Recent Developments in Biological Processing Technology for Palm Oil Mill Effluent Treatment—A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Characterisation of POME
3. Biological Processing Technologies for POME Treatment
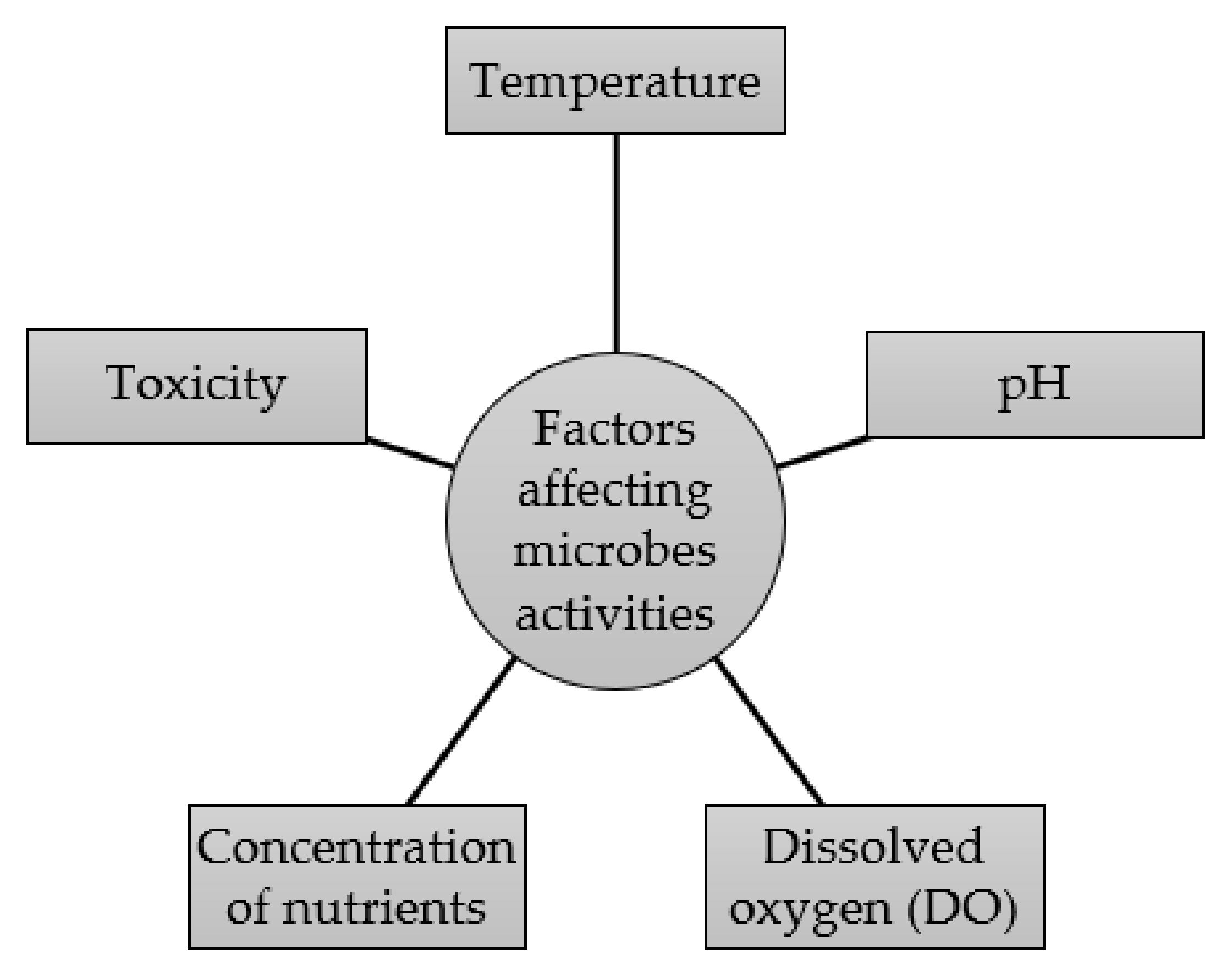
4. Factors Affecting Microorganism Activity during the Biological Processing Treatment
5. Biological Processing Technologies for POME Treatment
5.1. Upflow Anaerobic Sludge Blanket Reactor (UASBR)
5.2. Ultrasonic Membrane Anaerobic System (UMAS)
5.3. Membrane Anaerobic System
6. Biological Treatment of POME Using Microorganisms
6.1. Biological Treatment of POME Using Fungi
6.2. Biological Treatment of POME Using Bacteria
6.3. Biological Treatment of POME Using Microalgae
6.4. Biological Treatment of POME Using a Consortium of Microorganisms
| No. | Microorganism | Removal Efficiency | Parameter | Remarks | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COD % (mg/L) | BOD % (mg/L) | TSS % (mg/L) | OLR % (kg COD/m3 day) | Total Nitrogen | NH3-N % | Methane (CH₄) Gas Release (%) | Oil and Grease (mg/L) | °C | Working Volume (L) | HRT (day) | ||||
| 1. | Clostridium butyricum LS2 (1st) and Rhodopseudomonas palustris (2nd) | 93 | ND | ND | ND | ND | ND | ND | ND | 37 ± 1 | 1 | 4 | 1st stage: dark fermentation 2nd stage: photo fermentation Hydrogen production: 3.064 mL H2/mL | [119] |
| 2. | E. nidulans + A. niger + A. fumigatus | 91.43 | 94.34 | ND | ND | ND | ND | ND | ND | 30 | 0.25 | 5 | POME treatment | [93] |
| 3. | Consortium of bacteria and fungi: Micrococcus luteus 101 PB, Stenotrophomonas maltophilia 102 PB, Bacillus cereus 103 PB, Providencia vermicola 104 PB, Klebsiella pneumonia 105 PB, Bacillus subtilis 106 PB, Aspergillus fumigatus 107 PF, Aspergillus nomius 108 PF, Aspergillus niger 109 PF, and Meyerozyma guilliermondii 110 PF | 91.06 | 90.23 | 92.23 | ND | ND | ND | ND | ND | ND | 1 | 50 | POME treatment | [152] |
| 4. | Bacillus cereus 103 PB and Bacillus subtilis 106 PB | 90.64 | 93.11 | ND | ND | ND | ND | ND | ND | 37 | 0.25 | 5 | POME treatment | [113] |
| 5. | Consortium of B. subtilis and A. niger | 90.3 (1000 ± 100) | ND | ND | ND | 1920 ± 75 | 780 ± 20 | ND | ND | 40 | 0.095 | 7 | POME treatment | [31] |
| 6. | Bacillus toyonensis strain BCT-71120 and Stenotrophomonas rhizophila strain ep10 | 86 | 94 | 80 | ND | ND | ND | 41.05 | ND | ND | 3 | 18 | Production of methane using anaerobic consortium bacteria | [155] |
| 7. | Co-culture of yeast (Lipomyces starkeyi) and bacteria (Bacillus cereus) | 83.66 ± 1.9 | 77.34 | 71.43 | ND | 65.30 | 76.59 | ND | 79.23 | 30 | 0.2 | 6 | Microbial lipid accumulation: 2.27 g/L | [95] |
| 8. | Consortium of Scenedesmus sp. UKM9 and Chlorella sp. UKM2 | 71.00 | ND | ND | ND | ND | ND | ND | ND | 25 ± 2 | 2 | 25 | Integrated 2 stage treatment of POME treatment | [156] |
| 9. | Klebsiella variicola and Pseudomonas aeruginosa | 69.28 | ND | ND | ND | ND | ND | ND | ND | ND | 0.02 | 11 | Electricity generation using MFC: 12.21 W/m3 | [157] |
| 10. | Pseudomonas sp. on Chlorella sorokiniana CY-1 | 53.70 | ND | ND | ND | 55.6 | ND | ND | ND | 25 | ND | 5 | Lipid production | [123] |
| 11. | Consortium of microalgae: Chlorella sorokiniana UKM2, Coelastrella sp. UKM4 and Chlorella pyrenoidosa UKM7 | 27.55 (2845 ± 159) | 20.59 (725 ± 66) | ND | ND | 22.27 (506 ± 82) | −4.10 (279 ± 14) | ND | ND | 25 ± 1 | 1.8 | 7 | Anaerobic pond in Dominion Square Palm Oil Mill, Gambang, Pahang, Malaysia (APDI) | [158] |
| 12. | Consortium of microalgae: Chlorella sorokiniana UKM2, Coelastrella sp. UKM4 and Chlorella pyrenoidosa UKM7 | 25.97 (3352 ± 193) | 22.65 (731 ± 52) | ND | ND | 25.09 (241 ± 17) | 5.58 (254 ± 33) | ND | ND | 25 ± 1 | 1.8 | 7 | Anaerobic pond at the Sime Darby Palm Oil Mill, Carey Island, Selangor, Malaysia (APCI) | [158] |
| 13. | Consortium of microalgae: Chlorella sorokiniana UKM2, Coelastrella sp. UKM4 and Chlorella pyrenoidosa UKM7 | 15.93 (1953 ± 131) | 13.03 (661 ± 41) | ND | ND | 13.43 (464 ± 25) | −5.70 (241 ± 17) | ND | ND | 25 ± 1 | 1.8 | 7 | Facultative pond in Sime Darby Palm Oil Mill, Port Dickson, Negeri Sembilan, Malaysia (FPPD) | [158] |
| 14. | Natural microflora anaerobic POME sludge | 13 | ND | ND | ND | ND | ND | ND | ND | 30 ± 2 | 0.1 | 6 | Bioelectricity generation: 85.12 mW/m2 | [159] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| POME | Palm oil mill effluent |
| EFB | Empty fruit bunch |
| BOD | Biochemical oxygen demand |
| COD | Chemical oxygen demand |
| TSS | Total suspended solid |
| HRT | Hydraulic retention time |
| UASBR | Upflow anaerobic sludge blanket reactor |
| UMAS | Ultrasonic membrane anaerobic system |
| MAS | Membrane anaerobic system |
References
- Oil World. OIL WORLD ISTA Mielke GmbH: Independent Global Market Analyses & Forecasts Since 1958. (2021, July 16). Retrieved. Available online: https://www.oilworld.biz/p/monthly-july-16-2021 (accessed on 12 December 2021).
- MPOB-Malaysian Palm Oil Board. Production of Crude Palm Oil 2021. Economic and Industry Development Division. (2021, November). Available online: https://bepi.mpob.gov.my/index.php/en/production/production-2021/production-of-crude-oil-palm-2021 (accessed on 12 December 2021).
- Ghulam Khadir, A.P.; Hishamuddin, E.; Soh, K.L.; Ong-Abdullah, M.; Mohamed Salleh, K.; Zanal Bidin, M.N.I.; Sundram, S.; Azizul Hasan, Z.A.; Idris, Z. Oil Palm Economic Performance in Malaysia and R&D Progress in 2019. J. Oil Palm Res. 2020, 32, 159–190. [Google Scholar] [CrossRef]
- Madaki, Y.S.; Seng, L. Palm oil mill effluent (POME) from Malaysia palm oil mills: Waste or resource. Int. J. Sci. Environ. Technol. 2013, 2, 1138–1155. [Google Scholar]
- Abdullah, N.; Sulaiman, F. The oil palm wastes in Malaysia. In Biomass now—Sustainable Growth and Use; IntechOpen: London, UK, 2013; Volume 1, pp. 75–93. [Google Scholar]
- Environmental Quality Act 1974 (No. 127 of 1974). Available online: https://www.informea.org/en/legislation/environmental-quality-act-1974-no-127-1974 (accessed on 12 December 2021).
- Rana, S.; Singh, L.; Wahid, Z.; Liu, H. A recent overview of palm oil mill effluent management via bioreactor configurations. Curr. Pollut. Rep. 2017, 3, 254–267. [Google Scholar] [CrossRef]
- Lee, Z.S.; Chin, S.Y.; Lim, J.W.; Witoon, T.; Cheng, C.K. Treatment technologies of palm oil mill effluent (POME) and olive mill wastewater (OMW): A brief review. Environ. Technol. Innov. 2019, 15, 100377. [Google Scholar] [CrossRef]
- Low, T.J.; Mohammad, S.; Sudesh, K.; Baidurah, S. Utilization of banana (Musa sp.) fronds extract as an alternative carbon source for poly (3-hydroxybutyrate) production by Cupriavidus necator H16. Biocatal. Agricul. Biotechnol. 2021, 34, 102048. [Google Scholar] [CrossRef]
- Sen, K.Y.; Hussin, M.H.; Baidurah, S. Biosynthesis of poly (3-hydroxybutyrate) (PHB) by Cupriavidus necator from various pretreated molasses as carbon source. Biocatal. Agric. Biotechnol. 2019, 17, 51–59. [Google Scholar] [CrossRef]
- Sen, K.Y.; Baidurah, S. Renewable biomass feedstocks for production of sustainable biodegradable polymer. Curr. Opin. Green Sustain. Chem. 2020, 27, 1–6. [Google Scholar] [CrossRef]
- Boey, J.Y.; Mohamad, L.; Khok, Y.S.; Tay, G.S.; Baidurah, S. A Review of the Applications and Biodegradation of Polyhydroxyalkanoates and Poly (lactic acid) and Its Composites. Polymers 2021, 13, 1544. [Google Scholar] [CrossRef]
- Kassim, M.A.; Meng, T.K.; Serri, N.A.; Yusoff, S.B.; Shahrin, N.A.M.; Seng, K.Y.; Bakar, M.H.A.; Keong, L.C. Sustainable Biorefinery Concept for Industrial Bioprocessing. In Biorefinery Production Technologies for Chemicals and Energy, 1st ed.; Kuila, A., Mukhopadhyay, M., Eds.; Wiley & Sons: Hoboken, NJ, USA; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 15–53. [Google Scholar]
- Mohamad, L.; Hasan, H.A.; Sudesh, K.; Baidurah, S. Dual application of black soldier fly (Hermetia illucens): Protein-rich animal feed and biological extraction agent for polyhydroxybutyrate. Malays. J. Microbiol. 2021, 17, 624–634. [Google Scholar] [CrossRef]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef]
- Kristanti, R.A.; Hadibarata, T.; Yuniarto, A.; Muslim, A. Palm Oil Industries in Malaysia and Possible Treatment Technologies for Palm Oil Mill Effluent: A Review. Environ. Res. Eng. Manag. 2021, 77, 50–65. [Google Scholar] [CrossRef]
- Kongnoo, A.; Suksaroj, T.; Intharapat, P.; Promtong, T.; Suksaroj, C. Decolorization and organic removal from palm oil mill effluent by Fenton’s process. Environ. Eng. Sci. 2012, 29, 855–859. [Google Scholar] [CrossRef]
- Sethupathi, S. Removal of Residue Oil from Palm Oil Mill Effluent (POME) Using Chitosan; Universiti Sains Malaysia: Penang, Malaysia, 2004; Volume 51. [Google Scholar]
- Wu, T.Y.; Mohammad, A.W.; Jahim, J.M.; Anuar, N. Pollution control technologies for the treatment of palm oil mill effluent (POME) through end-of-pipe processes. J. Environ. Manag. 2010, 91, 1467–1490. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Baidurah, S.; Kobayashi, T.; Ismail, N.; Leh, C.P. Palm Oil Mill Effluent Treatment Processes—A Review. Processes 2021, 9, 739. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Din, M.F.M.; Rezania, S.; Khademi, T.; Kumar, A. Palm oil mill effluent as an environmental pollutant. Palm Oil 2018, 13, 13–28. [Google Scholar]
- The Commissioner of Law Revision, Malaysia; Federal Subsidiary Legislation, Division of Environment. Environmental Quality (Prescribed Premises) (Crude Palm Oil) (Amendment) Regulations 1982. Available online: https://www.doe.gov.my/wp-content/uploads/2021/11/Environmental_Quality_Prescribed_Premises_Crude_Palm_Oil_Amendment_Regulations_1982_-_P.U.A_183-82.pdf (accessed on 13 October 2021).
- Samer, M. Biological and chemical wastewater treatment processes. In Wastewater Treatment Engineering; IntechOpen: London, UK, 2015; Volume 150. [Google Scholar]
- Mohammad, S.; Baidurah, S.; Kamimura, N.; Matsuda, S.; Bakar, N.A.S.A.; Muhamad, N.N.I.; Ahmad, A.H.; Dominic, D.; Kobayashi, T. Fermentation of Palm Oil Mill Effluent in the Presence of Lysinibacillus sp. LC 556247 to Produce Alternative Biomass Fuel. Sustainability 2021, 13, 11915. [Google Scholar] [CrossRef]
- Muralikrishna, I.V.; Manickam, V.; Muralikrishna, I.V.; Manickam, V. Chapter Twelve-Wastewater Treatment Technologies; Environmental Management: Oxford, UK, 2017. [Google Scholar]
- Gray, N.F. Water Technology, Second Edition: An Introduction for Environmental Scientists and Engineers, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2005. [Google Scholar]
- Rittmann, B.E. Aerobic biological treatment. Water treatment processes. Environ. Sci. Technol. 1987, 21, 128–136. [Google Scholar] [CrossRef]
- Veolia Water Technologies. Aerobic Wastewater Treatment. Veolia Water Technologies UK. Available online: https://www.veoliawatertechnologies.co.uk/technologies/aerobic-treatment (accessed on 20 December 2021).
- Gao, W.J.; Leung, K.T.; Qin, W.S.; Liao, B.Q. Effects of temperature and temperature shock on the performance and microbial community structure of a submerged anaerobic membrane bioreactor. Bioresour. Technol. 2011, 102, 8733–8740. [Google Scholar] [CrossRef]
- LaPara, T.M.; Nakatsu, C.H.; Pantea, L.M.; Alleman, J.E. Aerobic biological treatment of a pharmaceutical wastewater: Effect of temperature on COD removal and bacterial community development. Water Res. 2001, 35, 4417–4425. [Google Scholar] [CrossRef]
- Nwuche, C.O.; Aoyagi, H.; Ogbonna, J.C. Treatment of palm oil mill effluent by a microbial consortium developed from compost soils. In International Scholarly Research Notices; Hindawi: New York, NY, USA, 2014. [Google Scholar]
- Sánchez-Clemente, R.; Igeño, M.I.; Población, A.G.; Guijo, M.I.; Merchán, F.; Blasco, R. Study of pH changes in media during bacterial growth of several environmental strains. Multidiscip. Digit. Publ. Inst. Proc. 2018, 2, 1297. [Google Scholar] [CrossRef] [Green Version]
- Beales, N. Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: A review. Compr. Rev. Food Sci. Food Saf. 2004, 3, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calviño, D.; Bååth, E. Growth response of the bacterial community to pH in soils differing in pH. Fed. Eur. Microbiol. Soc. Microbiol. Ecol. 2010, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Tajuddin, R.M.; Ismail, A.F.; Salim, M.R. Effects of oxygen concentration on microbial growth in aerated palm oil mill effluent using oxygen enriched air membrane system. In Regional Symposium on Membrane Science and Technology; Johor Bahru: Johor, Malaysia, 2004. [Google Scholar]
- Liao, B.Q.; Lin, H.J.; Langevin, S.P.; Gao, W.J.; Leppard, G.G. Effects of temperature and dissolved oxygen on sludge properties and their role in bioflocculation and settling. Water Res. 2011, 45, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yang, A.; Zhang, G.; Wang, H. Effects of dissolved oxygen concentration on photosynthetic bacteria wastewater treatment: Pollutants removal, cell growth and pigments production. Bioresour. Technol. 2017, 241, 993–997. [Google Scholar] [CrossRef]
- Wilbanks, B.; Trinh, C.T. Comprehensive characterization of toxicity of fermentative metabolites on microbial growth. Biotechnol. Biofuels 2017, 10, 262. [Google Scholar] [CrossRef] [Green Version]
- Riemann, B.; Lindgaard-Jørgensen, P. Effects of toxic substances on natural bacterial assemblages determined by means of [3H] thymidine incorporation. Appl. Environ. Microbiol. 1990, 56, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Shehata, T.E.; Marr, A.G. Effect of nutrient concentration on the growth of Escherichia coli. J. Bacteriol. 1971, 107, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Ghufran, R.; Abd Wahid, Z. Effect of COD loading rate on an upflow anaerobic sludge blanket reactor during anaerobic digestion of palm oil mill effluent with butyrate. J. Environ. Eng. Landsc. Manag. 2012, 20, 256–264. [Google Scholar] [CrossRef]
- Abdurahman, N.H.; Azhari, N.H.; Rosli, Y.M. Ultrasonic membrane anaerobic system (UMAS) for palm oil mill effluent (POME) treatment. Int. Perspect. Water Qual. Manag. Pollut. Control 2013, 1, 36–40. [Google Scholar]
- Abdurahman, N.H.; Rosli, Y.M.; Azhari, N.H. Development of a membrane anaerobic system (MAS) for palm oil mill effluent (POME) treatment. Desalination 2011, 266, 208–212. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ahmad, D.; Aziz, M.E.B.A. Aerobic treatment of palm oil mill effluent. J. Environ. Manag. 2007, 82, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Badroldin, N.A.; Latiff, A.A.; Karim, A.T.; Fulazzaky, M.A. Palm Oil Mill Effluent (POME) Treatment using Hybrid Upflow Anaerobic Sludge Blanket (HUASB) Reactors: Impact on COD Removal and Organic Loading Rates. In Proceedings of the Engineering Postgraduate Conference (EPC), Selangor, Malaysia, 20–21 October 2008. [Google Scholar]
- Yacob, S.; Hassan, M.A.; Shirai, Y.; Wakisaka, M.; Subash, S. Baseline study of methane emission from anaerobic ponds of palm oil mill effluent treatment. Sci. Total Environ. 2006, 366, 187–196. [Google Scholar] [CrossRef]
- Najafpour, G.D.; Zinatizadeh, A.A.L.; Mohamed, A.R.; Isa, M.H.; Nasrollahzadeh, H. High-rate anaerobic digestion of palm oil mill effluent in an upflow anaerobic sludge-fixed film bioreactor. Process Biochem. 2006, 41, 370–379. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, X.; Ni, J.; Borthwick, A. Palm oil mill effluent treatment using a two-stage microbial fuel cells system integrated with immobilized biological aerated filters. Bioresour. Technol. 2010, 101, 2729–2734. [Google Scholar] [CrossRef]
- Chaisri, R.; Boonsawang, P.; Prasertsan, P.; Chaiprapat, S. Effect of organic loading rate on methane and volatile fatty acids productions from anaerobic treatment of palm oil mill effluent in UASB and UFAF reactors. Warasan Songkhla Nakharin Sakha Witthayasat Lae Technol. 2007, 2, 311. [Google Scholar]
- Yuniarto, A.; Noor, Z.Z.; Ujang, Z.; Olsson, G.; Aris, A.; Hadibarata, T. Bio-fouling reducers for improving the performance of an aerobic submerged membrane bioreactor treating palm oil mill effluent. Desalination 2013, 316, 146–153. [Google Scholar] [CrossRef]
- Choi, W.H.; Shin, C.H.; Son, S.M.; Ghorpade, P.A.; Kim, J.J.; Park, J.Y. Anaerobic treatment of palm oil mill effluent using combined high-rate anaerobic reactors. Bioresour. Technol. 2013, 141, 138–144. [Google Scholar] [CrossRef]
- Wang, J.; Mahmood, Q.; Qiu, J.P.; Li, Y.S.; Chang, Y.S.; Li, X.D. Anaerobic treatment of palm oil mill effluent in pilot-scale anaerobic EGSB reactor. In BioMed Research International; Hindawi: New York, NY, USA, 2015. [Google Scholar]
- Zinatizadeh, A.A.; Mirghorayshi, M. Effect of temperature on the performance of an up-flow anaerobic sludge fixed film (UASFF) bioreactor treating palm oil mill effluent (POME). Waste Biomass Valorization 2019, 10, 349–355. [Google Scholar] [CrossRef]
- Loh, S.K.; Lai, M.E.; Ngatiman, M.; Lim, W.S.; Choo, Y.M.; Zhang, Z.; Salimon, J. Zero discharge treatment technology of palm oil mill effluent. J. Oil Palm Res. 2013, 25, 273–281. [Google Scholar]
- Chan, Y.J.; Chong, M.F.; Law, C.L. Biological treatment of anaerobically digested palm oil mill effluent (POME) using a Lab-Scale Sequencing Batch Reactor (SBR). J. Environ. Manag. 2010, 91, 1738–1746. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.A.N.; Lina, C.H.I.; Xiuhua, L.O.N.G.; Zhijian, M.E.I.; Zhang, Z. Startup and operation of anaerobic EGSB reactor treating palm oil mill effluent. J. Environ. Sci. 2008, 20, 658–663. [Google Scholar] [CrossRef]
- Poh, P.E.; Chong, M.F. Upflow anaerobic sludge blanket-hollow centered packed bed (UASB-HCPB) reactor for thermophilic palm oil mill effluent (POME) treatment. Biomass Bioenergy 2014, 67, 231–242. [Google Scholar] [CrossRef]
- Najafpour, G.; Yieng, H.A.; Younesi, H.; Zinatizadeh, A. Effect of organic loading on performance of rotating biological contactors using palm oil mill effluents. Process Biochem. 2005, 40, 2879–2884. [Google Scholar] [CrossRef]
- Chan, Y.J.; Chong, M.F.; Law, C.L. Optimization on thermophilic aerobic treatment of anaerobically digested palm oil mill effluent (POME). Biochem. Eng. J. 2011, 55, 193–198. [Google Scholar] [CrossRef]
- Al-Mamun, A.; Idris, A. Treatment of POME by pilot plant anaerobic fluidised bed reactor. IIUM Eng. J. 2008, 9, 9–18. [Google Scholar] [CrossRef]
- Malakahmad, A.; Abd Lahin, F.; Yee, W. Biodegradation of high-strength palm oil mill effluent (POME) through anaerobes partitioning in an integrated baffled reactor inoculated with anaerobic pond sludge. Water Air Soil Pollut. 2014, 225, 1–9. [Google Scholar] [CrossRef]
- Habeeb, S.A.; Latiff, A.A.A.; Daud, Z.; Ahmad, Z. A biodegradation and treatment of palm oil mill effluent (POME) using a hybrid up-flow anaerobic sludge bed (HUASB) reactor. Int. J. Energy Environ. 2011, 2, 653–660. [Google Scholar]
- Malakahmad, A.; Yee, W. Production of energy from palm oil mill effluent during start-up of carrier anaerobic baffled reactor (CABR) equipped with polymeric media. J. Jpn. Inst. Energy 2014, 93, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Fun, C.W.; Haq, M.R.U.; Kutty, S.R.M. Treatment of palm oil mill effluent using biological sequencing batch reactor system. WIT Trans. Ecol. Environ. 2007, 104, 8. [Google Scholar]
- Tong, S.L.; Jaafar, A.B. POME Biogas capture, upgrading and utilization. Palm Oil Eng. Bull. 2006, 78, 11–17. [Google Scholar]
- Irvan, I.; Trisakti, B.; Wongistani, V.; Tomiuchi, Y. Methane Emission from Digestion of Palm Oil Mill Effluent (POME) in a Thermophilic Anaerobic Reactor. Int. J. Sci. Eng. 2012, 3, 32–35. [Google Scholar]
- Vijayaraghavan, K.; Ahmad, D. Biohydrogen generation from palm oil mill effluent using anaerobic contact filter. Int. J. Hydrog. Energy 2006, 31, 1284–1291. [Google Scholar] [CrossRef]
- Choorit, W.; Wisarnwan, P. Effect of temperature on the anaerobic digestion of palm oil mill effluent. Electron. J. Biotechnol. 2007, 10, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Baranitharan, E.; Khan, M.R.; Prasad, D.M.R.; Salihon, J.B. Bioelectricity generation from palm oil mill effluent in microbial fuel cell using polacrylonitrile carbon felt as electrode. Water Air Soil Pollut. 2013, 224, 1–11. [Google Scholar] [CrossRef]
- Mohammadi, P.; Ibrahim, S.; Annuar, M.S.M.; Khashij, M.; Mousavi, S.A.; Zinatizadeh, A. Optimization of fermentative hydrogen production from palm oil mill effluent in an up-flow anaerobic sludge blanket fixed film bioreactor. Sustain. Environ. Res. 2017, 27, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Wong, Y.S.; Teng, T.T.; Ong, S.A.; Norhashimah, M.; Rafatullah, M.; Leong, J.Y. Methane gas production from palm oil wastewater—An anaerobic methanogenic degradation process in continuous stirrer suspended closed anaerobic reactor. J. Taiwan Inst. Chem. Eng. 2014, 45, 896–900. [Google Scholar] [CrossRef]
- Fang, C.; Sompong, O.; Boe, K.; Angelidaki, I. Comparison of UASB and EGSB reactors performance, for treatment of raw and deoiled palm oil mill effluent (POME). J. Hazard. Mater. 2011, 189, 229–234. [Google Scholar] [CrossRef]
- Singh, L.; Siddiqui, M.F.; Ahmad, A.; Rahim, M.H.A.; Sakinah, M.; Wahid, Z.A. Application of polyethylene glycol immobilized Clostridium sp. LS2 for continuous hydrogen production from palm oil mill effluent in upflow anaerobic sludge blanket reactor. Biochem. Eng. J. 2013, 70, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Singh, L.; Wahid, Z.A.; Siddiqui, M.F.; Ahmad, A.; Rahim, M.H.A.; Sakinah, M. Application of immobilized upflow anaerobic sludge blanket reactor using Clostridium LS2 for enhanced biohydrogen production and treatment efficiency of palm oil mill effluent. Int. J. Hydrog. Energy 2013, 38, 2221–2229. [Google Scholar] [CrossRef]
- Sompong, O.; Prasertsan, P.; Intrasungkha, N.; Dhamwichukorn, S.; Birkeland, N.K. Improvement of biohydrogen production and treatment efficiency on palm oil mill effluent with nutrient supplementation at thermophilic condition using an anaerobic sequencing batch reactor. Enzym. Microb. Technol. 2007, 41, 583–590. [Google Scholar]
- Islam, M.A.; Khan, M.R.; Yousuf, A.; Wai, W.C.; Cheng, C.K. Electricity generation form pretreated palm oil mill effluent using Klebsiella variicola as an inoculum in Microbial fuel cell. In Proceedings of the 2016 4th International Conference on the Development in the in Renewable Energy Technology (ICDRET), Dhaka, Bangladesh, 7–9 January 2016; IEEE: Dhaka, Bangladesh, 2016; pp. 1–4. [Google Scholar]
- Ismail, M.H.S.; Dalang, S.; Syam, S.; Izhar, S. A study on zeolite performance in waste treating ponds for treatment of palm oil mill effluent. J. Water Resour. Prot. 2013, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Badiei, M.; Jahim, J.M.; Anuar, N.; Abdullah, S.R.S. Effect of hydraulic retention time on biohydrogen production from palm oil mill effluent in anaerobic sequencing batch reactor. Int. J. Hydrog. Energy 2011, 36, 5912–5919. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.A.N.; Xiangli, Q.I.A.O.; Lina, C.H.I.; Xiangjun, N.I.U.; Zhijian, M.E.I.; Zhang, Z. Integration of biological method and membrane technology in treating palm oil mill effluent. J. Environ. Sci. 2008, 20, 558–564. [Google Scholar] [CrossRef]
- Latif, M.A.; Ahmad, A.; Ghufran, R.; Wahid, Z.A. Effect of temperature and organic loading rate on upflow anaerobic sludge blanket reactor and CH4 production by treating liquidized food waste. Environ. Prog. Sustain. Energy 2012, 31, 114–121. [Google Scholar] [CrossRef]
- Misevičius, A.; Baltrėnas, P. Experimental investigation of biogas production using biodegradable municipal waste. J. Environ. Eng. Landsc. Manag. 2011, 19, 167–177. [Google Scholar] [CrossRef]
- Bal, A.S.; Dhagat, N.N. Upflow anaerobic sludge blanket reactor a review. Indian J. Environ. Health 2001, 43, 1–82. [Google Scholar]
- Basri, M.F.; Yacob, S.; Hassan, M.A.; Shirai, Y.; Wakisaka, M.; Zakaria, M.R.; Phang, L.Y. Improved biogas production from palm oil mill effluent by a scaled-down anaerobic treatment process. World J. Microbiol. Biotechnol. 2010, 26, 505–514. [Google Scholar] [CrossRef]
- Hansen, C.L.; Cheong, D.Y. Fermentation, biogas and biohydrogen production from solid food processing. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Woodhead Publishing: Cambridge, UK, 2007; pp. 611–648. [Google Scholar]
- Shafie, N.F.A.; Mansor, U.Q.A.; Yahya, A.; Som, A.M.; Nour, A.H.; Hassan, Z.; RMA, R. The performance study of Ultrasonic-assisted Membrane Anaerobic System (UMAS) for Chemical Oxygen Demand (COD) removal efficiency and methane gas production in Palm Oil Mill Effluent (POME) treatment. In Proceedings of the 4th IET Clean Energy and Technology Conference (CEAT 2016), Kuala Lumpur, Malaysia, 12–15 November 2016; pp. 1–5. [Google Scholar] [CrossRef]
- Zouari, N.; Al Jabiri, H. Improvement by Micro-Aeration of Anaerobic Digestion of Slaughterhouse Wastewater at 38 °C. Int. J. Innov. Sci. Eng. Technol. 2015, 4, 807–816. [Google Scholar]
- Chiemchaisri, C.; Wong, Y.K.; Urase, T.; Yamamoto, A.K. Organic stabilization and nitrogen removal in membrane separation bioreactor for domestic wastewater treatment. Water Sci. Technol. 1992, 25, 231–240. [Google Scholar] [CrossRef]
- Oswal, N.; Sarma, P.M.; Zinjarde, S.S.; Pant, A. Palm oil mill effluent treatment by a tropical marine yeast. Bioresour. Technol. 2002, 85, 35–37. [Google Scholar] [CrossRef]
- Karim, M.I.A.; Kamil, A.Q.A. Biological treatment of palm oil mill effluent using Trichoderma viride. Biol. Wastes 1989, 27, 143–152. [Google Scholar] [CrossRef]
- Iwuagwu, J.O.; Ugwuanyi, J.O. Treatment and valorization of palm oil mill effluent through production of food grade yeast biomass. J. Waste Manag. 2014, 2014, 439071. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.Z.; Jamal, P.; Nadzir, M.M. Bioconversion of palm oil mill effluent for citric acid production: Statistical optimization of fermentation media and time by central composite design. World J. Microbiol. Biotechnol. 2008, 24, 1177–1185. [Google Scholar] [CrossRef]
- Ibegbulam-Njoku, P.N.; Achi, O.K. Use of fungi in bioremediation of palm oil mill effluent (POME). Int. J. Adv. Res. Technol. 2014, 3, 1–8. [Google Scholar]
- Lanka, S.; Pydipalli, M. Reduction of organic load from palm oil mill effluent (POME) using selected fungal strains isolated from POME dump sites. Afr. J. Biotechnol. 2018, 17, 1138–1145. [Google Scholar]
- Prasertsan, P.; Binmaeil, H. Treatment of palm oil mill effluent by thermotolerant polymer-producing fungi. J. Water Environ. Technol. 2018, 16, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Karim, A.; Islam, M.A.; Khalid, Z.B.; Yousuf, A.; Khan, M.M.R.; Faizal, C.K.M. Microbial lipid accumulation through bioremediation of palm oil mill effluent using a yeast-bacteria co-culture. Renew. Energy 2021, 176, 106–114. [Google Scholar] [CrossRef]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Efficient concomitant production of lipids and carotenoids by oleaginous red yeast Rhodotorula glutinis cultured in palm oil mill effluent and application of lipids for biodiesel production. Biotechnol. Bioprocess Eng. 2011, 16, 23–33. [Google Scholar] [CrossRef]
- Nwuche, C.O.; Aoyagi, H.; Ogbonna, J.C. Citric acid production from cellulase-digested palm oil mill effluent. Asian J. Biotechnol. 2013, 5, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.J.; Ma, Y.Y.; Cheng, B.Q.; Gao, Q.; Hua, Q. Metabolic engineering Yarrowia lipolytica for a dual biocatalytic system to produce fatty acid ethyl esters from renewable feedstock in situ and in one pot. Appl. Microbiol. Biotechnol. 2021, 105, 8561–8573. [Google Scholar] [CrossRef]
- Zinjarde, S.S.; Pant, A. Crude oil degradation by free and immobilized cells of Yarrowia lipolytica NCIM 3589. J. Environ. Sci. Health Part A 2000, 35, 755–763. [Google Scholar] [CrossRef]
- Gottardi, D.; Siroli, L.; Vannini, L.; Patrignani, F.; Lanciotti, R. Recovery and valorization of agri-food wastes and by-products using the non-conventional yeast Yarrowia lipolytica. Trends Food Sci. Technol. 2021, 115, 74–86. [Google Scholar] [CrossRef]
- Lotti, M.; Alberghina, L. Lipases: Molecular structure and function. In Industrial Enzymes; Springer: Dordrecht, The Netherlands, 2007; pp. 263–281. [Google Scholar]
- Sharma, R.; Chisti, Y.; Banerjee, U.C. Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 2001, 19, 627–662. [Google Scholar] [CrossRef] [Green Version]
- Louhasakul, Y.; Cheirsilp, B.; Prasertsan, P. Valorization of palm oil mill effluent into lipid and cell-bound lipase by marine yeast Yarrowia lipolytica and their application in biodiesel production. Waste Biomass Valorization 2016, 7, 417–426. [Google Scholar] [CrossRef]
- Schuster, A.; Schmoll, M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799. [Google Scholar] [CrossRef] [Green Version]
- Jaklitsch, W.M. European species of Hypocrea Part, I. The green-spored species. Stud. Mycol. 2009, 63, 1–91. [Google Scholar] [CrossRef] [Green Version]
- Amira, R.D.; Roshanida, A.R.; Rosli, M.I.; Zahrah, M.S.F.; Anuar, J.M.; Adha, C.N. Bioconversion of empty fruit bunches (EFB) and palm oil mill effluent (POME) into compost using Trichoderma virens. Afr. J. Biotechnol. 2011, 10, 18775–18780. [Google Scholar]
- Sabaratnam, A.A.S.V. Enzymatic hydrolysis of palm oil mill effluent solid using mixed cellulases from locally isolated fungi. Res. J. Microbiol. 2008, 3, 474–481. [Google Scholar]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Siddiquee, S.; Shafawati, S.N.; Naher, L. Effective composting of empty fruit bunches using potential Trichoderma strains. Biotechnol. Rep. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- Afraa, R.; Sushant, S.; Ali, F. Assessment of The Composting Process and Compost’s Utilization. Vegetos 2016, 29, 1–7. [Google Scholar]
- Jameel, A.T.; Olanrewaju, A.A. Aerobic biodegradation of oil and grease in palm oil mill effluent using consortium of microorganisms. In Current Research and Development in Biotechnology Engineering at International Islamic University Malaysia (IIUM), 3rd ed.; IIUM Press: Kuala Lumpur, Malaysia, 2011; pp. 43–51. [Google Scholar]
- Jameel, A.T.; Muyibi, S.A.; Olanrewaju, A.A. Comparative study of bioreactors used for palm oil mill effluent treatment based on chemical oxygen removal efficiencies. In Current Research and Development in Biotechnology Engineering at International Islamic University Malaysia (IIUM), 3rd ed.; IIUM Press: Kuala Lumpur, Malaysia, 2011; pp. 277–284. [Google Scholar]
- Bala, J.D.; Lalung, J.; Ismail, N. Studies on the reduction of organic load from palm oil mill effluent (POME) by bacterial strains. Int. J. Recycl. Org. Waste Agric. 2015, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bunrung, S.; Prasertsan, S.; Prasertsan, P. Decolorization of biogas effluent from palm oil mill using combined biological and physical methods. Agric. Nat. Resour. 2014, 48, 95–104. [Google Scholar]
- Izah, S.C. Feed potentials of Saccharomyces cerevisiae biomass cultivated in palm oil and cassava mill effluents. J. Bacteriol. Mycol. 2018, 6, 287–293. [Google Scholar] [CrossRef]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Karim, A.; Islam, M.A.; Yousuf, A.; Khan, M.M.R.; Faizal, C.K.M. Microbial lipid accumulation through bioremediation of palm oil mill wastewater by Bacillus cereus. ACS Sustain. Chem. Eng. 2019, 7, 14500–14508. [Google Scholar] [CrossRef]
- Mishra, P.; Thakur, S.; Singh, L.; Krishnan, S.; Sakinah, M.; Ab Wahid, Z. Fermentative hydrogen production from indigenous mesophilic strain Bacillus anthracis PUNAJAN 1 newly isolated from palm oil mill effluent. Int. J. Hydrog. Energy 2017, 42, 16054–16063. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.; Thakur, S.; Singh, L.; Ab Wahid, Z.; Sakinah, M. Enhanced hydrogen production from palm oil mill effluent using two stage sequential dark and photo fermentation. Int. J. Hydrog. Energy 2016, 41, 18431–18440. [Google Scholar] [CrossRef] [Green Version]
- Bala, J.D.; Lalung, J.; Ismail, N. Biodegradation of palm oil mill effluent (POME) by bacterial. Int. J. Sci. Res. Publ. 2014, 4, 502–511. [Google Scholar]
- Soleimaninanadegani, M.; Manshad, S. Enhancement of biodegradation of palm oil mill effluents by local isolated microorganisms. In International Scholarly Research Notices; Hindawi: New York, NY, USA, 2014. [Google Scholar]
- Cheah, W.Y.; Show, P.L.; Juan, J.C.; Chang, J.S.; Ling, T.C. Enhancing biomass and lipid productions of microalgae in palm oil mill effluent using carbon and nutrient supplementation. Energy Convers. Manag. 2018, 164, 188–197. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Juan, J.C.; Chang, J.S.; Ling, T.C. Waste to energy: The effects of Pseudomonas sp. on Chlorella sorokiniana biomass and lipid productions in palm oil mill effluent. Clean Technol. Environ. Policy 2018, 20, 2037–2045. [Google Scholar] [CrossRef]
- Yousuf, A.; Sannino, F.; Addorisio, V.; Pirozzi, D. Microbial conversion of olive oil mill wastewaters into lipids suitable for biodiesel production. J. Agric. Food Chem. 2010, 58, 8630–8635. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Pei, H.; Ma, G.; Zhang, S.; Mu, R. Improving productivity and quality of biodiesel from Chlorella vulgaris SDEC-3M through customized process designs. Energy Convers. Manag. 2016, 129, 100–107. [Google Scholar] [CrossRef]
- Singh, R.P.; Ibrahim, M.H.; Esa, N.; Iliyana, M.S. Composting of waste from palm oil mill: A sustainable waste management practice. Rev. Environ. Sci. BioTechnol. 2010, 9, 331–344. [Google Scholar] [CrossRef]
- Rout, P.R.; Dash, R.R.; Bhunia, P.; Rao, S. Role of Bacillus cereus GS-5 strain on simultaneous nitrogen and phosphorous removal from domestic wastewater in an inventive single unit multi-layer packed bed bioreactor. Bioresour. Technol. 2018, 262, 251–260. [Google Scholar] [CrossRef]
- Islam, M.A.; Rahman, M.; Yousuf, A.; Cheng, C.K.; Wai, W.C. Performance of Klebsiella oxytoca to generate electricity from POME in microbial fuel cell. MATEC Web Conf. 2016, 38, 03004. [Google Scholar] [CrossRef] [Green Version]
- Barrios-Camacho, H.; Aguilar-Vera, A.; Beltran-Rojel, M.; Aguilar-Vera, E.; Duran-Bedolla, J.; Rodriguez-Medina, N.; Lozano-Aguirre, L.; Perez-Carrascal, O.M.; Rojas, J.; Garza-Ramos, U. Molecular epidemiology of Klebsiella variicola obtained from different sources. Sci. Rep. 2019, 9, 10610. [Google Scholar] [CrossRef]
- Duran-Bedolla, J.; Garza-Ramos, U.; Rodríguez-Medina, N.; Aguilar Vera, A.; Barrios-Camacho, H. Exploring the environmental traits and applications of Klebsiella variicola. Braz. J. Microbiol. 2021, 52, 2233–2245. [Google Scholar] [CrossRef]
- Islam, M.A.; Karim, A.; Woon, C.W.; Ethiraj, B.; Cheng, C.K.; Yousuf, A.; Khan, M.M.R. Augmentation of air cathode microbial fuel cell performance using wild type Klebsiella variicola. RSC Adv. 2017, 7, 4798–4805. [Google Scholar] [CrossRef] [Green Version]
- Ditfurth, H.V. Im Anfang war der Wasserstoff; Hoffmann und Campe: Hamburg, Germany, 1972. [Google Scholar]
- Ashfaq, A.; Bhat, A.H.; Azizul, B. Immobilized Chlorella vulgaris for efficient palm oil mill effluent treatment and heavy metals removal. Desalination Water Treat. 2017, 81, 105–117. [Google Scholar]
- Mohammed, A.K.; Ali, S.A.; Ali, I.F. Using locally isolated Chlorella vulgaris in wastewater treatment. Eng. Tech. J. 2016, 34, 762–768. [Google Scholar]
- Kumar, P.; Kuppusamy, S.; Yusop, H.M.; Isa, S.; Alwi, S. POME treatment using Spirulina platensis Geitler. Int. J. Curr. Sci. 2011, 1, 11–13. [Google Scholar]
- Haruna, S.; Mohamad, S.E.; Jamaluddin, H. Potential of treating unsterilized Palm Oil Mill Effluent (POME) using freshwater microalgae. Pak. J. Biotechnol. 2017, 14, 221–225. [Google Scholar]
- Rajkumara, R.; Takriffab, M.S. Nutrient removal from anaerobically treated palm oil mill effluent by Spirulina platensis and Scenedesmus dimorphus. Pharm. Lett. 2015, 7, 416–421. [Google Scholar]
- Kamyab, H.; Md Din, M.F.; Lee, C.T.; Keyvanfar, A.; Shafaghat, A.; Majid, M.Z.A.; Ponraj, M.; Yun, T.X. Lipid production by microalgae Chlorella pyrenoidosa cultivated in palm oil mill effluent (POME) using hybrid photo bioreactor (HPBR). Desalination Water Treat. 2015, 55, 3737–3749. [Google Scholar] [CrossRef]
- Kamyab, H.; Din, M.F.M.; Keyvanfar, A.; Abd Majid, M.Z.; Talaiekhozani, A.; Shafaghat, A.; Lee, C.T.; Shiun, L.J.; Ismail, H.H. Efficiency of microalgae Chlamydomonas on the removal of pollutants from palm oil mill effluent (POME). Energy Procedia 2015, 75, 2400–2408. [Google Scholar] [CrossRef] [Green Version]
- Udaiyappan, A.F.M.; Hasan, H.A.; Takriff, M.S.; Abdullah, S.R.S.; Yasin, N.H.M.; Ji, B. Cultivation and application of Scenedesmus sp. strain UKM9 in palm oil mill effluent treatment for enhanced nutrient removal. J. Clean. Prod. 2021, 294, 126295. [Google Scholar] [CrossRef]
- Ding, G.T.; Yaakob, Z.; Takriff, M.S.; Salihon, J.; Abd Rahaman, M.S. Biomass production and nutrients removal by a newly-isolated microalgal strain Chlamydomonas sp. in palm oil mill effluent (POME). Int. J. Hydrog. Energy 2016, 41, 4888–4895. [Google Scholar] [CrossRef]
- Burlew, J.S. Algal culture from laboratory to pilot plant. In Algal Culture from Laboratory to Pilot Plant; Carnegie Institution of Washington: Washington, DC, USA, 1953. [Google Scholar]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef] [Green Version]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Impact of microalgae characteristics on their conversion to biofuel. Part I: Focus on cultivation and biofuel production. Biofuels Bioprod. Biorefining 2012, 6, 105–113. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, K.; Manjari, A.S.C. Determination of Spirulina platensis’s ability to improve the DO content in Waste Water. Int. J. Sci. Adv. Res. Technol. 2019, 5, 382–384. [Google Scholar]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Comparative study on the growth performance of Spirulina platensis on modifying culture media. Energy Rep. 2019, 5, 327–336. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Lee, C.T.; Khademi, T.; Kumar, A.; Yadav, K.K.; Rezania, S.; Kumar, S.; Ebrahim, S.S. Improved production of lipid contents by cultivating Chlorella pyrenoidosa in heterogeneous organic substrates. Clean Technol. Environ. Policy 2019, 21, 1969–1978. [Google Scholar] [CrossRef]
- Hadiyanto, H. Valorization of Microalgae for Palm Oil Liquid Waste Processing and as a Source of Alternative Energy and Food. In Proceedings of the Chemical and Process Engineering; Polish Academy of Sciences Committee of Chemical and Process Engineering: Warsaw, Poland, 2013; pp. 1–11. [Google Scholar]
- Chen, J.; Wei, J.; Ma, C.; Yang, Z.; Li, Z.; Yang, X.; Wang, M.; Zhang, H.; Hu, J.; Zhang, C. Photosynthetic bacteria-based technology is a potential alternative to meet sustainable wastewater treatment requirement. Environ. Int. 2020, 137, 105417. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.K.; Abou-Shanab, R.A.; Kim, S.H.; Salama, E.S.; Lee, S.H.; Kabra, A.N.; Lee, Y.S.; Hong, S.; Jeon, B.H. Cultivation of microalgae species in tertiary municipal wastewater supplemented with CO2 for nutrient removal and biomass production. Ecol. Eng. 2013, 58, 142–148. [Google Scholar] [CrossRef]
- Wu, T.Y.; Mohammad, A.W.; Jahim, J.M.; Anuar, N. A holistic approach to managing palm oil mill effluent (POME): Biotechnological advances in the sustainable reuse of POME. Biotechnol. Adv. 2009, 27, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Bala, J.D.; Lalung, J.; Al-Gheethi, A.A.; Kaizar, H.; Ismail, N. Reduction of organic load and biodegradation of palm oil mill effluent by aerobic indigenous mixed microbial consortium isolated from Palm Oil Mill Effluent (POME). Water Conserv. Sci. Eng. 2018, 3, 139–156. [Google Scholar] [CrossRef]
- Van Hamme, J.D.; Odumeru, J.A.; Ward, O.P. Community dynamics of a mixed-bacterial culture growing on petroleum hydrocarbons in batch culture. Can. J. Microbiol. 2000, 46, 441–450. [Google Scholar] [CrossRef]
- Cao, S.G.; Yang, H.; Ma, L.; Guo, S.Q. Enhancing enzymatic properties by the immobilization method. Appl. Biochem. Biotechnol. 1996, 59, 7–14. [Google Scholar] [CrossRef]
- Said, M.; Sitanggang, A.S.; Julianda, R.; Estuningsih, S.P.; Fudholi, A. Production of methane as bio-fuel from palm oil mill effluent using anaerobic consortium bacteria. J. Clean. Prod. 2021, 282, 124424. [Google Scholar] [CrossRef]
- Hariz, H.B.; Takriff, M.S. Palm oil mill effluent treatment and CO2 sequestration by using microalgae—sustainable strategies for environmental protection. Environ. Sci. Pollut. Res. 2017, 24, 20209–20240. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Ong, H.R.; Ethiraj, B.; Cheng, C.K.; Khan, M.M.R. Optimization of co-culture inoculated microbial fuel cell performance using response surface methodology. J. Environ. Manag. 2018, 225, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.T.; Yasin, N.H.M.; Takriff, M.S.; Kamarudin, K.F.; Salihon, J.; Yaakob, Z.; Hakimi, N.I.N.M. Phycoremediation of palm oil mill effluent (POME) and CO2 fixation by locally isolated microalgae: Chlorella sorokiniana UKM2, Coelastrella sp. UKM4 and Chlorella pyrenoidosa UKM7. J. Water Process Eng. 2020, 35, 101202. [Google Scholar] [CrossRef]
- Nor, M.H.M.; Mubarak, M.F.M.; Elmi, H.S.A.; Ibrahim, N.; Wahab, M.F.A.; Ibrahim, Z. Bioelectricity generation in microbial fuel cell using natural microflora and isolated pure culture bacteria from anaerobic palm oil mill effluent sludge. Bioresour. Technol. 2015, 190, 458–465. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Binupriya, A.R.; Baik, S.H.; Yun, S.E. Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium isolated from hydrocarbon contaminated areas. CLEAN Soil Air Water 2008, 36, 92–96. [Google Scholar] [CrossRef]
- El-Masry, M.H.; El-Bestawy, E.; Nawal, I. Bioremediation of vegetable oil and grease from polluted wastewater using a sand biofilm system. World J. Microbiol. Biotechnol. 2004, 20, 551–557. [Google Scholar] [CrossRef]
| Parameters | Concentration Range | ||
|---|---|---|---|
| [20] | [21] | [22] | |
| Chemical oxygen demand (COD) | 15,000–100,000 | 51,000 | 100 |
| Biochemical oxygen demand (BOD) | 10,250–43,750 | 25,000 | 50 |
| Total suspended solids (TSS) | 5000–54,000 | 18,000 | 400 |
| Ammoniacal nitrogen | 4–80 | 35 | 100 |
| Oil and grease | 130–18,000 | 6000 | 50 |
| Total nitrogen | 180–1400 | 750 | 200 |
| pH | 3.4–5.2 | 4.2 | 5.0 |
| No. | Biological Technique | Removal Efficiency or Concentration | Parameter | Remarks | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COD % (mg/L) | BOD % (mg/L) | TSS % (mg/L) | OLR % (kg COD/m3 day) | Total Nitrogen (mg/L) | NH3-N % | Methane (CH₄) Gas Release (%) | Oil and Grease (mg/L) | °C | Working Volume (L) | HRT (day) | ||||
| 1. | Upflow anaerobic sludge blanket reactor (UASBR) | 99 | ND | ND | ND | ND | ND | 70–80 | ND | 37 | 4.7 | 7.2 | Biogas production: 20.17 11−1 d−1 | [41] |
| 2. | Ultrasonic membrane anaerobic system (UMAS) | 98.5 | ND | ND | 0.5 | ND | ND | 79 | ND | 30 | 200 | 480.3 | POME treatment | [42] |
| 3. | Membrane anaerobic system (MAS) | 98.4 | ND | ND | 1 | ND | ND | 72 | ND | ND | 50 | 600.4 | POME treatment | [43] |
| 4. | Aerobic oxidation (activated sludge reactor) | 98 | 93 | ND | ND | 58 | ND | ND | 24 | ND | 91 | 60 | Treatment of anaerobically digested POME | [44] |
| 5. | Hybrid upflow anaerobic aludge bed (HUASB) reactor | 98 (663) | ND | 1387 | 5.5 | 75 | 23.4 | ND | ND | 24 ± 1 | ND | 47 | POME treatment | [45] |
| 6. | Anaerobic pond | 97.8 (1204 ± 292) | ND | ND | 1.4 | ND | ND | 54.4 | ND | ND | ND | 40 | POME treatment | [46] |
| 7. | Upflow anaerobic sludge fixed-film (UASFF) | 97 | ND | ND | ND | ND | ND | 74.2–80.1 | ND | 38 | 4.38 | 3 | POME treatment | [47] |
| 8. | An integrated system of two-stage microbial fuel cells (MFCs) and immobilized biological aerated filters (I-BAFs) | 96.5 | ND | ND | ND | ND | 93.6 | ND | ND | 35 ± 1 | 2.36 | 48 | Direct electricity generation (input value) | [48] |
| 9. | Upflow anaerobic sludge blanket (UASB) | 96.3 | ND | ND | ND | ND | ND | ND | ND | 28.0 ± 2.0 | 10.0 | 20.0 | Anaerobic POME treatment for methane production: 0.012 L CH4/g COD degraded | [49] |
| 10. | Aerobic submerged membrane bioreactor (ASMBR) | 96–98 | ND | ND | ND | ND | ND | ND | ND | ND | 20 | 8 | Improved with the addition of bio-fouling reducers | [50] |
| 11. | Combined high-rate anaerobic reactors | 95.6 | ND | ND | 13 | ND | ND | 59.5–78.2 | ND | 36 ± 1 | 2 | 2.4 | POME treatment | [51] |
| 12. | Anaerobic expanded granular sludge bed (EGSB) reactor | 94.89 | ND | ND | ND | ND | ND | ND | 65–70 | ND | ND | 9.8 | Inoculum from open anaerobic ponds of POME | [52] |
| 13. | Upflow anaerobic sludge fixed-film (UASFF) bioreactor | 94 | ND | ND | ND | ND | ND | (0.331) | 94 | 50 | 3.65 | 1.5 | POME treatment | [53] |
| 14. | Anaerobic bioreactor | 93.7 (2523 ± 19) | 800 ± 16 (2.0) | 37.9 | ND | 327 ± 11(3.4) | 220 ± 8 | ND | ND | 35 ± 3 | ND | 100 | Biogas generation: 474.6 ± 97.4 m3 day−1 | [54] |
| 15. | Lab scale sequencing batch reactor (activated sludge) | 93.2 ± 1.2 (906 ± 140) | 95.5 ± 1 (62 ± 28) | 97.2 ± 1.3 (363 ± 190) | 1.8–4.2 | ND | ND | ND | ND | 28 ± 1 | 1.8 | 15 | POME treatment | [55] |
| 16. | Anaerobic expanded granular sludge bed (EGSB) bioreactor | 93 (1959) | ND | 26,704 | ND | 560 | 64.4 | 43 | 3856 | 35 | 12 | 3 | POME treatment | [56] |
| 17. | Upflow anaerobic filter (UFAF) reactor | 91.6 | ND | ND | ND | ND | ND | ND | ND | 28.0 ± 2.0 | 5.0 | 13.5 | Anaerobic POME treatment for methane production: 0.482 L CH4/g COD degraded | [49] |
| 18. | Anaerobic expanded granular sludge bed (EGSB) reactor | 91 | ND | ND | 17.5 | ND | ND | 70 | ND | 35 | 20.5 | 2 | POME treatment | [56] |
| 19. | Upflow anaerobic sludge blanket-hollow centered packed bed (UASB-HCPB) reactor | 90 | 90 | 80 | 27.65 | ND | ND | 60 | ND | 55 | 5 | 2 | POME treatment | [57] |
| 20. | Aerobic oxidation (activated sludge reactor) | 89 | 82 | ND | ND | 3.0 | ND | ND | 112 | ND | 91 | 60 | POME treatment | [44] |
| 21. | Rotating biological contactors (RBC) | 88 | ND | ND | ND | 80 | ND | ND | ND | ND | 61 | 5 | Innoculated with S. cerevisiae | [58] |
| 22. | Lab-scale sequencing batch reactor | 86 | 87 | 89 | ND | ND | ND | ND | ND | 50 | 1.8 | 2.5 | Thermophilic aerobic treatment system of anaerobically digested POME | [59] |
| 23. | Anaerobic fluidised bed reactor | 85.00 | 91.00 | 89.00 | 4.0 | ND | ND | ND | ND | ND | 2 | 17 | POME treatment | [60] |
| 24. | Integrated baffled reactor | 83 (7735.0 ± 227.5) | ND | 24,400 | 7.64 | ND | ND | 75–54 | ND | 32 ± 2 | ND | 6 | POME treatment by inoculation with anaerobic pond sludge | [61] |
| 25. | Hybrid upflow anaerobic sludge blanket (HUASB) reactor | 82 | ND | 80 | ND | 87 | ND | ND | ND | 37 ± 1 | 7.22 | 40 | POME treatment | [62] |
| 26. | Carrier anaerobic baffled reactor (CABR) | 82 | ND | ND | 11.38 | ND | ND | 75–54 | ND | ND | ND | 26 | POME treatment by inoculation with anaerobic pond sludge and biogas production | [63] |
| 27. | Biological sequencing batch reactor | 82 | ND | 62 | ND | ND | ND | ND | ND | ND | 50 | 28–36 | POME treatment | [64] |
| 28. | Continuous stirred tank reactor (CSTR) | 80 | ND | ND | 3.33 | ND | ND | 62.5 | ND | ND | ND | 18 | POME treatment | [65] |
| 29. | Continuous stirred-tank reactor (CSTR) | 77 | ND | ND | ND | ND | ND | ND | ND | 55 | 1 | 8 | POME treatment by thermophilic anaerobic reaction Methane emission: 6.05–9.82 L/day | [66] |
| 30. | Anaerobic contact filter | 73 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 7 | Biohydrogen generation: 56 L | [67] |
| 31. | Anaerobic digestion using continuous stirred tank reactors | 71.10 | ND | ND | ND | ND | ND | 71.04 | ND | 37 | 1.6 | 7 | Biogas production | [68] |
| 32. | Aerobic bioreactor | 71.1 (681 ± 11) | 25 ± 9 (36.0) | ND | ND | 14 ± 1 (7.1) | ND | ND | ND | 35 ± 3 | ND | 100 | POME treatment | [54] |
| 33. | MFC | 70 (964) | ND | ND | ND | ND | ND | ND | ND | 25–28 | 0.45 | 15 | Treatment with (polacrylonitrile carbon felt) and bioelectricity generation: 22 mW/m2 | [69] |
| 34. | Upflow anaerobic sludge blanket fixed-film (UASB-FF) bioreactor | 68 | ND | ND | ND | ND | ND | ND | ND | 200 | 2.55 | 1.5 | Hydrogen gas: 0.31 L H2/g COD | [70] |
| 35. | Upflow anaerobic filter (UFAF) reactor | 66.3 | ND | ND | ND | ND | ND | ND | ND | 28.0 ± 2.0 | 5.0 | 1.50 | POME treatment for methane production: 0.107 l CH4/g COD degraded | [49] |
| 36. | Continuous stirred-tank reactor (CSTR) | 66.09 | ND | ND | ND | ND | ND | 48.05 | ND | 35 | 4.5 | 12 | Anaerobic methanogenic degradation of POME | [71] |
| 37. | Upflow anaerobic sludge blanket (UASB) | 65 | ND | ND | ND | ND | ND | 58 | ND | 55 | 1.2 | 5 | POME treatment | [72] |
| 38. | Upflow anaerobic sludge blanket (UASB) | 62.5 | ND | ND | ND | ND | ND | ND | ND | 28.0 ± 2.0 | 10.0 | 2.86 | Anaerobic POME treatment for methane production: 0.013 L CH4/g COD degraded | [49] |
| 39. | Upflow anaerobic sludge blanket (UASB) | 62 | ND | ND | 5.0 | ND | ND | ND | ND | 37 | 5 | 12 | Continuous hydrogen production: 0.35 L H2/g COD removed | [73] |
| 40. | Upflow anaerobic sludge blanket (UASB) | 62 | ND | ND | ND | ND | ND | ND | ND | 37 | 5 | 0.33 | POME treatment using Clostridium LS2 for enhanced hydrogen production: 67% | [74] |
| 41. | Anaerobic sequencing batch reactor | 62.2 ± 2.8 (26,500) | ND | 93.6 ± 1.1 | ND | ND | ND | ND | ND | 60 ± 1 | 2 | 4 | POME treatment for hydrogen production: 6.1 ± 0.03 LH2POME/d | [75] |
| 42. | Membrane bioreactor | 53.4 (486 ± 5) | 18 ± 5 (27.8) | 93.4 | ND | 28 ± 1 (3.6) | ND | ND | ND | 35 ± 3 | ND | 100 | POME treatment | [54] |
| 43. | Expanded granular sludge bed reactor | 53 | ND | ND | ND | ND | ND | 59 | ND | 55 | 1.0 | 5 | POME treatment | [72] |
| 44. | Microbial fuel cell (MFC) | 48.63 (31,980) | 46.54 (14,080) | 75.27 (2882) | ND | ND | 57.69 (11) | ND | ND | ND | 0.02 | 10 | Bioelectricity generation: 207.28 mW/m3 | [76] |
| 45. | Microbial fuel cell (MFC) | 45.21 (33,200) | 45 (13,200) | 70.91 (2920) | ND | ND | 56.52 (10) | ND | ND | 25–28 | 0.45 | 15 | Bioelectricity generation: 45 mW/m2 | [69] |
| 46. | Anaerobic ponding system | 41.2 | 77.8 | ND | ND | ND | ND | ND | ND | ND | ND | 18 | Zeolite performance for POME treatment | [77] |
| 47. | Anaerobic sequencing batch reactor (ASBR) | 37 | ND | ND | ND | ND | ND | ND | ND | 37 | 2 | 4 | POME treatment | [78] |
| 48. | Aerobic inner-circulation biofilm reactor | 22 (1439) | ND | 22,579 | ND | 238 | 0 | ND | 258 | 25 | 5 | 10 | POME treatment | [79] |
| No. | Fungi | Removal Efficiency | Parameter | Remarks | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COD % (mg/L) | BOD % (mg/L) | TSS % (mg/L) | OLR % (kg COD/m3 day) | Total Nitrogen | NH3-N % | Methane (CH₄) Gas Release (%) | Oil and Grease (mg/L) | °C | Working Volume (L) | HRT (day) | ||||
| 1. | Yarrowia lipolytica NCIM 3589 | 97.80 | ND | ND | ND | ND | ND | ND | ND | 30 | ND | 4 | POME treatment | [88] |
| 2. | Yarrowia lipolytica NCIM 3589 | 97.40 | ND | ND | ND | ND | ND | ND | ND | 30 | ND | 2 | POME treatment | [88] |
| 3. | Trichoderma viride ATCC 32086 | 95.00 (44.0–56.0) | ND | ND | ND | ND | ND | ND | ND | 28 ± 2 | 0.3 | 10–14 | POME treatment | [89] |
| 4. | Saccharomyces sp. L31 | 83 | ND | ND | ND | ND | ND | ND | ND | 28 ± 2 | 0.025 | 4 | Production of value-added feed grade yeast biomass | [90] |
| 5. | Aspergillus niger A 103 | 82.1 | ND | ND | ND | ND | ND | ND | ND | 32 | 0.1 | 7 | Production of citric acid: 5.2 g/L | [91] |
| 6. | Candida rugosa | 80.7 | 71.8 | 67.6 | ND | ND | ND | ND | 85.2 | 30 | 0.1 | 7 | POME treatment supplemented with soybean | [92] |
| 7. | Emericella nidulans NFCCI 3643 | 80.28 | 88.23 | ND | ND | ND | ND | ND | 87.34 | 30 | 0.25 | 5 | POME treatment | [93] |
| 8. | Pichia sp. SP5 | 73 | ND | ND | ND | ND | ND | ND | ND | 28 ± 2 | 0.025 | 3 | Production of value-added yeast biomass | [90] |
| 9. | Rhizopus oryzae ST 29 | 72.5 | ND | ND | ND | ND | ND | ND | 98.6 | 45 | 5 | 4 | POME treatment | [94] |
| 10. | Lipomyces starkeyi ATCC 56304 | 69.01 ± 2.3 | ND | ND | ND | ND | ND | ND | ND | 30 | 0.2 | 6 | Microbial lipid accumulation | [95] |
| 11. | Rhodotorula glutinis TISTR 5159 | 66.85 ± 1.57 | ND | ND | ND | ND | ND | ND | ND | 30 | 1 | 14 | Supplemented with Tween 20 surfactant for production of lipids (38.15%) and carotenoids (125.94 mg/L). | [96] |
| 12. | Aspergillus niger ATCC 9642 | 63.00 (6260 ± 40) | ND | ND | ND | ND | ND | ND | ND | 30 | 1 | 7 | Production of citric acid: 0.78 ± 0.02 g/L | [97] |
| 13. | Aspergillus niger | 52 (4055) | ND | ND | ND | ND | ND | ND | ND | 40 | 0.095 | 7 | POME treatment | [31] |
| 14. | Geotricum candidium | 49.1 | 79.8 | 91.8 | ND | ND | ND | ND | 83.6 | 30 | 0.1 | 7 | POME treatment supplemented with soybean | [92] |
| No. | Bacteria | Removal Efficiency | Parameter | Remarks | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COD % (mg/L) | BOD % (mg/L) | TSS % (mg/L) | OLR % (kg COD/m3 day) | Total Nitrogen | NH3-N % | Methane (CH₄) Gas Release (%) | Oil and Grease (mg/L) | °C | Working Volume (L) | HRT (day) | ||||
| 1. | Bacillus cereus MF661883 | 79.35 (4859 ± 605) | 72.65 (4054 ± 368) | 65.91 (5101 ± 327) | ND | 41.76 (191 ± 36) | 36.92 (41 ± 11) | ND | 74.17 (910 ± 458) | 35 | 0.2 | 6 | POME treatment at 50% dilution | [117] |
| 2. | Bacillus cereus 103 PB | 78.60 | 90.98 | ND | ND | ND | ND | ND | ND | 37 | 0.25 | 5 | POME treatment | [113] |
| 3. | Klebsiella variicola | 74 | ND | ND | ND | ND | ND | ND | ND | ND | 0.02 | 12 | Electricity generation from pretreated POME using MFC: 1648.70 mW/m3. | [76] |
| 4. | Bacillus cereus | 74.35 ± 1.7 | ND | ND | ND | ND | ND | ND | ND | 30 | 0.2 | 6 | Microbial lipid accumulation | [95] |
| 5. | Klebsiella oxytoca | 73.40 | 47.51 | 65.59 | ND | ND | 64.28 | ND | ND | ND | 0.02 | 10 | Continuous feeding of POME. Electricity generation using MFC: 207.28 mW/m3 | [76] |
| 6. | Micrococcus luteus 101 PB | 67.19 | ND | ND | ND | ND | ND | ND | ND | 37 | 0.25 | 5 | POME treatment | [113] |
| 7. | Bacillus subtilis 106 PB | 64.08 | 90.98 | ND | ND | ND | ND | ND | ND | 37 | 0.25 | 5 | POME treatment | [113] |
| 8. | Clostridium sp. LS2 | 62 | ND | ND | ND | ND | ND | ND | ND | 37 | 5 | 0.33 | POME treatment and hydrogen production using UASB | [74] |
| 9. | Stenotrophomonas maltophilia 102 PB | 61.92 | ND | ND | ND | ND | ND | ND | ND | 37 | 0.25 | 5 | POME treatment | [113] |
| 10. | Lysinibacillus sp. LC 556247 | 50.83 | 71.73 | 42.99 | ND | 12.80 ± 0.08 | ND | ND | 12.03 ± 0.02 | 35 ± 2 | 0.3 | 5 | POME treatment | [23] |
| 11. | Bacillus anthracis strain PUNAJAN 1 | 47.44 | 39.00 | ND | ND | ND | ND | ND | 27 | 35 ± 1 | 1 | 2 | Hydrogen production: 236 mL g COD | [118] |
| 12. | Clostridium butyricum LS2 | 42 | 39 | ND | ND | ND | ND | ND | ND | 37 ± 1 | 1 | 4 | Hydrogen production: 0.784 mL /mL | [119] |
| 13. | Bacillus cerius 103 PB | ND | ND | 71.63 | ND | ND | ND | ND | 85.14 | 37 | 0.25 | 5 | POME treatment | [120] |
| No. | Microalgae | Removal efficiency | Parameter | Remarks | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COD % (mg/L) | BOD % (mg/L) | TSS % (mg/L) | OLR % (kg COD/m3 day) | Total Nitrogen (mg/L) | NH3-N (mg/L) | Methane (CH₄) Gas Release (%) | Oil and Grease (mg/L) | °C | Working Volume (L) | HRT (day) | ||||
| 1. | Chlorella vulgaris | 95–99.9 | 97–99.9 | ND | ND | 78–98 | ND | ND | ND | ND | ND | 14 | POME treatment by immobilization of C. vulgaris in alginate beads and biodiesel production. | [133] |
| 2. | Streptomyces platensis | 93.57 (182) | 97.18 (42) | 86.98 (25) | ND | ND | ND | ND | ND | ND | 0.1 | 6 | POME treatment | [135] |
| 3. | Chlorella sorokiniana | 90 | ND | ND | ND | 71 | ND | ND | ND | 25–30 | 1 | 15 | POME treatment at 80% dilution | [136] |
| 4. | S. platensis | 84.9 | 78.3 | ND | ND | 91.0 (57.9) | 93.8 (19.8) | ND | ND | ND | 500 | 18 | Nutrient removal | [137] |
| 5. | S. dimorphus | 79 | 71.5 (148.8) | ND | ND | 87.5 | 88.5 (37.0) | ND | ND | ND | 500 | 18 | Nutrient removal | [137] |
| 6. | Chlorella pyrenoidosa | 71.43 | ND | ND | ND | ND | ND | ND | ND | 28 ± 1 | 5 | 14 | Hybrid photo bioreactor | [138] |
| 7. | Chlamydomonas incerta | 67.35 | ND | ND | ND | ND | ND | ND | ND | ND | 0.1 | 28 | POME treatment | [139] |
| 8. | Scenedesmus sp. strain UKM9 | 57 | 86.5 | ND | ND | ND | 100 | ND | ND | 30 ± 5 | 1.8 | 30 | POME treatment | [140] |
| 9. | Chlamydomonas sp. UKM6 | 29 (1450 ± 62) | ND | ND | ND | 43.50 (165 ± 12) | 58.58 (84.5 ± 7.2) | ND | ND | 25 ± 1 | 1.8 | 10 | Biomass production and nutrient removal | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominic, D.; Baidurah, S. Recent Developments in Biological Processing Technology for Palm Oil Mill Effluent Treatment—A Review. Biology 2022, 11, 525. https://doi.org/10.3390/biology11040525
Dominic D, Baidurah S. Recent Developments in Biological Processing Technology for Palm Oil Mill Effluent Treatment—A Review. Biology. 2022; 11(4):525. https://doi.org/10.3390/biology11040525
Chicago/Turabian StyleDominic, Debbie, and Siti Baidurah. 2022. "Recent Developments in Biological Processing Technology for Palm Oil Mill Effluent Treatment—A Review" Biology 11, no. 4: 525. https://doi.org/10.3390/biology11040525
APA StyleDominic, D., & Baidurah, S. (2022). Recent Developments in Biological Processing Technology for Palm Oil Mill Effluent Treatment—A Review. Biology, 11(4), 525. https://doi.org/10.3390/biology11040525







