Diaphragmatic Activity and Respiratory Function Following C3 or C6 Unilateral Spinal Cord Contusion in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Unilateral Cervical Spinal Cord Contusion
2.3. Breathing Recording Using Plethysmography
2.4. Electrophysiological Recording of the Diaphragm
2.5. Tissue Processing
2.6. Immunohistofluorescence
2.7. Data Processing and Statistical Analyses
3. Results
3.1. Physiologic Effect of Cervical Contusion
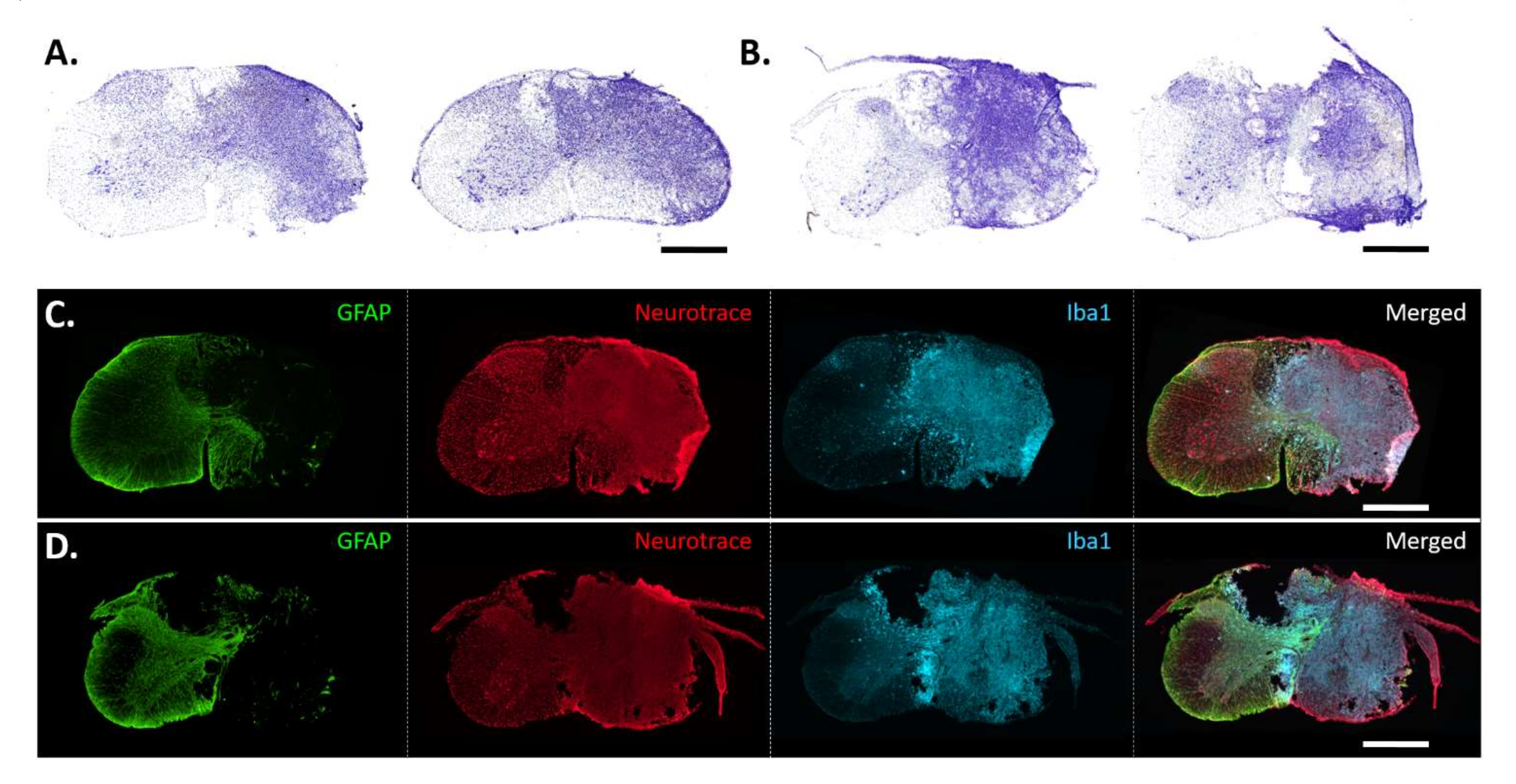
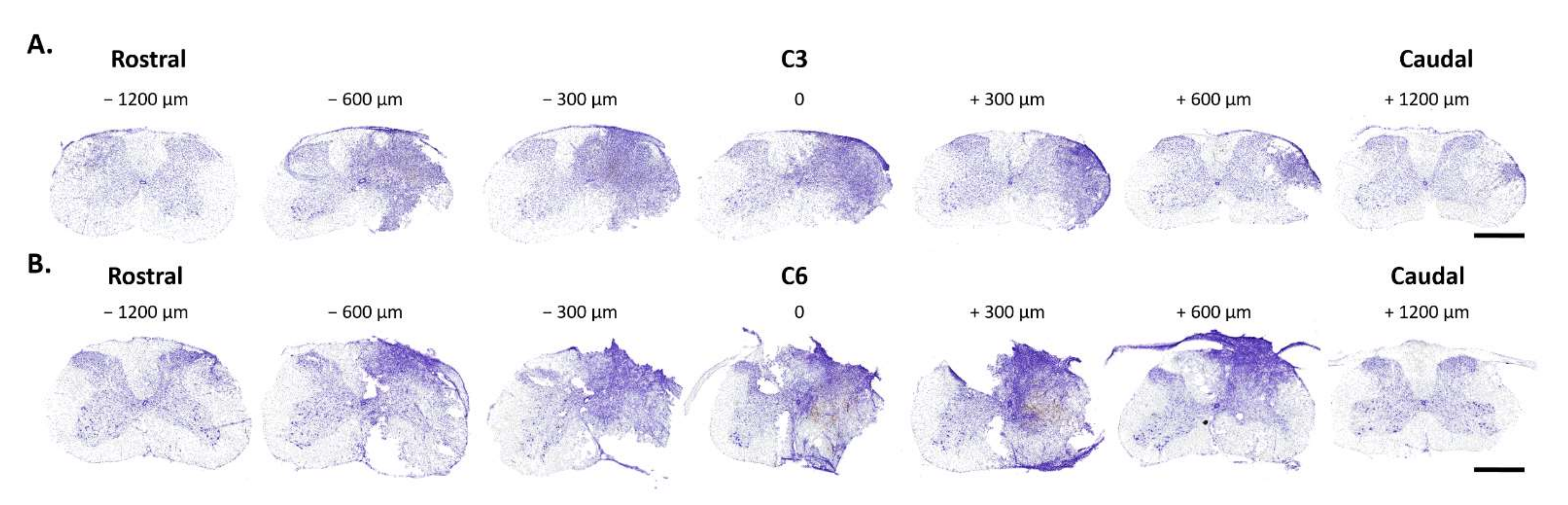
3.2. Histological Analysis of C3 and C6 Hemicontusions
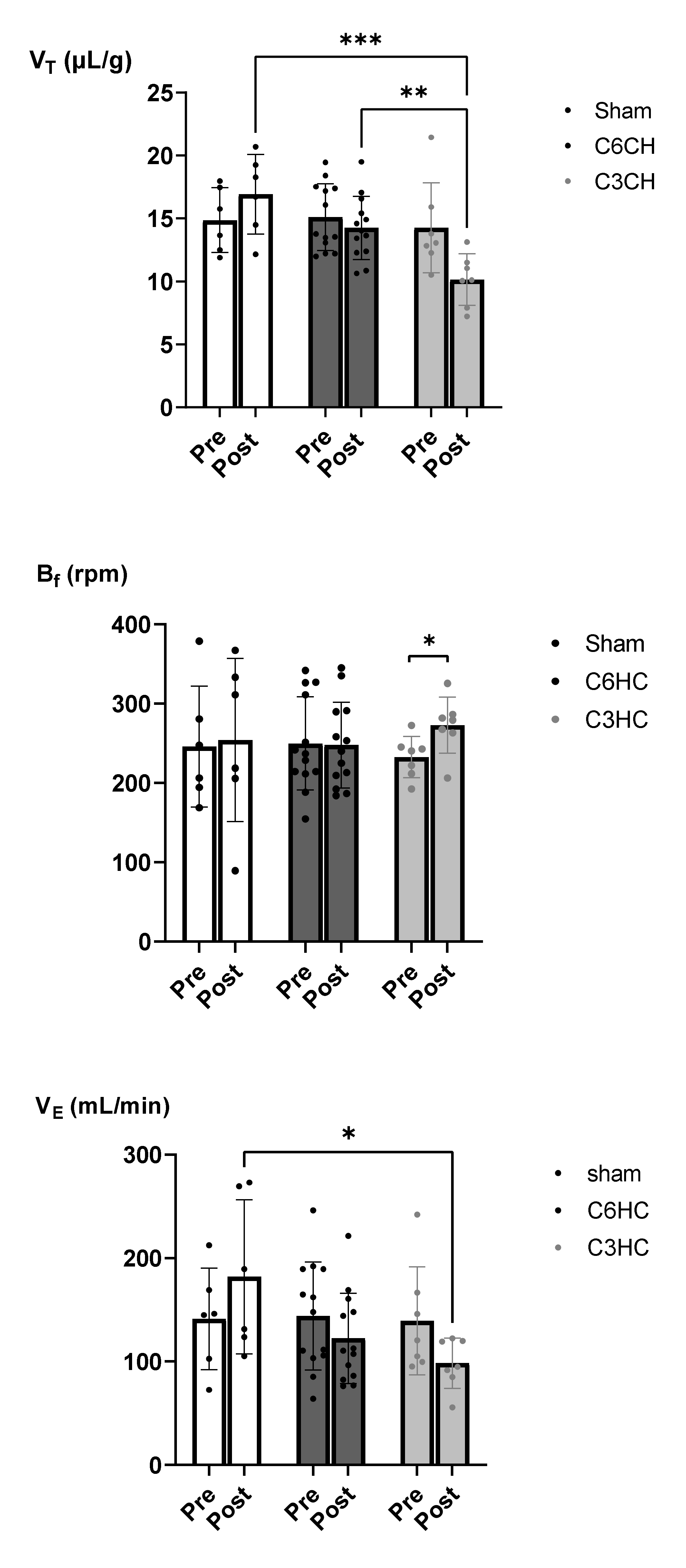
3.3. Respiratory Function Following C3 or C6 Hemicontusion
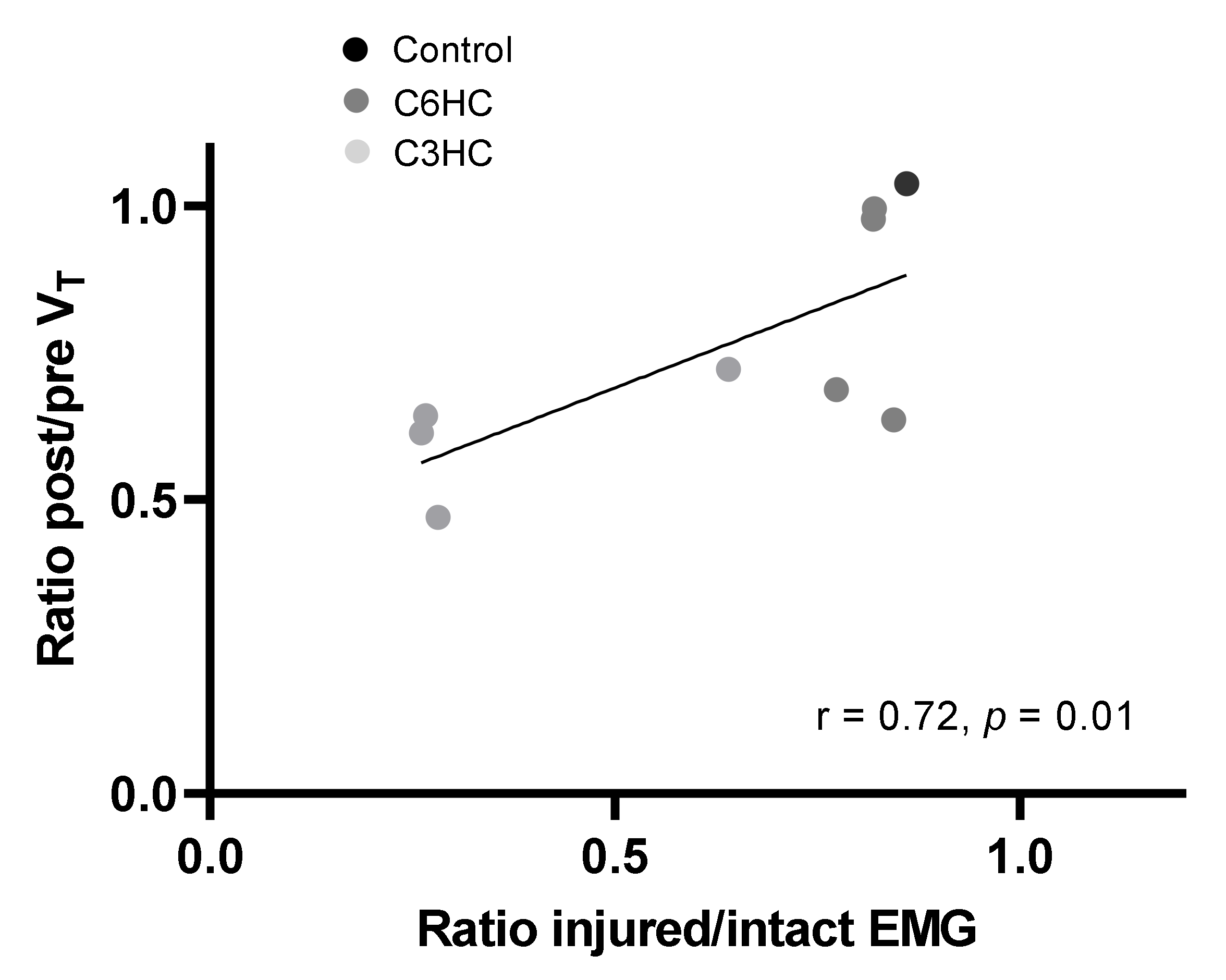
3.4. Diaphragmatic Activity Following C3 or C6 Hemicontusion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OMS. Spinal Cord Injury. Available online: http://www.who.int/mediacentre/factsheets/fs384/en/ (accessed on 19 November 2013).
- Van den Berg, M.E.; Castellote, J.M.; Mahillo-Fernandez, I.; de Pedro-Cuesta, J. Incidence of spinal cord injury worldwide: A systematic review. Neuroepidemiology 2010, 34, 184–192; discussion 192. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.B.; Dijkers, M.; DeVivo, M.J.; Poczatek, R.B. A demographic profile of new traumatic spinal cord injuries: Change and stability over 30 years. Arch. Phys. Med. Rehabil. 2004, 85, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Winslow, C.; Rozovsky, J. Effect of spinal cord injury on the respiratory system. Am. J. Phys. Med. Rehabil. 2003, 82, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.F.; Lv, Y.; Zhou, F.; Tian, Y.; Ji, H.Q.; Zhang, Z.S.; Guo, Y. Development and validation of a risk prediction model for tracheostomy in acute traumatic cervical spinal cord injury patients. Eur. Spine J. 2015, 24, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Sankari, A.; Bascom, A.; Oomman, S.; Badr, M.S. Sleep disordered breathing in chronic spinal cord injury. J. Clin. Sleep Med. 2014, 10, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Mantilla, C.B.; Sieck, G.C. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir. Physiol. Neurobiol. 2011, 179, 57–63. [Google Scholar] [CrossRef] [Green Version]
- DeVivo, M.J.; Black, K.J.; Stover, S.L. Causes of death during the first 12 years after spinal cord injury. Arch. Phys. Med. Rehabil. 1993, 74, 248–254. [Google Scholar] [CrossRef]
- Le Pimpec-Barthes, F.; Legras, A.; Arame, A.; Pricopi, C.; Boucherie, J.C.; Badia, A.; Panzini, C.M. Diaphragm pacing: The state of the art. J. Thorac. Dis. 2016, 8, S376–S386. [Google Scholar] [CrossRef] [Green Version]
- Assouad, J.; Masmoudi, H.; Gonzalez-Bermejo, J.; Morelot-Panzini, C.; Diop, M.; Grunenwald, D.; Similowski, T. Diaphragm pacing after bilateral implantation of intradiaphragmatic phrenic stimulation electrodes through a transmediastinal endoscopic minimally invasive approach: Pilot animal data. Eur. J. Cardiothorac. Surg. 2012, 42, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Senjaya, F.; Midha, R. Nerve transfer strategies for spinal cord injury. World Neurosurg. 2013, 80, e319–e326. [Google Scholar] [CrossRef]
- Gauthier, P.; Baussart, B.; Stamegna, J.C.; Tadie, M.; Vinit, S. Diaphragm recovery by laryngeal innervation after bilateral phrenicotomy or complete C2 spinal section in rats. Neurobiol. Dis. 2006, 24, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Pizzolato, C.; Gunduz, M.A.; Palipana, D.; Wu, J.; Grant, G.; Hall, S.; Dennison, R.; Zafonte, R.D.; Lloyd, D.G.; Teng, Y.D. Non-invasive approaches to functional recovery after spinal cord injury: Therapeutic targets and multimodal device interventions. Exp. Neurol. 2021, 339, 113612. [Google Scholar] [CrossRef] [PubMed]
- Bezdudnaya, T.; Lane, M.A.; Marchenko, V. Paced breathing and phrenic nerve responses evoked by epidural stimulation following complete high cervical spinal cord injury in rats. J. Appl. Physiol. 2018, 125, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rothi, E.J.; Lee, K.Z. Intermittent hypoxia and respiratory recovery in pre-clinical rodent models of incomplete cervical spinal cord injury. Exp. Neurol. 2021, 342, 113751. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic potential of intermittent hypoxia: A matter of dose. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 307, R1181–R1197. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.T.; Vinit, S.; Lee, K.Z. Functional role of carbon dioxide on intermittent hypoxia induced respiratory response following mid-cervical contusion in the rat. Exp. Neurol. 2021, 339, 113610. [Google Scholar] [CrossRef]
- Randelman, M.; Zholudeva, L.V.; Vinit, S.; Lane, M.A. Respiratory Training and Plasticity after Cervical Spinal Cord Injury. Front. Cell. Neurosci. 2021, 15, 700821. [Google Scholar] [CrossRef]
- Vinit, S.; Lovett-Barr, M.R.; Mitchell, G.S. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir. Physiol. Neurobiol. 2009, 169, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Tester, N.J.; Fuller, D.D.; Fromm, J.S.; Spiess, M.R.; Behrman, A.L.; Mateika, J.H. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am. J. Respir. Crit. Care Med. 2014, 189, 57–65. [Google Scholar] [CrossRef]
- Sandhu, M.S.; Rymer, W.Z. Brief exposure to systemic hypoxia enhances plasticity of the central nervous system in spinal cord injured animals and man. Curr. Opin. Neurol. 2021, 34, 819–824. [Google Scholar] [CrossRef]
- Sutor, T.; Cavka, K.; Vose, A.K.; Welch, J.F.; Davenport, P.; Fuller, D.D.; Mitchell, G.S.; Fox, E.J. Single-session effects of acute intermittent hypoxia on breathing function after human spinal cord injury. Exp. Neurol. 2021, 342, 113735. [Google Scholar] [CrossRef] [PubMed]
- Goshgarian, H.G. Invited Review: The crossed phrenic phenomenon: A model for plasticity in the respiratory pathways following spinal cord injury. J. Appl. Physiol. 2003, 94, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Vinit, S.; Gauthier, P.; Stamegna, J.-C.; Kastner, A. High Cervical Lateral Spinal Cord Injury Results in Long-Term Ipsilateral Hemidiaphragm Paralysis. J. Neurotrauma 2006, 23, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Warren, P.M.; Campanaro, C.; Jacono, F.J.; Alilain, W.J. Mid-cervical spinal cord contusion causes robust deficits in respiratory parameters and pattern variability. Exp. Neurol. 2018, 306, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Michel-Flutot, P.; Mansart, A.; Deramaudt, B.T.; Jesus, I.; Lee, K.; Bonay, M.; Vinit, S. Permanent diaphragmatic deficits and spontaneous respiratory plasticity in a mouse model of incomplete cervical spinal cord injury. Respir. Physiol. Neurobiol. 2021, 284, 103568. [Google Scholar] [CrossRef] [PubMed]
- Minor, K.H.; Akison, L.K.; Goshgarian, H.G.; Seeds, N.W. Spinal cord injury-induced plasticity in the mouse—The crossed phrenic phenomenon. Exp. Neurol. 2006, 200, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Mantilla, C.B.; Greising, S.M.; Stowe, J.M.; Zhan, W.-Z.; Sieck, G.C. TrkB kinase activity is critical for recovery of respiratory function after cervical spinal cord hemisection. Exp. Neurol. 2014, 261, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Vinit, S.; Kastner, A. Descending bulbospinal pathways and recovery of respiratory motor function following spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 115–122. [Google Scholar] [CrossRef]
- Cheriyan, T.; Ryan, D.J.; Weinreb, J.H.; Cheriyan, J.; Paul, J.C.; Lafage, V.; Kirsch, T.; Errico, T.J. Spinal cord injury models: A review. Spinal Cord 2014, 52, 588–595. [Google Scholar] [CrossRef]
- Alilain, W.J.; Horn, K.P.; Hu, H.; Dick, T.E.; Silver, J. Functional regeneration of respiratory pathways after spinal cord injury. Nature 2011, 475, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Goshgarian, H.G. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghali, M.G.Z. The crossed phrenic phenomenon. Neural Regen. Res. 2017, 12, 845–864. [Google Scholar] [CrossRef] [PubMed]
- Zholudeva, L.V.; Iyer, N.; Qiang, L.; Spruance, V.M.; Randelman, M.L.; White, N.W.; Bezdudnaya, T.; Fischer, I.; Sakiyama-Elbert, S.E.; Lane, M.A. Transplantation of Neural Progenitors and V2a Interneurons after Spinal Cord Injury. J. Neurotrauma 2018, 35, 2883–2903. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Z.; Liou, L.M.; Vinit, S. Diaphragm Motor-Evoked Potential Induced by Cervical Magnetic Stimulation following Cervical Spinal Cord Contusion in the Rat. J. Neurotrauma 2021, 38, 2122–2140. [Google Scholar] [CrossRef] [PubMed]
- Khurram, O.U.; Fogarty, M.J.; Rana, S.; Vang, P.; Sieck, G.C.; Mantilla, C.B. Diaphragm muscle function following midcervical contusion injury in rats. J. Appl. Physiol. 2019, 126, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Liao, W.L.; Newton, K.M.; Onario, R.C.; King, A.M.; Desilets, F.C.; Woodard, E.J.; Eichler, M.E.; Frontera, W.R.; Sabharwal, S.; et al. Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J. Neurosci. 2005, 25, 4550–4559. [Google Scholar] [CrossRef] [Green Version]
- Golder, F.J.; Fuller, D.D.; Lovett-Barr, M.R.; Vinit, S.; Resnick, D.K.; Mitchell, G.S. Breathing patterns after mid-cervical spinal contusion in rats. Exp. Neurol. 2011, 231, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.A.; Lee, K.Z.; Salazar, K.; O’Steen, B.E.; Bloom, D.C.; Fuller, D.D.; Reier, P.J. Respiratory function following bilateral mid-cervical contusion injury in the adult rat. Exp. Neurol. 2012, 235, 197–210. [Google Scholar] [CrossRef] [Green Version]
- Wen, M.H.; Lee, K.Z. Diaphragm and Intercostal Muscle Activity after Mid-Cervical Spinal Cord Contusion in the Rat. J. Neurotrauma 2018, 35, 533–547. [Google Scholar] [CrossRef]
- Nicaise, C. Degeneration of Phrenic Motor Neurons Induces Long-Term Diaphragm Deficits following Mid-Cervical Spinal Contusion in Mice. J. Neurotrauma 2012, 29, 2748–2760. [Google Scholar] [CrossRef] [Green Version]
- Nicaise, C.; Hala, T.J.; Frank, D.M.; Parker, J.L.; Authelet, M.; Leroy, K.; Brion, J.-P.; Wright, M.C.; Lepore, A.C. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Exp. Neurol. 2012, 235, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Vandeweerd, J.-M.; Hontoir, F.; De Knoop, A.; De Swert, K.; Nicaise, C. Retrograde Neuroanatomical Tracing of Phrenic Motor Neurons in Mice. J. Vis. Exp. 2018, 132, e56758. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Lane, M.A.; Lee, K.Z.; Reier, P.J.; Fuller, D.D. The phrenic motor nucleus in the adult mouse. Exp. Neurol. 2010, 226, 254–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robac, A.; Neveu, P.; Hugede, A.; Garrido, E.; Nicol, L.; Delarue, Q.; Guérout, N. Repetitive Trans Spinal Magnetic Stimulation Improves Functional Recovery and Tissue Repair in Contusive and Penetrating Spinal Cord Injury Models in Rats. Biomedicines 2021, 9, 1827. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhao, T.; Li, J.; Li, L.; Hu, R.; Hu, S.; Feng, H.; Lin, J. Antisense vimentin cDNA combined with chondroitinase ABC reduces glial scar and cystic cavity formation following spinal cord injury in rats. Biochem. Biophys. Res. Commun. 2008, 377, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Von Boxberg, Y.; Salim, C.; Soares, S.; Baloui, H.; Alterio, J.; Ravaille-Veron, M.; Nothias, F. Spinal cord injury-induced up-regulation of AHNAK, expressed in cells delineating cystic cavities, and associated with neoangiogenesis. Eur. J. Neurosci. 2006, 24, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Michel-Flutot, P.; Jesus, I.; Vanhee, V.; Bourcier, C.H.; Emam, L.; Ouguerroudj, A.; Lee, K.Z.; Zholudeva, L.V.; Lane, M.A.; Mansart, A.; et al. Effects of Chronic High-Frequency rTMS Protocol on Respiratory Neuroplasticity Following C2 Spinal Cord Hemisection in Rats. Biology 2022, 11, 473. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Z.; Kong, G.; Lin, J.; Liu, J.; Yang, Z.; Li, R.; Wu, X.; Alaeiilkhchi, N.; Jiang, H.; et al. Anatomical and behavioral outcomes following a graded hemi-contusive cervical spinal cord injury model in mice. Behav. Brain Res. 2022, 419, 113698. [Google Scholar] [CrossRef]
- Chiu, T.-T.; Lee, K.-Z. Impact of cervical spinal cord injury on the relationship between the metabolism and ventilation in rats. J. Appl. Physiol. 2021, 131, 1799–1814. [Google Scholar] [CrossRef]
- Baussart, B.; Stamegna, J.C.; Polentes, J.; Tadié, M.; Gauthier, P. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol. Dis. 2006, 22, 562–574. [Google Scholar] [CrossRef]
- Goshgarian, H.G. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp. Neurol. 1981, 72, 211–225. [Google Scholar] [CrossRef]
- Tian, G.F.; Peever, J.H.; Duffin, J. Bötzinger-complex expiratory neurons monosynaptically inhibit phrenic motoneurons in the decerebrate rat. Exp. Brain Res. 1998, 122, 149–156. [Google Scholar] [CrossRef] [PubMed]

| Variables | Sham | C3HC | C6HC | |
|---|---|---|---|---|
| Weight (g) | day of surgery | 38 (9) | 41 ± 5 | 37 ± 3 |
| day 1 | - | 38 ± 3 | 35 ± 2 | |
| day 2 | - | 36 ± 4 | 34 ± 2 | |
| day 5 | - | 33 ± 3 | 33 ± 3 | |
| day 7 | 39 (12) | 34 ± 3 | 34 ± 3 | |
| VT (µL/g) | pre | 14.7 (5.2) | 14.3 ± 3.6 | 14.9 ± 2.6 |
| 7 d post | 17.5 (5.7) | 10.1 #ab ± 2.1 | 14.2 ± 2.5 | |
| VT (mL) | pre | 0.56 (0.11) | 0.59 ± 0.2 | 0.56 ± 0.1 |
| 7 d post | 0.62 (0.20) | 0.36 * ± 0.1 | 0.48 * ± 0.1 | |
| Bf (rpm) | pre | 226 (117) | 233 ± 25 | 250 ± 59 |
| 7 d post | 264 (165) | 273 * ± 36 | 248 ± 54 | |
| VE (mL/min) | pre | 146 (85) | 139 ± 52 | 144 ± 52 |
| 7 d post | 160 (151) | 98 #a ± 24 | 122 ± 44 | |
| Ti (ms) | pre | 107 (55) | 111 ± 12 | 98 ± 21 |
| 7 d post | 98 * (58) | 96 ± 14 | 90 ± 18 | |
| Te (ms) | pre | 168 (82) | 179 ± 26 | 171 ± 47 |
| 7 d post | 155 (90) | 150 * ± 38 | 179 ± 47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajjig, A.; Michel-Flutot, P.; Migevent, T.; Cayetanot, F.; Bodineau, L.; Vinit, S.; Vivodtzev, I. Diaphragmatic Activity and Respiratory Function Following C3 or C6 Unilateral Spinal Cord Contusion in Mice. Biology 2022, 11, 558. https://doi.org/10.3390/biology11040558
Bajjig A, Michel-Flutot P, Migevent T, Cayetanot F, Bodineau L, Vinit S, Vivodtzev I. Diaphragmatic Activity and Respiratory Function Following C3 or C6 Unilateral Spinal Cord Contusion in Mice. Biology. 2022; 11(4):558. https://doi.org/10.3390/biology11040558
Chicago/Turabian StyleBajjig, Afaf, Pauline Michel-Flutot, Tiffany Migevent, Florence Cayetanot, Laurence Bodineau, Stéphane Vinit, and Isabelle Vivodtzev. 2022. "Diaphragmatic Activity and Respiratory Function Following C3 or C6 Unilateral Spinal Cord Contusion in Mice" Biology 11, no. 4: 558. https://doi.org/10.3390/biology11040558
APA StyleBajjig, A., Michel-Flutot, P., Migevent, T., Cayetanot, F., Bodineau, L., Vinit, S., & Vivodtzev, I. (2022). Diaphragmatic Activity and Respiratory Function Following C3 or C6 Unilateral Spinal Cord Contusion in Mice. Biology, 11(4), 558. https://doi.org/10.3390/biology11040558







