Modeling of Valeriana wallichii Habitat Suitability and Niche Dynamics in the Himalayan Region under Anticipated Climate Change
Abstract
:Simple Summary
Abstract
1. Introduction
- (i)
- Study the role of different bioclimatic variables on the habitat distribution of V. wallichii;
- (ii)
- Model the current distribution range of V. wallichii in the Himalayan biodiversity hotspots;
- (iii)
- Model the climate change-driven shifting patterns in the distribution of V. wallichii at different spatiotemporal scales;
- (iv)
- Predicting the extent and rate of potential range expansion/contraction of V. wallichii and evaluating the niche dynamics using the models generated to formulate different management strategies.
2. Materials and Methods
2.1. Distribution Data
2.2. Bioclimatic Variables and Their Importance
2.3. Modeling Technique
2.4. Species Range Change
2.5. Niche Overlap
3. Results
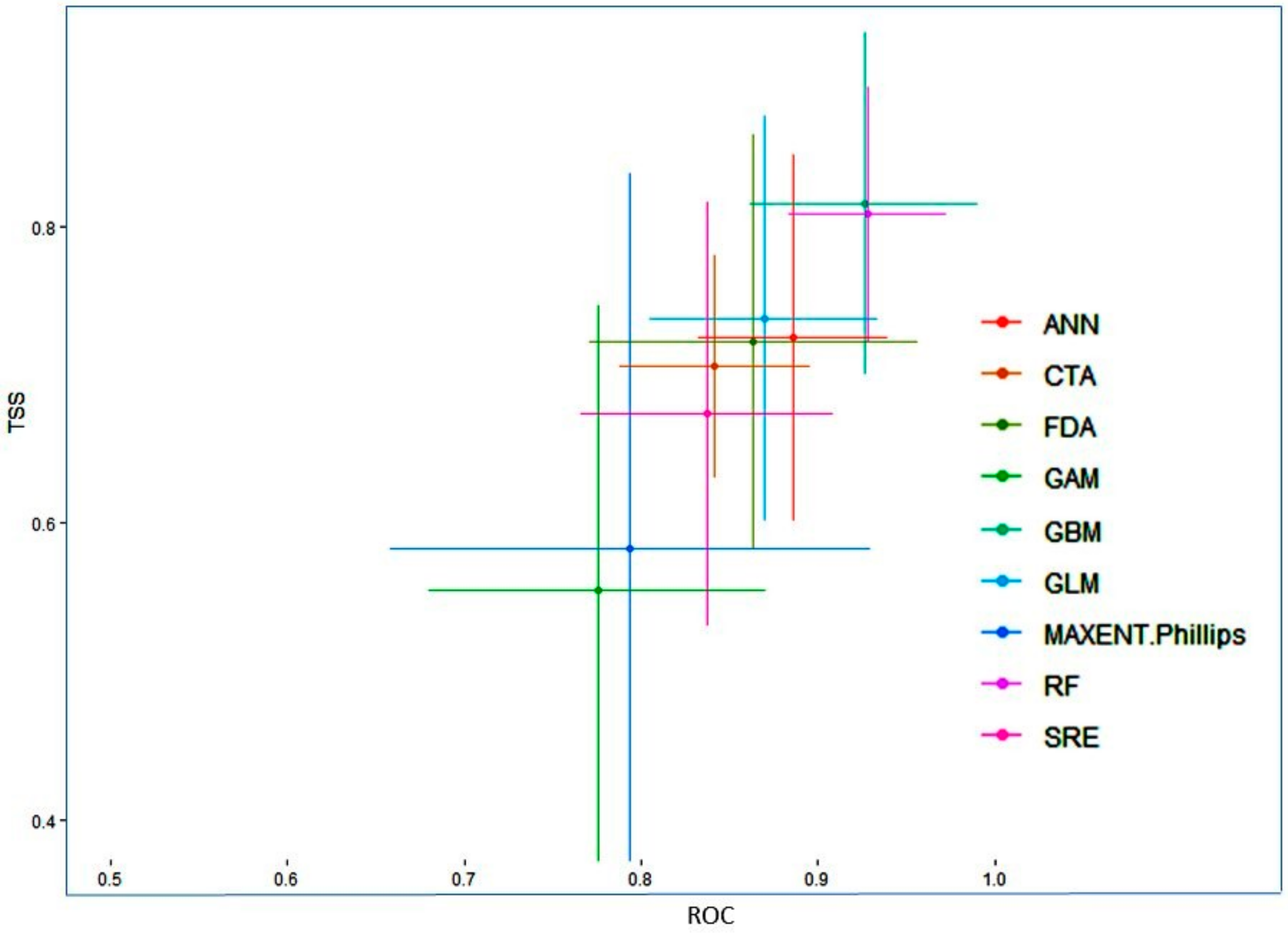
3.1. Model Evaluation and Variable Contribution
3.2. Current and Future Habitat Distribution
3.3. Species Range Shift under Future Climatic Conditions
3.4. Niche Dynamics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area Under Curve |
| TSS | True Skill Statistics |
| RCP | Representative Concentration Pathway |
| PCA | Principal Component Analysis |
| IUCN | International Union for Conservation of Nature and Natural Resources |
| ANN | Artifical Neural Network |
| CTA | Cluster Tree Analysis |
| GAM | Generalized Additive Model |
| GBM | Generalized Boosted Model |
| GLM | Generalized Linear Model |
| MaxEnt | Maximum Entropy |
| RF | Random Forest |
| SRE | Surface Range Envelope |
| FDA | Flexible Discriminant Analysis |
References
- Cruz, R.V.; Harasawa, H.; Lal, M.; Wu, S.; Anokhin, Y.; Punsalmaa, B.; Honda, Y.; Jafari, M.; Li, C.; Huu, N.N. Asia climate change 2007: Impacts, adaptation and vulnerability. In Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 469–506. [Google Scholar]
- Shrestha, U.B.; Gautam, S.; Bawa, K.S. Widespread Climate Change in the Himalayas and Associated Changes in Local Ecosystems. PLoS ONE 2012, 7, e36741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.; Zhang, H.; Wang, Y.; Zhu, Y. Assessing the impact of climate variability and human activities on streamflow variation. Hydr. Earth Syst. Sci. 2016, 20, 1547–1560. [Google Scholar] [CrossRef] [Green Version]
- Wani, I.A.; Verma, S.; Kumari, P.; Charles, B.; Hashim, M.J.; El-Serehy, H.A. Ecological assessment and environmental niche modelling of Himalayan rhubarb (Rheum webbianum Royle) in northwest Himalaya. PLoS ONE 2021, 16, e0259345. [Google Scholar] [CrossRef] [PubMed]
- Iannella, M.; Cerasoli, F.; Alessandro, D.P.; Console, G.; Biondi, M. Coupling GIS spatial analysis and Ensemble Niche Modelling to investigate climate change-related threats to the Sicilian pond turtle Emystrinacris, an endangered species from the Mediterranean. Peer J. 2018, 6, e4969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.C.; Li, H.C.; Shih, H.J.; Liu, K.F. Potential impact of climate change and extreme events on slope land hazard—A case study of Xindian watershed in Taiwan. Nat. Hazards Earth Syst. Sci. 2018, 18, 3283–3296. [Google Scholar] [CrossRef] [Green Version]
- Halloy, S.R.; Mark, A.F. Climate-change effects on alpine plant biodiversity: A New Zealand perspective on quantifying the threat. Arc. Ant. Alp. Res. 2003, 35, 248–254. [Google Scholar] [CrossRef]
- Thullier, W.; Richardson, D.M.; Pysek, P.; Midgley, G.F.; Huges, G.O.; Rouget, M. Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Glob. Change Biol. 2005, 11, 2234–2250. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, M.; Bhardwaj, A.; Singh, S.; Ranhotra, P.S.; Bhattacharyya, A.; Pal, A.K.; Roy, I.; Martín-Torres, F.J.; Zorzano, M.-P. Himalayan glaciers experienced significant mass loss during later phases of little ice age. Sci. Rep. 2017, 7, 10305. [Google Scholar] [CrossRef]
- Van de Ven, C.M.; Weiss, S.B.; Ernst, W.G. Plant species distributions under present conditions and forecasted for warmer climates in an arid mountain range. Earth Interact. 2007, 11, 1–33. [Google Scholar] [CrossRef]
- Engler, R.; Randin, C.F.; Thuiller, W.; Dullinger, S.; Zimmermann, N.E.; Araujo, M.B.; Guisan, A. 21st century climate change threatens mountain flora unequally across Europe. Glob. Change Biol. 2011, 17, 2330–2341. [Google Scholar] [CrossRef]
- Tovar, C.; Arnillas, C.A.; Cuesta, F.; Buytaert, W. Diverging Responses of Tropical Andean Biomes under Future Climate Conditions. PLoS ONE 2013, 8, e63634. [Google Scholar] [CrossRef] [Green Version]
- Cuena-Lombra~na, A.; Fois, M.; Fenu, G.; Cogoni, D.; Bacchetta, G. The impact of climatic variations on the reproductive success of Gentiana lutea L. in a Mediterranean mountain area. Int. J. Biometeorol. 2018, 62, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Khuroo, A.A.; Hamid, M.; Charles, B.; Rashid, I. Predicting invasion potential and niche dynamics of Parthenium hysterophorus (Congress grass) in India under projected climate change. Biodivers. Conserv. 2019, 28, 2319–2344. [Google Scholar] [CrossRef]
- Malhi, Y.; Franklin, J.; Seddon, N.; Solan, M.; Turner, M.G.; Field, C.B.; Knowlton, N. Climate change and ecosystems: Threats, opportunities and solutions. Phil. Trans. R. Soc. 2020, 375, 20190104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taleshi, H.; Jalali, S.G.; Alavi, S.J.; Hosseini, S.M.; Naimi, B.; Zimmermann, N.E. Climate change impacts on the distribution and diversity of major tree species in the temperate forests of Northern Iran. Reg. Environ. Change 2019, 19, 2711–2728. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Bobrowski, M.; Weidinger, J.; Schwab, N.; Schickhoff, U. Searching for ecology in species distribution models in the Himalayas. Ecol. Modell. 2021, 458, 109693. [Google Scholar] [CrossRef]
- Rew, J.; Cho, Y.; Moon, J.; Hwang, E. Habitat Suitability Estimation Using a Two-Stage Ensemble Approach. Remote Sens. 2020, 12, 1475. [Google Scholar] [CrossRef]
- Tingley, M.; Darling, E.; Wilcove, D. Fine- and coarse-filter conservation strategies in a time of climate change. Ann. N. Y. Acad. Sci. 2014, 1322, 92–109. [Google Scholar] [CrossRef]
- Singh, L.; Tariq, K.M.; Chandra, S.; Bhatt, I.D.; Nandi, S.K. Ecological niche modelling: An important tool for predicting Suitable habitat and conservation of the himalayan medicinal Herbs. Envis Bull. Himal. Ecol. 2017, 25, 154. [Google Scholar]
- Guisan, A.; Zimmermann, N.E. Predictive Habitat distribution models in ecology. Ecol. Modell. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.M.; Butchart, S.H.M.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akçakaya, H.R.; et al. Assessing species vulnerability to climate change. Nat. Clim. Change 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Serra-Diaz, J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Cengic, M.; Rost, J.; Remenska, D.; Janse, J.H.; Huijbregts, M.A.J.; Schipper, A.M. On the importance of predictor choice, modelling technique, and number of pseudo-absences for bioclimatic envelope model performance. Ecol. Evol. 2020, 10, 12307–12317. [Google Scholar] [CrossRef]
- Condro, A.A.; Prasetyo, L.B.; Rushayati, S.B.; Santikayasa, I.P.; Iskandar, E. Predicting Hotspots and Prioritizing Protected Areas for Endangered Primate Species in Indonesia under Changing Climate. Biology 2021, 10, 154. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Ann. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Gaston, K.J. Species Richness: Measure and Measurement. Biodiversity: A Biology of Numbers and Difference; Blackwell Science: Oxford, UK, 1996; pp. 77–111. [Google Scholar]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Williams, S.E. Biodiversity redistribution under climate change: Impacts one ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Wani, I.A.; Kumar, V.; Verma, S.; Jan, A.T.; Rather, I.A. Dactylorhiza hatagirea D. Don soo: A critically endangered perennial orchid From the North-West Himalayas. Plants 2020, 9, 1644. [Google Scholar] [CrossRef]
- Wani, I.A.; Verma, S.; Ahmad, P.; El-Serehy, H.; Hashim, M.J. Reproductive biology of rheum webbianum Royle, A vulnerable medicinal her from the alpines of North Western Himalaya. Front. Plant Sci. 2022, 13, 699645. [Google Scholar] [CrossRef]
- Rodríguez, J.; Brotons, L.; Bustamante, J.; Seoane, J. The application of predictive modeling of species distribution to biodiversity conservation. Divers. Distrib. 2007, 13, 243–251. [Google Scholar] [CrossRef]
- Rashid, I.; Romshoo, S.A.; Chaturvedi, R.K.; Ravindranath, N.H.; Sukumar, R.; Jayaraman, M.; Lakshmi, T.V.; Sharma, J. Projected climate change impacts on vegetation distribution over Kashmir Himalayas. Clim. Chan. 2015, 132, 601–613. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Cianfrani, C.; Lay, G.L.; Maiorano, L.; Satizábal, H.F.; Loy, A.; Guisan, A. Adapting global conservation strategies to climate change at the European scale: The otter as a flagship species. Biol. Conserv. 2011, 144, 2068–2080. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Buja, A. Flexible Discriminant Analysis by Optimal Scoring. J. Am. Stat. Assoc. 1994, 89, 1255–1270. [Google Scholar] [CrossRef]
- Kumari, P.; Khajuria, A.; Wani, I.A.; Khan, S.; Verma, S. Effect of floral size reduction on pollination and reproductive efficiency of female flowers of Valeriana wallichii, a threatened medicinal plant. Nat. Acad. Sci. Lett. 2020, 44, 75–79. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of consensus methods in predictive species distribution modelling. Divers Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Beaumont, L.J.; Graham, E.; Duursma, D.E.; Wilson, P.D.; Cabrelli, A.; Baumgartner, J.B.; Hallgren, W.; Laffan, S.W. Which species distribution models are more (or less) likely to project broad-scale, climate induced shifts in species ranges? Ecol. Modell. 2016, 342, 135–146. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef] [Green Version]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef] [Green Version]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental Niche Equivalency Versus Conservatism: Quantitative Approaches to Niche Evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef] [PubMed]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. Ecospat: An R package to support spatial analyses and modelling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Zhong, Y.; Xue, Z.; Jiang, M.; Liu, B.; Wang, G. The application of species distribution modeling in wetland restoration: A case study in the Songnen Plain, Northeast China. Ecol. Indic. 2021, 121, 107137. [Google Scholar] [CrossRef]
- Mushtaq, S.; Reshi, Z.A.; Shah, M.; Charles, B. Modelled distribution of an invasive alien plant species differs at different spatio-temporal scales under changing climate: A case study of Parthenium hysterophorus L. Trop. Ecol. 2021, 62, 10. [Google Scholar] [CrossRef]
- Davies, T.J.; Purvis, A.; Gittleman, J.L. Quaternary climate change and the geographic ranges of mammals. Amer. Natural. 2009, 174, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, M.; Mace, G.M.; Gittleman, J.L.; Jones, K.E.; Bielby, J.; Purvis, A. The predictability of extinction: Biological and external correlates of decline in mammals. Proc. Royal Soc. Biol. Sci. 2008, 275, 1441–1448. [Google Scholar] [CrossRef] [Green Version]
- Purvis, A.; Gittleman, J.L.; Cowlishaw, G.; Mace, G.M. Predicting extinction risk in declining species. Proc. Royal Soc. Biol. Sci. 2000, 267, 1947–1952. [Google Scholar] [CrossRef] [Green Version]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Telwala, Y.; Brook, B.W.; Manish, K.; Pandit, M.K. Climate-Induced Elevational Range Shifts and Increase in Plant Species Richness in a Himalayan Biodiversity Epicentre. PLoS ONE 2013, 8, e57103. [Google Scholar] [CrossRef] [Green Version]
- Palacios, C.R.; John, C.W. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Nat. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef]
- Tewari, V.P.; Verma, R.K.; Von Gadow, K. Climate change effects in the Western Himalayan ecosystems of India: Evidence and strategies. For. Ecosyst. 2017, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Dubey, V.K.; Johnson, J.A.; Rawal, Y.K.; Sivakumar, K. Is there always space at the top? Ensemble modeling reveals climate-driven high-altitude squeeze for the vulnerable snow trout Schizothorax richardsonii in Himalaya. Ecol. Indic. 2021, 120, 106900. [Google Scholar] [CrossRef]
- Dechen, L.; Gabriele, C.; Sommer, S.; Phuntsho, T.; Namgay, W.; Sonam, W.; Arpat, O. Modeling distribution and habitat suitability for the snow leopard in Bhutan. Front. Conserv. Sci. 2021, 781085. [Google Scholar]
- Pant, G.; Maraseni, T.; Apan, A.; Allen, B.L. Predicted declines in suitable habitat for greater one-horned rhinoceros (Rhinoceros unicornis) under future climate and land use change scenarios. Ecol. Evol. 2021, 11, 18288–18304. [Google Scholar] [CrossRef] [PubMed]
- Amanda, M.W.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Konowalik, K.; Nosol, A. Evaluation metrics and validation of presence-only species distribution models based on distributional maps with varying coverage. Sci. Rep. 2021, 11, 1482. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Wonkka, C.L.; Treglia, M.L.; Grant, W.E.; Smeins, F.E.; Rogers, W.E. Species distribution modelling for conservation of an endangered endemic orchid. AoB Plants 2020, 7, plv039. [Google Scholar] [CrossRef] [Green Version]
- Salam, N.; Reshi, Z.; Shah, M. Habitat suitability modelling for Lagotis cashmeriana (ROYLE) RUPR., a threatened species endemic to Kashmir Himalayan alpines. Geo. Ecol. Lands 2020, 1–11. [Google Scholar] [CrossRef]
- Ye, P.; Zhang, G.; Zhao, X.; Chen, H.; Si, Q.; Wu, J. Potential geographical distribution and environmental explanations of rare and endangered plant species through combined modeling: A case study of Northwest Yunnan, China. Ecol. Evol. 2021, 11, 13052–13067. [Google Scholar] [CrossRef]
- Zangiabadi, S.; Zaremaivan, H.; Brotons, L.; Mostafavi, H.; Ranjbar, H. Using climatic variables alone overestimate climate change impacts on predicting distribution of an endemic species. PLoS ONE 2021, 16, e0256918. [Google Scholar] [CrossRef]
- Meier, E.; Kienast, F.; Pearman, P.B.; Svenning, J.C.; Thuiller, W.; Araújo, M.B.; Guisan, A.; Zimmermann, N.E. Biotic and abiotic variables show little redundancy in explaining tree species distributions. Ecography 2010, 33, 1038–1048. [Google Scholar] [CrossRef]
- Page, N.V.; Shanker, K. Environment and dispersal influence changes in species composition at different scales in woody plants of the Western Ghats, India. J. Veg. Sci. 2018, 29, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Wani, I.A.; Verma, S.; Mushtaq, S.; Alsahli, A.A.; Alyemeni, M.A.; Tariq, M.; Pant, S. Ecological analysis and environmental niche modelling of Dactylorhiza hatagirea (D. Don) Soo: A conservation approach for critically endangered medicinal orchid. Saud. J. Biol. Sci. 2021, 28, 2109–2122. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, H.K.; Bisht, A.K.; Bhatt, I.D.; Bhatt, A.; Gallacher, D.; Santo, A. Population change of Trillium govanianum (Melanthiaceae) amid altered indigenous harvesting practices in the Indian Himalayas. J. Ethnopharmacol. 2018, 213, 302–310. [Google Scholar] [CrossRef]
- Tariq, M.; Nandi, S.K.; Bhatt, I.D.; Bhavsar, D.; Roy, A.; Pande, V. Phytosociological and niche distribution study of Paris polyphylla Smith, an important medicinal herb of Indian Himalayan region. Trop. Ecol. 2021, 62, 163–173. [Google Scholar] [CrossRef]
- Dhyani, A.; Kadaverugu, R.; Nautiyal, B.P.; Nautiyal, M.C. Predicting the potential distribution of a critically endangered medicinal plant Lilium polyphyllum in Indian Western Himalayan Region. Reg. Environ. Chang. 2021, 21, 30. [Google Scholar] [CrossRef]
- Dikshit, A.; Sarkar, R.; Pradhan, B.; Acharya, S.; Dorji, K. Estimating rainfall thresholds for landslide occurrence in the Bhutan Himalayas. Water 2019, 11, 1616. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, R.; Roy, P. Vegetation response to climate change in Himalayan hill ranges: A remote sensing perspective. In Plant Diversity in the Himalaya Hotspot Region; Bishen Singh Mahendra Pal Singh Publishers and Distributors: Dehradun, India, 2018; ISBN 978-81-211-0946-8. [Google Scholar]
- Hamid, M.; Khuroo, A.A.; Charles, B.; Ahmad, R.; Singh, C.P.; Aravind, N.A. Impact of climate change on the distribution range and niche dynamics of Himalayan birch, a typical treeline species in Himalayas. Biodivers. Conserv. 2019, 28, 2345–2370. [Google Scholar] [CrossRef]
- Dimri, A.P.; Dash, S.K. Wintertime climatic trends in the western Himalayas. Clim. Chang. 2012, 111, 775–800. [Google Scholar] [CrossRef]
- Dash, S.K.; Jenamani, R.K.; Kalsi, S.R.; Panda, S.K. Some evidence of climate change in twentieth-century India. Clim. Chang. 2007, 85, 299–321. [Google Scholar] [CrossRef]
- Sontakke, N.A.; Singh, H.N.; Singh, N. Monitoring physiographic rainfall variation for sustainable Management of Water Bodies in India. In Natural and Anthropogenic Disasters: Vulnerability, Preparedness and Mitigation; Jha, M.K., Ed.; Springer: Amsterdam, The Netherlands, 2009; pp. 293–331. [Google Scholar]
- Wei, B.; Wang, R.; Hou, K.; Wang, X.; Wu, W. Predicting the current and future cultivation regions of Carthamus tinctorius L. using MaxEnt model under climate change in China. Glob. Ecol. Conserv. 2018, 16, e00477. [Google Scholar] [CrossRef]
- Parmesan, C.; Hanley, M.E. Plants and climate change: Complexities and surprises. Ann. Bot. 2015, 116, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Hamilton, H. Ecological Niche Transferability Using Invasive Species as a Case Study. PLoS ONE 2015, 10, e0119891. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, H.; Zhao, W.; Zhao, Q. Niche shifts and the potential distribution of Phenacoccus solenopsis (Hemiptera: Pseudococcidae) under climate change. PLoS ONE 2017, 12, e0180913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pili, A.N.; Tingley, R.; Sy, E.Y.; Diesmos, M.L.L.; Diesmos, A.C. Niche shifts and environmental non-equilibrium undermine the usefulness of ecological niche models for invasion risk assessments. Sci. Rep. 2020, 10, 7972. [Google Scholar] [CrossRef] [PubMed]






| Site No. | Site | Coordinates | Altitude |
|---|---|---|---|
| 1 | Manyal Gali, J&K | 33°33′ N 74°22′ E | 1903 m.a.s.l |
| 2 | Dera Ki Gali, J&K | 33°35′ N 74°21′ E | 2126 m.a.s.l |
| 3 | Bafliaz, J&K | 33°21′ N 74°21′ E | 1566 m.a.s.l |
| 4 | Noorichamb, J&K | 33°36′ N 74°25′ E | 1834 m.a.s.l |
| 5 | Bakori, J&K | 33°21′ N 74°31′ E | 1637 m.a.s.l |
| 6 | Budhal, J&K | 33°22′ N 74°38′ E | 1781 m.a.s.l |
| 7 | Patnitop, J&K | 33°05′ N 75°19′ E | 2072 m.a.s.l |
| 8 | Batote, J&K | 33°01′ N 75°39′ E | 1656 m.a.s.l |
| 9 | Amiranagar, J&K | 33°00′ N 75°05′ E | 1498 m.a.s.l |
| 10 | Neota, J&K | 33°02′ N 75°03′ E | 1327 m.a.s.l |
| 11 | Drudhoo, J&K | 33°15′ N 75°45′ E | 1366 m.a.s.l |
| 12 | Nai Basti, J&K | 33°01′ N 75°39′ E | 1370 m.a.s.l |
| 13 | Dranga, J&K | 33°01′ N 75°40′ E | 1383 m.a.s.l |
| 14 | Narnoo 1, J&K | 33°0′ N 75°40′ E | 1378 m.a.s.l |
| 15 | Narnoo 2, J&K | 33°06′ N 75°40′ E | 1459 m.a.s.l |
| 16 | Kursari 1, J&K | 33°0′ N 75°41′ E | 1468 m.a.s.l |
| 17 | Kursari 2, J&K | 33°0′ N 75°41′ E | 1434 m.a.s.l |
| 18 | Kursari 3, J&K | 33°0′ N 75°41′ E | 1459 m.a.s.l |
| 19 | Kursari 4, J&K | 33°0′ N 75°41′ E | 1468 m.a.s.l |
| 20 | Khelani, J&K | 33°03′ N 75°38′E | 1274 m.a.s.l |
| 21 | Gatha, J&K | 32°59′ N 75°42′ E | 1480 m.a.s.l |
| 22 | Randa, J&K | 32°59′ N 75°43′ E | 1583 m.a.s.l |
| 23 | Wazir Kotli, J&K | 32°58′ N 75°43′ E | 1606 m.a.s.l |
| 24 | Singhasan Pull, J&K | 32°59′ N 75°43′ E | 1645 m.a.s.l |
| 25 | Kapra, J&K | 33°07′ N 75°24′ E | 1740 m.a.s.l |
| 26 | Powerhouse, J&K | 32°56′ N 75°43′ E | 1885 m.a.s.l |
| 27 | Bhadrote, J&K | 32°56′ N 75°43′ E | 1898 m.a.s.l |
| 28 | MushDev Nallah, J&K | 32°56′ N 75°45′ E | 1941 m.a.s.l |
| 29 | Atalgarh, J&K | 32°56′ N 75°45′ E | 1941 m.a.s.l |
| 30 | Haliyan 1, J&K | 32°55′ N 75°42′ E | 1774 m.a.s.l |
| 31 | Haliyan 2, J&K | 32°58′ N 75°42′ E | 1706 m.a.s.l |
| 32 | Haliyan 3, J&K | 33°01′ N 75°41′ E | 2664 m.a.s.l |
| 33 | Panaja, J&K | 32°57′ N 75°43′ E | 1763 m.a.s.l |
| 34 | Qilla Mohalla, J&K | 32°58′ N 75°42′ E | 1718 m.a.s.l |
| 35 | Almora, Uttrakhand | 29°37′ N 79°32′ E | 1870 m.a.s.l |
| 36 | Chakrata, Uttrakhand | 33°33′ N 74°24′ E | 1781 m.a.s.l |
| 37 | Kund, J&K | 33°33′ N 74°23′ E | 2159 m.a.s.l |
| 38 | Cha, J&K | 33°33′ N 74°24′ E | 2440 m.a.s.l |
| 39 | Tungwali, J&K | 33°34′ N 74°24′ E | 2858 m.a.s.l |
| 40 | Sapanwali, J&K | 33°33′ N 74°23′ E | 2263 m.a.s.l |
| 41 | Azamtabad, J&K | 33°33′ N 74°23′ E | 2124 m.a.s.l |
| 42 | Thajwas, J&K | 33°16′ N 75°17′ E | 3108 m.a.s.l |
| Bioclimatic Variables | bio_1 | bio_2 | bio_3 | bio_4 | bio_5 | bio_6 | bio_7 | bio_8 | bio_9 | bio_10 | bio_11 | bio_12 | bio_13 | bio_14 | bio_15 | bio_16 | bio_17 | bio_18 | bio_19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| bio_1 | 1 | ||||||||||||||||||
| bio_2 | 0.63 | ||||||||||||||||||
| bio_3 | 0.52 | 0.56 | |||||||||||||||||
| bio_4 | −0.39 | −0.05 | −0.78 | ||||||||||||||||
| bio_5 | 0.91 | 0.71 | 0.24 | 0 | |||||||||||||||
| bio_6 | 0.94 | 0.51 | 0.67 | −0.63 | 0.75 | ||||||||||||||
| bio_7 | −0.08 | 0.25 | −0.63 | 0.91 | 0.31 | −0.38 | |||||||||||||
| bio_8 | 0.46 | 0.18 | 0.39 | −0.4 | 0.32 | 0.53 | −0.32 | ||||||||||||
| bio_9 | 0.92 | 0.5 | 0.32 | −0.27 | 0.88 | 0.84 | 0.01 | 0.23 | |||||||||||
| bio_10 | 0.92 | 0.67 | 0.25 | −0.02 | 0.99 | 0.77 | 0.26 | 0.34 | 0.88 | ||||||||||
| bio_11 | 0.95 | 0.55 | 0.68 | −0.63 | 0.76 | 0.99 | −0.35 | 0.51 | 0.85 | 0.78 | |||||||||
| bio_12 | 0.33 | −0.26 | 0.42 | −0.7 | 0 | 0.48 | −0.7 | 0.41 | 0.24 | 0.06 | 0.48 | ||||||||
| bio_13 | 0.18 | −0.28 | 0.48 | −0.74 | −0.15 | 0.39 | −0.78 | 0.38 | 0.06 | −0.1 | 0.37 | 0.91 | |||||||
| bio_14 | 0.04 | −0.08 | −0.66 | 0.59 | 0.27 | −0.14 | 0.59 | −0.2 | 0.2 | 0.28 | −0.14 | −0.38 | −0.65 | ||||||
| bio_15 | 0.06 | −0.21 | 0.57 | −0.77 | −0.25 | 0.31 | −0.81 | 0.35 | −0.06 | −0.23 | 0.29 | 0.72 | 0.91 | −0.84 | |||||
| bio_16 | 0.21 | −0.26 | 0.5 | −0.73 | −0.12 | 0.41 | −0.77 | 0.36 | 0.1 | −0.06 | 0.4 | 0.93 | 0.99 | −0.63 | 0.89 | ||||
| bio_17 | 0.26 | −0.23 | −0.36 | −0.01 | 0.27 | 0.19 | 0.1 | −0.04 | 0.43 | 0.26 | 0.21 | 0.16 | −0.13 | 0.66 | −0.32 | −0.12 | |||
| bio_18 | 0.29 | −0.06 | 0.6 | −0.68 | −0.02 | 0.44 | −0.68 | 0.48 | 0.12 | 0.03 | 0.45 | 0.9 | 0.88 | −0.6 | 0.76 | 0.9 | −0.2 | ||
| bio_19 | 0.32 | −0.03 | −0.32 | 0.07 | 0.38 | 0.19 | 0.25 | −0.11 | 0.51 | 0.36 | 0.23 | 0.04 | −0.29 | 0.68 | −0.46 | −0.26 | 0.94 | −0.25 | 1 |
| Variable | Description |
|---|---|
| BIO-1 | (Annual Mean Temperature) |
| BIO-2 | (Mean Diurnal Range) |
| BIO-3 | (Isothermality) |
| BIO-7 | (Temperature Annual Range) |
| BIO-8 | (Mean Temperature of Wettest Quarter) |
| BIO-12 | (Annual Mean Precipitation) |
| BIO-14 | (Precipitation of Driest Month) |
| BIO-17 | (Precipitation of Driest Quarter) |
| Bioclimatic Variable | GLM | GBM | GAM | CTA | ANN | SRE | FDA | RF | MAXENT. Phillips | Mean |
|---|---|---|---|---|---|---|---|---|---|---|
| bio_01 | 0.80 | 0.14 | 0.66 | 0.27 | 0.60 | 0.48 | 0.39 | 0.06 | 0.69 | 0.45 |
| bio_02 | 0.59 | 0.02 | 0.70 | 0.00 | 0.43 | 0.34 | 0.02 | 0.03 | 0.27 | 0.27 |
| bio_03 | 0.19 | 0.08 | 0.61 | 0.09 | 0.16 | 0.21 | 0.01 | 0.05 | 0.12 | 0.17 |
| bio_07 | 0.37 | 0.08 | 0.65 | 0.07 | 0.20 | 0.19 | 0.06 | 0.04 | 0.29 | 0.22 |
| bio_08 | 0.09 | 0.01 | 0.41 | 0.02 | 0.38 | 0.22 | 0.26 | 0.02 | 0.54 | 0.22 |
| bio_12 | 0.49 | 0.02 | 0.65 | 0.08 | 0.59 | 0.34 | 0.08 | 0.02 | 0.49 | 0.31 |
| bio_14 | 0.07 | 0.06 | 0.55 | 0.21 | 0.13 | 0.23 | 0.15 | 0.11 | 0.30 | 0.20 |
| bio_17 | 0.54 | 0.13 | 0.82 | 0.64 | 0.78 | 0.36 | 0.25 | 0.06 | 0.57 | 0.46 |
| Scenario | Ensemble Type | Loss | Absent | Stable | Gain | Percent Loss | Percent Gain | Range Change (%) |
|---|---|---|---|---|---|---|---|---|
| RCP4.5 2050 | Committee averaging | 22,334 | 895,491 | 16,690 | 6514 | 57.231 | 16.692 | −40.539 |
| RCP4.5 2070 | Committee averaging | 34,439 | 898,663 | 4585 | 3342 | 88.251 | 8.564 | −79.687 |
| RCP8.5 2050 | Committee averaging | 34,801 | 898,511 | 4223 | 3494 | 89.178 | 8.953 | −80.225 |
| RCP8.5 2070 | Committee averaging | 38,196 | 900,519 | 828 | 1486 | 97.878 | 3.808 | −94.070 |
| RCP4.5 2050 | Weighted mean | 25,408 | 885,378 | 21,537 | 8706 | 54.123 | 18.545 | −35.578 |
| RCP4.5 2070 | Weighted mean | 39,793 | 889,563 | 7152 | 4521 | 84.765 | 9.630 | −75.135 |
| RCP8.5 2050 | Weighted mean | 38,494 | 889,352 | 8451 | 4732 | 81.998 | 10.080 | −71.918 |
| RCP8.5 2070 | Weighted mean | 45,560 | 891,102 | 1385 | 2982 | 97.050 | 6.352 | −90.698 |
| Pair | PC1 (%) | PC2 (%) | Overlap (D) | Equivalency Test (p-Value) | Similarity Test (p-Value) |
|---|---|---|---|---|---|
| Current vs. RCP 4.5 2050 | 45.11 | 33.6 | 0.66 | 0.45545 | 0.0198 |
| Current vs. RCP 4.5 2070 | 43.08 | 25.59 | 0.60 | 0.55446 | 0.05941 |
| Current vs. RCP 8.5 2050 | 45.76 | 32.77 | 0.65 | 0.52475 | 0.0297 |
| Current vs. RCP 8.5 2070 | 44.65 | 33.28 | 0.42 | 0.46436 | 0.0495 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, P.; Wani, I.A.; Khan, S.; Verma, S.; Mushtaq, S.; Gulnaz, A.; Paray, B.A. Modeling of Valeriana wallichii Habitat Suitability and Niche Dynamics in the Himalayan Region under Anticipated Climate Change. Biology 2022, 11, 498. https://doi.org/10.3390/biology11040498
Kumari P, Wani IA, Khan S, Verma S, Mushtaq S, Gulnaz A, Paray BA. Modeling of Valeriana wallichii Habitat Suitability and Niche Dynamics in the Himalayan Region under Anticipated Climate Change. Biology. 2022; 11(4):498. https://doi.org/10.3390/biology11040498
Chicago/Turabian StyleKumari, Priyanka, Ishfaq Ahmad Wani, Sajid Khan, Susheel Verma, Shazia Mushtaq, Aneela Gulnaz, and Bilal Ahamad Paray. 2022. "Modeling of Valeriana wallichii Habitat Suitability and Niche Dynamics in the Himalayan Region under Anticipated Climate Change" Biology 11, no. 4: 498. https://doi.org/10.3390/biology11040498






