COVID-19-Associated Myocarditis: An Evolving Concern in Cardiology and Beyond
Abstract
:Simple Summary
Abstract
1. Introduction
2. Incidence and Clinical Relevance of Myocarditis in the Setting of COVID-19 Infection
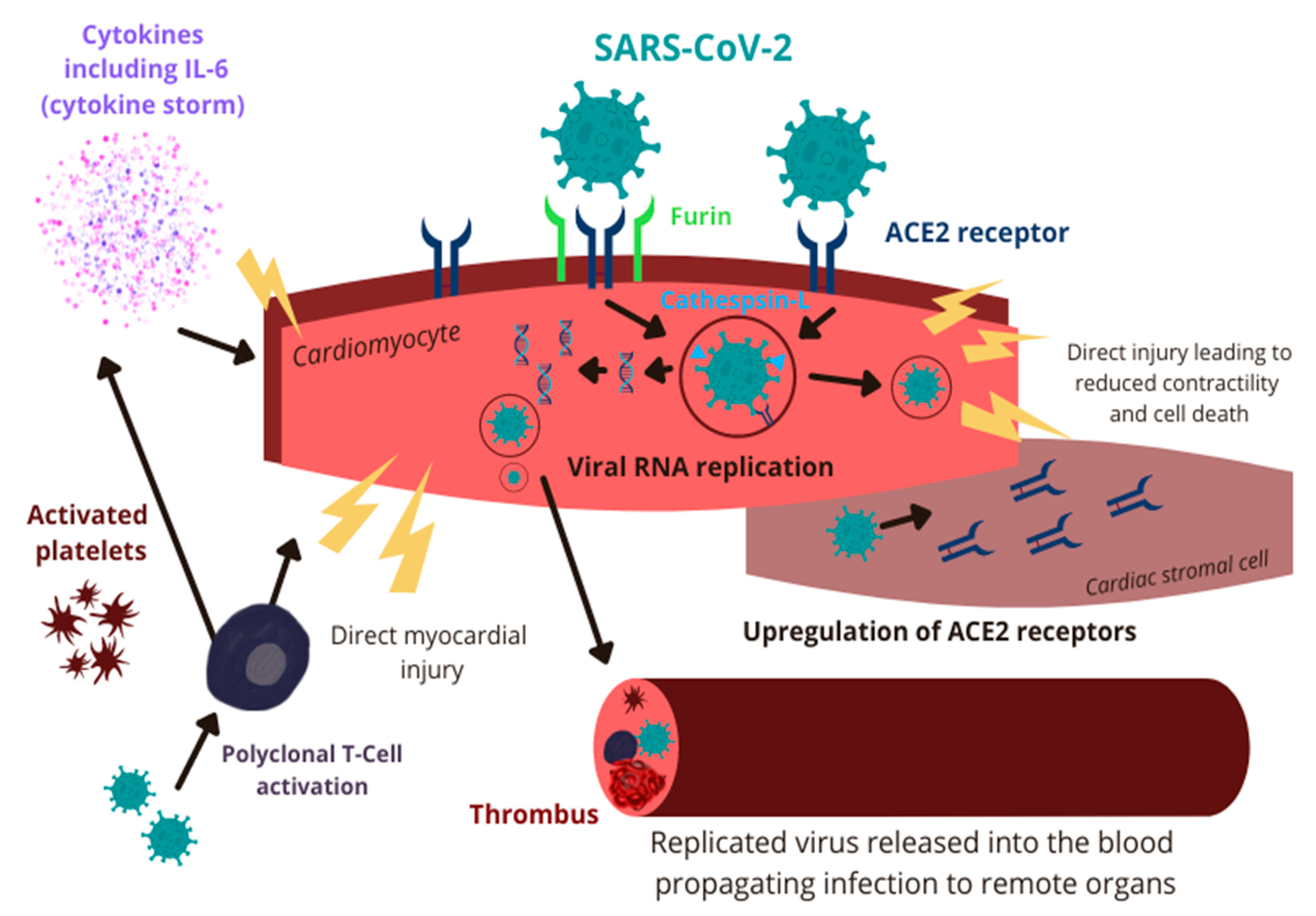
3. Pathophysiology of COVID-19-Associated Myocarditis
4. Serum Biomarkers in the Diagnosis of COVID-19-Associated Myocarditis
4.1. Cardiac Troponins
4.2. Circulating Natriuretic Peptides
4.3. Other Potential Biomarkers to Detect Myocarditis in COVID-19 Infection
5. Histopathological Testing for COVID-19-Associated Myocarditis
6. Imaging Studies to Detect and Monitor COVID-19-Associated Myocarditis
7. Myocarditis in COVID-19 Vaccine Recipients
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 8 February 2022).
- JHU, J.H.U. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available online: https://coronavirus.jhu.edu/map.html (accessed on 8 February 2022).
- Gao, Z.; Xu, Y.; Sun, C.; Wang, X.; Guo, Y.; Qiu, S.; Ma, K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2021, 54, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020. China CDC Wkly. 2020, 2, 113–122. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Correction to Lancet Respir. Med. 2020; published online Feb 21. https://doi.org/10.1016/S2213-2600(20)30079-5. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [Green Version]
- Myers, L.C.; Parodi, S.M.; Escobar, G.J.; Liu, V.X. Characteristics of Hospitalized Adults With COVID-19 in an Integrated Health Care System in California. JAMA 2020, 323, 2195–2198. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zeron, P.; Mariette, X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat. Rev. Rheumatol. 2021, 17, 315–332. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Spinato, G.; Fabbris, C.; Polesel, J.; Cazzador, D.; Borsetto, D.; Hopkins, C.; Boscolo-Rizzo, P. Alterations in Smell or Taste in Mildly Symptomatic Outpatients With SARS-CoV-2 Infection. JAMA 2020, 323, 2089–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Pere, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Matthay, M.A.; Calfee, C.S. Is a “Cytokine Storm” Relevant to COVID-19? JAMA Intern. Med. 2020, 180, 1152–1154. [Google Scholar] [CrossRef]
- Calabrese, L.H. Cytokine storm and the prospects for immunotherapy with COVID-19. Cleve. Clin. J. Med. 2020, 87, 389–393. [Google Scholar] [CrossRef]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Carretta, D.M.; Silva, A.M.; D’Agostino, D.; Topi, S.; Lovero, R.; Charitos, I.A.; Wegierska, A.E.; Montagnani, M.; Santacroce, L. Cardiac Involvement in COVID-19 Patients: A Contemporary Review. Infect. Dis. Rep. 2021, 13, 48. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I. Errors in Statistical Numbers and Data. JAMA Cardiol. 2020, 5, 1308. [Google Scholar] [CrossRef]
- Sawalha, K.; Abozenah, M.; Kadado, A.J.; Battisha, A.; Al-Akchar, M.; Salerno, C.; Hernandez-Montfort, J.; Islam, A.M. Systematic Review of COVID-19 Related Myocarditis: Insights on Management and Outcome. Cardiovasc. Revasc. Med. 2021, 23, 107–113. [Google Scholar] [CrossRef]
- Salabei, J.K.; Asnake, Z.T.; Ismail, Z.H.; Charles, K.; Stanger, G.T.; Abdullahi, A.H.; Abraham, A.T.; Okonoboh, P. COVID-19 and the Cardiovascular System: An Update. Am. J. Med. Sci. 2022. [Google Scholar] [CrossRef]
- Murk, W.; Gierada, M.; Fralick, M.; Weckstein, A.; Klesh, R.; Rassen, J.A. Diagnosis-wide analysis of COVID-19 complications: An exposure-crossover study. CMAJ 2021, 193, E10–E18. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernan, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, T.K.; Kompaniyets, L.; Lavery, A.M.; Hsu, J.; Ko, J.Y.; Yusuf, H.; Romano, S.D.; Gundlapalli, A.V.; Oster, M.E.; Harris, A.M. Association Between COVID-19 and Myocarditis Using Hospital-Based Administrative Data—United States, March 2020-January 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.J.R.; Harrison, S.L.; Fazio-Eynullayeva, E.; Underhill, P.; Lane, D.A.; Lip, G.Y.H. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur. J. Clin. Invest. 2021, 51, e13679. [Google Scholar] [CrossRef]
- Rathore, S.S.; Rojas, G.A.; Sondhi, M.; Pothuru, S.; Pydi, R.; Kancherla, N.; Singh, R.; Ahmed, N.K.; Shah, J.; Tousif, S.; et al. Myocarditis associated with Covid-19 disease: A systematic review of published case reports and case series. Int. J. Clin. Pract. 2021, 75, e14470. [Google Scholar] [CrossRef]
- Jaiswal, V.; Sarfraz, Z.; Sarfraz, A.; Mukherjee, D.; Batra, N.; Hitawala, G.; Yaqoob, S.; Patel, A.; Agarwala, P.; Ruchika; et al. COVID-19 Infection and Myocarditis: A State-of-the-Art Systematic Review. J. Prim. Care Community Health 2021, 12, 21501327211056800. [Google Scholar] [CrossRef]
- Merugu, G.P.; Nesheiwat, Z.; Balla, M.; Patel, M.; Fatima, R.; Sheikh, T.; Kotturi, V.; Bommana, V.; Pulagam, G.; Kaminski, B. Predictors of mortality in 217 COVID-19 patients in Northwest Ohio, United States: A retrospective study. J. Med. Virol. 2021, 93, 2875–2882. [Google Scholar] [CrossRef]
- Moayed, M.S.; Rahimi-Bashar, F.; Vahedian-Azimi, A.; Sathyapalan, T.; Guest, P.C.; Jamialahmadi, T.; Sahebkar, A. Cardiac Injury in COVID-19: A Systematic Review. Adv. Exp. Med. Biol. 2021, 1321, 325–333. [Google Scholar] [CrossRef]
- Chang, W.T.; Toh, H.S.; Liao, C.T.; Yu, W.L. Cardiac Involvement of COVID-19: A Comprehensive Review. Am. J. Med. Sci. 2021, 361, 14–22. [Google Scholar] [CrossRef]
- Piccioni, A.; Saviano, A.; Cicchinelli, S.; Franza, L.; Rosa, F.; Zanza, C.; Santoro, M.C.; Candelli, M.; Covino, M.; Nannini, G.; et al. Microbiota and Myopericarditis: The New Frontier in the Car-Diological Field to Prevent or Treat Inflammatory Cardiomyo-Pathies in COVID-19 Outbreak. Biomedicines 2021, 9, 1234. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.; Gui, M.; Wang, X.; Xiang, Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018, 14, e1007236. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Nakayama, T.; Wu, C.T.; Goltsev, Y.; Jiang, S.; Gall, P.A.; Liao, C.K.; Shih, L.C.; Schurch, C.M.; McIlwain, D.R.; et al. ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs. Nat. Commun. 2020, 11, 5453. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef]
- The Task Force for the management of COVID-19 of the European Society of Cardiology. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 1-epidemiology, pathophysiology, and diagnosis. Eur. Heart J. 2021, 43, 1033–1058. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–30119. [Google Scholar] [CrossRef] [Green Version]
- Wakui, H.; Tamura, K.; Tanaka, Y.; Matsuda, M.; Bai, Y.; Dejima, T.; Masuda, S.; Shigenaga, A.; Maeda, A.; Mogi, M.; et al. Cardiac-specific activation of angiotensin II type 1 receptor-associated protein completely suppresses cardiac hypertrophy in chronic angiotensin II-infused mice. Hypertension 2010, 55, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Paradis, P.; Dali-Youcef, N.; Paradis, F.W.; Thibault, G.; Nemer, M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc. Natl. Acad. Sci. USA 2000, 97, 931–936. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Bansal, V.; Feschotte, C. A Single-Cell RNA Expression Map of Human Coronavirus Entry Factors. Cell Rep. 2020, 32, 108175. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gai, S.; Wang, X.; Zeng, J.; Sun, C.; Zhao, Y.; Zheng, Z. Single-cell analysis of SARS-CoV-2 receptor ACE2 and spike protein priming expression of proteases in the human heart. Cardiovasc. Res. 2020, 116, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Bojkova, D.; Wagner, J.U.G.; Shumliakivska, M.; Aslan, G.S.; Saleem, U.; Hansen, A.; Luxan, G.; Gunther, S.; Pham, M.D.; Krishnan, J.; et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc. Res. 2020, 116, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Buchrieser, J.; Dufloo, J.; Hubert, M.; Monel, B.; Planas, D.; Rajah, M.M.; Planchais, C.; Porrot, F.; Guivel-Benhassine, F.; Van der Werf, S.; et al. Syncytia formation by SARS-CoV-2-infected cells. EMBO J. 2020, 39, e106267. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Mulay, A.; Konda, B.; Garcia, G.; Yao, C.; Beil, S.; Sen, C.; Purkayastha, A.; Kolls, J.K.; Pociask, D.A.; Pessina, P.; et al. SARS-CoV-2 infection of primary human lung epithelium for COVID-19 modeling and drug discovery. bioRxiv 2020. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- The Task Force for the management of COVID-19 of the European Society of Cardiology. ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 2-care pathways, treatment, and follow-up. Eur. Heart J. 2021, 43, 1059–1103. [Google Scholar] [CrossRef]
- Tanaka, T.; Kanda, T.; McManus, B.M.; Kanai, H.; Akiyama, H.; Sekiguchi, K.; Yokoyama, T.; Kurabayashi, M. Overexpression of interleukin-6 aggravates viral myocarditis: Impaired increase in tumor necrosis factor-alpha. J. Mol. Cell Cardiol. 2001, 33, 1627–1635. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; White, H.D.; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur. Heart J. 2007, 28, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.K.; Bergheanu, S.C.; Hasan-Ali, H.; Liem, S.S.; van der Laarse, A.; Wolterbeek, R.; Atsma, D.E.; Schalij, M.J.; Jukema, J.W. Usefulness of peak troponin-T to predict infarct size and long-term outcome in patients with first acute myocardial infarction after primary percutaneous coronary intervention. Am. J. Cardiol. 2009, 103, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Arruda-Olson, A.M.; Roger, V.L.; Jaffe, A.S.; Hodge, D.O.; Gibbons, R.J.; Miller, T.D. Troponin T levels and infarct size by SPECT myocardial perfusion imaging. JACC Cardiovasc. Imaging 2011, 4, 523–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younger, J.F.; Plein, S.; Barth, J.; Ridgway, J.P.; Ball, S.G.; Greenwood, J.P. Troponin-I concentration 72 h after myocardial infarction correlates with infarct size and presence of microvascular obstruction. Heart 2007, 93, 1547–1551. [Google Scholar] [CrossRef] [Green Version]
- Pruszczyk, P.; Bochowicz, A.; Torbicki, A.; Szulc, M.; Kurzyna, M.; Fijalkowska, A.; Kuch-Wocial, A. Cardiac troponin T monitoring identifies high-risk group of normotensive patients with acute pulmonary embolism. Chest 2003, 123, 1947–1952. [Google Scholar] [CrossRef] [Green Version]
- Becattini, C.; Vedovati, M.C.; Agnelli, G. Prognostic value of troponins in acute pulmonary embolism: A meta-analysis. Circulation 2007, 116, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Velmahos, G.C.; Karaiskakis, M.; Salim, A.; Toutouzas, K.G.; Murray, J.; Asensio, J.; Demetriades, D. Normal electrocardiography and serum troponin I levels preclude the presence of clinically significant blunt cardiac injury. J. Trauma. 2003, 54, 45–50. [Google Scholar] [CrossRef]
- Brandt, R.R.; Filzmaier, K.; Hanrath, P. Circulating cardiac troponin I in acute pericarditis. Am. J. Cardiol. 2001, 87, 1326–1328. [Google Scholar] [CrossRef]
- Stein, R.; Gupta, B.; Agarwal, S.; Golub, J.; Bhutani, D.; Rosman, A.; Eng, C. Prognostic implications of normal (<0.10 ng/ml) and borderline (0.10 to 1.49 ng/ml) troponin elevation levels in critically ill patients without acute coronary syndrome. Am. J. Cardiol. 2008, 102, 509–512. [Google Scholar] [CrossRef]
- Iser, D.M.; Thompson, A.J.; Sia, K.K.; Yeomans, N.D.; Chen, R.Y. Prospective study of cardiac troponin I release in patients with upper gastrointestinal bleeding. J. Gastroenterol. Hepatol. 2008, 23, 938–942. [Google Scholar] [CrossRef]
- Maeder, M.; Fehr, T.; Rickli, H.; Ammann, P. Sepsis-associated myocardial dysfunction: Diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest 2006, 129, 1349–1366. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.K.; Kristensen, S.R.; Bak, S.; Atar, D.; Hoilund-Carlsen, P.F.; Mickley, H. Frequency and significance of troponin T elevation in acute ischemic stroke. Am. J. Cardiol. 2007, 99, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Kelley, W.E.; Januzzi, J.L.; Christenson, R.H. Increases of cardiac troponin in conditions other than acute coronary syndrome and heart failure. Clin. Chem. 2009, 55, 2098–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauer, B.; Niederau, C.; Kuhl, U.; Schannwell, M.; Pauschinger, M.; Strauer, B.E.; Schultheiss, H.P. Cardiac troponin T in patients with clinically suspected myocarditis. J. Am. Coll. Cardiol. 1997, 30, 1354–1359. [Google Scholar] [CrossRef] [Green Version]
- Chaligne, C.; Mageau, A.; Ducrocq, G.; Ou, P.; Alexandra, J.F.; Mutuon, P.; Papo, T.; Sacre, K. Acute myocarditis revealing autoimmune and inflammatory disorders: Clinical presentation and outcome. Int. J. Cardiol. 2022, 351, 84–88. [Google Scholar] [CrossRef]
- Lauer, B.; Niederau, C.; Kuhl, U.; Schannwell, M.; Pauschinger, M.; Strauer, B.E.; Schultheiss, H.P. Cardiac troponin T in the diagnosis and follow up of suspected myocarditis. Dtsch. Med. Wochenschr. 1998, 123, 409–417. [Google Scholar] [CrossRef]
- Smith, S.C.; Ladenson, J.H.; Mason, J.W.; Jaffe, A.S. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997, 95, 163–168. [Google Scholar] [CrossRef]
- Sandoval, Y.; Januzzi, J.L., Jr.; Jaffe, A.S. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 1244–1258. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Rajpal, S.; Tong, M.S.; Borchers, J.; Zareba, K.M.; Obarski, T.P.; Simonetti, O.P.; Daniels, C.J. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering From COVID-19 Infection. JAMA Cardiol. 2021, 6, 116–118. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, P.; Tang, D.; Zhu, T.; Han, R.; Zhan, C.; Liu, W.; Zeng, H.; Tao, Q.; Xia, L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2020, 13, 2330–2339. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.S.; Drazner, M.H. Biomarkers of Cardiac Stress and Cytokine Release Syndrome in COVID-19: A Review. Curr. Heart Fail. Rep. 2021, 18, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Palmisano, A.; Natale, L.; Ligabue, G.; Peretto, G.; Lovato, L.; Vignale, D.; Fiocchi, F.; Marano, R.; Russo, V. Cardiac Magnetic Resonance Characterization of Myocarditis-Like Acute Cardiac Syndrome in COVID-19. JACC Cardiovasc. Imaging 2020, 13, 2462–2465. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [Green Version]
- Kermali, M.; Khalsa, R.K.; Pillai, K.; Ismail, Z.; Harky, A. The role of biomarkers in diagnosis of COVID-19—A systematic review. Life Sci. 2020, 254, 117788. [Google Scholar] [CrossRef]
- Garcia de Guadiana-Romualdo, L.; Morell-Garcia, D.; Rodriguez-Fraga, O.; Morales-Indiano, C.; Maria Lourdes Padilla Jimenez, A.; Gutierrez Revilla, J.I.; Urrechaga, E.; Alamo, J.M.; Hernando Holgado, A.M.; Lorenzo-Lozano, M.D.C.; et al. Cardiac troponin and COVID-19 severity: Results from BIOCOVID study. Eur. J. Clin. Invest. 2021, 51, e13532. [Google Scholar] [CrossRef]
- Hodges, G.; Pallisgaard, J.; Schjerning Olsen, A.M.; McGettigan, P.; Andersen, M.; Krogager, M.; Kragholm, K.; Kober, L.; Gislason, G.H.; Torp-Pedersen, C.; et al. Association between biomarkers and COVID-19 severity and mortality: A nationwide Danish cohort study. BMJ Open 2020, 10, e041295. [Google Scholar] [CrossRef]
- Henein, M.Y.; Mandoli, G.E.; Pastore, M.C.; Ghionzoli, N.; Hasson, F.; Nisar, M.K.; Islam, M.; Bandera, F.; Marrocco-Trischitta, M.M.; Baroni, I.; et al. Biomarkers Predict In-Hospital Major Adverse Cardiac Events in COVID-19 Patients: A Multicenter International Study. J. Clin. Med. 2021, 10, 5863. [Google Scholar] [CrossRef]
- Imazio, M.; Klingel, K.; Kindermann, I.; Brucato, A.; De Rosa, F.G.; Adler, Y.; De Ferrari, G.M. COVID-19 pandemic and troponin: Indirect myocardial injury, myocardial inflammation or myocarditis? Heart 2020, 106, 1127–1131. [Google Scholar] [CrossRef]
- Mueller, C.; Giannitsis, E.; Jaffe, A.S.; Huber, K.; Mair, J.; Cullen, L.; Hammarsten, O.; Mills, N.L.; Mockel, M.; Krychtiuk, K.; et al. Cardiovascular biomarkers in patients with COVID-19. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.S.; Kotecha, T.; Razvi, Y.; Chacko, L.; Brown, J.T.; Jeetley, P.S.; Goldring, J.; Jacobs, M.; Lamb, L.E.; Negus, R.; et al. COVID-19: Myocardial Injury in Survivors. Circulation 2020, 142, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, C.; Collins, L.F.; Malani, P. Long-term Health Consequences of COVID-19. JAMA 2020, 324, 1723–1724. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Hamm, C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart 2006, 92, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [Green Version]
- Caro-Codon, J.; Rey, J.R.; Buno, A.; Iniesta, A.M.; Rosillo, S.O.; Castrejon-Castrejon, S.; Rodriguez-Sotelo, L.; Martinez, L.A.; Marco, I.; Merino, C.; et al. Characterization of NT-proBNP in a large cohort of COVID-19 patients. Eur. J. Heart Fail. 2021, 23, 456–464. [Google Scholar] [CrossRef]
- Yoo, J.; Grewal, P.; Hotelling, J.; Papamanoli, A.; Cao, K.; Dhaliwal, S.; Jacob, R.; Mojahedi, A.; Bloom, M.E.; Marcos, L.A.; et al. Admission NT-proBNP and outcomes in patients without history of heart failure hospitalized with COVID-19. ESC Heart Fail. 2021, 8, 4278–4287. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, F.; Bai, L.; Bai, L.; Huang, Z.; Peng, Y. Association between NT-proBNP Level and the Severity of COVID-19 Pneumonia. Cardiol. Res. Pract. 2021, 2021, 5537275. [Google Scholar] [CrossRef]
- Pranata, R.; Huang, I.; Lukito, A.A.; Raharjo, S.B. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19: Systematic review and meta-analysis. Postgrad. Med. J. 2020, 96, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Sotgia, S.; Carru, C.; Mangoni, A.A. B-Type Natriuretic Peptide Concentrations, COVID-19 Severity, and Mortality: A Systematic Review and Meta-Analysis With Meta-Regression. Front Cardiovasc. Med. 2021, 8, 690790. [Google Scholar] [CrossRef] [PubMed]
- Ghany, R.; Palacio, A.; Chen, G.; Dawkins, E.; McCarter, D.; Forbes, E.; Chung, B.; Tamariz, L. Prior cardiovascular risk and screening echocardiograms predict hospitalization and severity of coronavirus infection among elderly medicare patients. Am. J. Prev. Cardiol. 2020, 3, 100090. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Xie, L.; Liu, R.; Yang, J.; Liu, F.; Wu, K.; Chen, L.; Hou, W.; Feng, Y.; Zhu, C. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J. Med. Virol. 2020, 92, 819–823. [Google Scholar] [CrossRef]
- Tajbakhsh, A.; Gheibi Hayat, S.M.; Taghizadeh, H.; Akbari, A.; Inabadi, M.; Savardashtaki, A.; Johnston, T.P.; Sahebkar, A. COVID-19 and cardiac injury: Clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev. Anti. Infect. Ther. 2021, 19, 345–357. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef] [Green Version]
- Gauchotte, G.; Venard, V.; Segondy, M.; Cadoz, C.; Esposito-Fava, A.; Barraud, D.; Louis, G. SARS-Cov-2 fulminant myocarditis: An autopsy and histopathological case study. Int. J. Legal. Med. 2021, 135, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Bulfamante, G.P.; Perrucci, G.L.; Falleni, M.; Sommariva, E.; Tosi, D.; Martinelli, C.; Songia, P.; Poggio, P.; Carugo, S.; Pompilio, G. Evidence of SARS-CoV-2 Transcriptional Activity in Cardiomyocytes of COVID-19 Patients without Clinical Signs of Cardiac Involvement. Biomedicines 2020, 8, 626. [Google Scholar] [CrossRef]
- Basso, C.; Leone, O.; Rizzo, S.; De Gaspari, M.; van der Wal, A.C.; Aubry, M.C.; Bois, M.C.; Lin, P.T.; Maleszewski, J.J.; Stone, J.R. Pathological features of COVID-19-associated myocardial injury: A multicentre cardiovascular pathology study. Eur. Heart J. 2020, 41, 3827–3835. [Google Scholar] [CrossRef]
- Fox, S.E.; Li, G.; Akmatbekov, A.; Harbert, J.L.; Lameira, F.S.; Brown, J.Q.; Vander Heide, R.S. Unexpected Features of Cardiac Pathology in COVID-19 Infection. Circulation 2020, 142, 1123–1125. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Brauninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.P.; et al. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Bois, M.C.; Boire, N.A.; Layman, A.J.; Aubry, M.C.; Alexander, M.P.; Roden, A.C.; Hagen, C.E.; Quinton, R.A.; Larsen, C.; Erben, Y.; et al. COVID-19-Associated Nonocclusive Fibrin Microthrombi in the Heart. Circulation 2021, 143, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Schaller, T.; Hirschbuhl, K.; Burkhardt, K.; Braun, G.; Trepel, M.; Markl, B.; Claus, R. Postmortem Examination of Patients With COVID-19. JAMA 2020, 323, 2518–2520. [Google Scholar] [CrossRef] [PubMed]
- Halushka, M.K.; Vander Heide, R.S. Myocarditis is rare in COVID-19 autopsies: Cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. 2021, 50, 107300. [Google Scholar] [CrossRef]
- Kyto, V.; Saukko, P.; Lignitz, E.; Schwesinger, G.; Henn, V.; Saraste, A.; Voipio-Pulkki, L.M. Diagnosis and presentation of fatal myocarditis. Hum. Pathol. 2005, 36, 1003–1007. [Google Scholar] [CrossRef]
- Mele, D.; Flamigni, F.; Rapezzi, C.; Ferrari, R. Myocarditis in COVID-19 patients: Current problems. Intern. Emerg. Med. 2021, 16, 1123–1129. [Google Scholar] [CrossRef]
- Tanacli, R.; Doeblin, P.; Gotze, C.; Zieschang, V.; Faragli, A.; Stehning, C.; Korosoglou, G.; Erley, J.; Weiss, J.; Berger, A.; et al. COVID-19 vs. Classical Myocarditis Associated Myocardial Injury Evaluated by Cardiac Magnetic Resonance and Endomyocardial Biopsy. Front. Cardiovasc. Med. 2021, 8, 737257. [Google Scholar] [CrossRef]
- Kociol, R.D.; Cooper, L.T.; Fang, J.C.; Moslehi, J.J.; Pang, P.S.; Sabe, M.A.; Shah, R.V.; Sims, D.B.; Thiene, G.; Vardeny, O.; et al. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e69–e92. [Google Scholar] [CrossRef]
- Skouri, H.N.; Dec, G.W.; Friedrich, M.G.; Cooper, L.T. Noninvasive imaging in myocarditis. J. Am. Coll. Cardiol. 2006, 48, 2085–2093. [Google Scholar] [CrossRef] [Green Version]
- Hiramitsu, S.; Morimoto, S.; Kato, S.; Uemura, A.; Ohtsuki, M.; Kato, Y.; Sugiura, A.; Miyagishima, K.; Mori, N.; Yoda, R.; et al. Significance of transient left ventricular wall thickening in acute lymphocytic myocarditis. Heart Vessels. 2007, 22, 25–29. [Google Scholar] [CrossRef]
- Shillcutt, S.K.; Thomas, W.R.; Sullivan, J.N.; Duhachek-Stapelman, A. Fulminant myocarditis: The role of perioperative echocardiography. Anesth. Analg. 2015, 120, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Cipriani, M.; Lilliu, M.; Sormani, P.; Varrenti, M.; Raineri, C.; Petrella, D.; Garascia, A.; Pedrotti, P.; Roghi, A.; et al. Survival and Left Ventricular Function Changes in Fulminant Versus Nonfulminant Acute Myocarditis. Circulation 2017, 136, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Halabe, A.; Sutton, R.A. Primary hyperparathyroidism and idiopathic hypercalciuria. Miner. Electrolyte Metab. 1987, 13, 235–241. [Google Scholar] [PubMed]
- Silverio, A.; Di Maio, M.; Scudiero, F.; Russo, V.; Esposito, L.; Attena, E.; Pezzullo, S.; Parodi, G.; D’Andrea, A.; Damato, A.; et al. Clinical conditions and echocardiographic parameters associated with mortality in COVID-19. Eur. J. Clin. Invest. 2021, 51, e13638. [Google Scholar] [CrossRef]
- Dweck, M.R.; Bularga, A.; Hahn, R.T.; Bing, R.; Lee, K.K.; Chapman, A.R.; White, A.; Salvo, G.D.; Sade, L.E.; Pearce, K.; et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 949–958. [Google Scholar] [CrossRef]
- Adeboye, A.; Alkhatib, D.; Butt, A.; Yedlapati, N.; Garg, N. A Review of the Role of Imaging Modalities in the Evaluation of Viral Myocarditis with a Special Focus on COVID-19-Related Myocarditis. Diagnostics 2022, 12, 549. [Google Scholar] [CrossRef]
- Logstrup, B.B.; Nielsen, J.M.; Kim, W.Y.; Poulsen, S.H. Myocardial oedema in acute myocarditis detected by echocardiographic 2D myocardial deformation analysis. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1018–1026. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, H.; Zhu, S.; Xie, Y.; Wang, B.; He, L.; Zhang, D.; Zhang, Y.; Yuan, H.; Wu, C.; et al. Prognostic Value of Right Ventricular Longitudinal Strain in Patients With COVID-19. JACC Cardiovasc. Imaging 2020, 13, 2287–2299. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.; Croft, L.B.; Ro, R.; Anastasius, M.; Zhao, W.; Giustino, G.; Argulian, E.; Goldman, M.E.; Sharma, S.K.; Kini, A.; et al. Biventricular strain by speckle tracking echocardiography in COVID-19: Findings and possible prognostic implications. Future Cardiol. 2021, 17, 663–667. [Google Scholar] [CrossRef]
- Citro, R.; Pontone, G.; Bellino, M.; Silverio, A.; Iuliano, G.; Baggiano, A.; Manka, R.; Iesu, S.; Vecchione, C.; Asch, F.M.; et al. Role of multimodality imaging in evaluation of cardiovascular involvement in COVID-19. Trends Cardiovasc. Med. 2021, 31, 8–16. [Google Scholar] [CrossRef]
- Motwani, M.; Kidambi, A.; Greenwood, J.P.; Plein, S. Advances in cardiovascular magnetic resonance in ischaemic heart disease and non-ischaemic cardiomyopathies. Heart 2014, 100, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Kligerman, S. Imaging of Pericardial Disease. Radiol. Clin. North Am. 2019, 57, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Trinchero, R. Myopericarditis: Etiology, management, and prognosis. Int. J. Cardiol. 2008, 127, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ojha, V.; Verma, M.; Pandey, N.N.; Mani, A.; Malhi, A.S.; Kumar, S.; Jagia, P.; Roy, A.; Sharma, S. Cardiac Magnetic Resonance Imaging in Coronavirus Disease 2019 (COVID-19): A Systematic Review of Cardiac Magnetic Resonance Imaging Findings in 199 Patients. J. Thorac. Imaging 2021, 36, 73–83. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Faron, A.; Isaak, A.; Dabir, D.; Kuetting, D.; Feisst, A.; Schmeel, F.C.; Sprinkart, A.M.; Thomas, D. Comparison of Original and 2018 Lake Louise Criteria for Diagnosis of Acute Myocarditis: Results of a Validation Cohort. Radiol. Cardiothorac. Imaging 2019, 1, e190010. [Google Scholar] [CrossRef]
- Greulich, S.; Klingel, K. COVID-19 and Myocarditis: Findings from Cardiac Magnetic Resonance Imaging and Endomyocardial Biopsies. Hamostaseologie 2021, 41, 366–370. [Google Scholar] [CrossRef]
- Daniels, C.J.; Rajpal, S.; Greenshields, J.T.; Rosenthal, G.L.; Chung, E.H.; Terrin, M.; Jeudy, J.; Mattson, S.E.; Law, I.H.; Borchers, J.; et al. Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes With Recent SARS-CoV-2 Infection: Results From the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021, 6, 1078–1087. [Google Scholar] [CrossRef]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Im, D.J.; Youn, J.C.; Chang, S.; Suh, Y.J.; Hong, Y.J.; Kim, Y.J.; Hur, J.; Choi, B.W. Myocardial Extracellular Volume Fraction with Dual-Energy Equilibrium Contrast-enhanced Cardiac CT in Nonischemic Cardiomyopathy: A Prospective Comparison with Cardiac MR Imaging. Radiology 2016, 280, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.; Han, K.; Youn, J.C.; Im, D.J.; Kim, J.Y.; Suh, Y.J.; Hong, Y.J.; Hur, J.; Kim, Y.J.; Choi, B.W.; et al. Utility of Dual-Energy CT-based Monochromatic Imaging in the Assessment of Myocardial Delayed Enhancement in Patients with Cardiomyopathy. Radiology 2018, 287, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Farina, D.; Vaccher, F.; Ferrandino, G.; Bellini, D.; Carbone, I. Myocarditis: Imaging up to date. Radiol. Med. 2020, 125, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Biere, L.; Piriou, N.; Ernande, L.; Rouzet, F.; Lairez, O. Imaging of myocarditis and inflammatory cardiomyopathies. Arch. Cardiovasc. Dis. 2019, 112, 630–641. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, P.; Bax, J.J.; Blom, N.; Yu, Y.; Wang, Y.; Han, X.; Wang, Y.; Van Der Wall, E.E. 99mTc-MIBI myocardial perfusion imaging in myocarditis. Nucl. Med. Commun. 2003, 24, 779–783. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Helio, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart. J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Lawal, I.; Sathekge, M. F-18 FDG PET/CT imaging of cardiac and vascular inflammation and infection. Br. Med. Bull. 2016, 120, 55–74. [Google Scholar] [CrossRef]
- Nensa, F.; Kloth, J.; Tezgah, E.; Poeppel, T.D.; Heusch, P.; Goebel, J.; Nassenstein, K.; Schlosser, T. Feasibility of FDG-PET in myocarditis: Comparison to CMR using integrated PET/MRI. J. Nucl. Cardiol. 2018, 25, 785–794. [Google Scholar] [CrossRef]
- Al-Ali, D.; Elshafeey, A.; Mushannen, M.; Kawas, H.; Shafiq, A.; Mhaimeed, N.; Mhaimeed, O.; Mhaimeed, N.; Zeghlache, R.; Salameh, M.; et al. Cardiovascular and haematological events post COVID-19 vaccination: A systematic review. J. Cell. Mol. Med. 2022, 26, 636–653. [Google Scholar] [CrossRef]
- Parra-Lucares, A.; Toro, L.; Weitz-Munoz, S.; Ramos, C. Cardiomyopathy Associated with Anti-SARS-CoV-2 Vaccination: What Do We Know? Viruses 2021, 13, 2493. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Costa, L.; Ruscitti, P.; Navarini, L.; Del Puente, A.; Giacomelli, R.; Scarpa, R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun. Rev. 2020, 19, 102524. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e899. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, P.D. High affinity binding of SARS-CoV-2 spike protein enhances ACE2 carboxypeptidase activity. J. Biol. Chem. 2020, 295, 18579–18588. [Google Scholar] [CrossRef]
| Diagnostic Modality | Expected Finding in COVID-19 Associated Myocarditis | Relative Specificity |
|---|---|---|
| Cardiac troponin | Elevated [70,71] | +++ |
| Brain-type natriuretic peptide | Elevated [78,80,81] | ++ |
| C-reactive protein | Elevated [78,80,81] | + |
| Interleukin-6 | Elevated [78,80,81] | + |
| Lactate dehydrogenase | Elevated [78,80,81] | + |
| Transthoracic echocardiogram | LV dysfunction, normal LVIDd, increased wall thickness, pericardial effusion, possible LV thrombus [111,112,113,114,115,117,118,123] | ++ |
| Cardiac MRI | Presence of LGE, edema, LV dysfunction, possible pericardial effusion [126,127] | ++++ |
| Cardiac multidetector CT | Increased myocardial extracellular volume [133,134] | +++ |
| Endomyocardial biopsy | Interstitial edema, lymphocytic infiltrate, increased macrophage presence, myocyte necrosis [76,100,101,102,103,114] | +++++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraser, M.; Agdamag, A.C.C.; Maharaj, V.R.; Mutschler, M.; Charpentier, V.; Chowdhury, M.; Alexy, T. COVID-19-Associated Myocarditis: An Evolving Concern in Cardiology and Beyond. Biology 2022, 11, 520. https://doi.org/10.3390/biology11040520
Fraser M, Agdamag ACC, Maharaj VR, Mutschler M, Charpentier V, Chowdhury M, Alexy T. COVID-19-Associated Myocarditis: An Evolving Concern in Cardiology and Beyond. Biology. 2022; 11(4):520. https://doi.org/10.3390/biology11040520
Chicago/Turabian StyleFraser, Meg, Arianne Clare C. Agdamag, Valmiki R. Maharaj, Melinda Mutschler, Victoria Charpentier, Mohammed Chowdhury, and Tamas Alexy. 2022. "COVID-19-Associated Myocarditis: An Evolving Concern in Cardiology and Beyond" Biology 11, no. 4: 520. https://doi.org/10.3390/biology11040520
APA StyleFraser, M., Agdamag, A. C. C., Maharaj, V. R., Mutschler, M., Charpentier, V., Chowdhury, M., & Alexy, T. (2022). COVID-19-Associated Myocarditis: An Evolving Concern in Cardiology and Beyond. Biology, 11(4), 520. https://doi.org/10.3390/biology11040520






