Seasonal Paspalum vaginatum Physiological Characteristics Change with Agricultural Byproduct Biochar in Sandy Potting Soil
Abstract
:Simple Summary
Abstract
1. Introduction
- (i)
- Evaluate the effect of biochar type from three different local feedstocks (almond shells, walnut shells, and peanut hull) on soil fertility.
- (ii)
- Evaluate the role of biochar addition into sandy loam soil and on P. vaginatum physiological characteristics in dry climates.
2. Materials and Methods
2.1. X-ray Diffraction and FTIR
2.2. Biochar Preparation
2.3. Experimental Design
2.4. Gas Exchange and Chlorophyll Pigment Content
2.5. Relative Water Content (RWC)
2.6. Leaf Stomatal Traits
2.7. Anatomical Characters
2.8. Chemometric Analyses
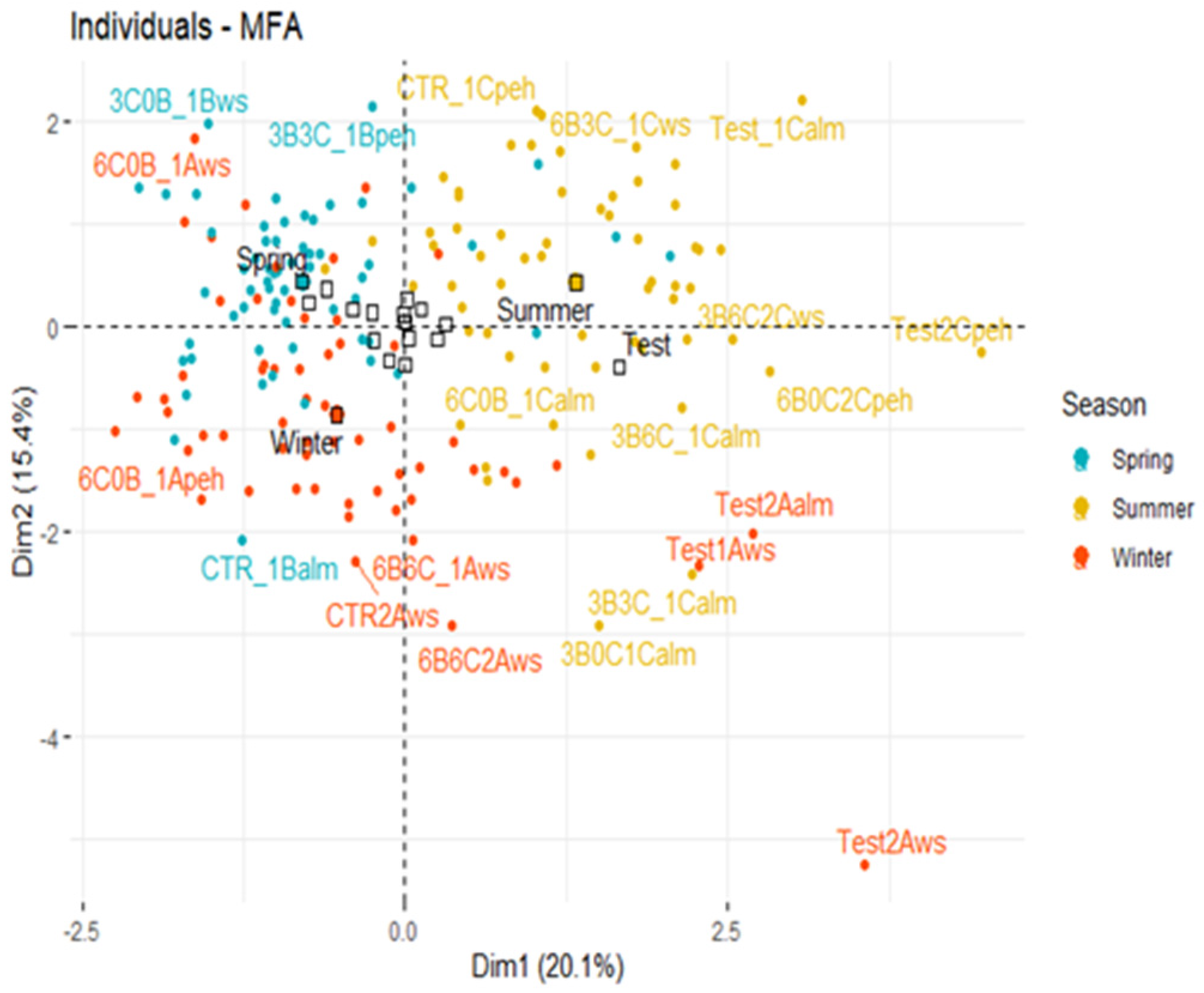
3. Results and Discussion
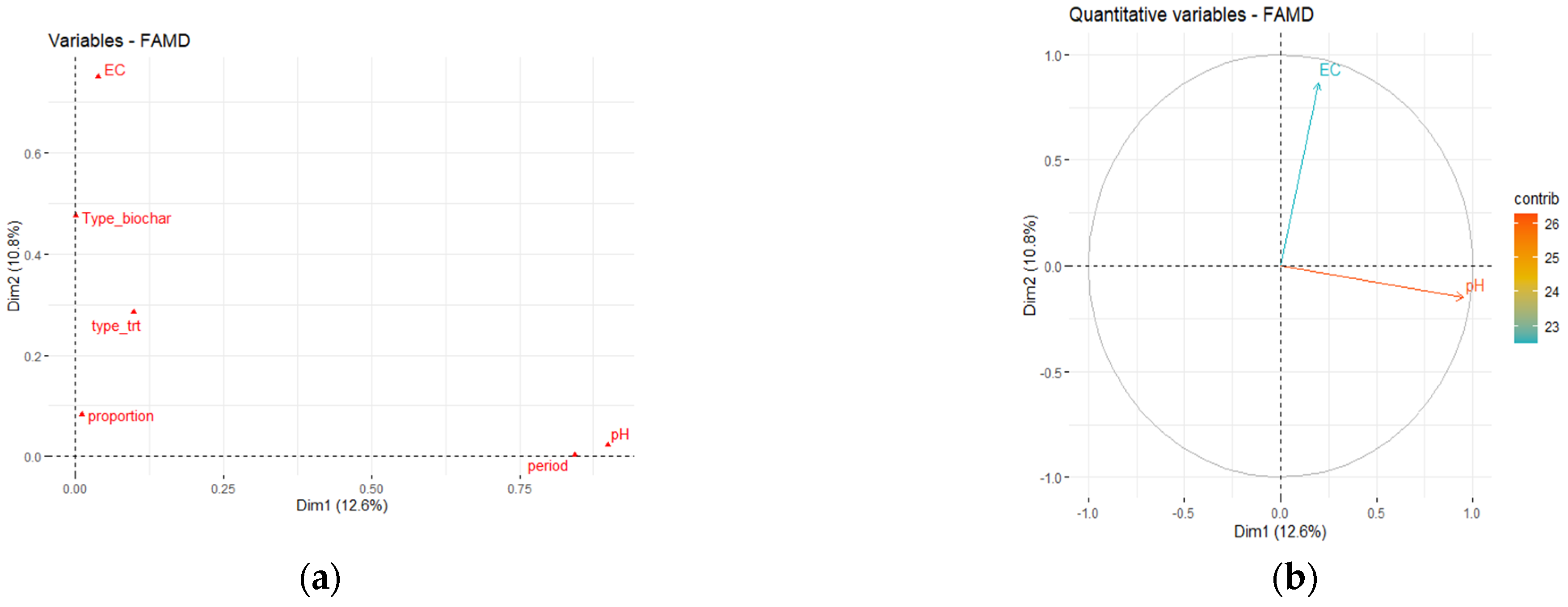
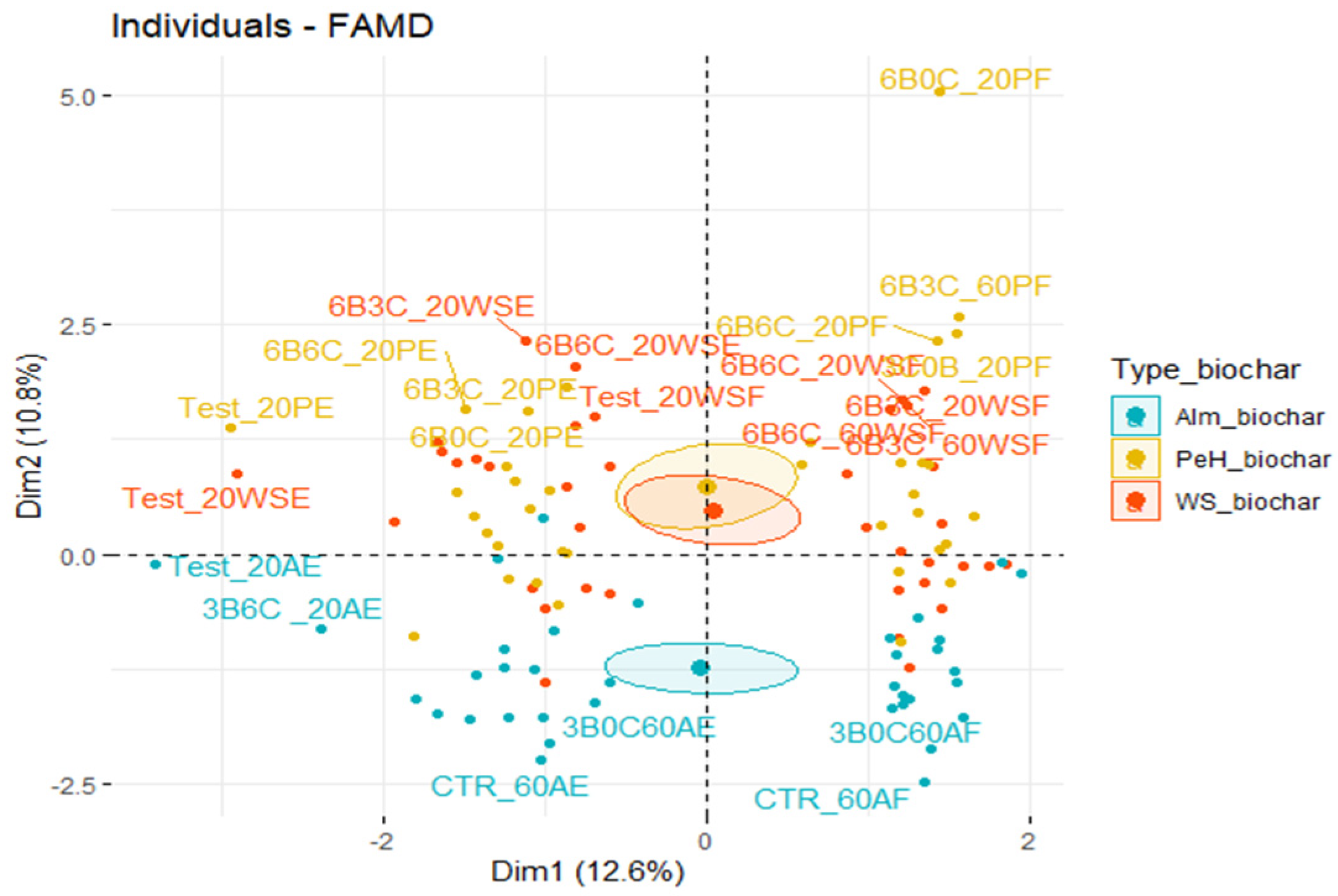
3.1. Effect of Three Biochars on Soil Properties
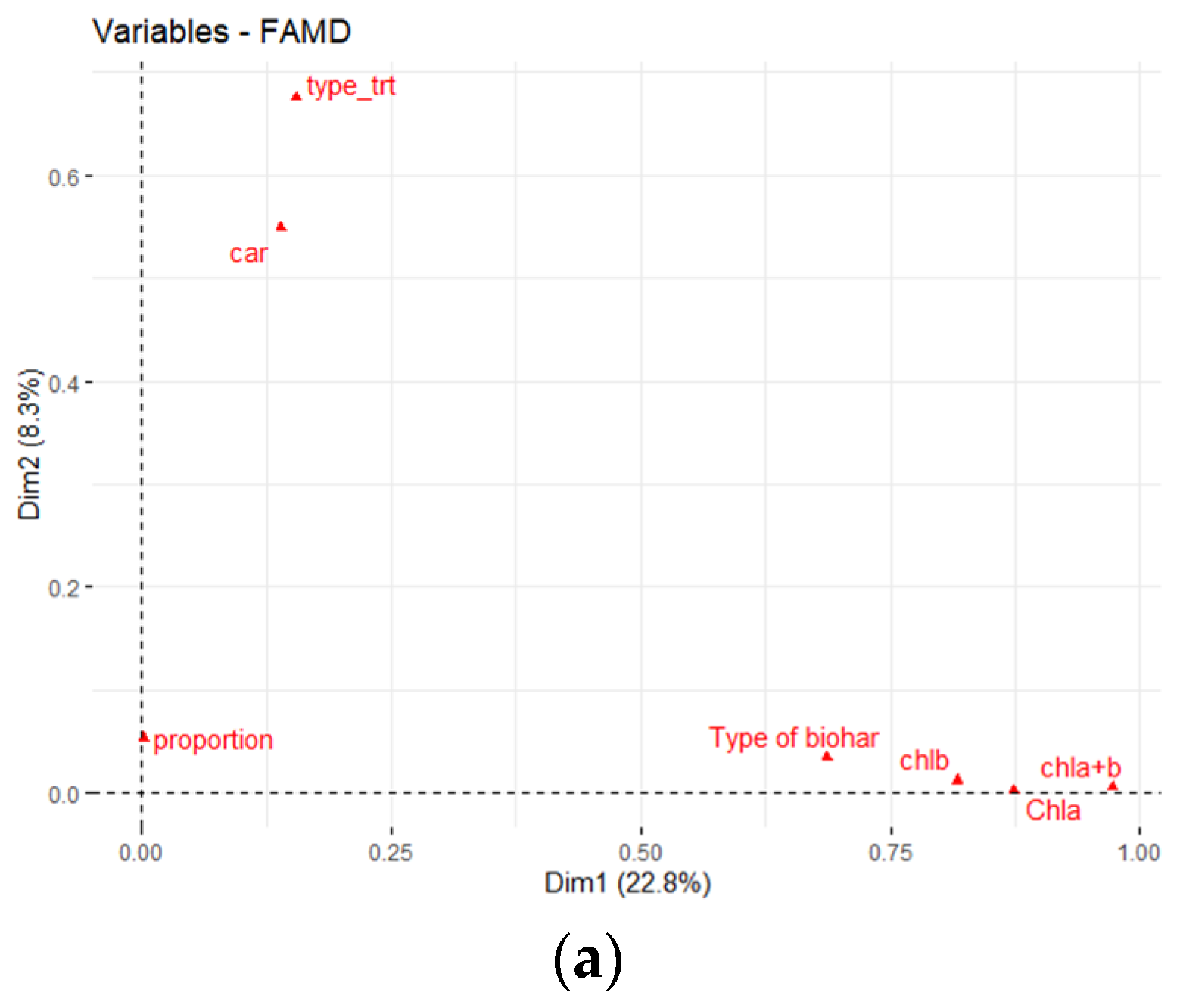
3.2. Effect of Three Biochars on Pigment Content of P. vaginatum
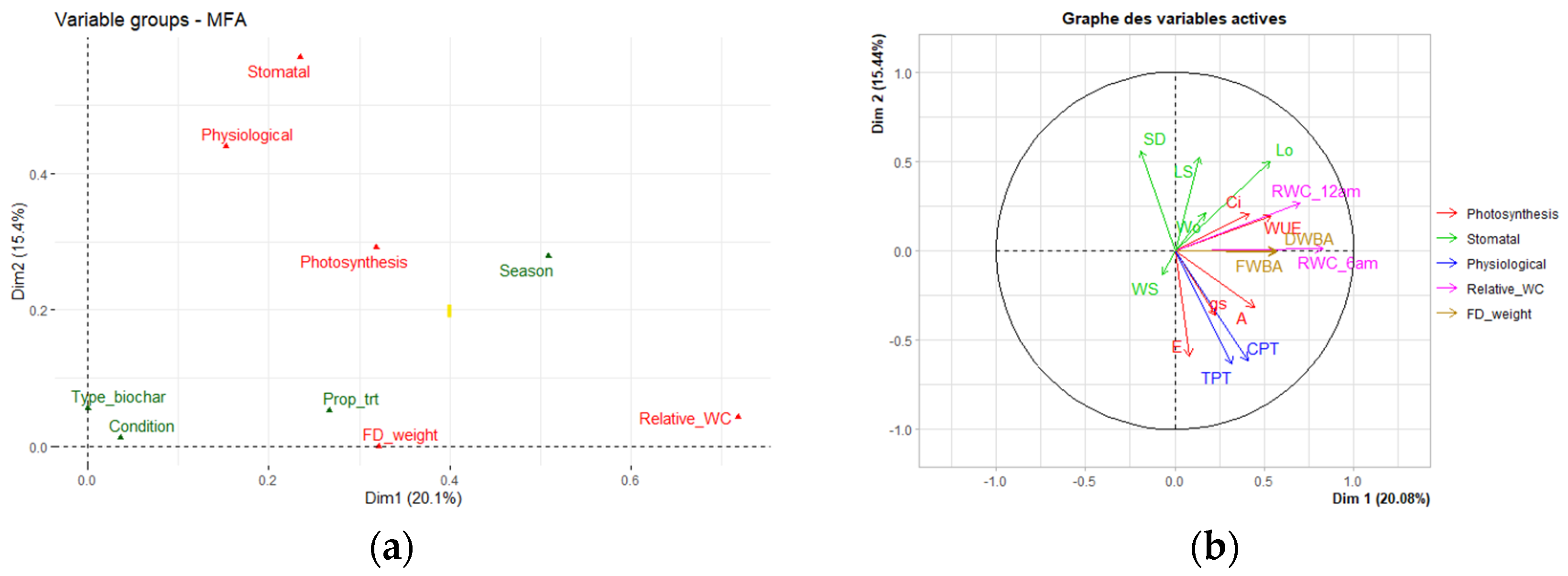
3.3. Effect of Three Biochars on Physiological Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reeves, D.W. The role of soil organic matter in maintaining soil quality in continuous cropping systems. Soil Tillage Res. 1997, 43, 131–167. [Google Scholar] [CrossRef]
- Idowu, O.J.; Flynn, R. Understanding Soil Health for Production Agriculture in New Mexico, Guide A-148; New Mexico State University: Las Cruces, NM, USA, 2013. [Google Scholar]
- Fetjah, D.; Ainlhout, L.F.E.; Ihssane, B.; Houari, A.; Idardare, Z.; Bouqbis, L. Biological, Physico-Chemical and Morphological Analyses of Four Biochars Derived from Agricultural Waste. J. Ecol. Eng. 2021, 22, 36–46. [Google Scholar] [CrossRef]
- Lal, R. Soil Carbon Sequestration Impacts on Global Climate Change and Food Security. Science 2004, 304, 1623–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Lehmann, J.; Pereira da Silva, J.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant. Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Steiner, C.; Glaser, B.; Teixeira, W.G.; Lehmann, J.; Blum, W.; Zech, W. Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J. Plant. Nutr. Soil Sci. 2008, 171, 893–899. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Steiner, C.; Teixeira, W.; Lehmann, J.; Nehls, T.; de Macêdo, J.; Blum, W.; Zech, W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant. Soil 2007, 291, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Morley, J. Compost and Charcoal. Natl. Greenskeeper 1929, 3, 8–26. [Google Scholar]
- Brockhoff, S.R.; Christians, N.E.; Killorn, R.J.; Horton, R.; Davis, D.D. Physical and Mineral-Nutrition Properties of Sand-Based Turfgrass Root Zones Amended with Biochar. Agron. J. 2010, 102, 1627–1631. [Google Scholar] [CrossRef]
- Carey, D.E.; McNamara, P.J.; Zitomer, D.H. Biochar from pyrolysis of biosolids for nutrient adsorption and turfgrass cultivation. Water Environ. Res. 2015, 87, 2098–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fetjah, D.; Ainlhout, L.F.Z.; Idardare, Z.; Ihssane, B.; Bouqbis, L. Effect of Banana-Waste Biochar and Compost Mixtures on Growth Responses and Physiological Traits of Seashore Paspalum Subjected to Six Different Water Conditions. Sustainability 2022, 14, 1541. [Google Scholar] [CrossRef]
- Aziz, S.; Yaseen, L.; Jamal, A.; Farooq, U.; Qureshi, Z.; Tauseef, I.; Haleem, S.K.; Ali, M.I. Fabrication of Biochar from Organic Wastes and its Effect on Wheat Growth and Soil Microflora. Pol. J. Environ. Stud. 2020, 29, 1069–1076. [Google Scholar] [CrossRef]
- Kazemipour, M.; Ansari, M.; Tajrobehkar, S. Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot stone. J. Hazard. Mater. 2008, 150, 322–327. [Google Scholar] [CrossRef]
- Yang, J.; Qiu, K. Preparation of activated carbons from walnut shells via vacuum chemical activation and their application for methylene blue removal. Chem. Eng. J. 2010, 165, 209–217. [Google Scholar] [CrossRef]
- Jones, D.L.; Rousk, J.; Edwards-jones, G.; Deluca, T.H.; Murphy, D.V. Soil Biology & Biochemistry Biochar-mediated changes in soil quality and plant growth in a three year fi eld trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar]
- Yunus, N.A.; Ani, M.H.; Salleh, H.M.; Zamani, R.; Rashid, A.B.D. Reduction of Iron Ore / Empty Fruit Bunch Char Briquette Composite. ISI J. Int. 2015, 53, 1749–1755. [Google Scholar] [CrossRef] [Green Version]
- Shahidah, N.; Adib, M.; Fairuz, S. Suitability of Biochar Produced from Biomass Waste as Soil Amendment. Procedia Soc. Behav. Sci. 2015, 195, 2457–2465. [Google Scholar]
- Blakemore, L.C.; Searle, P.L.; Daly, B.K. Methods for Chemical Analysis of Soils; New Zealand Soil Bureau scientific report; Department of Scientific and Industrial Research: Auckland, New Zealand, 1987; Volume 80, pp. 72–76. [Google Scholar] [CrossRef]
- Fetjah, D.; Ihssane, B.; Idardare, Z.; Ainlhout, L.F.E.; Bouqbis, L. Limiting the Hurtful Oxidative Stress and Seasonal Physiological Adaptations in Seashore Paspalum through the Use of Banana Waste Biochar and Compost Mixtures. J. Ecol. Eng. 2022, 23, 216–227. [Google Scholar] [CrossRef]
- Nikpour-Rashidabad, N.; Tavasolee, A.; Torabian, S.; Farhangi-Abriz, S. The effect of biochar on the physiological, morphological and anatomical characteristics of mung bean roots after exposure to salt stress. Arch. Biol. Sci. 2019, 71, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Sairam, R.K.; Rao, K.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Pagès, J. Factorial Analysis of Mixed Data. J. Appl. Stat. Rev. Stat. Appliquée 2004, 52, 93–111. [Google Scholar]
- Pagès, J. Multiple Factor Analysis Applied to Qualitative Variables and Mixed Data. Rev. Stat. Appl. 2002, 4, 5–37. [Google Scholar]
- Castaldi, S.; Riondino, M.; Baronti, S.; Esposito, F.R.; Marzaioli, R.; Rutigliano, F.A.; Vaccari, F.P.; Miglietta, F. Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes. Chemosphere 2011, 85, 1464–1471. [Google Scholar] [CrossRef]
- Fetjah, D.; Ainlhout, L.F.E.; Ihssane, B.; Bouqbis, L. Effect of Banana Waste Biochar on Physiological Responses and Growth of Seashore Paspalum. J. Ecol. Eng. 2021, 22, 1–10. [Google Scholar] [CrossRef]
- Xu, C.; Hosseini-bai, S.; Hao, Y.; Wallace, H. Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ. Sci. Pollut. Res. Int. 2015, 22, 6112–6125. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Bashir, S.; Saleem, M.H.; Chen, C.; Peng, D.; Siddique, K.H. Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant Physiol. Biochem. 2019, 138, 121–129. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.W.; Huchzermeyer, B.; Ansari, R.; Zulfiqar, F.; Gul, B. Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol. 2020, 22, 259–266. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Cross, A.; Sohi, S.P. The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol. Biochem. 2011, 43, 2127–2134. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Yang, S.; Wang, Y. Impacts of biochar addition on rice yield and soil properties in a cold waterlogged paddy for two crop seasons. F. Crop. Res. 2016, 191, 161–167. [Google Scholar] [CrossRef]
- Fu, Q.S.; Yang, R.C.; Wang, H.S.; Zhao, B.; Zhou, C.L.; Ren, S.X.; Guo, Y.D. Leaf morphological and ultrastructural performance of eggplant (Solanum melongena L.) in response to water stress. Photosynthetica 2013, 51, 109–114. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanure, M.M.; da Costa, L.M.; Huiz, H.A.; Fernandes, R.B.; Cecon, P.R.; Junior, J.D.; da Luz, J.M. Soil Soil water retention, physiological characteristics, and growth of maize plants in response to biochar application to soil. Soil Tillage Res. 2019, 192, 164–173. [Google Scholar] [CrossRef]
- Paneque, M.; De la Rosa, J.M.; Franco-Navarro, J.D.; Colmenero-Flores, J.M.; Knicker, H. Effect of biochar amendment on morphology, productivity and water relations of sunflower plants under non-irrigation conditions. Catena 2016, 147, 280–287. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fetjah, D.; Idardare, Z.; Ihssane, B.; Ainlhout, L.F.Z.; Bouqbis, L. Seasonal Paspalum vaginatum Physiological Characteristics Change with Agricultural Byproduct Biochar in Sandy Potting Soil. Biology 2022, 11, 560. https://doi.org/10.3390/biology11040560
Fetjah D, Idardare Z, Ihssane B, Ainlhout LFZ, Bouqbis L. Seasonal Paspalum vaginatum Physiological Characteristics Change with Agricultural Byproduct Biochar in Sandy Potting Soil. Biology. 2022; 11(4):560. https://doi.org/10.3390/biology11040560
Chicago/Turabian StyleFetjah, Dounia, Zaina Idardare, Bouchaib Ihssane, Lalla Fatima Zohra Ainlhout, and Laila Bouqbis. 2022. "Seasonal Paspalum vaginatum Physiological Characteristics Change with Agricultural Byproduct Biochar in Sandy Potting Soil" Biology 11, no. 4: 560. https://doi.org/10.3390/biology11040560





