Guru, S.A.; Sarwat, M. Efficacy and Safety of Filgrastim and Its Biosimilars to Prevent Febrile Neutropenia in Cancer Patients: A Prospective Study and Meta-Analysis. Biology 2021, 10, 1069. [Google Scholar] [CrossRef]
- Cornes, P.; Gascon, P.; Chan, S.; Hameed, K.; Mitchell, C.R.; Field, P.; Latymer, M.; Arantes, L.H., Jr. Systematic Review and Meta-analysis of Short- versus Long-Acting Granulocyte Colony-Stimulating Factors for Reduction of Chemotherapy-Induced Febrile Neutropenia. Adv. Ther. 2018, 35, 1816–1829. [Google Scholar] [CrossRef] [Green Version]
- Grigg, A.; Solal-Celigny, P.; Hoskin, P.; Taylor, K. Open-label, Randomized Study of Pegfilgrastim vs. Daily Filgrastim as an Adjunct to Chemotherapy in Elderly Patients with Non-Hodgkin’s Lymphoma. Leuk. Lymphoma 2003, 44, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Del Giglio, A.; Eniu, A.; Ganea-Motan, D.; Topuzov, E.; Lubenau, H. XM02 is superior to placebo and equivalent to Neupogen™ in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in cycle 1 in breast cancer patients receiving docetaxel/doxorubicin chemotherapy. BMC Cancer 2008, 8, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engert, A.; Griskevicius, L.; Zyuzgin, Y.; Lubenau, H.; Del Giglio, A. XM02, the first granulocyte colony-stimulating factor biosimilar, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with non-Hodgkin lymphoma receiving chemotherapy. Leuk. Lymphoma 2009, 50, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Hashimoto, K.; Nishikawa, K. Clinical safety and efficacy of “filgrastim biosimilar 2” in Japanese patients in a post-marketing surveillance study. J. Infect. Chemother. 2018, 24, 363–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, L.; Liu, Z.; Doan, Q.; Bernal, M.; Dubois, R.; Lyman, G. Comparison of pegfilgrastim with filgrastim on febrile neutropenia, grade IV neutropenia and bone pain: A meta-analysis of randomized controlled trials. Curr. Med. Res. Opin. 2007, 23, 2283–2295. [Google Scholar] [CrossRef]

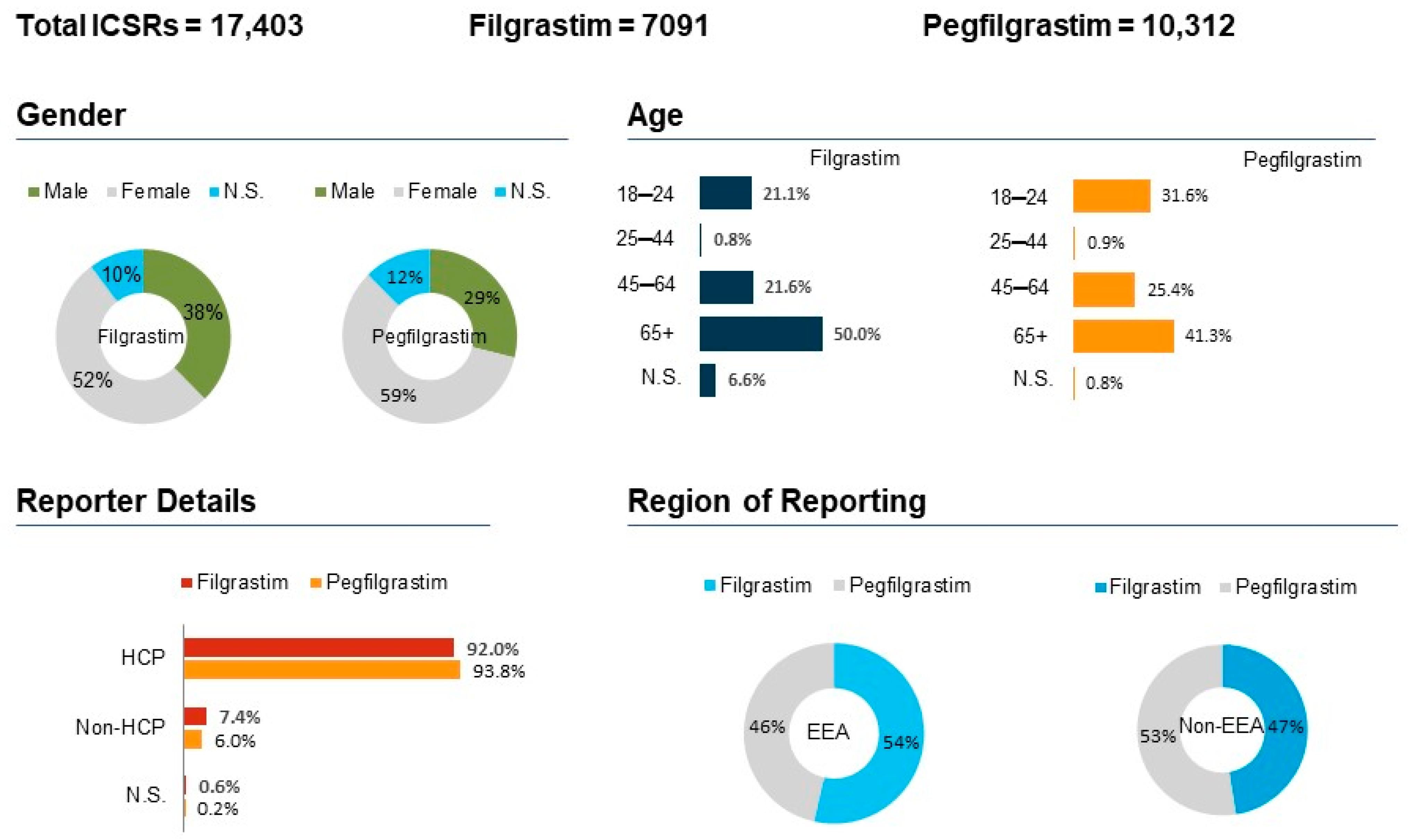


| System Organ Class | PT Term | Filgrastim ICSRs (n = 7091) | Pegfilgrastim ICSRs (n = 10,312) |
|---|---|---|---|
| General disorders and administration site conditions (n = 6084) | [n = 2600] | [n = 3484] | |
| Pyrexia | 7.8% (559) | 6.8% (705) | |
| Drug Ineffectiveness | 5.7% (407) | 5.4% (559) | |
| Death | 3.4% (244) | 5.3% (548) | |
| Blood and lymphatic system disorders (n = 4908) | [n = 1766] | [n = 3142] | |
| Neutropenia | 8.1% (579) | 10.9% (1129) | |
| Febrile Neutropenia | 7.4% (526) | 13.4% (1391) | |
| Thrombocytopenia | 3.1% (221) | 1.9% (199) | |
| Musculoskeletal and connective tissue disorders (n = 2662) | [n = 1191] | [n = 1471] | |
| Bone Pain | 6.6% (473) | 6.9% (719) | |
| Back Pain | 4.2% (303) | 2.3% (240) | |
| Arthralgia | 3.0% (214) | 2.5% (267) | |
| Investigations (n = 2740) | [n = 1123] | [n = 1617] | |
| WBC count decreased | 3.2% (229) | 3.3% (346) | |
| Platelet count decreased | 2.1% (155) | 1.3% (135) | |
| Neutrophil count decreased | 1.7% (123) | 2.3% (244) |
| ICSRs | ROR (95% CI) | p Value |
|---|---|---|
| General disorders and administration site conditions | 1.14 (1.06–1.21) | <0.0001 |
| Blood and lymphatic system disorders | 0.75 (0.70–0.80) | <0.0001 |
| Musculoskeletal and connective tissue disorders | 1.21 (1.18–1.32) | <0.0001 |
| Investigations | 1.01 (0.93–1.10) | 0.78 |
| ADRs | ROR (95% CI) | p Value |
| Pyrexia | 1.16 (1.04–1.30) | <0.05 |
| Bone Pain | 0.95 (0.84–1.07) | 0.456 |
| Back Pain | 1.87 (1.57–2.22) | <0.0001 |
| Neutropenia | 0.72 (0.65–0.80) | <0.0001 |
| Febrile Neutropenia | 0.51 (0.46–0.57) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastogi, S.; Kalaiselvan, V.; Bin Jardan, Y.A.; Zameer, S.; Sarwat, M. Comparative Study of Adverse Drug Reactions Associated with Filgrastim and Pegfilgrastim Using the EudraVigilance Database. Biology 2022, 11, 340. https://doi.org/10.3390/biology11020340
Rastogi S, Kalaiselvan V, Bin Jardan YA, Zameer S, Sarwat M. Comparative Study of Adverse Drug Reactions Associated with Filgrastim and Pegfilgrastim Using the EudraVigilance Database. Biology. 2022; 11(2):340. https://doi.org/10.3390/biology11020340
Chicago/Turabian StyleRastogi, Shruti, Vivekanandan Kalaiselvan, Yousef A. Bin Jardan, Saima Zameer, and Maryam Sarwat. 2022. "Comparative Study of Adverse Drug Reactions Associated with Filgrastim and Pegfilgrastim Using the EudraVigilance Database" Biology 11, no. 2: 340. https://doi.org/10.3390/biology11020340
APA StyleRastogi, S., Kalaiselvan, V., Bin Jardan, Y. A., Zameer, S., & Sarwat, M. (2022). Comparative Study of Adverse Drug Reactions Associated with Filgrastim and Pegfilgrastim Using the EudraVigilance Database. Biology, 11(2), 340. https://doi.org/10.3390/biology11020340