Smoking-, Alcohol-, and Age-Related Alterations of Blood Monocyte Subsets and Circulating CD4/CD8 T Cells in Head and Neck Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Blood Collection and Patient Data
2.3. Staining of Monocyte Subsets in Whole Blood
2.4. Staining of T-Cell Subsets in Isolated PBMC
2.5. FACS Analysis
2.6. Statistical Analysis
3. Results
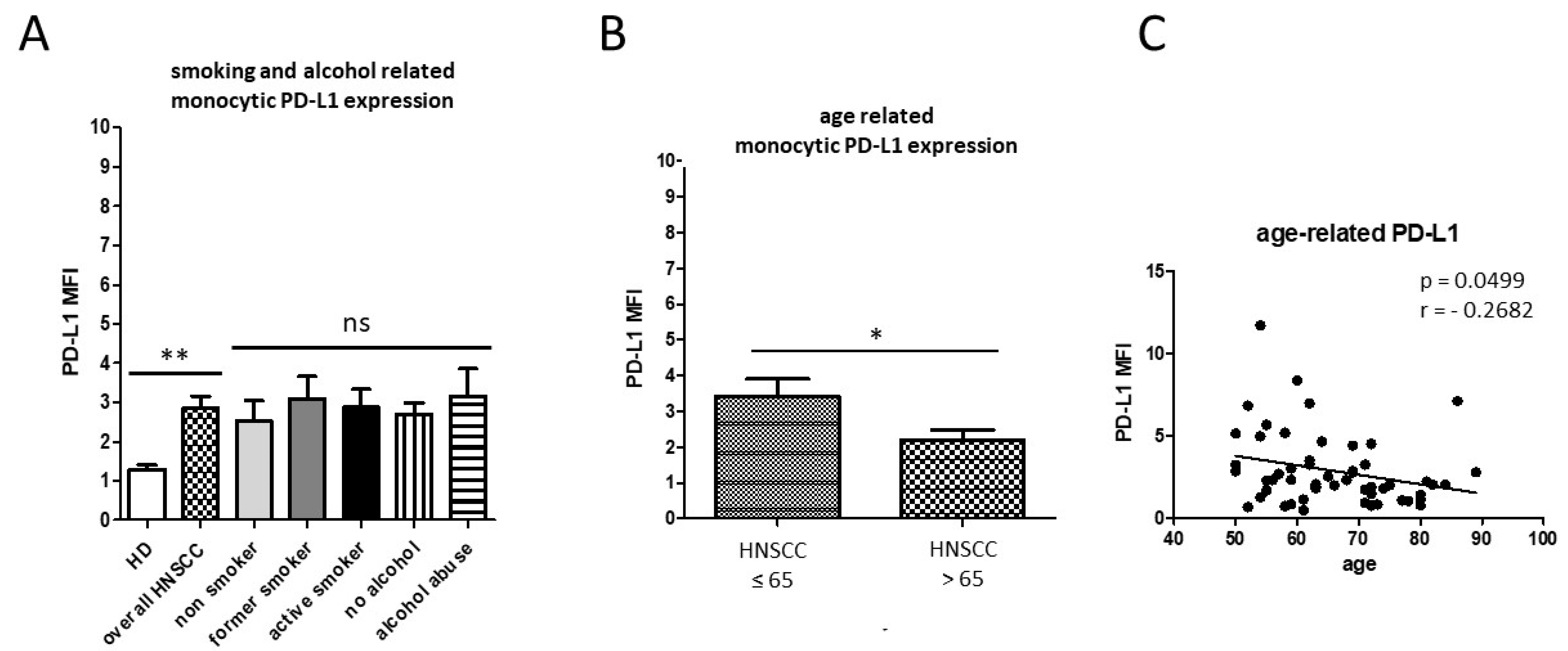
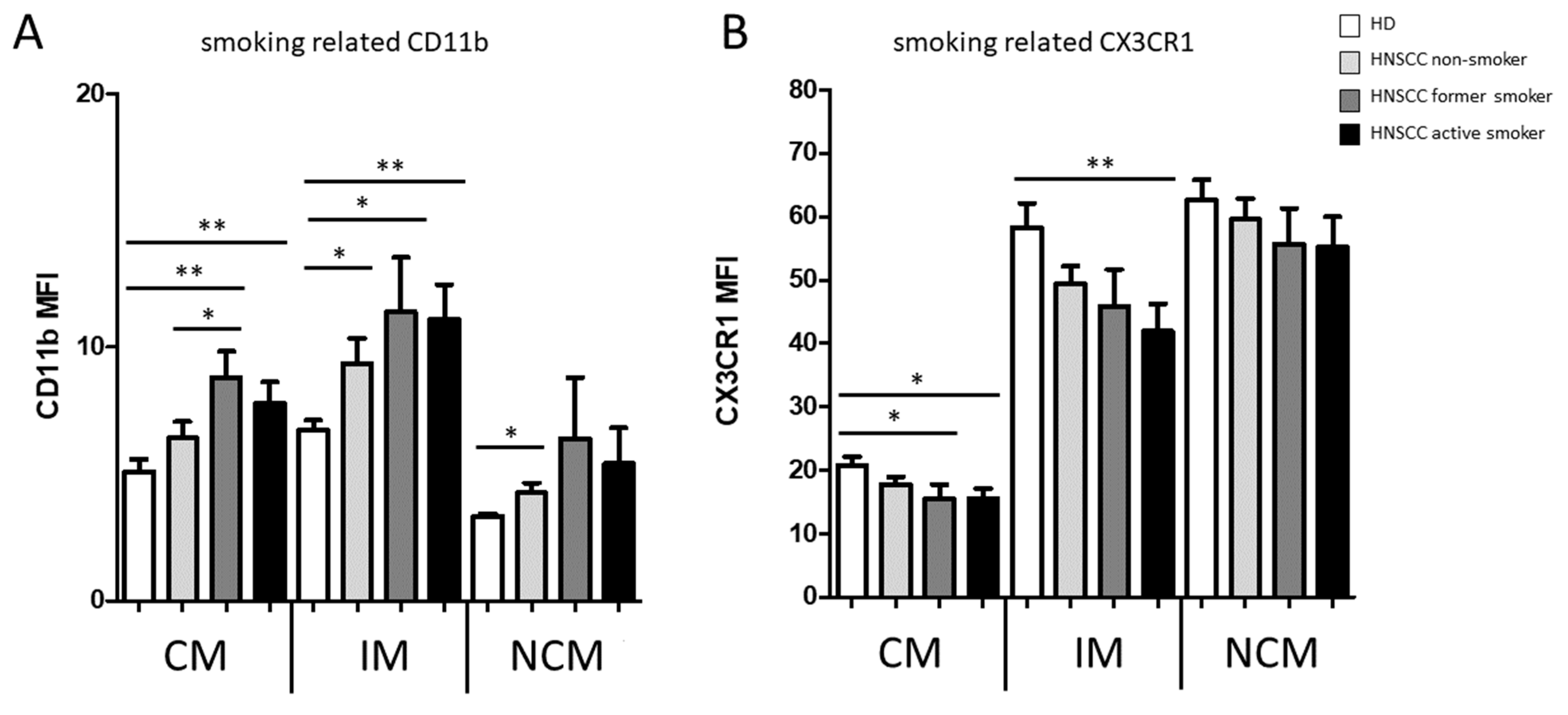
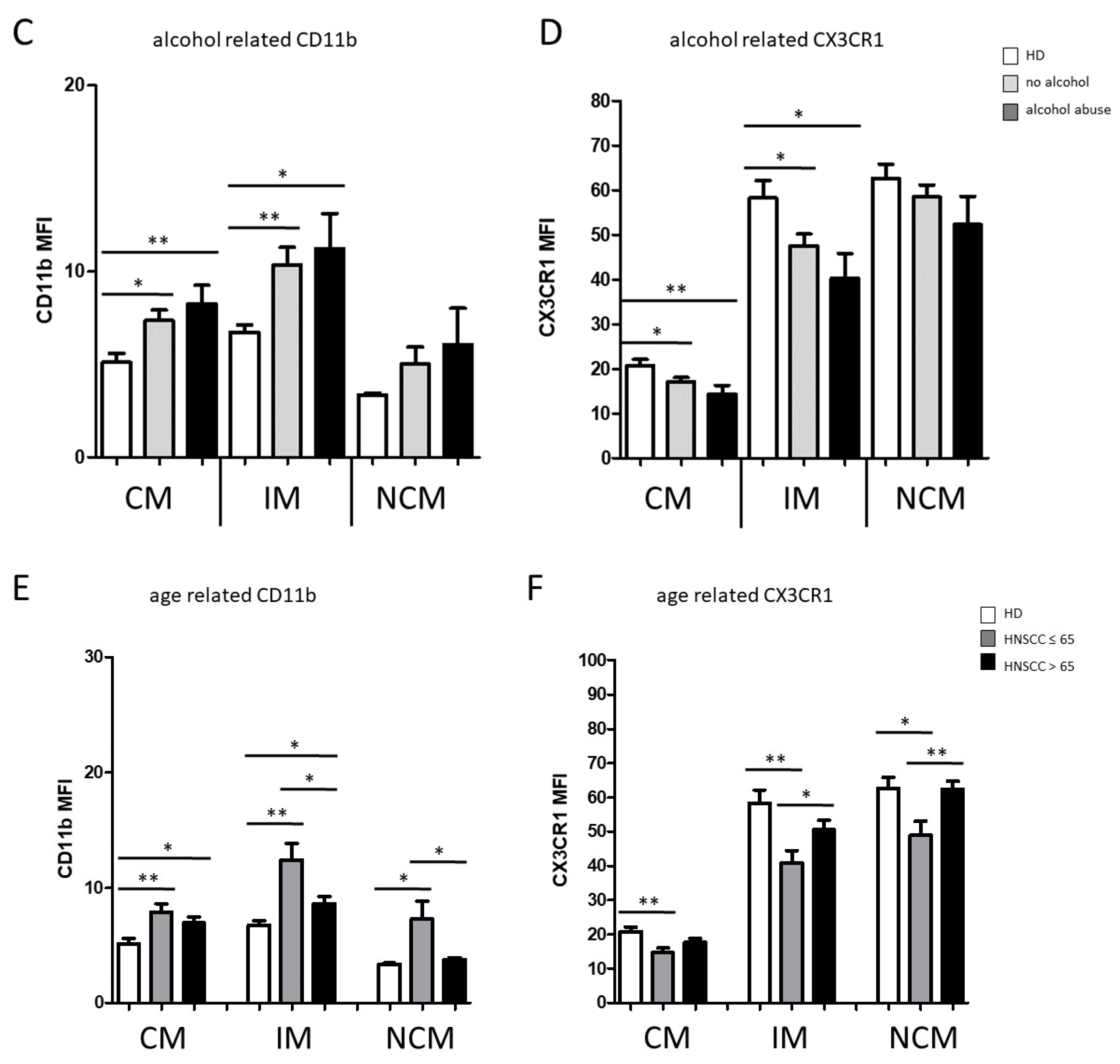
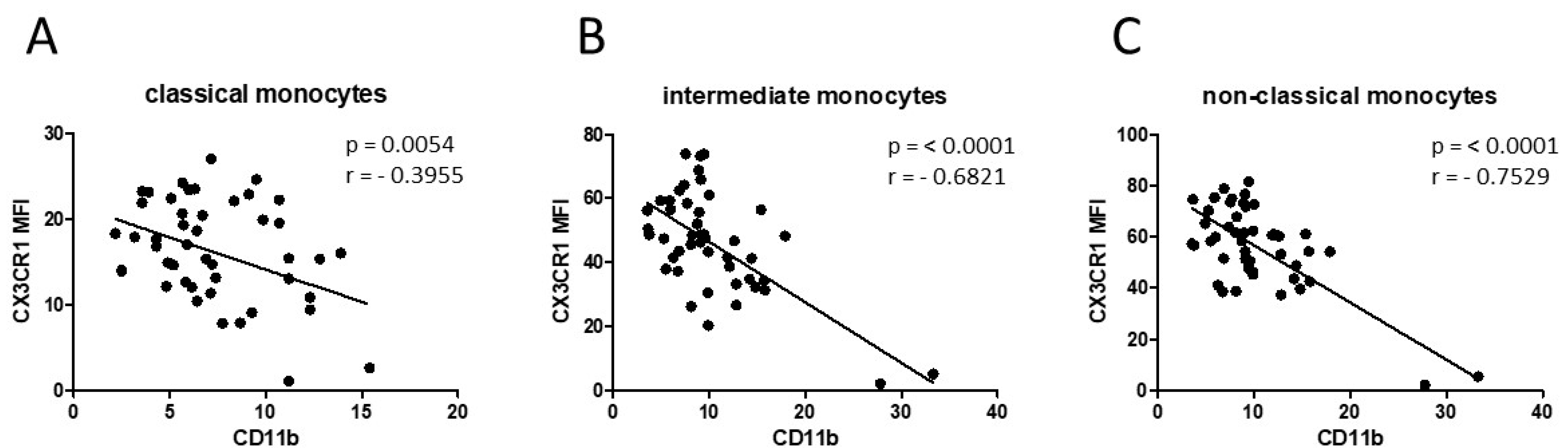
3.1. Smoking-, Alcohol-, and Age-Related Monocyte Subset Distribution
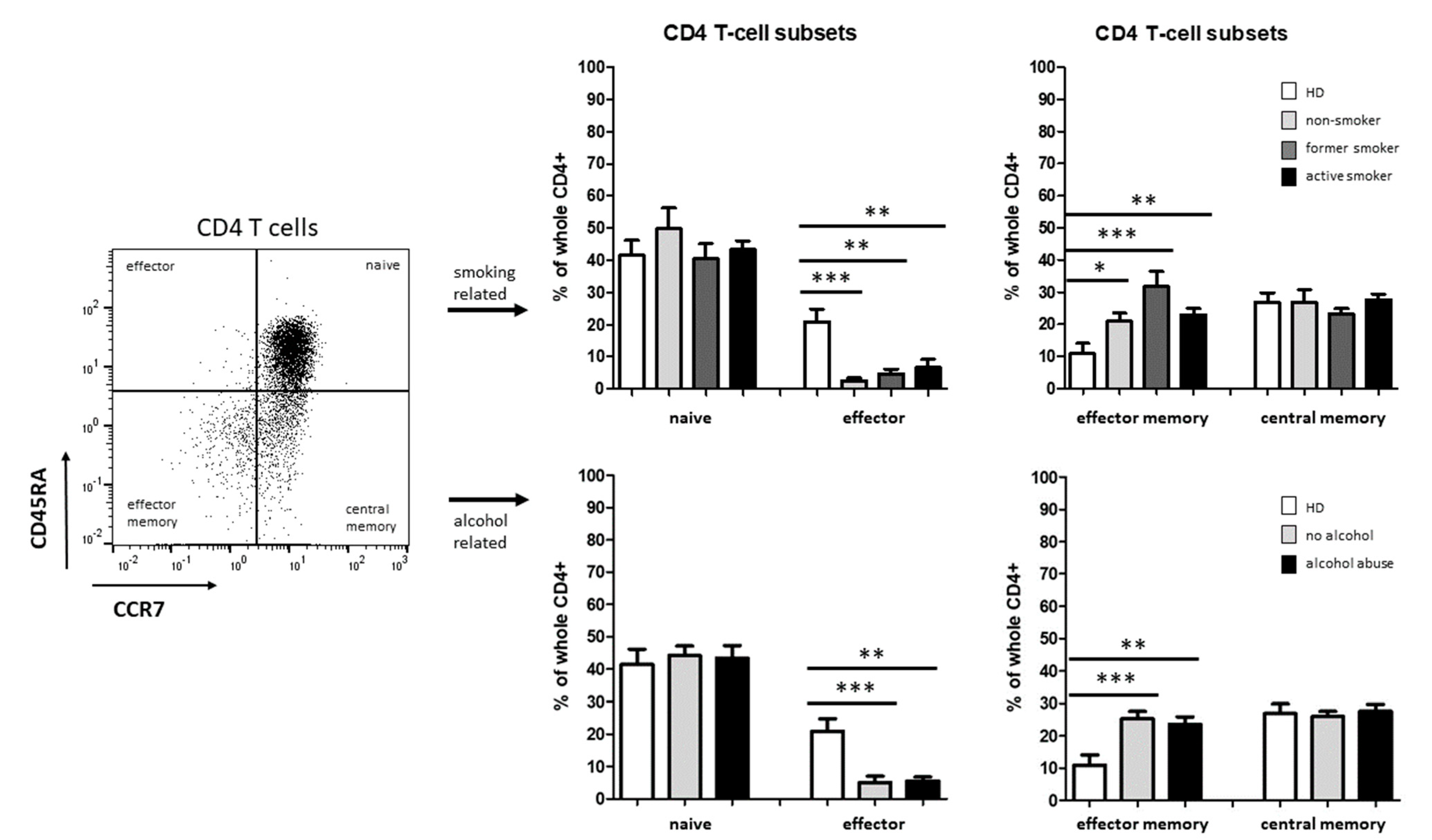
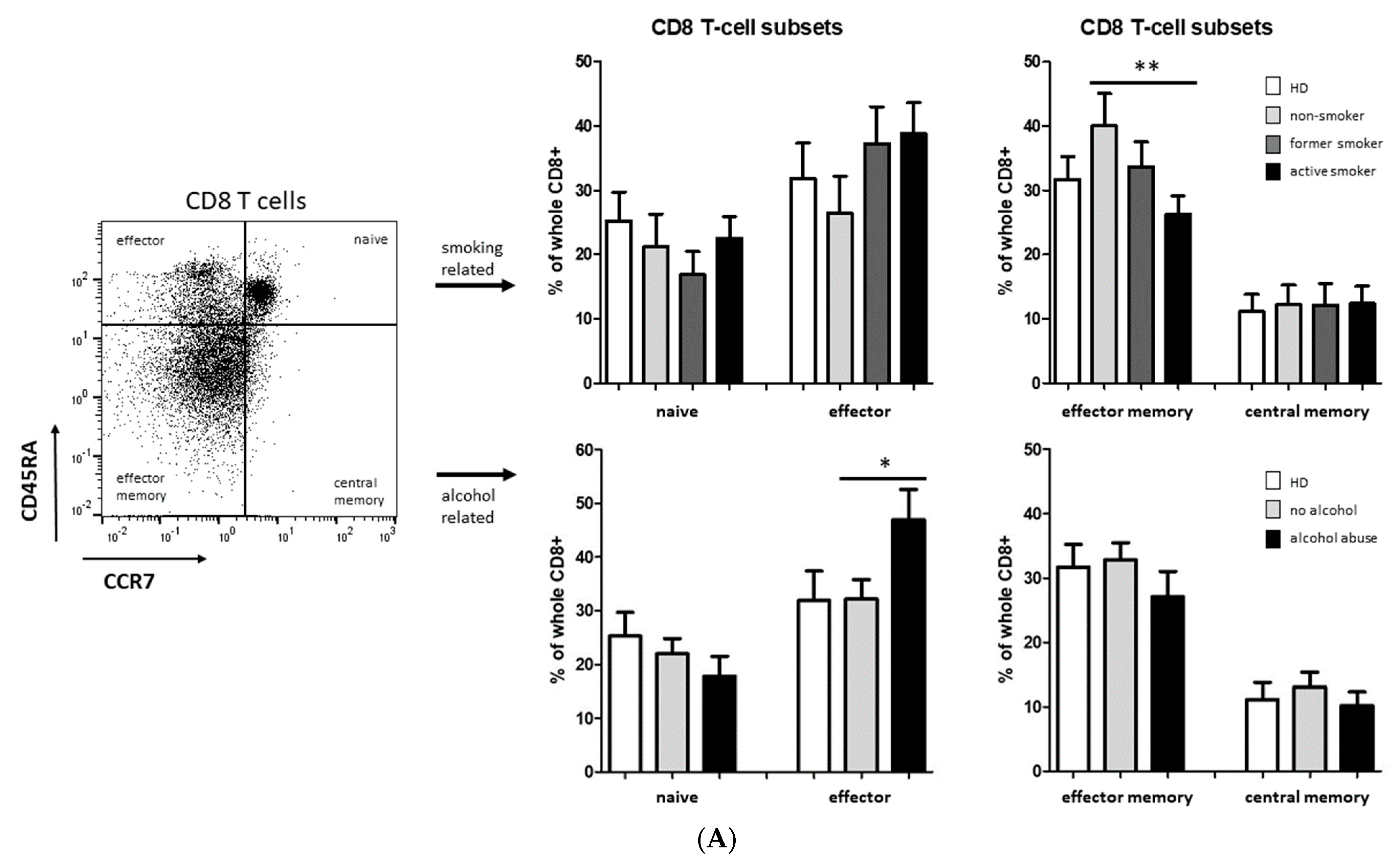
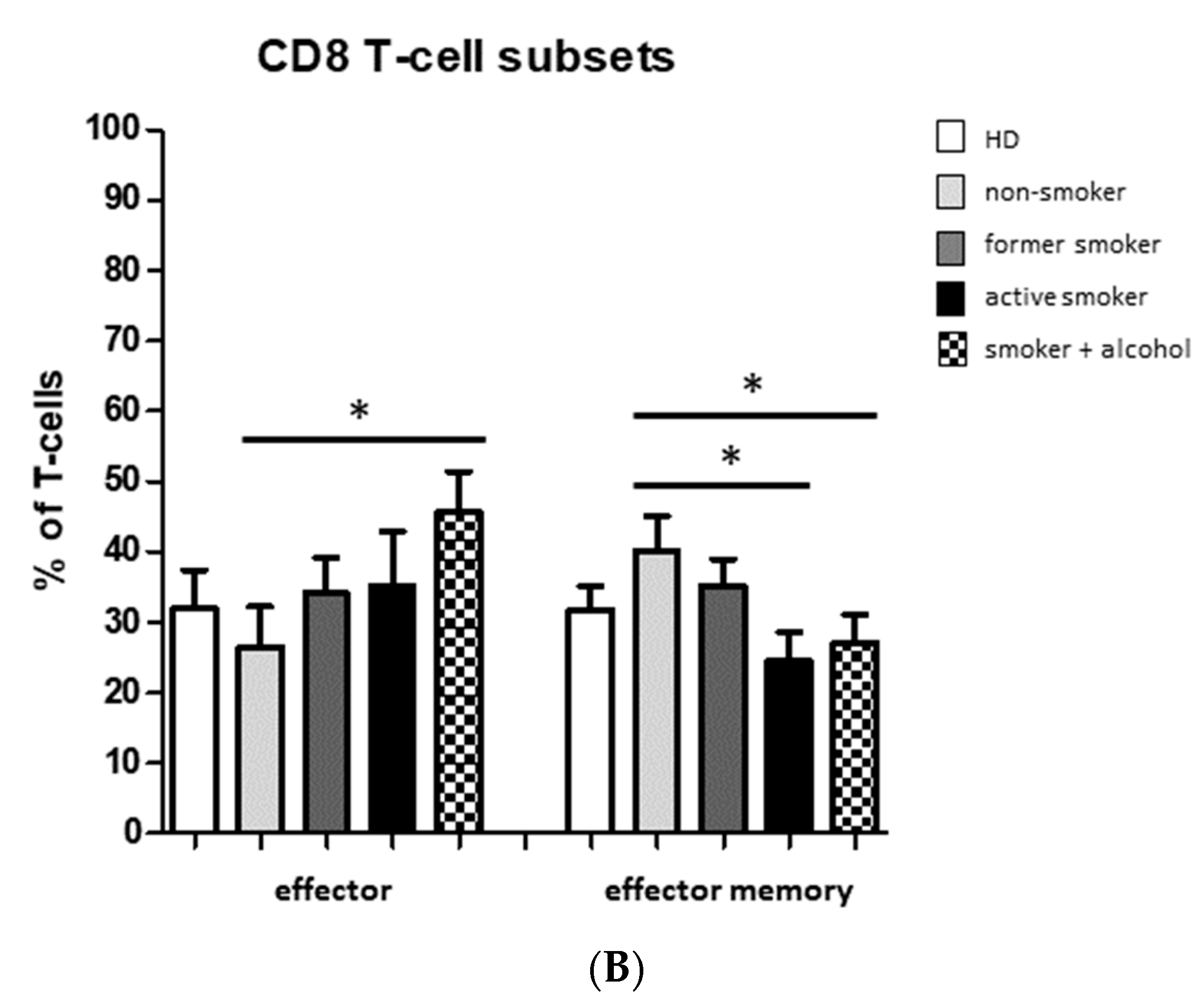
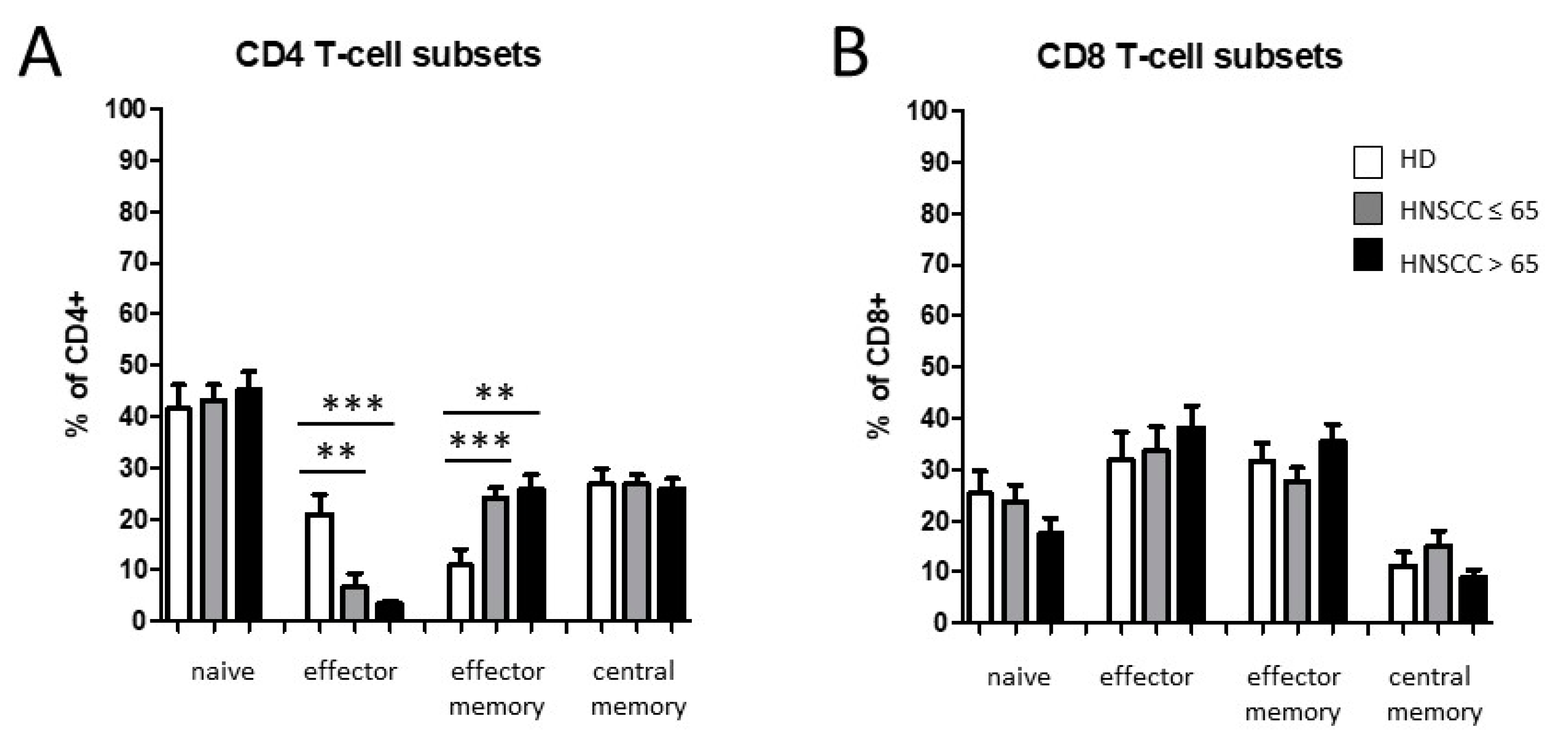
3.2. Smoking-, Alcohol-, and Age-Related Distribution of T-Cell Subsets
4. Discussion
4.1. Smoking-, Alcohol-, and Age-Related Alteration of Monocyte Subsets
4.2. Alteration of Circulating T Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, P.; Li, S.; Zhang, T.; Cui, F.; Shi, J.H.; Zhao, F.; Sheng, X. Characterization of Molecular Subtypes in Head and Neck Squamous Cell Carcinoma With Distinct Prognosis and Treatment Responsiveness. Front. Cell Dev. Biol. 2021, 9, 711348. [Google Scholar] [CrossRef] [PubMed]
- Cogliano, V.J.; Baan, R.; Straif, K.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; et al. Preventable exposures associated with human cancers. J. Natl. Cancer Inst. 2011, 103, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- Dobrossy, L. Epidemiology of head and neck cancer: Magnitude of the problem. Cancer Metastasis Rev. 2005, 24, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Auguste, A.; Joachim, C.; Deloumeaux, J.; Gaete, S.; Michineau, L.; Herrmann-Storck, C.; Duflo, S.; Luce, D. Head and neck cancer risk factors in the French West Indies. BMC Cancer 2021, 21, 1071. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Gregoire, V.; Lefebvre, J.L.; Licitra, L.; Felip, E.; EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21 (Suppl. S5), v184–v186. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef] [Green Version]
- Schrank, T.; Weir, W.; Lal, A.; Landess, L.; Lenze, N.; Hackman, T. Quantifying smoking exposure, genomic correlates, and related risk of treatment failure in p16+ squamous cell carcinoma of the oropharynx. Laryngoscope Investig. Otolaryngol. 2021, 6, 1376–1382. [Google Scholar] [CrossRef]
- Blot, W.J.; McLaughlin, J.K.; Winn, D.M.; Austin, D.F.; Greenberg, R.S.; Preston-Martin, S.; Bernstein, L.; Schoenberg, J.B.; Stemhagen, A.; Fraumeni, J.F., Jr. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988, 48, 3282–3287. [Google Scholar]
- Wyss, A.; Hashibe, M.; Chuang, S.C.; Lee, Y.C.; Zhang, Z.F.; Yu, G.P.; Winn, D.M.; Wei, Q.; Talamini, R.; Szeszenia-Dabrowska, N.; et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am. J. Epidemiol. 2013, 178, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Piaggeschi, G.; Rolla, S.; Rossi, N.; Brusa, D.; Naccarati, A.; Couvreur, S.; Spector, T.D.; Roederer, M.; Mangino, M.; Cordero, F.; et al. Immune Trait Shifts in Association With Tobacco Smoking: A Study in Healthy Women. Front. Immunol. 2021, 12, 637974. [Google Scholar] [CrossRef] [PubMed]
- de la Iglesia, J.V.; Slebos, R.J.C.; Martin-Gomez, L.; Wang, X.; Teer, J.K.; Tan, A.C.; Gerke, T.A.; Aden-Buie, G.; van Veen, T.; Masannat, J.; et al. Effects of Tobacco Smoking on the Tumor Immune Microenvironment in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 1474–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praud, D.; Rota, M.; Rehm, J.; Shield, K.; Zatonski, W.; Hashibe, M.; La Vecchia, C.; Boffetta, P. Cancer incidence and mortality attributable to alcohol consumption. Int. J. Cancer 2016, 138, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Stickel, F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol. Chem. 2006, 387, 349–360. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef]
- Garro, A.J.; Espina, N.; Farinati, F.; Salvagnini, M. The effects of chronic ethanol consumption on carcinogen metabolism and on O6-methylguanine transferase-mediated repair of alkylated DNA. Alcohol. Clin. Exp. Res. 1986, 10, 73S–77S. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, L.; Lopez-Hoyos, M.; Munoz-Cacho, P.; Martinez-Taboada, V.M. Aging is associated with circulating cytokine dysregulation. Cell. Immunol. 2012, 273, 124–132. [Google Scholar] [CrossRef]
- De Maeyer, R.P.H.; Chambers, E.S. The impact of ageing on monocytes and macrophages. Immunol. Lett. 2021, 230, 1–10. [Google Scholar] [CrossRef]
- Seidler, S.; Zimmermann, H.W.; Bartneck, M.; Trautwein, C.; Tacke, F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007, 317, 666–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.L.; Yeap, W.H.; Tai, J.J.; Ong, S.M.; Dang, T.M.; Wong, S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2012, 53, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Zhang, Y.; Fullerton, J.N.; Boelen, L.; Rongvaux, A.; Maini, A.A.; Bigley, V.; Flavell, R.A.; Gilroy, D.W.; Asquith, B.; et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 2017, 214, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L. Blood Monocytes and Their Subsets: Established Features and Open Questions. Front. Immunol. 2015, 6, 423. [Google Scholar] [CrossRef] [PubMed]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar] [CrossRef] [PubMed]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte differentiation and antigen-presenting functions. Nat. Reviews. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef]
- Moniuszko, M.; Bodzenta-Lukaszyk, A.; Kowal, K.; Lenczewska, D.; Dabrowska, M. Enhanced frequencies of CD14++CD16+, but not CD14+CD16+, peripheral blood monocytes in severe asthmatic patients. Clin. Immunol. 2009, 130, 338–346. [Google Scholar] [CrossRef]
- Polasky, C.; Steffen, A.; Loyal, K.; Lange, C.; Bruchhage, K.L.; Pries, R. Redistribution of Monocyte Subsets in Obstructive Sleep Apnea Syndrome Patients Leads to an Imbalanced PD-1/PD-L1 Cross-Talk with CD4/CD8 T Cells. J. Immunol. 2021, 206, 51–58. [Google Scholar] [CrossRef]
- Nyugen, J.; Agrawal, S.; Gollapudi, S.; Gupta, S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J. Clin. Immunol. 2010, 30, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Merino, A.; Buendia, P.; Martin-Malo, A.; Aljama, P.; Ramirez, R.; Carracedo, J. Senescent CD14+CD16+ monocytes exhibit proinflammatory and proatherosclerotic activity. J. Immunol. 2011, 186, 1809–1815. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.M.; Hadadi, E.; Dang, T.M.; Yeap, W.H.; Tan, C.T.; Ng, T.P.; Larbi, A.; Wong, S.C. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis. 2018, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Landsman, L.; Bar-On, L.; Zernecke, A.; Kim, K.W.; Krauthgamer, R.; Shagdarsuren, E.; Lira, S.A.; Weissman, I.L.; Weber, C.; Jung, S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 2009, 113, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Panek, C.A.; Ramos, M.V.; Mejias, M.P.; Abrey-Recalde, M.J.; Fernandez-Brando, R.J.; Gori, M.S.; Salamone, G.V.; Palermo, M.S. Differential expression of the fractalkine chemokine receptor (CX3CR1) in human monocytes during differentiation. Cell. Mol. Immunol. 2015, 12, 669–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schittenhelm, L.; Hilkens, C.M.; Morrison, V.L. beta(2) Integrins As Regulators of Dendritic Cell, Monocyte, and Macrophage Function. Front. Immunol. 2017, 8, 1966. [Google Scholar] [CrossRef] [PubMed]
- Han, C.F.; Jin, J.; Xu, S.; Liu, H.B.; Li, N.; Cao, X.T. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat. Immunol. 2010, 11, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gordon, R.A.; Huynh, L.; Su, X.D.; Min, K.H.P.; Han, J.H.; Arthur, J.S.; Kalliolias, G.D.; Ivashkiv, L.B. Indirect Inhibition of Toll-like Receptor and Type I Interferon Responses by ITAM-Coupled Receptors and Integrins. Immunity 2010, 32, 518–530. [Google Scholar] [CrossRef] [Green Version]
- Caruntu, A.; Scheau, C.; Tampa, M.; Georgescu, S.R.; Caruntu, C.; Tanase, C. Complex Interaction Among Immune, Inflammatory, and Carcinogenic Mechanisms in the Head and Neck Squamous Cell Carcinoma. Adv. Exp. Med. Biol. 2021, 1335, 11–35. [Google Scholar] [CrossRef]
- Sakakura, K.; Takahashi, H.; Motegi, S.I.; Yokobori-Kuwabara, Y.; Oyama, T.; Chikamatsu, K. Immunological features of circulating monocyte subsets in patients with squamous cell carcinoma of the head and neck. Clin. Immunol. 2021, 225, 108677. [Google Scholar] [CrossRef]
- Tavenier, J.; Rasmussen, L.J.H.; Houlind, M.B.; Andersen, A.L.; Panum, I.; Andersen, O.; Petersen, J.; Langkilde, A.; Nehlin, J.O. Alterations of monocyte NF-kappa B p65/RelA signaling in a cohort of older medical patients, age-matched controls, and healthy young adults. Immun. Ageing 2020, 17, 25. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Kuang, D.M.; Peng, C.; Zhao, Q.; Wu, Y.; Chen, M.S.; Zheng, L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology 2010, 51, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Heeren, A.M.; Punt, S.; Bleeker, M.C.; Gaarenstroom, K.N.; van der Velden, J.; Kenter, G.G.; de Gruijl, T.D.; Jordanova, E.S. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2016, 29, 753–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.R.; Ha, S.J.; Hong, M.H.; Heo, S.J.; Koh, Y.W.; Choi, E.C.; Kim, E.K.; Pyo, K.H.; Jung, I.; Seo, D.; et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci. Rep. 2016, 6, 36956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Ge, S.; Sang, S.; Hu, C.; Deng, S. Evaluation of PD-L1 Expression Level in Patients With Non-Small Cell Lung Cancer by (18)F-FDG PET/CT Radiomics and Clinicopathological Characteristics. Front. Oncol. 2021, 11, 789014. [Google Scholar] [CrossRef]
- Cass, S.P.; Mekhael, O.; Thayaparan, D.; McGrath, J.J.C.; Revill, S.D.; Fantauzzi, M.F.; Wang, P.; Reihani, A.; Hayat, A.I.; Stevenson, C.S.; et al. Increased Monocyte-Derived CD11b(+) Macrophage Subpopulations Following Cigarette Smoke Exposure Are Associated With Impaired Bleomycin-Induced Tissue Remodelling. Front. Immunol. 2021, 12, 740330. [Google Scholar] [CrossRef]
- Stockfelt, M.; Christenson, K.; Andersson, A.; Bjorkman, L.; Padra, M.; Brundin, B.; Ganguly, K.; Asgeirsdottir, H.; Linden, S.; Qvarfordt, I.; et al. Increased CD11b and Decreased CD62L in Blood and Airway Neutrophils from Long-Term Smokers with and without COPD. J. Innate Immun. 2020, 12, 480–489. [Google Scholar] [CrossRef]
- McDermott, D.H.; Halcox, J.P.J.; Schenke, W.H.; Waclawiw, M.A.; Merrell, M.N.; Epstein, N.; Quyyumi, A.A.; Murphy, P.M. Association between polymorphism in the chemokine receptor CX3CR1 and coronary vascular endothelial dysfunction and atherosclerosis. Circ. Res. 2001, 89, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Tacke, F.; Alvarez, D.; Kaplan, T.J.; Jakubzick, C.; Spanbroek, R.; Llodra, J.; Garin, A.; Liu, J.H.; Mack, M.; van Rooijen, N.; et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Investig. 2007, 117, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Chen, T.; Wang, B.; Zhang, M.; An, H.; Guo, Z.; Yu, Y.; Qin, Z.; Cao, X. Chemoattraction, adhesion and activation of natural killer cells are involved in the antitumor immune response induced by fractalkine/CX3CL1. Immunol. Lett. 2003, 89, 1–7. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, M.; Wang, B.; Yuan, Z.; Guo, Z.; Chen, T.; Yu, Y.; Qin, Z.; Cao, X. Fractalkine transgene induces T-cell-dependent antitumor immunity through chemoattraction and activation of dendritic cells. Int. J. Cancer 2003, 103, 212–220. [Google Scholar] [CrossRef]
- Fernandes, C.J.; Morinaga, L.T.K.; Alves, J.L.; Castro, M.A.; Calderaro, D.; Jardim, C.V.P.; Souza, R. Cancer-associated thrombosis: The when, how and why. Eur. Respir. Rev. 2019, 28, 180119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of cancer-associated venous thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridman, W.H.; Pages, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.A.; Amir, E. HYPE or HOPE: The prognostic value of infiltrating immune cells in cancer. Br. J. Cancer 2017, 117, 451–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boffetta, P.; Hashibe, M. Alcohol and cancer. Lancet Oncol. 2006, 7, 149–156. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Chuang, S.C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009, 18, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Wight, A.J.; Ogden, G.R. Possible mechanisms by which alcohol may influence the development of oral cancer—A review. Oral. Oncol. 1998, 34, 441–447. [Google Scholar] [CrossRef]
- Browman, G.P.; Wong, G.; Hodson, I.; Sathya, J.; Russell, R.; McAlpine, L.; Skingley, P.; Levine, M.N. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N. Engl. J. Med. 1993, 328, 159–163. [Google Scholar] [CrossRef]
- Whincup, P.; Papacosta, O.; Lennon, L.; Haines, A. Carboxyhaemoglobin levels and their determinants in older British men. BMC Public Health 2006, 6, 189. [Google Scholar] [CrossRef] [Green Version]
- Rades, D.; Setter, C.; Schild, S.E.; Dunst, J. Effect of smoking during radiotherapy, respiratory insufficiency, and hemoglobin levels on outcome in patients irradiated for non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1134–1142. [Google Scholar] [CrossRef]
- Fleshner, N.; Garland, J.; Moadel, A.; Herr, H.; Ostroff, J.; Trambert, R.; O’Sullivan, M.; Russo, P. Influence of smoking status on the disease-related outcomes of patients with tobacco-associated superficial transitional cell carcinoma of the bladder. Cancer 1999, 86, 2337–2345. [Google Scholar] [CrossRef]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Turksma, A.W.; Bontkes, H.J.; van den Heuvel, H.; de Gruijl, T.D.; von Blomberg, B.M.; Braakhuis, B.J.; Leemans, C.R.; Bloemena, E.; Meijer, C.J.; Hooijberg, E. Effector memory T-cell frequencies in relation to tumour stage, location and HPV status in HNSCC patients. Oral Dis. 2013, 19, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Le Page, A.; Dupuis, G.; Larbi, A.; Witkowski, J.M.; Fulop, T. Signal transduction changes in CD4(+) and CD8(+) T cell subpopulations with aging. Exp. Gerontol. 2018, 105, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Jeske, S.S.; Schuler, P.J.; Doescher, J.; Theodoraki, M.N.; Laban, S.; Brunner, C.; Hoffmann, T.K.; Wigand, M.C. Age-related changes in T lymphocytes of patients with head and neck squamous cell carcinoma. Immun. Ageing 2020, 17, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | Patients (n = 64) | |
|---|---|---|
| n | % | |
| Gender | ||
| m | 47 | 73 |
| f | 17 | 27 |
| Age (years) | ||
| ≤65 | 38 | 59 |
| >65 | 26 | 41 |
| Tumor Site | ||
| pharynx | 37 | 58 |
| larynx | 13 | 20 |
| oral cavity | 14 | 22 |
| Tumor Stage | ||
| T1-T2 | 40 | 63 |
| T3-T4 | 24 | 37 |
| HPV Status | ||
| positive | 20 | 31 |
| negative | 44 | 69 |
| Alcohol Abuse | ||
| yes | 14 | 22 |
| no | 50 | 78 |
| Tobacco Consumption | ||
| yes | 45 | 70 |
| no | 19 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idel, C.; Loyal, K.; Rades, D.; Hakim, S.G.; Schumacher, U.; Bruchhage, K.-L.; Pries, R. Smoking-, Alcohol-, and Age-Related Alterations of Blood Monocyte Subsets and Circulating CD4/CD8 T Cells in Head and Neck Cancer. Biology 2022, 11, 658. https://doi.org/10.3390/biology11050658
Idel C, Loyal K, Rades D, Hakim SG, Schumacher U, Bruchhage K-L, Pries R. Smoking-, Alcohol-, and Age-Related Alterations of Blood Monocyte Subsets and Circulating CD4/CD8 T Cells in Head and Neck Cancer. Biology. 2022; 11(5):658. https://doi.org/10.3390/biology11050658
Chicago/Turabian StyleIdel, Christian, Kristin Loyal, Dirk Rades, Samer G. Hakim, Udo Schumacher, Karl-Ludwig Bruchhage, and Ralph Pries. 2022. "Smoking-, Alcohol-, and Age-Related Alterations of Blood Monocyte Subsets and Circulating CD4/CD8 T Cells in Head and Neck Cancer" Biology 11, no. 5: 658. https://doi.org/10.3390/biology11050658
APA StyleIdel, C., Loyal, K., Rades, D., Hakim, S. G., Schumacher, U., Bruchhage, K.-L., & Pries, R. (2022). Smoking-, Alcohol-, and Age-Related Alterations of Blood Monocyte Subsets and Circulating CD4/CD8 T Cells in Head and Neck Cancer. Biology, 11(5), 658. https://doi.org/10.3390/biology11050658






