Plasmonic Biosensors: Review
Abstract
Simple Summary
Abstract
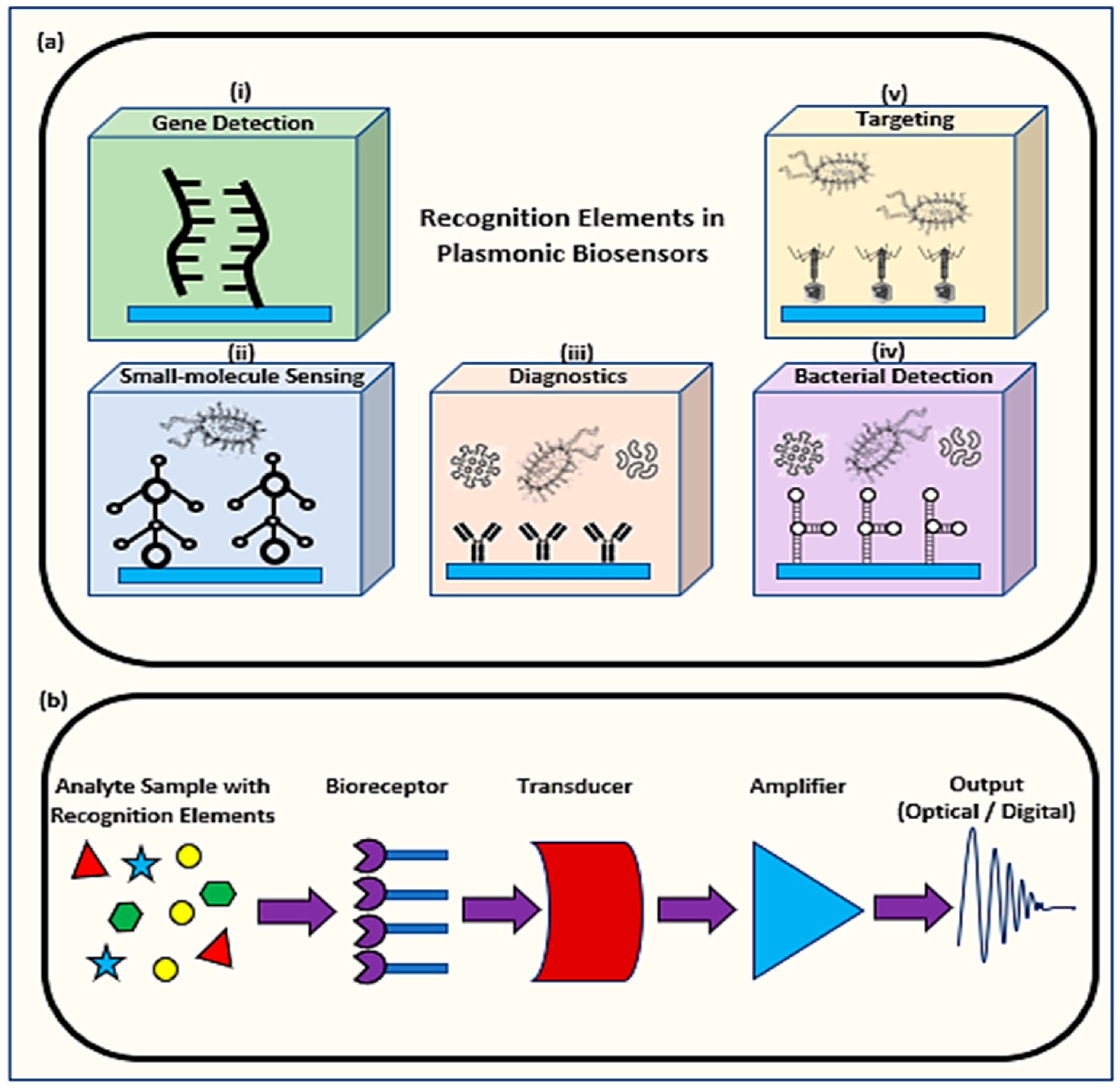
1. Introduction
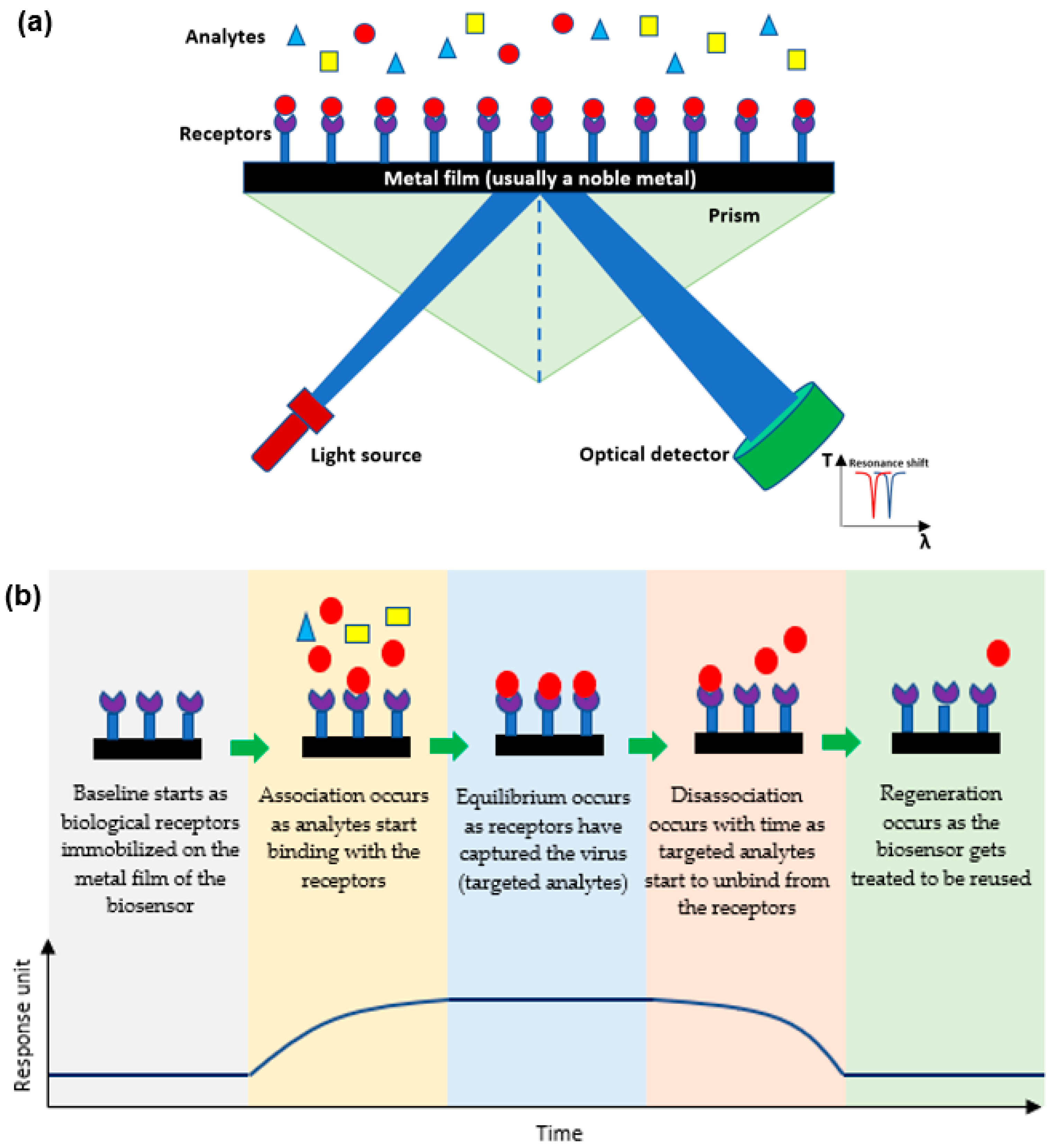
1.1. Mechanism of Plasmonic Biosensors
1.2. Determining the Efficiency of a Plasmonic Biosensor
1.2.1. Limit of Blank
1.2.2. Limit of Detection
1.2.3. Specificity
2. Applications of Plasmonic Optical Biosensors
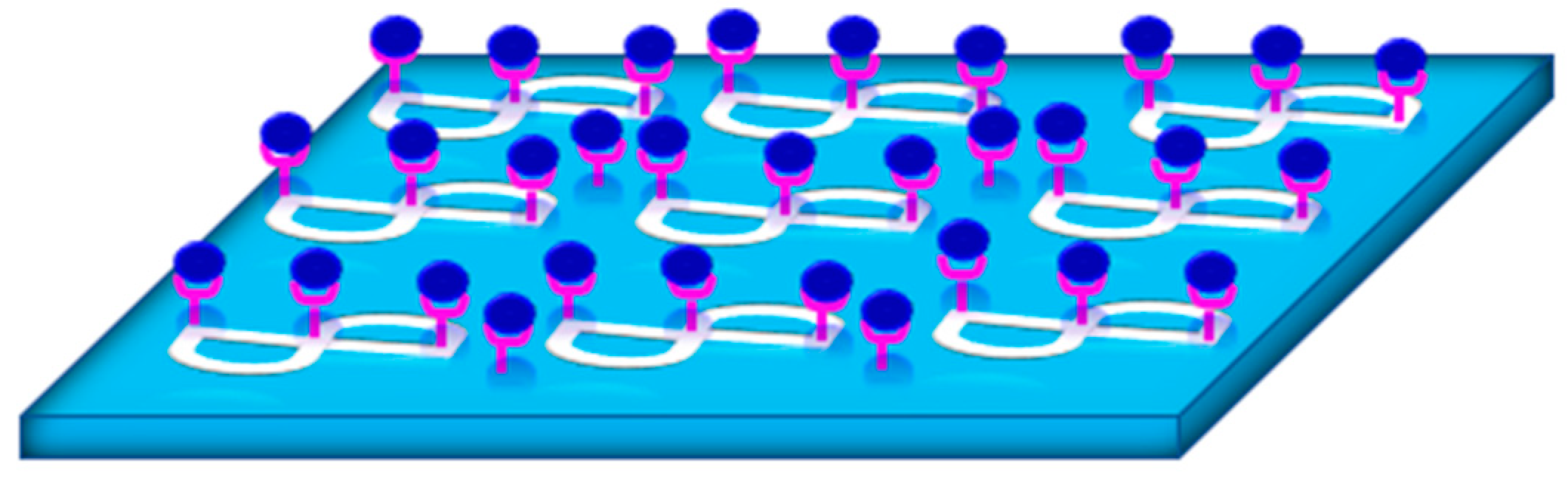
2.1. The Use of Plasmonic Biosensors for Viral Detection
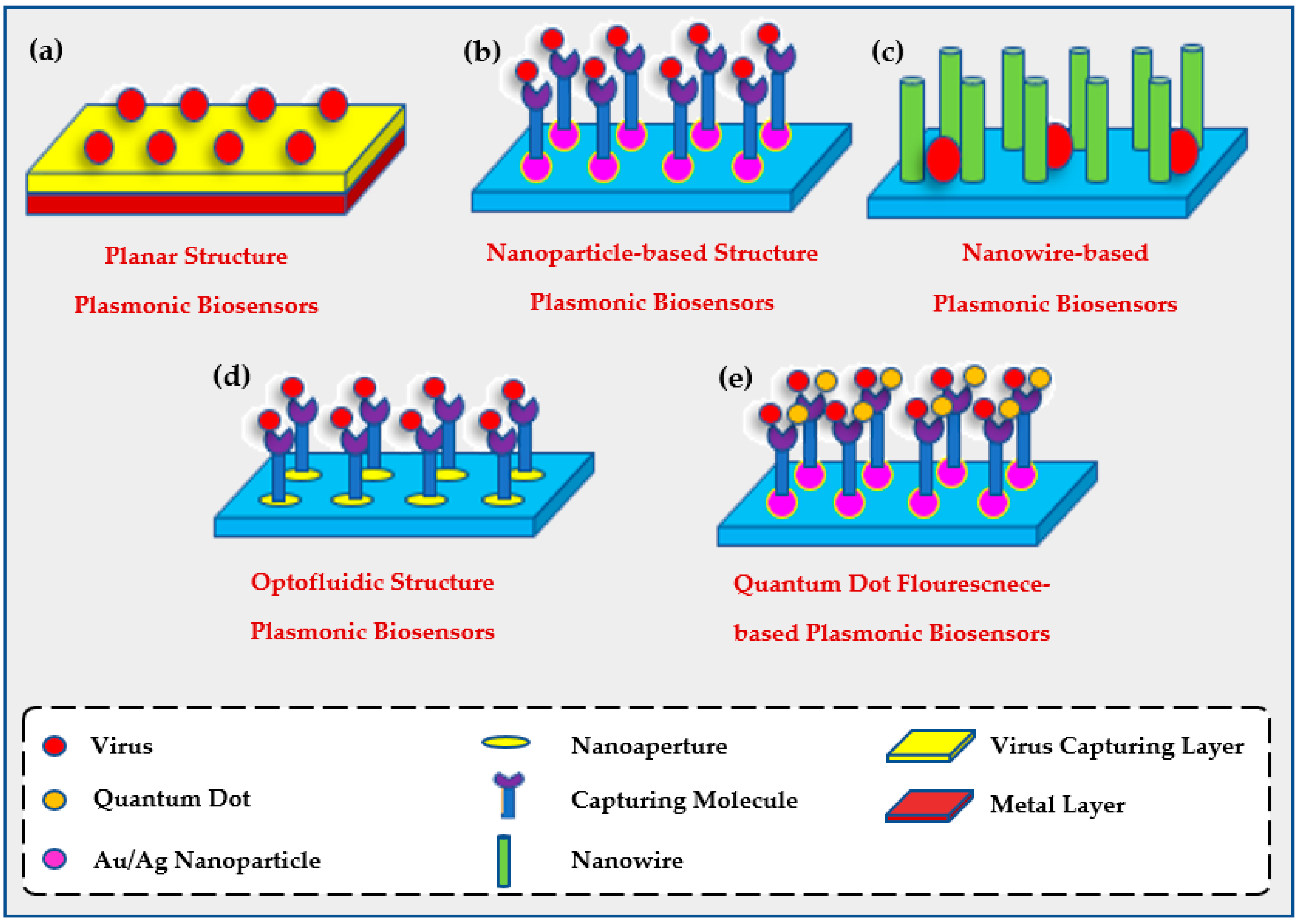
2.1.1. Planar Biosensors
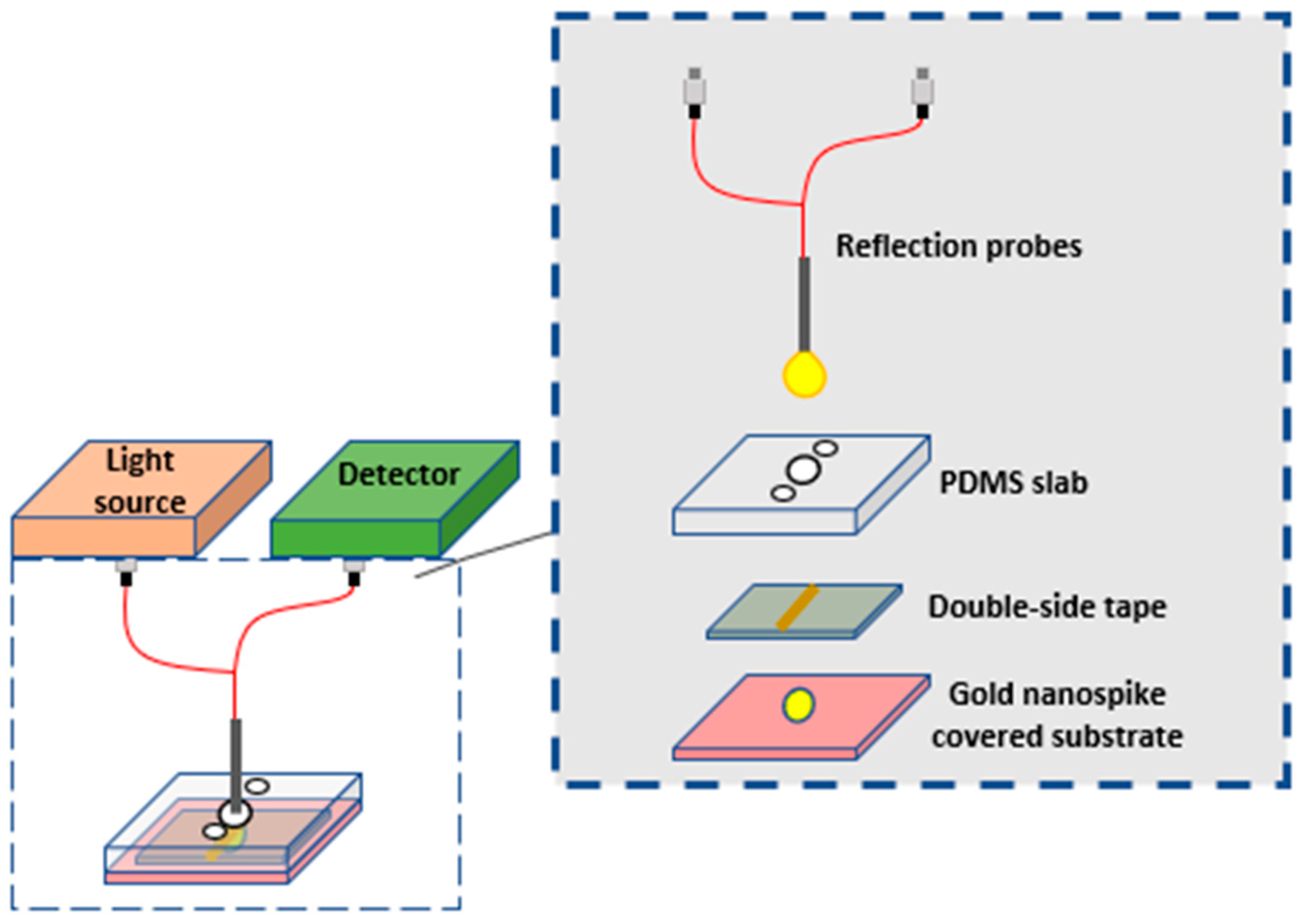
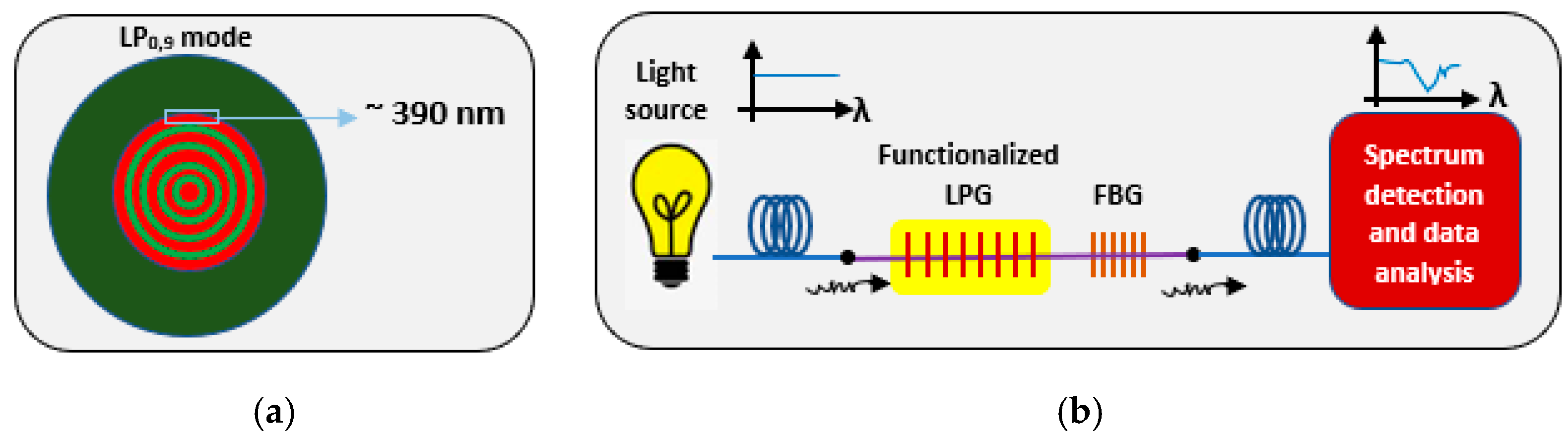
2.1.2. Opto-Fluidic Nano-Plasmonic Biosensors
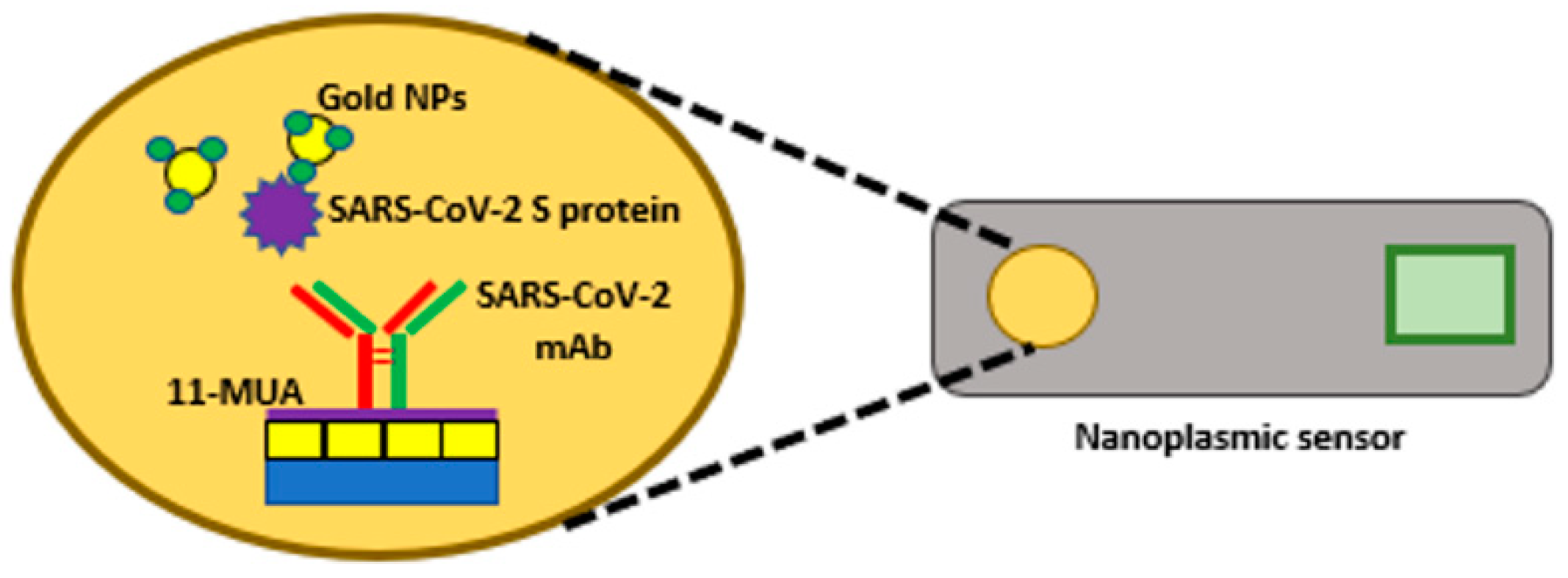
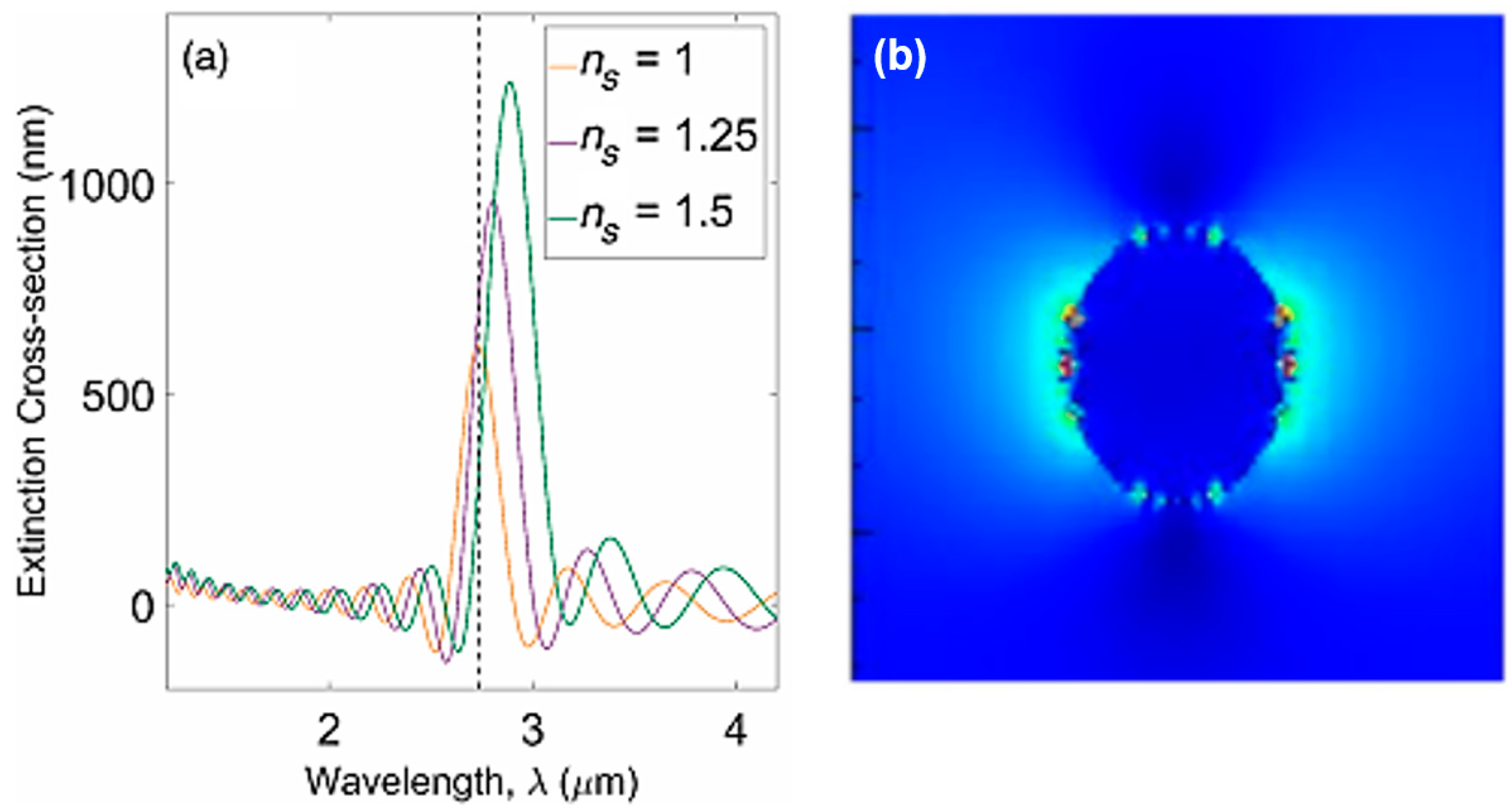
2.1.3. Nanoparticle-Based Biosensors
2.1.4. Quantum Dot Enhanced Fluorescent LSPR
2.1.5. Nanowire-Based Biosensors
2.2. The Use of Plasmonic Biosensors for Environmental Evaluation
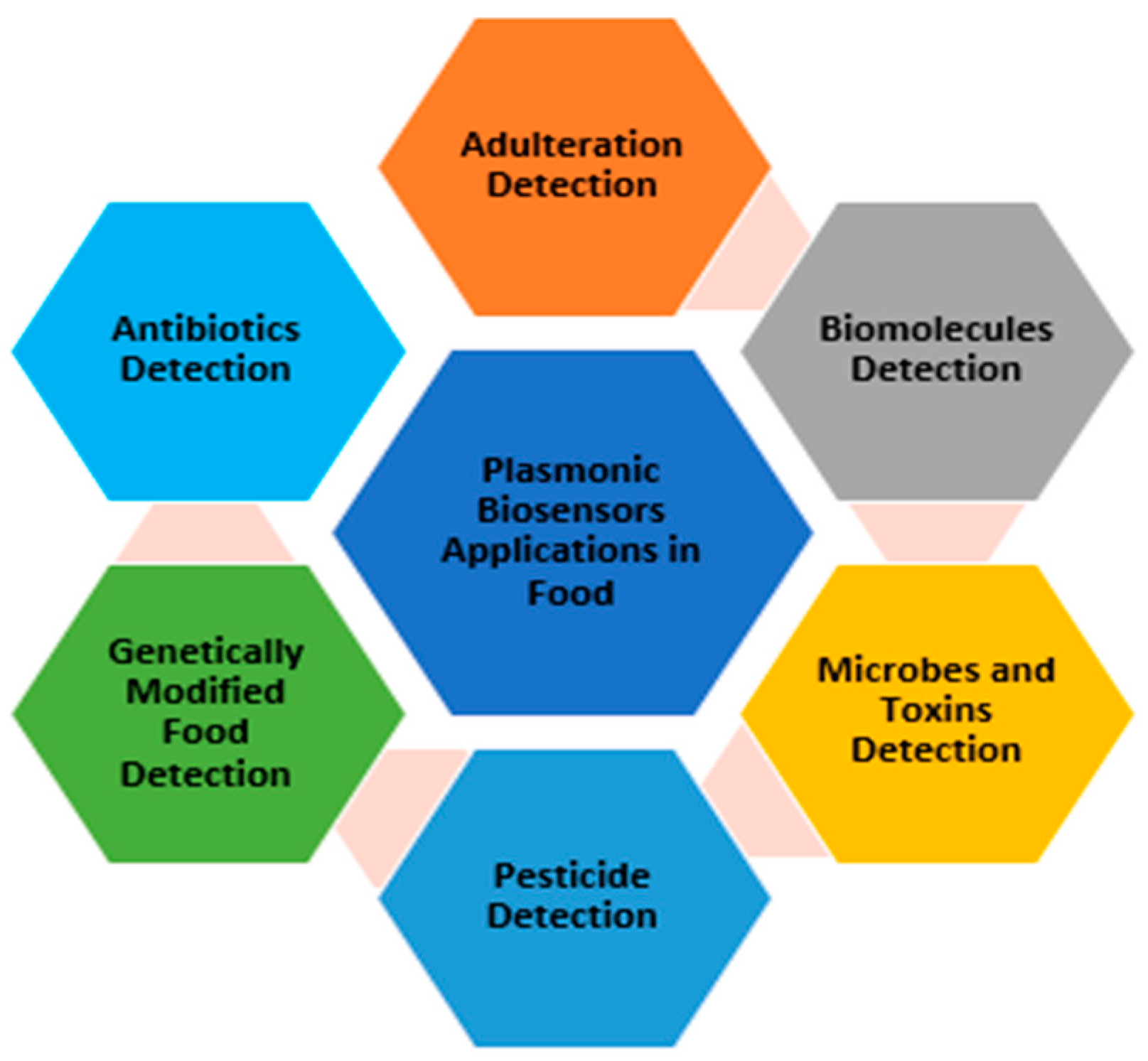
2.3. The Use of Plasmonic Biosensors for Food Analysis
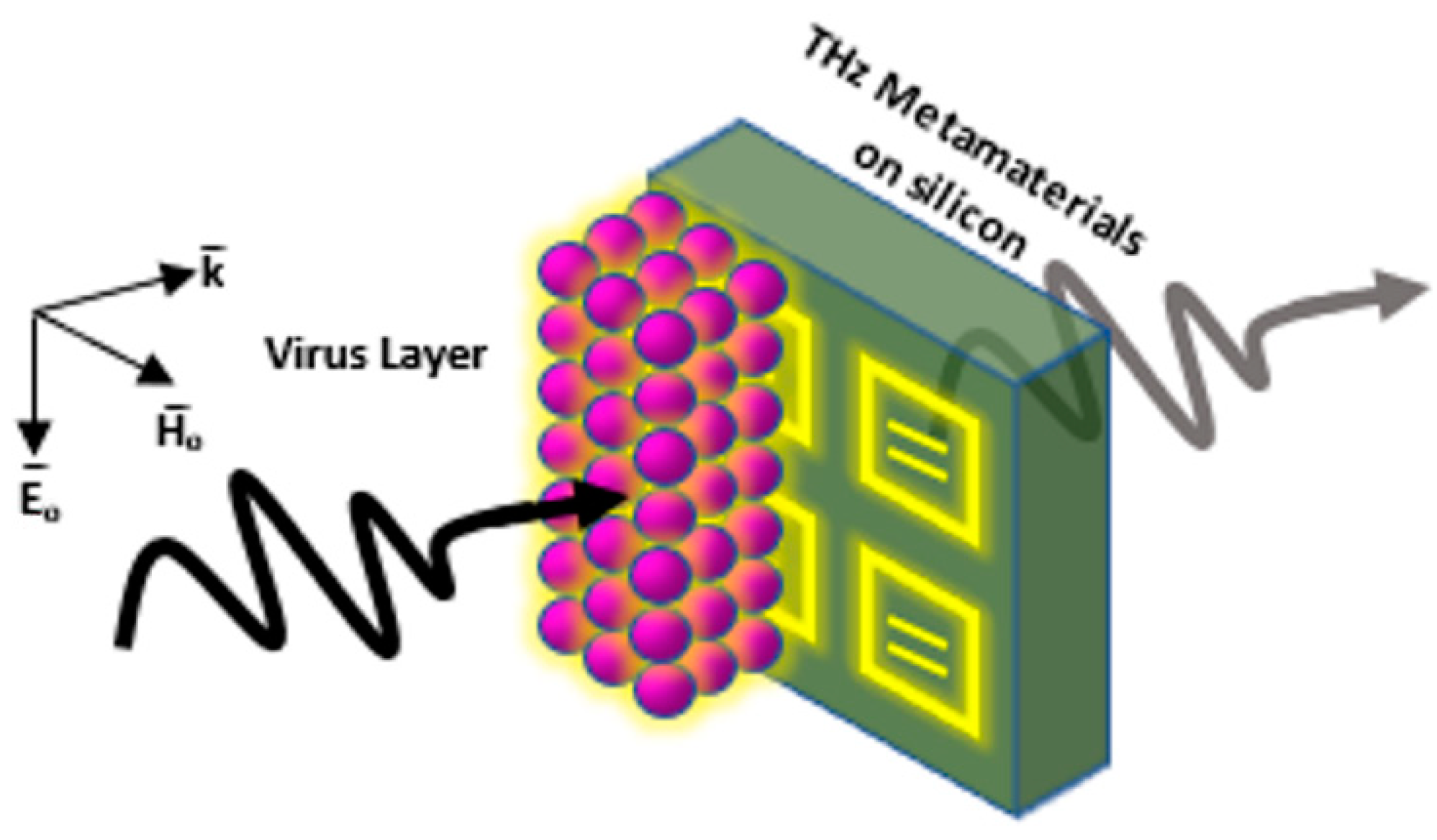
3. Introducing Metamaterials to Plasmonic Biosensors
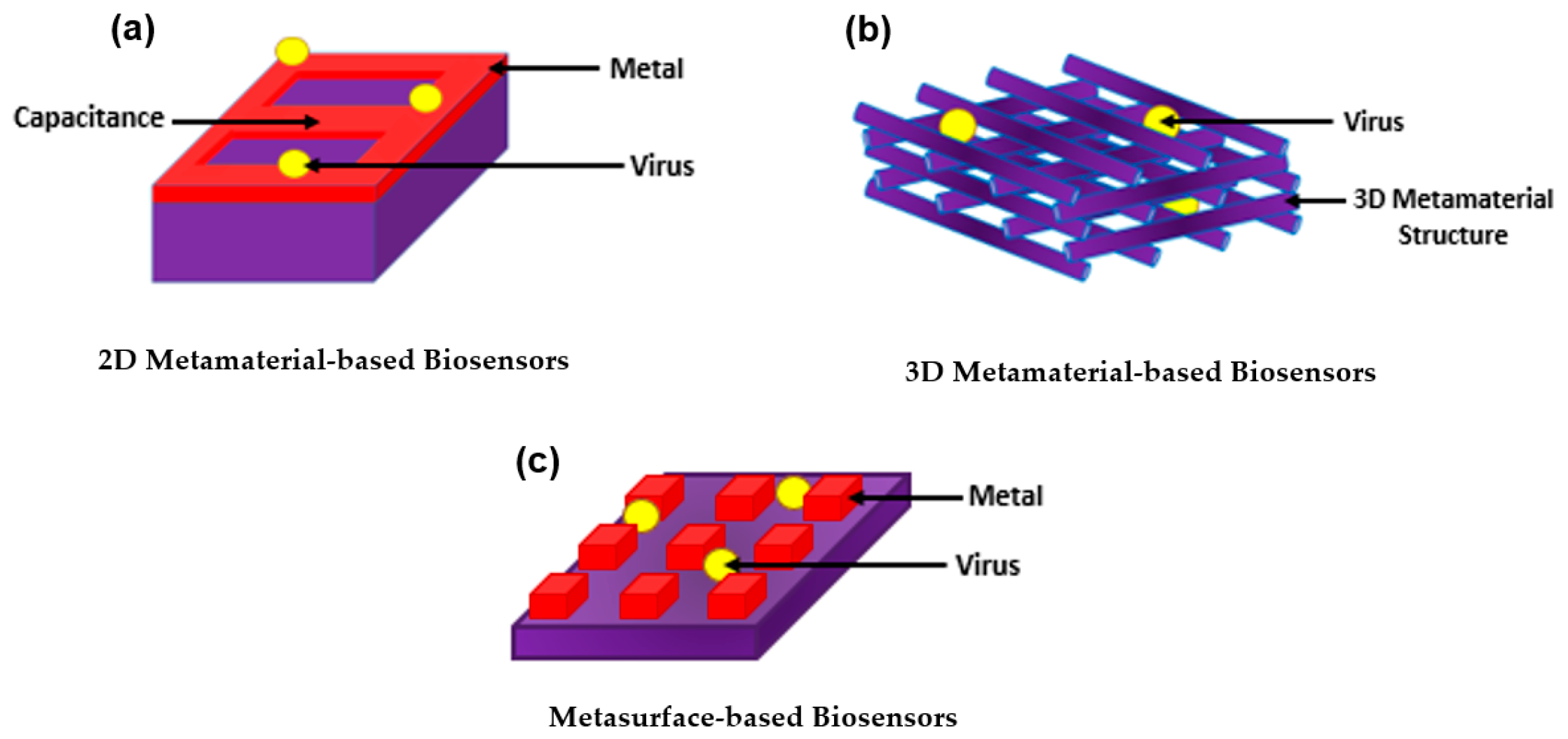
3.1. Two-Dimensional Metamaterial-Based Structures as Plasmonic Biosensors
3.2. Three-Dimensional Metamaterial-Based Structures as Plasmonic Biosensors
3.3. Metasurface-Based Structures as Plasmonic Biosensors
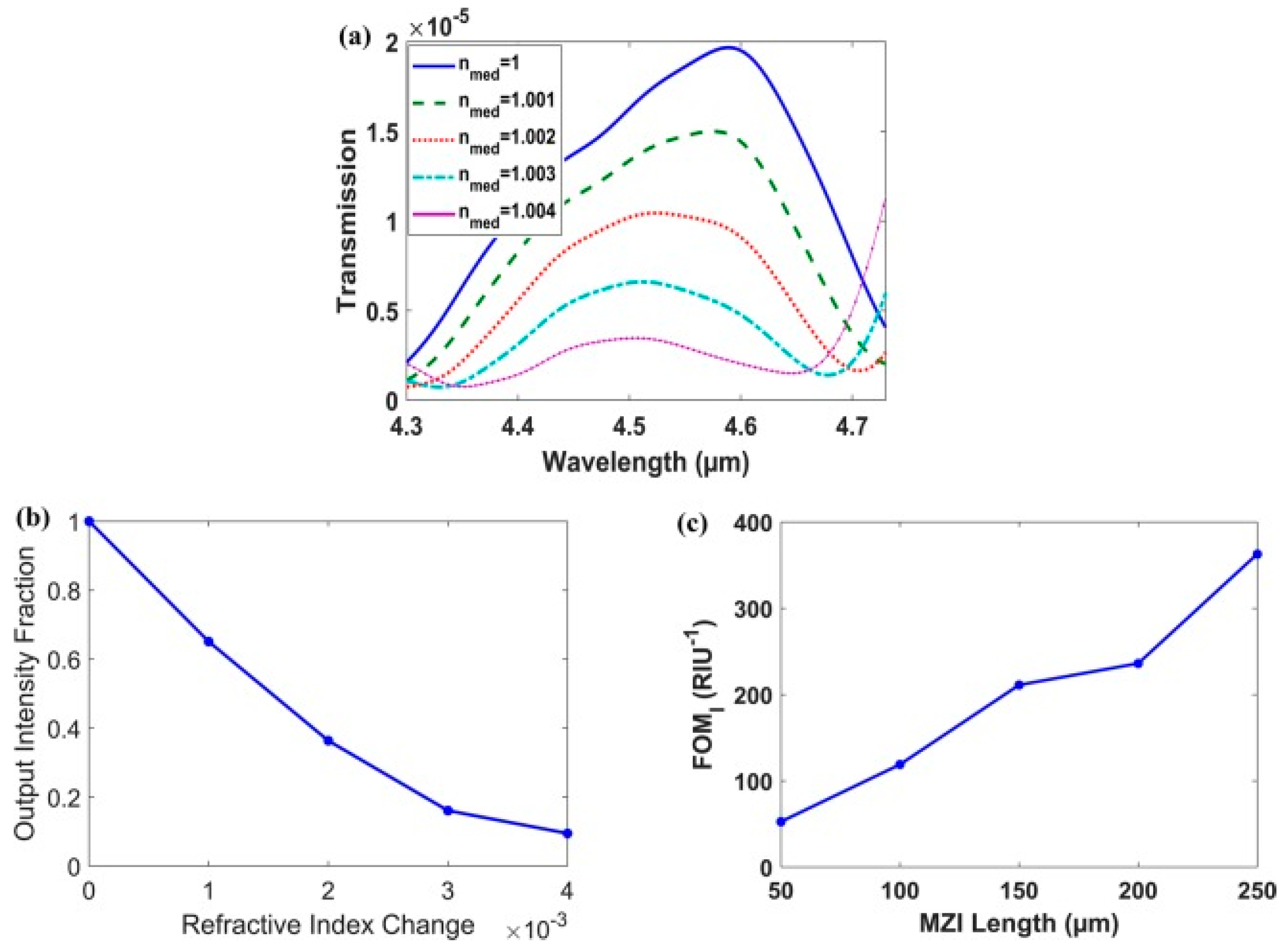
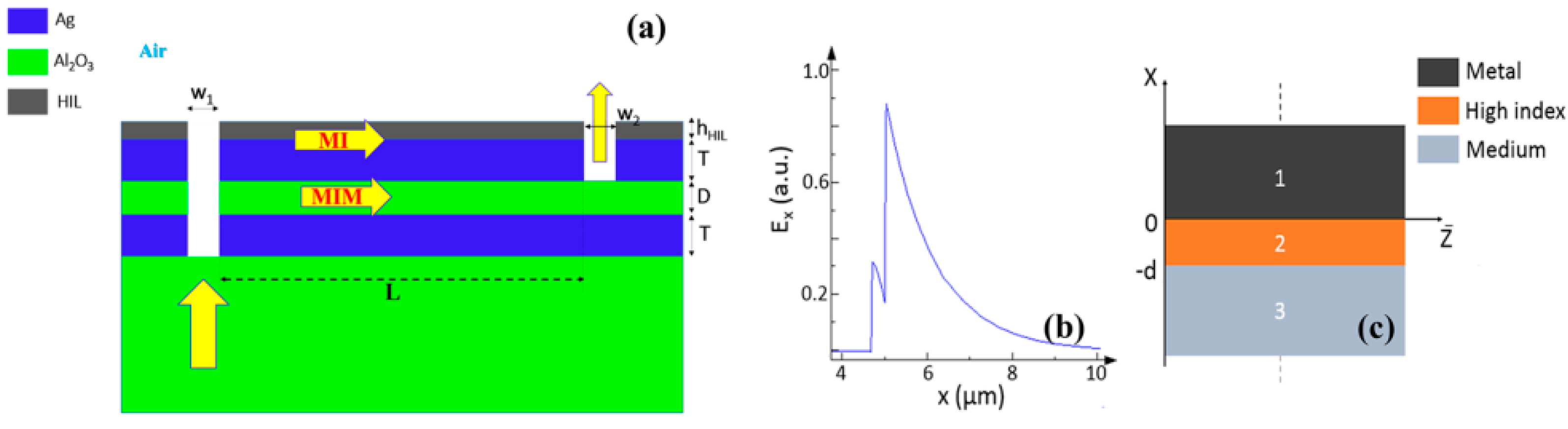
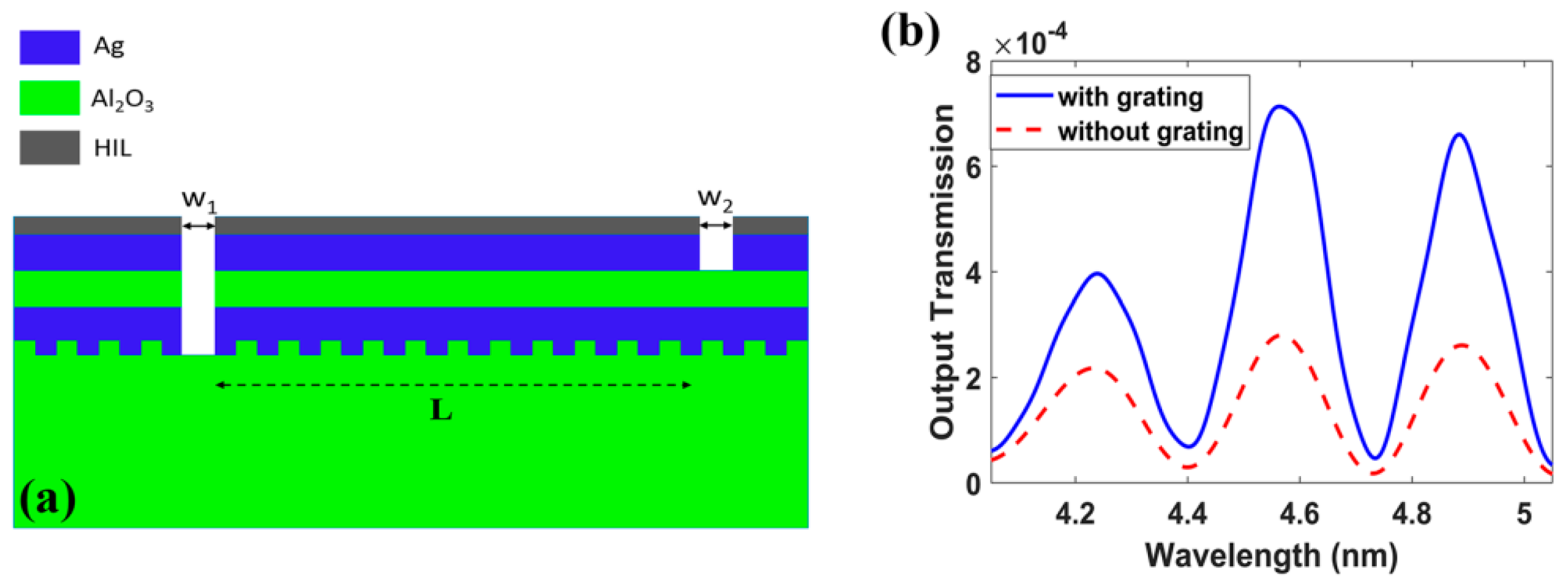
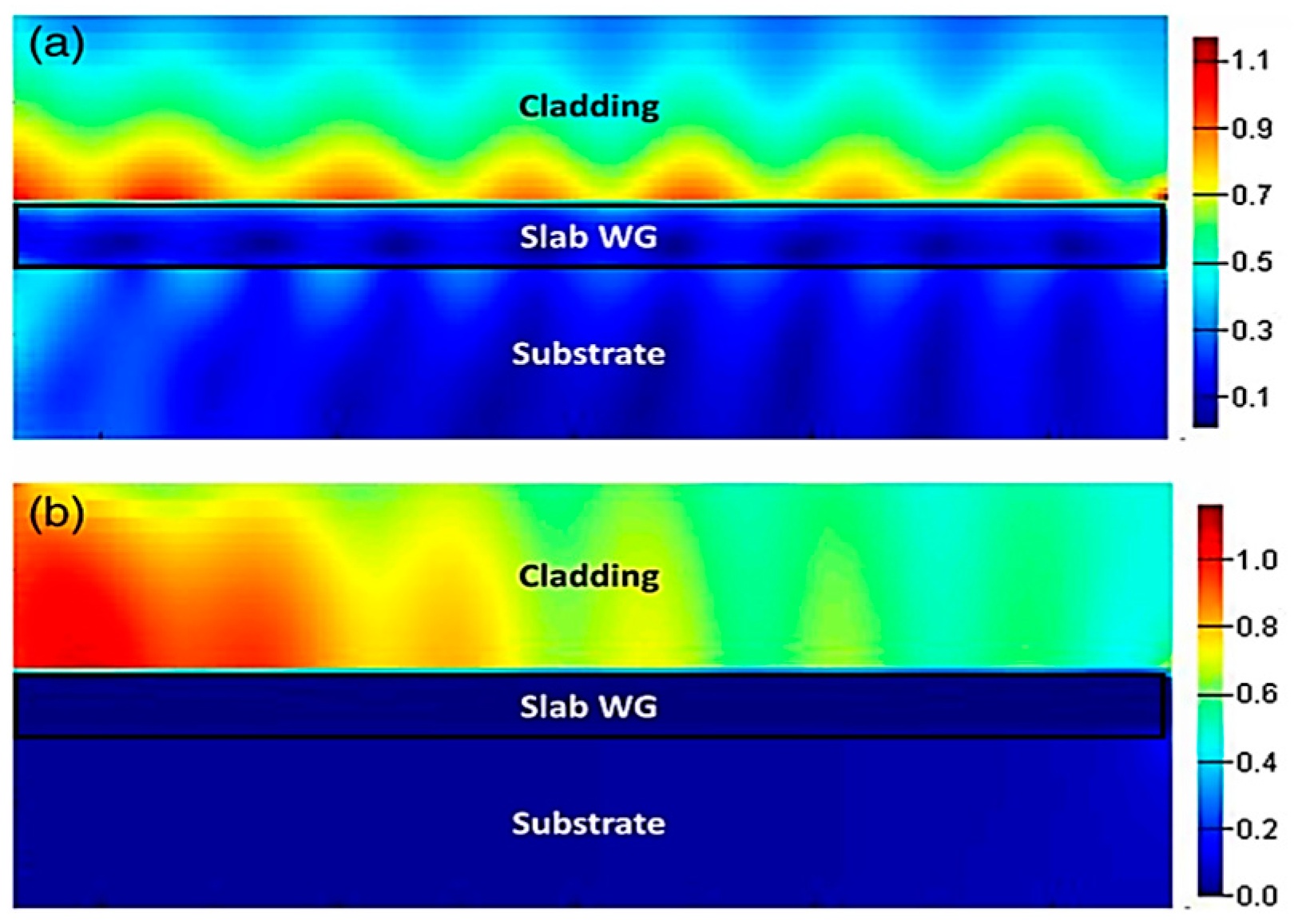
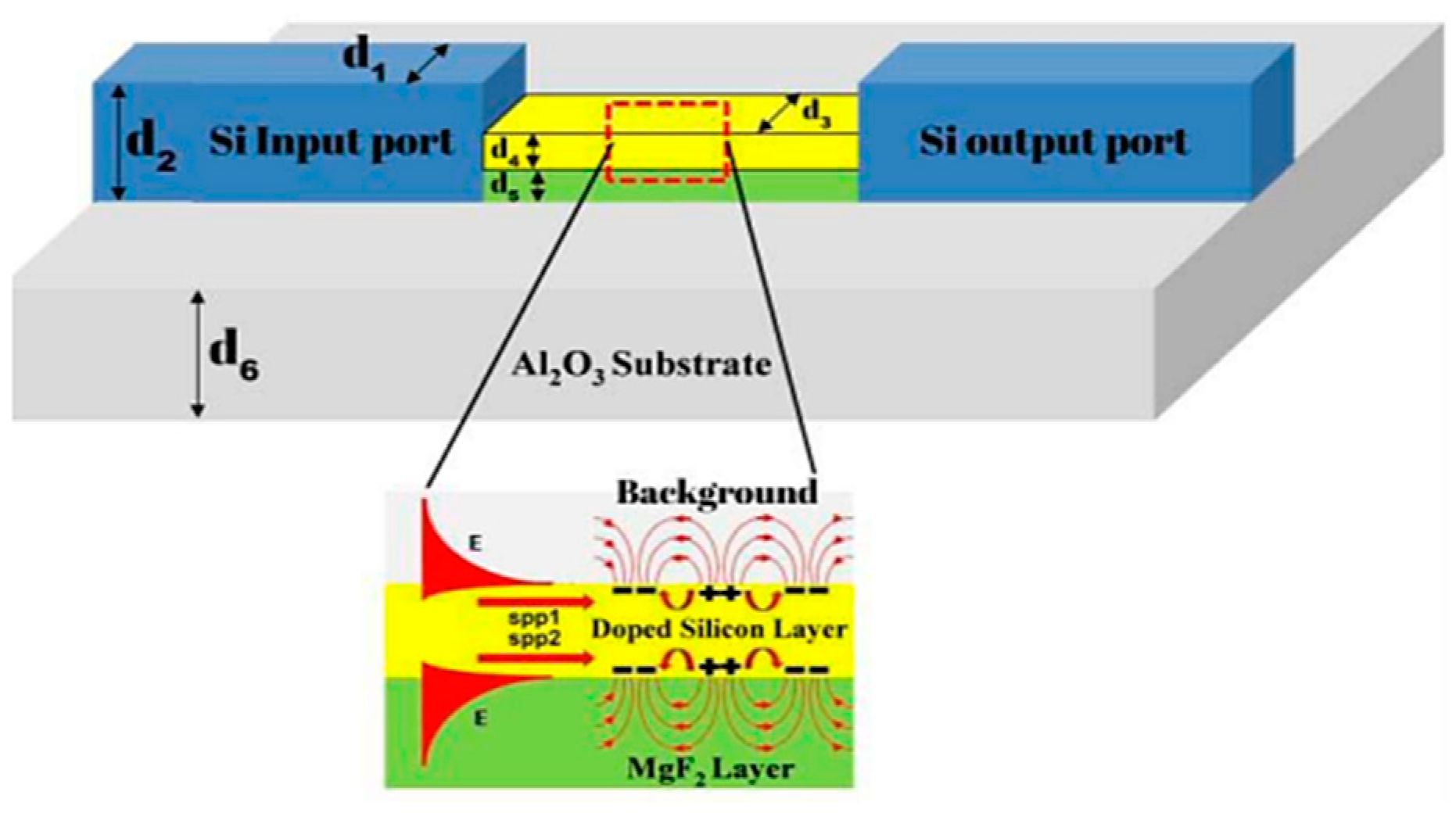
4. Lab-on-a-Chip (LoC) for Plasmonic Biosensors
5. Future Perspective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villalonga, A.; Pérez-Calabuig, A.M.; Villalonga, R. Electrochemical biosensors based on nucleic acid aptamers. Anal. Bioanal. Chem. 2020, 412, 55–72. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Chendake, A.D.; Ghosal, D.; Mathuriya, A.S.; Kumar, S.S.; Pandit, S. Advanced microbial fuel cell for biosensor applications to detect quality parameters of pollutants. In Bioremediation, Nutrients, and Other Valuable Product Recovery; Singh, L., Mahapatra, D.M., Thakur, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 125–139. [Google Scholar] [CrossRef]
- Bollella, P.; Katz, E. Biosensors—Recent Advances and Future Challenges. Sensors 2020, 20, 6645. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.; Lee, U.; Fermin, C.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef]
- Mauriz, E.; Lechuga, L.M. Plasmonic Biosensors for Single-Molecule Biomedical Analysis. Biosensors 2021, 11, 123. [Google Scholar] [CrossRef]
- Solhi, E.; Hasanzadeh, M. Critical role of biosensing on the efficient monitoring of cancer proteins/biomarkers using label-free aptamer based bioassay. Biomed. Pharmacother. 2020, 132, 110849. [Google Scholar] [CrossRef]
- Sezgintürk, M.K. Introduction to commercial biosensors. In Commercial Biosensors and Their Applications; Sezgintürk, M.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–28. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Wu, K.; Saha, R.; Su, D.; Krishna, V.D.; Liu, J.; Cheeran, M.C.-J.; Wang, J.-P. Magnetic-Nanosensor-Based Virus and Pathogen Detection Strategies before and during COVID-19. ACS Appl. Nano Mater. 2020, 3, 9560–9580. [Google Scholar] [CrossRef]
- Zhao, V.X.T.; Wong, T.I.; Zheng, X.T.; Tan, Y.N.; Zhou, X. Colorimetric biosensors for point-of-care virus detections. Mater. Sci. Energy Technol. 2020, 3, 237–249. [Google Scholar] [CrossRef]
- Kaya, S.I.; Karadurmus, L.; Ozcelikay, G.; Bakirhan, N.K.; Ozkan, S.A. Electrochemical virus detections with nanobiosensors. Nanosens. Smart Cities 2020, 303–326. [Google Scholar] [CrossRef]
- Antiochia, R. Paper-Based Biosensors: Frontiers in Point-of-Care Detection of COVID-19 Disease. Biosensors 2021, 11, 110. [Google Scholar] [CrossRef]
- Maddali, H.; Miles, C.E.; Kohn, J.; O’Carroll, D.M. Optical Biosensors for Virus Detection: Prospects for SARS-CoV-2/COVID-19. Chembiochem 2021, 22, 1176–1189. [Google Scholar] [CrossRef]
- Zanchetta, G.; Lanfranco, R.; Giavazzi, F.; Bellini, T.; Buscaglia, M. Emerging applications of label-free optical biosensors. Nanophotonics 2017, 6, 627–645. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X. Microfluidics-Based Plasmonic Biosensing System Based on Patterned Plasmonic Nanostructure Arrays. Micromachines 2021, 12, 826. [Google Scholar] [CrossRef]
- Liu, L.; Ye, K.; Jia, Z.; Xue, T.; Nie, A.; Xiang, J.; Mu, C.; Wang, B.; Wen, F.; Zhai, K.; et al. High-sensitivity and versatile plasmonic biosensor based on grain boundaries in polycrystalline 1L WS2 films. Biosens. Bioelectron. 2021, 194, 113596. [Google Scholar] [CrossRef]
- Li, X.; Soler, M.; Özdemir, C.I.; Belushkin, A.; Yesilköy, F.; Altug, H. Plasmonic nanohole array biosensor for label-free and real-time analysis of live cell secretion. Lab Chip 2017, 17, 2208–2217. [Google Scholar] [CrossRef]
- Armstrong, R.E.; Horáček, M.; Zijlstra, P. Plasmonic Assemblies for Real-Time Single-Molecule Biosensing. Small 2020, 16, 2003934. [Google Scholar] [CrossRef]
- Minopoli, A.; Della Ventura, B.; Lenyk, B.; Gentile, F.; Tanner, J.A.; Offenhäusser, A.; Mayer, D.; Velotta, R. Ultrasensitive antibody-aptamer plasmonic biosensor for malaria biomarker detection in whole blood. Nat. Commun. 2020, 11, 6134. [Google Scholar] [CrossRef]
- Mondal, B.; Zeng, S. Recent Advances in Surface Plasmon Resonance for Biosensing Applications and Future Prospects. 2020. Available online: https://hal-unilim.archives-ouvertes.fr/hal-02915657 (accessed on 18 March 2022).
- Duan, Q.; Liu, Y.; Chang, S.; Chen, H.; Chen, J. Surface Plasmonic Sensors: Sensing Mechanism and Recent Applications. Sensors 2021, 21, 5262. [Google Scholar] [CrossRef]
- Hill, R.T. Plasmonic Biosensors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 152–168. [Google Scholar] [CrossRef]
- Miyazaki, C.; Shimizu, F.; Ferreira, M. Surface Plasmon Resonance (SPR) for Sensors and Biosensors. In Nanocharacterization Techniques; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 183–200. [Google Scholar] [CrossRef]
- Chlebus, R.; Chylek, J.; Ciprian, D.; Hlubina, P. Surface Plasmon Resonance Based Measurement of the Dielectric Function of a Thin Metal Film. Sensors 2018, 18, 3693. [Google Scholar] [CrossRef] [PubMed]
- Kravets, V.G.; Wu, F.; Yu, T.; Grigorenko, A.N. Metal-Dielectric-Graphene Hybrid Heterostructures with Enhanced Surface Plasmon Resonance Sensitivity Based on Amplitude and Phase Measurements. Plasmonics 2022. [Google Scholar] [CrossRef]
- Zakaria, R.; Zainuddin, N.A.M.; Leong, T.C.; Rosli, R.; Rusdi, M.F.; Harun, S.W.; Sadegh Amiri, I. Investigation of Surface Plasmon Resonance (SPR) in MoS2- and WS2-Protected Titanium Side-Polished Optical Fiber as a Humidity Sensor. Micromachines 2019, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Derkachova, A.; Kolwas, K.; Demchenko, I. Dielectric Function for Gold in Plasmonics Applications: Size Dependence of Plasmon Resonance Frequencies and Damping Rates for Nanospheres. Plasmonics 2016, 11, 941–951. [Google Scholar] [CrossRef]
- Raza, S.; Stenger, N.; Kadkhodazadeh, S.; Fischer, S.V.; Kostesha, N.; Jauho, A.-P.; Burrows, A.; Wubs, M.; Mortensen, N.A. Blueshift of the surface plasmon resonance in silver nanoparticles studied with EELS. Nanophotonics 2013, 2, 131–138. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, T. Spectrometry. In Nano-Inspired Biosensors for Protein Assay with Clinical Applications; Li, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–264. [Google Scholar] [CrossRef]
- Hassan, M.M.; Sium, F.S.; Islam, F.; Choudhury, S.M. A review on plasmonic and metamaterial based biosensing platforms for virus detection. Sens. Bio-Sens. Res. 2021, 33, 100429. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, K.; Li, L.; Peng, W.; Yu, Q. Angle modulated surface plasmon resonance spectrometer for refractive index sensing with enhanced detection resolution. Opt. Commun. 2017, 382, 610–614. [Google Scholar] [CrossRef]
- Xu, K.; Zhou, R.; Takei, K.; Hong, M. Toward Flexible Surface-Enhanced Raman Scattering (SERS) Sensors for Point-of-Care Diagnostics. Adv. Sci. 2019, 6, 1900925. [Google Scholar] [CrossRef]
- Park, S.-G.; Mun, C.; Xiao, X.; Braun, A.; Kim, S.; Giannini, V.; Maier, S.A.; Kim, D.-H. Surface Energy-Controlled SERS Substrates for Molecular Concentration at Plasmonic Nanogaps. Adv. Funct. Mater. 2017, 27, 1703376. [Google Scholar] [CrossRef]
- Elsayed, M.; Gouda, A.; Ismail, Y.; Swillam, M. Silicon-Based SERS Substrates Fabricated by Electroless Etching. J. Lightwave Technol. 2017, 35, 3075–3081. [Google Scholar] [CrossRef]
- EP17A2|Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures, 2nd ed. Available online: https://www.clsi.orghttps://clsi.org/standards/products/method-evaluation/documents/ep17/ (accessed on 18 March 2022).
- Armbruster, D.A.; Pry, T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29, S49–S52. [Google Scholar] [PubMed]
- Shang, J.; Gao, X. Nanoparticle counting: Towards accurate determination of the molar concentration. Chem. Soc. Rev. 2014, 43, 7267–7278. [Google Scholar] [CrossRef] [PubMed]
- Zopf, D.; Jatschka, J.; Dathe, A.; Jahr, N.; Fritzsche, W.; Stranik, O. Hyperspectral imaging of plasmon resonances in metallic nanoparticles. Biosens. Bioelectron. 2016, 81, 287–293. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 22 July 2021).
- Louten, J. Detection and Diagnosis of Viral Infections. Essent. Hum. Virol. 2016, 111–132. [Google Scholar] [CrossRef]
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Laboratory Diagnosis of Virus Diseases. Fenner White’s Med. Virol. 2017, 135–154. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, C.-W.; Wang, J.; Oh, D.H. Advances in Rapid Detection Methods for Foodborne Pathogens. J. Microbiol. Biotechnol. 2014, 24, 297–312. [Google Scholar] [CrossRef]
- Bağcı, U.; Bray, M.; Caban, J.; Yao, J.; Mollura, D.J. Computer-Assisted Detection of Infectious Lung Diseases: A Review. Comput. Med. Imaging Graph. 2012, 36, 72–84. [Google Scholar] [CrossRef]
- Kumar, P. Methods for Rapid Virus Identification and Quantification. Mater. Methods 2013, 3, 13070. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ohmagari, N. Microbiological Testing for Coronavirus Disease 2019. JMA J. 2021, 4, 67–75. [Google Scholar] [CrossRef]
- Bruce, E.A.; Mills, M.G.; Sampoleo, R.; Perchetti, G.A.; Huang, M.-L.; Despres, H.W.; Schmidt, M.M.; Roychoudhury, P.; Shirley, D.J.; Jerome, K.R.; et al. Predicting infectivity: Comparing four PCR-based assays to detect culturable SARS-CoV-2 in clinical samples. EMBO Mol. Med. 2022, 14, e15290. [Google Scholar] [CrossRef]
- Ahmed, W.; Simpson, S.L.; Bertsch, P.M.; Bibby, K.; Bivins, A.; Blackall, L.L.; Bofill-Mas, S.; Bosch, A.; Brandão, J.; Choi, P.M.; et al. Minimizing errors in RT-PCR detection and quantification of SARS-CoV-2 RNA for wastewater surveillance. Sci. Total Environ. 2022, 805, 149877. [Google Scholar] [CrossRef] [PubMed]
- Toya, M.; Iida, Y.; Sugimoto, A. Imaging of Mitotic Spindle Dynamics in Caenorhabditis elegans Embryos. In Methods in Cell Biology; Cassimeris, L., Tran, P., Eds.; Microtubules: In vivo; Academic Press: Cambridge, MA, USA, 2010; Volume 97, pp. 359–372. [Google Scholar] [CrossRef]
- Noah, D.L.; Hill, H.; Hines, D.; White, E.L.; Wolff, M.C. Qualification of the Hemagglutination Inhibition Assay in Support of Pandemic Influenza Vaccine Licensure. Clin. Vaccine Immunol. 2009, 16, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, E.J.; Manguiat, K.; Wood, H.; Drebot, M. Two Detailed Plaque Assay Protocols for the Quantification of Infectious SARS-CoV-2. Curr. Protoc. Microbiol. 2020, 57, ecpmc105. [Google Scholar] [CrossRef] [PubMed]
- Cossarizza, A.; Chang, H.-D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar] [CrossRef]
- Tymm, C.; Zhou, J.; Tadimety, A.; Burklund, A.; Zhang, J.X.J. Scalable COVID-19 Detection Enabled by Lab-on-Chip Biosensors. Cell. Mol. Bioeng. 2020, 13, 313–329. [Google Scholar] [CrossRef]
- Chang, Y.-F.; Wang, W.-H.; Hong, Y.-W.; Yuan, R.-Y.; Chen, K.-H.; Huang, Y.-W.; Lu, P.-L.; Chen, Y.-H.; Chen, Y.-M.A.; Su, L.-C.; et al. Simple Strategy for Rapid and Sensitive Detection of Avian Influenza A H7N9 Virus Based on Intensity-Modulated SPR Biosensor and New Generated Antibody. Anal. Chem. 2018, 90, 1861–1869. [Google Scholar] [CrossRef]
- Shi, L.; Sun, Q.; He, J.; Xu, H.; Liu, C.; Zhao, C.; Xu, Y.; Wu, C.; Xiang, J.; Gu, D.; et al. Development of SPR biosensor for simultaneous detection of multiplex respiratory viruses. Biomed. Mater. Eng. 2015, 26 (Suppl. 1), S2207–S2216. [Google Scholar] [CrossRef]
- Tam, Y.J.; Zeenathul, N.A.; Rezaei, M.A.; Mustafa, N.H.; Azmi, M.L.M.; Bahaman, A.R.; Lo, S.C.; Tan, J.S.; Hani, H.; Rasedee, A. Wide dynamic range of surface-plasmon-resonance-based assay for hepatitis B surface antigen antibody optimal detection in comparison with ELISA. Biotechnol. Appl. Biochem. 2017, 64, 735–744. [Google Scholar] [CrossRef]
- Diao, W.; Tang, M.; Ding, S.; Li, X.; Cheng, W.; Mo, F.; Yan, X.; Ma, H.; Yan, Y. Highly sensitive surface plasmon resonance biosensor for the detection of HIV-related DNA based on dynamic and structural DNA nanodevices. Biosens. Bioelectron. 2018, 100, 228–234. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Chik, C.E.N.C.E.; Mahdi, M.A. Development of an optical sensor based on surface plasmon resonance phenomenon for diagnosis of dengue virus E-protein. Sens. Bio-Sens. Res. 2018, 20, 16–21. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kumar, J.S.; Singh, V.V.; Biswas, U.; Sarkar, S.S.; Alam, S.I.; Dash, P.K.; Boopathi, M.; Ganesan, K.; Jain, R. Surface plasmon resonance sensing of Ebola virus: A biological threat. Anal. Bioanal. Chem. 2020, 412, 4101–4112. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Mustapha Kamil, Y.; Daniyal, W.M.E.M.M.; Sadrolhosseini, A.R.; Mahdi, M.A. Sensitive Detection of Dengue Virus Type 2 E-Proteins Signals Using Self-Assembled Monolayers/Reduced Graphene Oxide-PAMAM Dendrimer Thin Film-SPR Optical Sensor. Sci. Rep. 2020, 10, 2374. [Google Scholar] [CrossRef] [PubMed]
- Raether, H. Surface plasmons on smooth surfaces. In Surface Plasmons on Smooth and Rough Surfaces and on Gratings; Raether, H., Ed.; Springer Tracts in Modern Physics; Springer: Berlin/Heidelberg, Germany, 1988; pp. 4–39. [Google Scholar] [CrossRef]
- Hassan, M.; Sium, F.; Islam, F.; Choudhury, S. A Review on Plasmonic Nano-Biosensors for Virus Detection with a Focus on Coronavirus. arXiv: Quantitative Methods. 2020. Available online: https://www.researchgate.net/publication/346555633_A_Review_on_Plasmonic_Nano-biosensors_for_Virus_Detection_with_a_Focus_on_Coronavirus (accessed on 20 February 2022).
- Leuermann, J.; Fernández-Gavela, A.; Torres-Cubillo, A.; Postigo, S.; Sánchez-Postigo, A.; Lechuga, L.M.; Halir, R.; Molina-Fernández, Í. Optimizing the Limit of Detection of Waveguide-Based Interferometric Biosensor Devices. Sensors 2019, 19, 3671. [Google Scholar] [CrossRef] [PubMed]
- Yanik, A.A.; Huang, M.; Kamohara, O.; Artar, A.; Geisbert, T.W.; Connor, J.H.; Altug, H. An optofluidic nanoplasmonic biosensor for direct detection of live viruses from biological media. Nano Lett. 2010, 10, 4962–4969. [Google Scholar] [CrossRef]
- Coskun, A.F.; Cetin, A.E.; Galarreta, B.C.; Alvarez, D.A.; Altug, H.; Ozcan, A. Lensfree optofluidic plasmonic sensor for real-time and label-free monitoring of molecular binding events over a wide field-of-view. Sci. Rep. 2014, 4, 6789. [Google Scholar] [CrossRef]
- Geng, Z.; Kan, Q.; Yuan, J.; Chen, H. A route to low-cost nanoplasmonic biosensor integrated with optofluidic-portable platform. Sens. Actuators B Chem. 2014, 195, 682–691. [Google Scholar] [CrossRef]
- Funari, R.; Chu, K.-Y.; Shen, A.Q. Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip. Biosens. Bioelectron. 2020, 169, 112578. [Google Scholar] [CrossRef]
- Jans, H.; Huo, Q. Gold nanoparticle-enabled biological and chemical detection and analysis. Chem. Soc. Rev. 2012, 41, 2849–2866. [Google Scholar] [CrossRef]
- Takemura, K. Surface Plasmon Resonance (SPR)- and Localized SPR (LSPR)-Based Virus Sensing Systems: Optical Vibration of Nano- and Micro-Metallic Materials for the Development of Next-Generation Virus Detection Technology. Biosensors 2021, 11, 250. [Google Scholar] [CrossRef]
- Inci, F.; Tokel, O.; Wang, S.; Gurkan, U.; Tasoglu, S.; Kuritzkes, D.; Demirci, U. Nanoplasmonic Quantitative Detection of Intact Viruses from Unprocessed Whole Blood. ACS Nano 2013, 7, 4733–4745. [Google Scholar] [CrossRef]
- Yu, H.; Kim, K.; Ma, K.; Lee, W.; Choi, J.-W.; Yun, C.-O.; Kim, D. Enhanced detection of virus particles by nanoisland-based localized surface plasmon resonance. Biosens. Bioelectron. 2013, 41, 249–255. [Google Scholar] [CrossRef]
- Jahanshahi, P.; Zalnezhad, E.; Sekaran, S.D.; Adikan, F.R.M. Rapid Immunoglobulin M-Based Dengue Diagnostic Test Using Surface Plasmon Resonance Biosensor. Sci. Rep. 2014, 4, 3851. [Google Scholar] [CrossRef]
- Valdez, J.; Bawage, S.; Gomez, I.; Singh, S.R. Facile and rapid detection of respiratory syncytial virus using metallic nanoparticles. J. Nanobiotechnol. 2016, 14, 13. [Google Scholar] [CrossRef]
- Lee, J.; Takemura, K.; Kato, C.N.; Suzuki, T.; Park, E.Y. Binary Nanoparticle Graphene Hybrid Structure-Based Highly Sensitive Biosensing Platform for Norovirus-Like Particle Detection. ACS Appl. Mater. Interfaces 2017, 9, 27298–27304. [Google Scholar] [CrossRef]
- Kim, J.; Oh, S.Y.; Shukla, S.; Hong, S.B.; Heo, N.S.; Bajpai, V.K.; Chun, H.S.; Jo, C.-H.; Choi, B.G.; Huh, Y.S.; et al. Heteroassembled gold nanoparticles with sandwich-immunoassay LSPR chip format for rapid and sensitive detection of hepatitis B virus surface antigen (HBsAg). Biosens. Bioelectron. 2018, 107, 118–122. [Google Scholar] [CrossRef]
- Heo, N.S.; Oh, S.Y.; Ryu, M.Y.; Baek, S.H.; Park, T.J.; Choi, C.; Huh, Y.S.; Park, J.P. Affinity Peptide-guided Plasmonic Biosensor for Detection of Noroviral Protein and Human Norovirus. Biotechnol. Bioprocess Eng. 2019, 24, 318–325. [Google Scholar] [CrossRef]
- Lee, T.; Kim, G.H.; Kim, S.M.; Hong, K.; Kim, Y.; Park, C.; Sohn, H.; Min, J. Label-free localized surface plasmon resonance biosensor composed of multi-functional DNA 3 way junction on hollow Au spike-like nanoparticles (HAuSN) for avian influenza virus detection. Colloids Surf. B Biointerfaces 2019, 182, 110341. [Google Scholar] [CrossRef]
- Austin Suthanthiraraj, P.P.; Sen, A.K. Localized surface plasmon resonance (LSPR) biosensor based on thermally annealed silver nanostructures with on-chip blood-plasma separation for the detection of dengue non-structural protein NS1 antigen. Biosens. Bioelectron. 2019, 132, 38–46. [Google Scholar] [CrossRef]
- Rippa, M.; Castagna, R.; Brandi, S.; Fusco, G.; Monini, M.; Chen, D.; Zhou, J.; Zyss, J.; Petti, L. Octupolar Plasmonic Nanosensor Based on Ordered Arrays of Triangular Au Nanopillars for Selective Rotavirus Detection. ACS Appl. Nano Mater. 2020, 3, 4837–4844. [Google Scholar] [CrossRef]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef]
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. ACS Nano 2020, 14, 7617–7627. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, T.; Nogueira Campos, M.G.; Smith, S.; Doomra, M.; Thwin, Z.; Santra, S. Quantum Dots. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–265. [Google Scholar] [CrossRef]
- Li, X.; Lu, D.; Sheng, Z.; Chen, K.; Guo, X.; Jin, M.; Han, H. A fast and sensitive immunoassay of avian influenza virus based on label-free quantum dot probe and lateral flow test strip. Talanta 2012, 100, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Takemura, K.; Adegoke, O.; Takahashi, N.; Kato, T.; Li, T.-C.; Kitamoto, N.; Tanaka, T.; Suzuki, T.; Park, E.Y. Versatility of a localized surface plasmon resonance-based gold nanoparticle-alloyed quantum dot nanobiosensor for immunofluorescence detection of viruses. Biosens. Bioelectron. 2017, 89, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Hennechart-Collette, C.; Dehan, O.; Laurentie, M.; Fraisse, A.; Martin-Latil, S.; Perelle, S. Detection of norovirus, hepatitis A and hepatitis E viruses in multicomponent foodstuffs. Int. J. Food Microbiol. 2021, 337, 108931. [Google Scholar] [CrossRef]
- Huang, L.; Ding, L.; Zhou, J.; Chen, S.; Chen, F.; Zhao, C.; Xu, J.; Hu, W.; Ji, J.; Xu, H.; et al. One-step rapid quantification of SARS-CoV-2 virus particles via low-cost nanoplasmonic sensors in generic microplate reader and point-of-care device. Biosens. Bioelectron. 2021, 171, 112685. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Ahmed Khan, F.S.; Mazari, S.A.; Mubarak, N.M.; Abro, R.; Ahmed, J.; Ahmed, M.; Baloch, H.; Sabzoi, N. Current applications of smart nanotextiles and future trends. In Nanosensors and Nanodevices for Smart Multifunctional Textiles; Ehrmann, A., Nguyen, T.A., Nguyen Tri, P., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 343–365. [Google Scholar] [CrossRef]
- Vo-Dinh, T.; Dhawan, A.; Norton, S.J.; Khoury, C.G.; Wang, H.-N.; Misra, V.; Gerhold, M.D. Plasmonic Nanoparticles and Nanowires: Design, Fabrication and Application in Sensing. J. Phys. Chem. C 2010, 114, 7480–7488. [Google Scholar] [CrossRef]
- Abel, B.; Coskun, S.; Mohammed, M.; Williams, R.; Unalan, H.E.; Aslan, K. Metal-Enhanced Fluorescence from Silver Nanowires with High Aspect Ratio on Glass Slides for Biosensing Applications. J. Phys. Chem. C 2015, 119, 675–684. [Google Scholar] [CrossRef]
- Zhang, A.; Zheng, G. Semiconductor nanowires for biosensors. In Semiconductor Nanowires; Arbiol, J., Xiong, Q., Eds.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Philadelphia, PA, USA, 2015; pp. 471–490. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Zhang, L.; Huang, M.J.; Luo, Z.H.H.; Tay, G.K.I.; Lim, E.-J.A.; Kang, T.G.; Chen, Y. Silicon nanowire biosensor for highly sensitive and rapid detection of Dengue virus. Sens. Actuators B Chem. 2010, 146, 138–144. [Google Scholar] [CrossRef]
- Kao, L.T.-H.; Shankar, L.; Kang, T.G.; Zhang, G.; Tay, G.K.I.; Rafei, S.R.M.; Lee, C.W.H. Multiplexed detection and differentiation of the DNA strains for influenza A (H1N1 2009) using a silicon-based microfluidic system. Biosens. Bioelectron. 2011, 26, 2006–2011. [Google Scholar] [CrossRef]
- Shen, F.; Wang, J.; Xu, Z.; Wu, Y.; Chen, Q.; Li, X.; Jie, X.; Li, L.; Yao, M.; Guo, X.; et al. Rapid Flu Diagnosis Using Silicon Nanowire Sensor. Nano Lett. 2012, 12, 3722–3730. [Google Scholar] [CrossRef]
- Ibarlucea, B.; Fawzul Akbar, T.; Kim, K.; Rim, T.; Baek, C.-K.; Ascoli, A.; Tetzlaff, R.; Baraban, L.; Cuniberti, G. Ultrasensitive detection of Ebola matrix protein in a memristor mode. Nano Res. 2018, 11, 1057–1068. [Google Scholar] [CrossRef]
- Daoudi, K.; Ramachandran, K.; Alawadhi, H.; Boukherroub, R.; Dogheche, E.; Khakani, M.A.E.; Gaidi, M. Ultra-sensitive and fast optical detection of the spike protein of the SARS-CoV-2 using AgNPs/SiNWs nanohybrid based sensors. Surf. Interfaces 2021, 27, 101454. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Marco, M.-P.; de Alda, M.J.L.; Barceló, D. Biosensors for environmental applications: Future development trends. Pure Appl. Chem. 2004, 76, 723–752. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; de Alda, M.J.L.; Marco, M.-P.; Barceló, D. Biosensors for environmental monitoring A global perspective. Talanta 2005, 65, 291–297. [Google Scholar] [CrossRef]
- Rogers, K.R. Recent advances in biosensor techniques for environmental monitoring. Anal. Chim. Acta 2006, 568, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.R.; Gerlach, C.L. Peer Reviewed: Environmental Biosensors: A Status Report. Environ. Sci. Technol. 1996, 30, 486A–491A. [Google Scholar] [CrossRef]
- Ju, H.; Kandimalla, V.B. Biosensors for pesticides. In Electrochemical Sensors, Biosensors and Their Biomedical Applications; Zhang, X., Ju, H., Wang, J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 31–56. [Google Scholar] [CrossRef]
- Suryan, S.K. Biosensors: A Tool for Environmental Monitoring and Analysis. In Advances in Environmental Biotechnology; Kumar, R., Sharma, A.K., Ahluwalia, S.S., Eds.; Springer: Singapore, 2017; pp. 265–288. [Google Scholar] [CrossRef]
- Gieva, E.; Nikolov, G.; Nikolova, B. Biosensors for Environmental Monitoring. In Proceedings of the Conference on Challenges in Higher Education & Research, Proceedings of the 12th Conference, Sozopol, Bulgaria, 17–19 November 2014; Volume 12. [Google Scholar]
- Gamal, R.; Shafaay, S.; Ismail, Y.; Swillam, M.A. Silicon plasmonics at midinfrared using silicon-insulator-silicon platform. JNP 2017, 11, 016006. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Cai, L.; Gao, Y.; Cai, Y. Optical fiber sensors for measurement of heavy metal ion concentration: A review. Measurement 2020, 158, 107742. [Google Scholar] [CrossRef]
- Chugh, S.; Ghosh, S.; Gulistan, A.; Rahman, B.M.A. Machine Learning Regression Approach to the Nanophotonic Waveguide Analyses. J. Lightwave Technol. JLT 2019, 37, 6080–6089. [Google Scholar] [CrossRef]
- Ghosh, S.; Dissanayake, K.; Sun, T.; Grattan, K.T.V.; Rahman, B.M.A. Calibration of Fiber Grating Heavy Metal Ion Sensor Using Artificial Neural Network. In Proceedings of the Conference on Lasers and Electro-Optics (2021), San Jose, CA, USA, 9–14 May 2021; Optical Society of America: Washington, DC, USA, 2021; p. JW1A.25. [Google Scholar] [CrossRef]
- Del Villar, I.; Matías, I.; Arregui, F.; Lalanne, P. Optimization of sensitivity in Long Period Fiber Gratings with overlay deposition. Opt. Express 2005, 13, 56–69. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, G.; Li, T.; Lou, X.; Zhu, L. A dual-channel optical fiber sensor based on surface plasmon resonance for heavy metal ions detection in contaminated water. Opt. Commun. 2020, 462, 124750. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, L.; Liu, L. Ultrahigh Sensitivity Mach−Zehnder Interferometer Sensor Based on a Weak One-Dimensional Field Confinement Silica Waveguide. Sensors 2021, 21, 6600. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Liu, L.; Huang, Q.; Lu, L.; Yang, H.; Liu, F.; Liu, P.; Ji, W.; Jiang, S.; Sun, J.; et al. Integrated Ultrahigh-Sensitivity Temperature Sensor Based on Asymmetric Mach–Zehnder Interferometer and Stress Deformation of Aluminum-SiO2. IEEE Trans. Instrum. Meas. 2021, 70, 1–7. [Google Scholar] [CrossRef]
- Gao, Y.; Gan, Q.; Xin, Z.; Cheng, X.; Bartoli, F. Plasmonic Mach-Zehnder Interferometer for Ultrasensitive On-Chip Biosensing. ACS Nano 2011, 5, 9836–9844. [Google Scholar] [CrossRef]
- Zeng, X.; Gao, Y.; Hu, H.-F.; Ji, D.; Gan, Q.; Bartoli, F. A metal-insulator-metal plasmonic Mach-Zehnder interferometer array for multiplexed sensing. J. Appl. Phys. 2013, 113, 133102. [Google Scholar] [CrossRef]
- Ayoub, A.B.; Ji, D.; Gan, Q.; Swillam, M.A. Silicon plasmonic integrated interferometer sensor for lab on chip applications. Opt. Commun. 2018, 427, 319–325. [Google Scholar] [CrossRef]
- Zeng, X.; Gao, Y.; Ji, D.; Zhang, N.; Song, H.; Gan, Q.; Bartoli, F. On-Chip Plasmonic Interferometer Array for Portable Multiplexed Biosensing System. In Proceedings of the Conference on Lasers and Electro-Optics (CLEO)-Laser Science to Photonic Applications, San Jose, CA, USA, 8–13 June 2014; Volume 2014, pp. 1–2. [Google Scholar] [CrossRef]
- El Shamy, R.S.; Khalil, D.; Swillam, M.A. Mid Infrared Optical Gas Sensor Using Plasmonic Mach-Zehnder Interferometer. Sci. Rep. 2020, 10, 1293. [Google Scholar] [CrossRef]
- Calaf, G.M.; Ponce-Cusi, R.; Aguayo, F.; Muñoz, J.P.; Bleak, T.C. Endocrine disruptors from the environment affecting breast cancer (Review). Oncol. Lett. 2020, 20, 19–32. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Zhu, Q.; Li, K.; Lu, Y.; Zhou, X.; Guo, T. Ultrasensitive detection of endocrine disruptors via superfine plasmonic spectral combs. Light Sci. Appl. 2021, 10, 181. [Google Scholar] [CrossRef]
- Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 30 December 2021).
- Manoharan, H.; Kalita, P.; Gupta, S.; Sai, V.V.R. Plasmonic biosensors for bacterial endotoxin detection on biomimetic C-18 supported fiber optic probes. Biosens. Bioelectron. 2019, 129, 79–86. [Google Scholar] [CrossRef]
- An, N.; Li, K.; Zhang, Y.; Wen, T.; Liu, W.; Liu, G.; Li, L.; Jin, W. A multiplex and regenerable surface plasmon resonance (MR-SPR) biosensor for DNA detection of genetically modified organisms. Talanta 2021, 231, 122361. [Google Scholar] [CrossRef] [PubMed]
- Hye Koh, E.; Moon, J.-Y.; Kim, S.-Y.; Lee, W.-C.; Park, S.-G.; Kim, D.-H.; Sang Jung, H. A cyclodextrin-decorated plasmonic gold nanosatellite substrate for selective detection of bipyridylium pesticides. Analyst 2021, 146, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.; Nikolic, M.V.; Vidic, J. Rapid point-of-need detection of bacteria and their toxins in food using gold nanoparticles. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5880–5900. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Paliwal, A.; Bassi, M.; Tomar, M.; Gupta, V.; Gulati, S. Investigation of Adulteration in Milk using Surface Plasmon Resonance. ECS J. Solid State Sci. Technol. 2021, 10, 091004. [Google Scholar] [CrossRef]
- Ravindran, N.; Kumar, S.; Yashini, M.; Rajeshwari, S.; Mamathi, C.A.; Nirmal Thirunavookarasu, S.N.; Sunil, C.K. Recent advances in Surface Plasmon Resonance (SPR) biosensors for food analysis: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–23. [Google Scholar] [CrossRef]
- Narsaiah, K.; Jha, S.N.; Bhardwaj, R.; Sharma, R.; Kumar, R. Optical biosensors for food quality and safety assurance—A review. J. Food Sci. Technol. 2012, 49, 383. [Google Scholar] [CrossRef]
- Wing Fen, Y.; Mahmood Mat Yunus, W. Surface plasmon resonance spectroscopy as an alternative for sensing heavy metal ions: A review. Sens. Rev. 2013, 33, 305–314. [Google Scholar] [CrossRef]
- Verma, R.; Gupta, B.D. Detection of heavy metal ions in contaminated water by surface plasmon resonance based optical fibre sensor using conducting polymer and chitosan. Food Chem. 2015, 166, 568–575. [Google Scholar] [CrossRef]
- Ashley, J.; Shahbazi, M.-A.; Kant, K.; Chidambara, V.A.; Wolff, A.; Bang, D.D.; Sun, Y. Molecularly imprinted polymers for sample preparation and biosensing in food analysis: Progress and perspectives. Biosens. Bioelectron. 2017, 91, 606–615. [Google Scholar] [CrossRef]
- Atar, N.; Eren, T.; Yola, M.L. A molecular imprinted SPR biosensor for sensitive determination of citrinin in red yeast rice. Food Chem. 2015, 184, 7–11. [Google Scholar] [CrossRef]
- Zhou, J.; Qi, Q.; Wang, C.; Qian, Y.; Liu, G.; Wang, Y.; Fu, L. Surface plasmon resonance (SPR) biosensors for food allergen detection in food matrices. Biosens. Bioelectron. 2019, 142, 111449. [Google Scholar] [CrossRef] [PubMed]
- Çakır, O.; Baysal, Z. Pesticide analysis with molecularly imprinted nanofilms using surface plasmon resonance sensor and LC-MS/MS: Comparative study for environmental water samples. Sens. Actuators B Chem. 2019, 297, 126764. [Google Scholar] [CrossRef]
- D’Aurelio, R.; Ashley, J.; Rodgers, T.L.; Trinh, L.; Temblay, J.; Pleasants, M.; Tothill, I.E. Development of a NanoMIPs-SPR-Based Sensor for β-Lactoglobulin Detection. Chemosensors 2020, 8, 94. [Google Scholar] [CrossRef]
- Ashley, J.; D’Aurelio, R.; Piekarska, M.; Temblay, J.; Pleasants, M.; Trinh, L.; Rodgers, T.L.; Tothill, I.E. Development of a β-Lactoglobulin Sensor Based on SPR for Milk Allergens Detection. Biosensors 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Yakes, B.J.; Deeds, J.; White, K.; Degrasse, S.L. Evaluation of surface plasmon resonance biosensors for detection of tetrodotoxin in food matrices and comparison to analytical methods. J. Agric. Food Chem. 2011, 59, 839–846. [Google Scholar] [CrossRef]
- Hodnik, V.; Anderluh, G. Toxin detection by surface plasmon resonance. Sensors 2009, 9, 1339–1354. [Google Scholar] [CrossRef]
- Chalyan, T.; Potrich, C.; Schreuder, E.; Falke, F.; Pasquardini, L.; Pederzolli, C.; Heideman, R.; Pavesi, L. AFM1 Detection in Milk by Fab’ Functionalized Si3N4 Asymmetric Mach-Zehnder Interferometric Biosensors. Toxins 2019, 11, 409. [Google Scholar] [CrossRef]
- Smith, D.R.; Padilla, W.J.; Vier, D.C.; Nemat-Nasser, S.C.; Schultz, S. Composite Medium with Simultaneously Negative Permeability and Permittivity. Phys. Rev. Lett. 2000, 84, 4184–4187. [Google Scholar] [CrossRef]
- Beruete, M.; Jáuregui-López, I. Terahertz Sensing Based on Metasurfaces. Adv. Opt. Mater. 2020, 8, 1900721. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Sun, H. Metamaterials application in sensing. Sensors 2012, 12, 2742–2765. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, C.; Xu, W.; Xie, L. Biological applications of terahertz technology based on nanomaterials and nanostructures. Nanoscale 2019, 11, 3445–3457. [Google Scholar] [CrossRef] [PubMed]
- Ahmadivand, A.; Gerislioglu, B.; Tomitaka, A.; Manickam, P.; Kaushik, A.; Bhansali, S.; Nair, M.; Pala, N. Extreme sensitive metasensor for targeted biomarkers identification using colloidal nanoparticles-integrated plasmonic unit cells. Biomed. Opt. Express 2018, 9, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-K.; Kang, J.-H.; Kwon, J.; Lee, J.-S.; Lee, S.; Woo, D.H.; Kim, J.H.; Song, C.-S.; Park, Q.-H.; Seo, M. Nano metamaterials for ultrasensitive Terahertz biosensing. Sci. Rep. 2017, 7, 8146. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Ozen, M.O.; Karaaslan, M.G.; Prator, C.A.; Thanh, C.; Kumar, S.; Torres, L.; Iyer, N.; Munter, S.; Southern, S.; et al. Tunable Fano-Resonant Metasurfaces on a Disposable Plastic-Template for Multimodal and Multiplex Biosensing. Adv. Mater. 2020, 32, e1907160. [Google Scholar] [CrossRef]
- Park, S.J.; Cha, S.H.; Shin, G.A.; Ahn, Y.H. Sensing viruses using terahertz nano-gap metamaterials. Biomed. Opt. Express 2017, 8, 3551–3558. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; El Kabbash, M.; Alapan, Y.; Ilker, E.I.; Hinczewski, M.; Gurkan, U.A.; Strangi, G. Hyperbolic metamaterials-based plasmonic biosensor for fluid biopsy with single molecule sensitivity. EPJ Appl. Metamater. 2017, 4, 1. [Google Scholar] [CrossRef][Green Version]
- Aristov, A.I.; Manousidaki, M.; Danilov, A.; Terzaki, K.; Fotakis, C.; Farsari, M.; Kabashin, A.V. 3D plasmonic crystal metamaterials for ultra-sensitive biosensing. Sci. Rep. 2016, 6, 25380. [Google Scholar] [CrossRef]
- Cheng, D.; He, X.; Huang, X.; Zhang, B.; Liu, G.; Shu, G.; Fang, C.; Wang, J.; Luo, Y. Terahertz biosensing metamaterial absorber for virus detection based on spoof surface plasmon polaritons. Int. J. RF Microw. Comput.-Aided Eng. 2018, 28, e21448. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B.; Manickam, P.; Kaushik, A.; Bhansali, S.; Nair, M.; Pala, N. Rapid Detection of Infectious Envelope Proteins by Magnetoplasmonic Toroidal Metasensors. ACS Sens. 2017, 2, 1359–1368. [Google Scholar] [CrossRef]
- Alam, M.M.; Malik, H.; Khan, M.I.; Pardy, T.; Kuusik, A.; Le Moullec, Y. A Survey on the Roles of Communication Technologies in IoT-Based Personalized Healthcare Applications. IEEE Access 2018, 6, 36611–36631. [Google Scholar] [CrossRef]
- Solanki, S.; Pandey, C.M.; Gupta, R.K.; Malhotra, B.D. Emerging Trends in Microfluidics Based Devices. Biotechnol. J. 2020, 15, 1900279. [Google Scholar] [CrossRef] [PubMed]
- Swillam, M.A.; Helmy, A.S. Feedback Effects in Plasmonic Slot Waveguides Examined Using a Closed Form Model. IEEE Photonics Technol. Lett. 2012, 24, 497–499. [Google Scholar] [CrossRef]
- Kotb, R.; Ismail, Y.; Swillam, M. Integrated metal-insulator-metal plasmonic nano resonator: An analytical approach. Prog. Electromagn. Res. Lett. 2013, 43, 83–94. [Google Scholar] [CrossRef]
- El-Zohary, S.E.; Azzazi, A.A.; Okamoto, H.; Okamoto, T.; Haraguchi, M.; Swillam, M.A. Resonance-based integrated plasmonic nanosensor for lab-on-chip applications. JNP 2013, 7, 073077. [Google Scholar] [CrossRef][Green Version]
- Swillam, M.; Helmy, A. Analysis and applications of 3D rectangular metallic waveguides. Opt. Express 2010, 18, 19831–19843. [Google Scholar] [CrossRef]
- Sherif, S.M.; Zografopoulos, D.C.; Shahada, L.A.; Beccherelli, R.; Swillam, M. Integrated plasmonic refractometric sensor using Fano resonance. J. Phys. D Appl. Phys. 2017, 50, 055104. [Google Scholar] [CrossRef]
- Gamal, R.; Ismail, Y.; Swillam, M.A. Optical biosensor based on a silicon nanowire ridge waveguide for lab on chip applications. J. Opt. 2015, 17, 045802. [Google Scholar] [CrossRef]
- Elsayed, M.Y.; Sherif, S.M.; Aljaber, A.S.; Swillam, M.A. Integrated Lab-on-a-Chip Optical Biosensor Using Ultrathin Silicon Waveguide SOI MMI Device. Sensors 2020, 20, 4955. [Google Scholar] [CrossRef]
- Luan, E.; Shoman, H.; Ratner, D.M.; Cheung, K.C.; Chrostowski, L. Silicon Photonic Biosensors Using Label-Free Detection. Sensors 2018, 18, 3519. [Google Scholar] [CrossRef]
- Zaki, A.O.; Kirah, K.; Swillam, M.A. Integrated optical sensor using hybrid plasmonics for lab on chip applications. J. Opt. 2016, 18, 085803. [Google Scholar] [CrossRef]
- Khalifa, A.E.; Swillam, M.A. Plasmonic silicon solar cells using titanium nitride: A comparative study. JNP 2014, 8, 084098. [Google Scholar] [CrossRef]
- Naik, G.V.; Shalaev, V.M.; Boltasseva, A. Alternative Plasmonic Materials: Beyond Gold and Silver. Adv. Mater. 2013, 25, 3264–3294. [Google Scholar] [CrossRef]
- Naik, G.V.; Kim, J.; Boltasseva, A. Oxides and nitrides as alternative plasmonic materials in the optical range [Invited]. Opt. Mater. Express OME 2011, 1, 1090–1099. [Google Scholar] [CrossRef]
- Lau, B.; Swillam, M.A.; Helmy, A.S. Hybrid orthogonal junctions: Wideband plasmonic slot-silicon waveguide couplers. Opt. Express OE 2010, 18, 27048–27059. [Google Scholar] [CrossRef]
- Gamal, R.; Ismail, Y.; Swillam, M.A. Silicon Waveguides at the Mid-Infrared. J. Lightwave Technol. JLT 2015, 33, 3207–3214. [Google Scholar] [CrossRef]
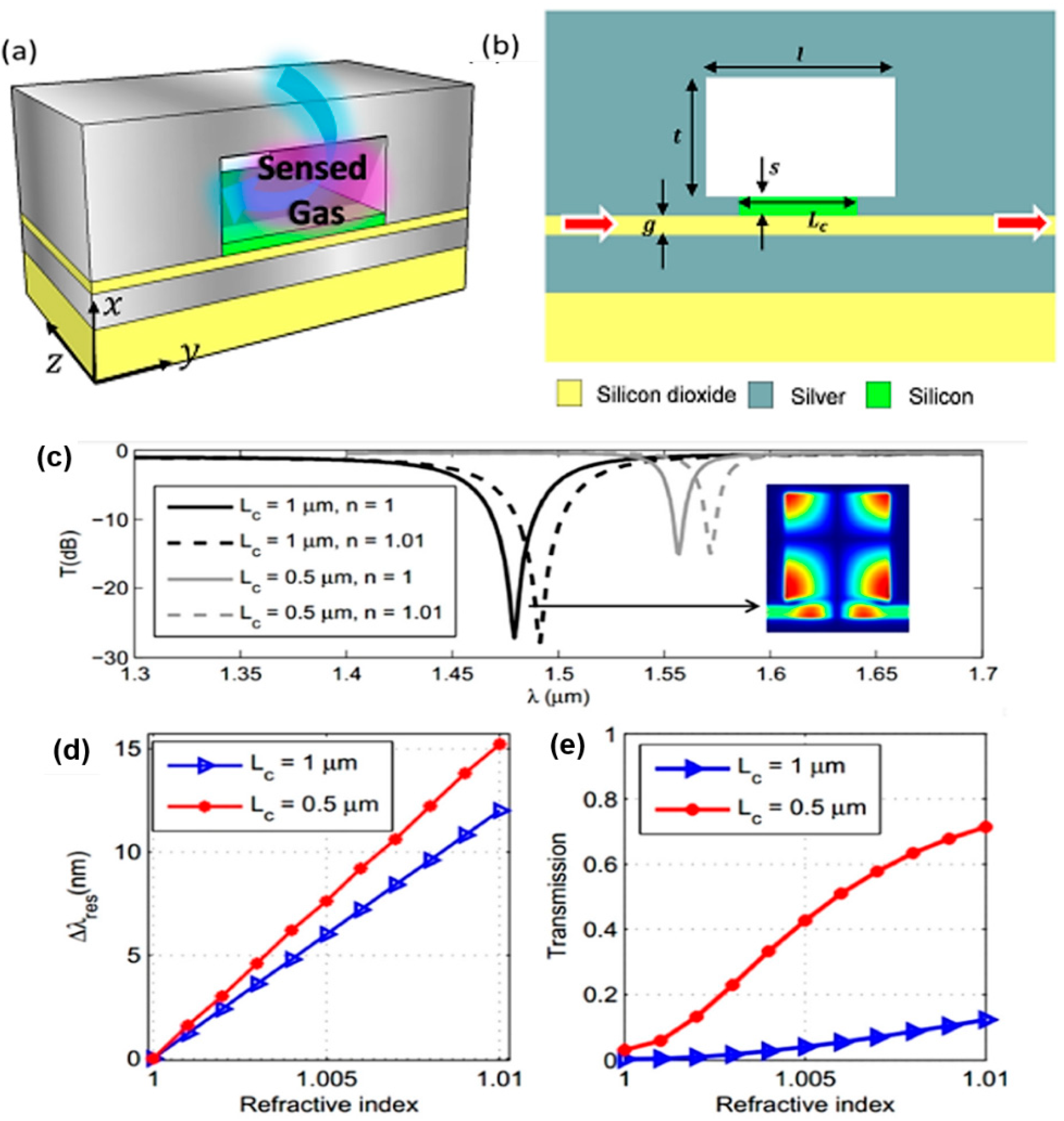
- Elsayed, M.Y.; Ismail, Y.; Swillam, M.A. Semiconductor plasmonic gas sensor using on-chip infrared spectroscopy. Appl. Phys. A 2017, 123, 113. [Google Scholar] [CrossRef]
- Sherif, S.M.; Swillam, M.A. Metal-less silicon plasmonic mid-infrared gas sensor. JNP 2016, 10, 026025. [Google Scholar] [CrossRef]
- Ayoub, A.B.; Swillam, M.A. Silicon Plasmonics On-Chip Mid-IR Gas Sensor. IEEE Photonics Technol. Lett. 2018, 30, 931–934. [Google Scholar] [CrossRef]
- Schwarz, B.; Reininger, P.; Ristanić, D.; Detz, H.; Andrews, A.M.; Schrenk, W.; Strasser, G. Monolithically integrated mid-infrared lab-on-a-chip using plasmonics and quantum cascade structures. Nat. Commun. 2014, 5, 4085. [Google Scholar] [CrossRef]
- Moon, G.; Choi, J.-R.; Lee, C.; Oh, Y.; Kim, K.H.; Kim, D. Machine learning-based design of meta-plasmonic biosensors with negative index metamaterials. Biosens. Bioelectron. 2020, 164, 112335. [Google Scholar] [CrossRef]
- Plasmonics Takes a Step Closer to Real-World Applications. Available online: https://spectrum.ieee.org/plasmonics-takes-a-step-closer-to-realworld-applications (accessed on 9 January 2022).
- Berini, P. Plasmon-polariton waves guided by thin lossy metal films of finite width: Bound modes of symmetric structures. Phys. Rev. B 2000, 61, 10484–10503. [Google Scholar] [CrossRef]
- Berini, P. Long-range surface plasmon polaritons. Adv. Opt. Photonics AOP 2009, 1, 484–588. [Google Scholar] [CrossRef]
- Li, X.; Shu, J.; Gu, W.; Gao, L. Deep neural network for plasmonic sensor modeling. Opt. Mater. Express OME 2019, 9, 3857–3862. [Google Scholar] [CrossRef]
- Bhardwaj, Y. Topological Insulator: A Next Generation; Central University of Rajasthan: Ajmer, Rajasthan, India, 2017. [Google Scholar] [CrossRef]
- Dowran, M.; Kumar, A.; Lawrie, B.J.; Pooser, R.C.; Marino, A.M. Quantum-enhanced plasmonic sensing. Opt. OPTICA 2018, 5, 628–633. [Google Scholar] [CrossRef]
- Lautner, G.; Balogh, Z.; Bardóczy, V.; Mészáros, T.; Gyurcsányi, R.E. Aptamer-based biochips for label-free detection of plant virus coat proteins by SPR imaging. Analyst 2010, 135, 918–926. [Google Scholar] [CrossRef]
- Ohannesian, N.; Misbah, I.; Lin, S.H.; Shih, W.-C. Plasmonic nano-aperture label-free imaging (PANORAMA). Nat. Commun. 2020, 11, 5805. [Google Scholar] [CrossRef]
- Sánchez-Purrà, M.; Carré-Camps, M.; de Puig, H.; Bosch, I.; Gehrke, L.; Hamad-Schifferli, K. Surface-Enhanced Raman Spectroscopy-Based Sandwich Immunoassays for Multiplexed Detection of Zika and Dengue Viral Biomarkers. ACS Infect. Dis. 2017, 3, 767–776. [Google Scholar] [CrossRef]
- Biosensor to Detect Coronavirus in Crowded Places. Available online: https://healthcare-in-europe.com/en/news/biosensor-to-detect-coronavirus-in-crowded-places.html (accessed on 5 November 2021).
- Li, D.; Zhou, H.; Hui, X.; He, X.; Mu, X. Plasmonic Biosensor Augmented by a Genetic Algorithm for Ultra-Rapid, Label-Free, and Multi-Functional Detection of COVID-19. Anal. Chem. 2021, 93, 9437–9444. [Google Scholar] [CrossRef]


| Current Methods for Virus Detection | Advantages | Limitations | Refs. |
|---|---|---|---|
| Immunofluorescence Assays | Numerous, simultaneous samples can be analyzed and stored for some time. | Fluorescent molecules bound to primary antibody is limited. Low sensitivity may result in false negatives. | [49] |
| Hemagglutination Assays | Low-cost instruments. Results within hours. Has standardization as it is recognized in labs worldwide. | Little specificity. Requires trained personnel. Analysis needed by qualified individuals. Difficult inter-laboratory comparison of results due to the several controlled variables. | [50] |
| Viral Plaque Assay | Available in most labs. Rapid results. | Absence of standardization. Involves costly repeat runs for accurate results. | [51] |
| Viral Flow Cytometry | Rapid results. Numerous cells analyzed instantly. | Requires highly trained personnel. Requires ongoing maintenance by service engineers. | [52] |
| ELISA | Accurate/fast results. Very sensitive process. Easily automated. | Expensive preparation method. Requires trained personnel. | [43] |
| CT | Combined assessment. Short acquisition time. | Expensive preparation method. Requires trained personnel. Exposure to gamma rays. | [44] |
| NAAT | Very sensitive process. Accurate and reliable | Requires trained personnel. Expensive detection kits. Time-consuming (2–3 days). False-positive cases. | [46,47,48] |
| Component | Other Methods | SPR | Refs. |
|---|---|---|---|
| Heavy metals | Atomic Absorption Spectroscopy
|
| [126,127] |
| Food Allergens | ELISA
|
| [126,127] |
| Citrinin (Mycotoxin) | HPLC and LC-MS
|
| [128,129] |
| Pesticides | LC-MS/MS
|
| [130,131] |
| β-Lactoglobulin | ELISA and LC-MS
|
| [132,133] |
| Tetrodotoxin (Fish toxin) | LC-MS/MS, ELISA, and HPLC
|
| [134,135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, M.E.; Othman, M.A.; Swillam, M.A. Plasmonic Biosensors: Review. Biology 2022, 11, 621. https://doi.org/10.3390/biology11050621
Hamza ME, Othman MA, Swillam MA. Plasmonic Biosensors: Review. Biology. 2022; 11(5):621. https://doi.org/10.3390/biology11050621
Chicago/Turabian StyleHamza, Mohga E., Muhammad A. Othman, and Mohamed A. Swillam. 2022. "Plasmonic Biosensors: Review" Biology 11, no. 5: 621. https://doi.org/10.3390/biology11050621
APA StyleHamza, M. E., Othman, M. A., & Swillam, M. A. (2022). Plasmonic Biosensors: Review. Biology, 11(5), 621. https://doi.org/10.3390/biology11050621







