Characterization and In Vitro Cytotoxicity Safety Screening of Fractionated Organosolv Lignin on Diverse Primary Human Cell Types Commonly Used in Tissue Engineering
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Lignin Preparation
2.2. Molecular Weight Determination of Lignin Fractions
2.3. Hydroxyl Content Analysis of Lignin Fractions
2.4. Glass Transition Temperature of Lignin Fractions
2.5. Solubility Parameters of the Lignin Fractions and Agarose
2.6. Preparation of the Low MW Fraction for In Vitro Cytotoxicity Safety Screening
2.7. Isolation and Culture of Human-Derived Cells
2.8. Isolation and Culture of Bovine-Derived Cells
2.9. WST-1 Assay
2.10. Lignin-Agarose Hydrogels
2.11. Hydrogel Stiffness
2.12. Viscoelastic Properties
2.13. Cell Viability of Chondrocytes on Lignin-Agarose Hydrogels
2.14. Statistics
3. Results
3.1. Structural Features and Physico-Chemical Characteristics of High and Low MW Lignin Fractions
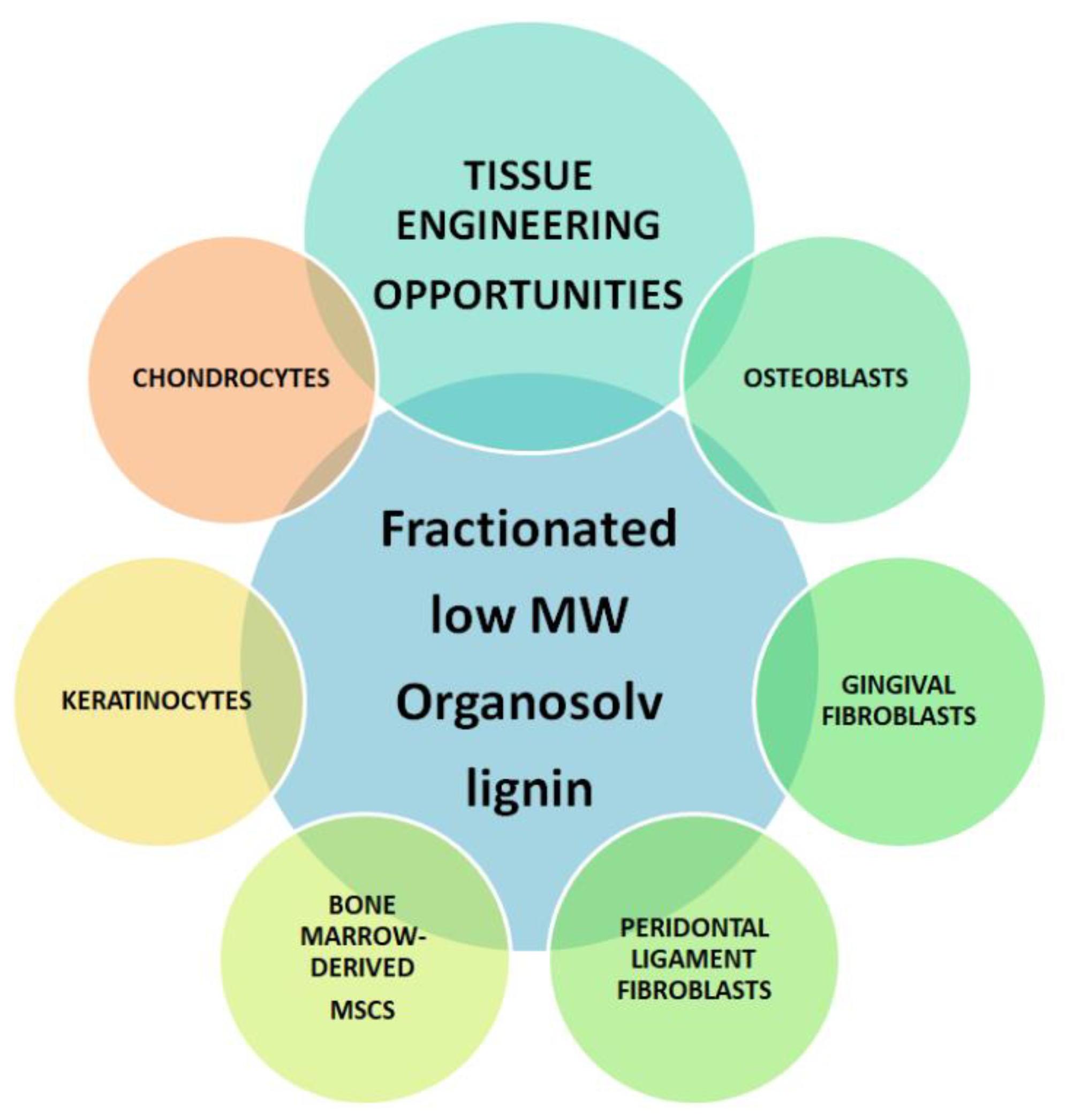
3.2. In Vitro Cytotoxicity Safety Screening of Organosolv Lignin with Diverse Primary Human Cells
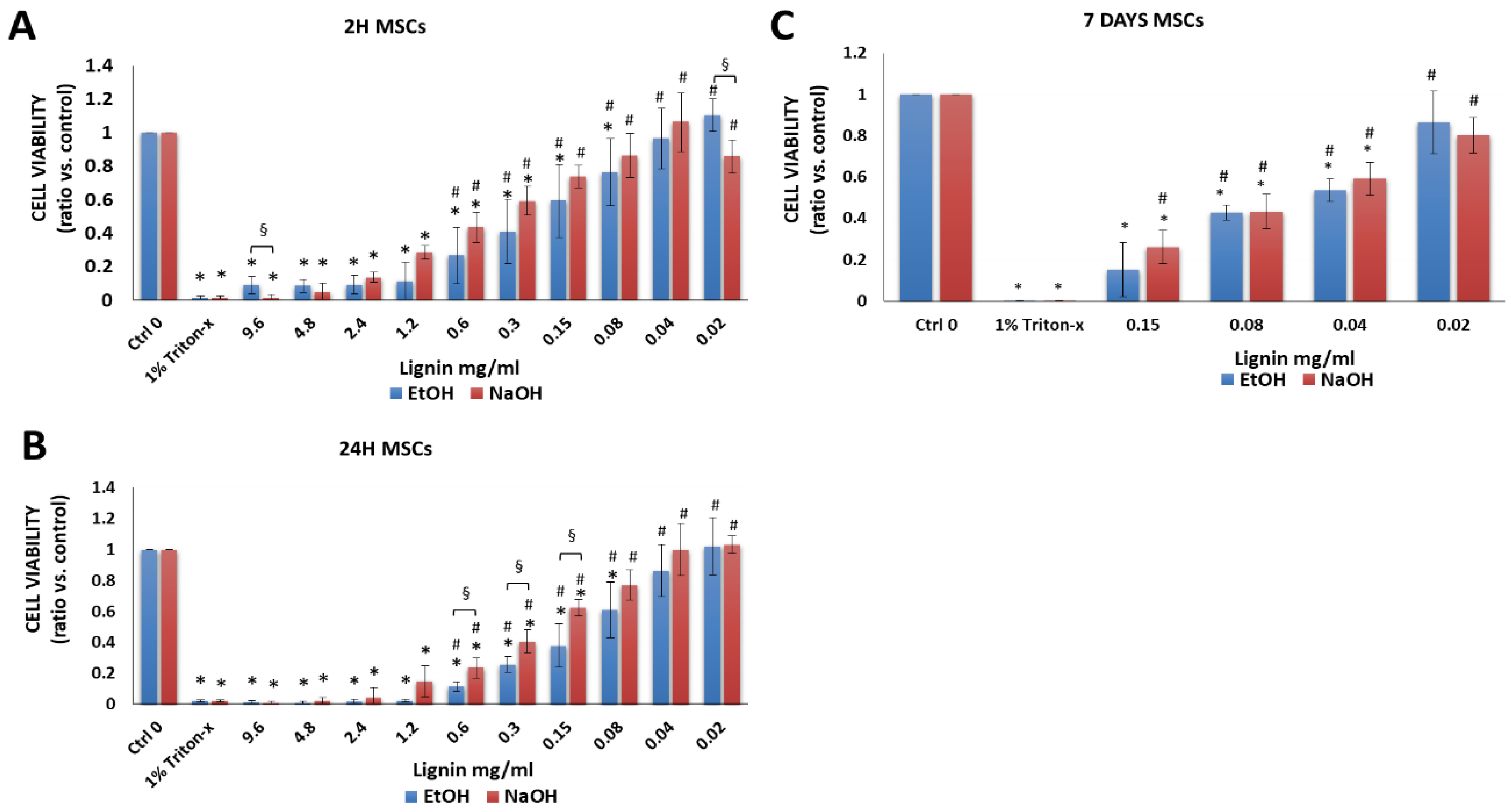
3.2.1. Bone Marrow-Derived MSCs
3.2.2. Chondrocytes
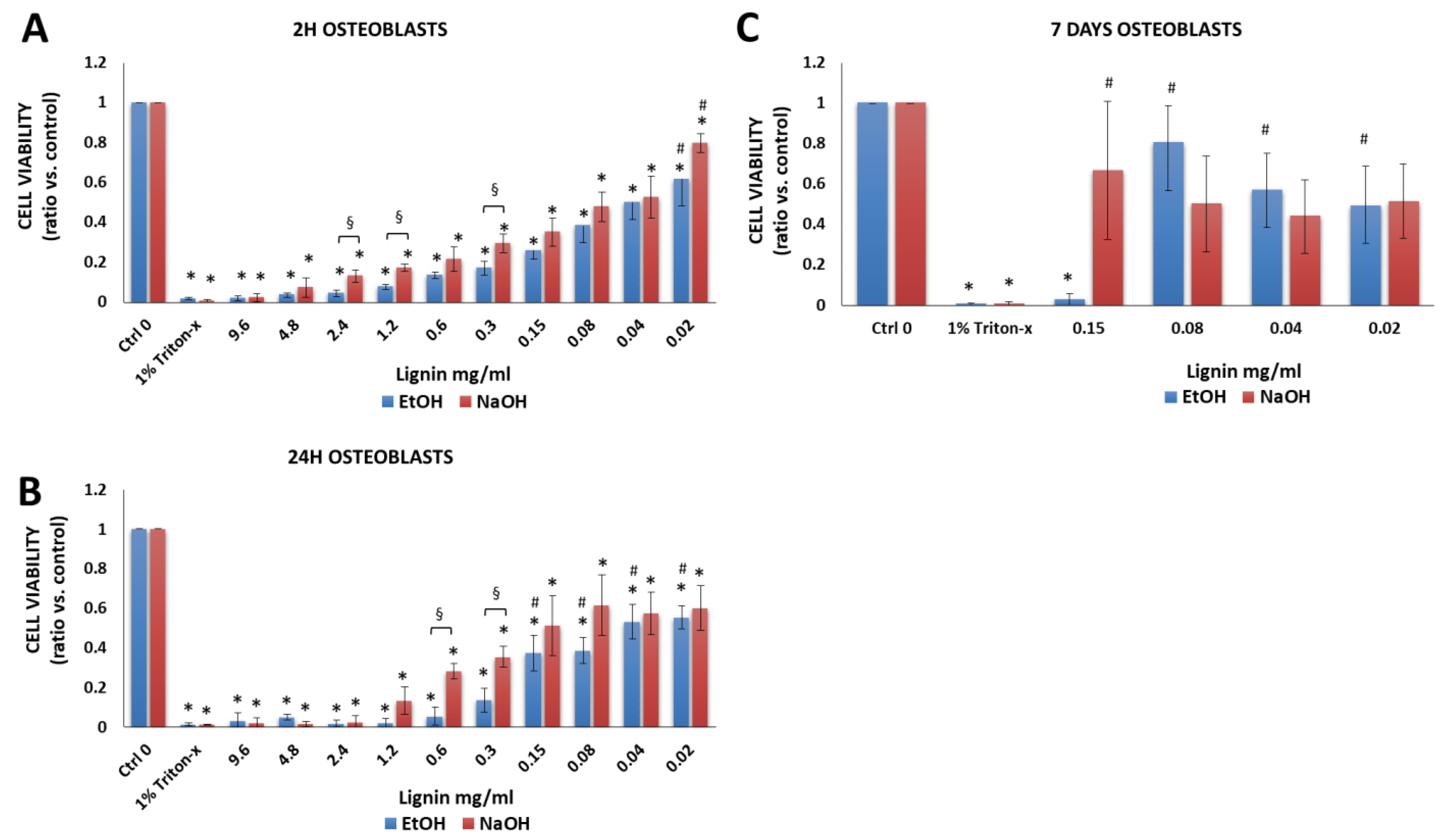
3.2.3. Osteoblasts
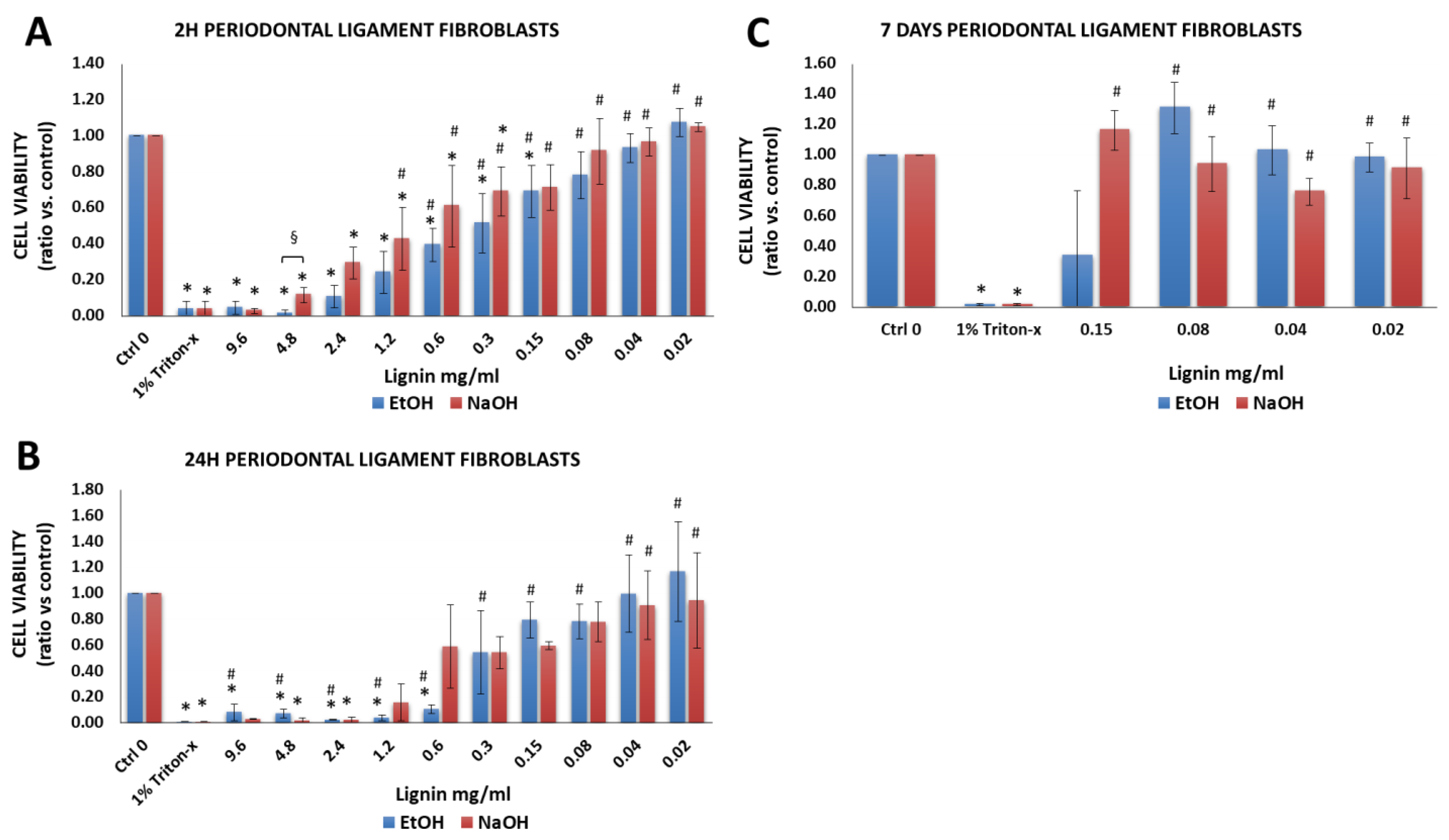
3.2.4. Periodontal Ligament Fibroblasts
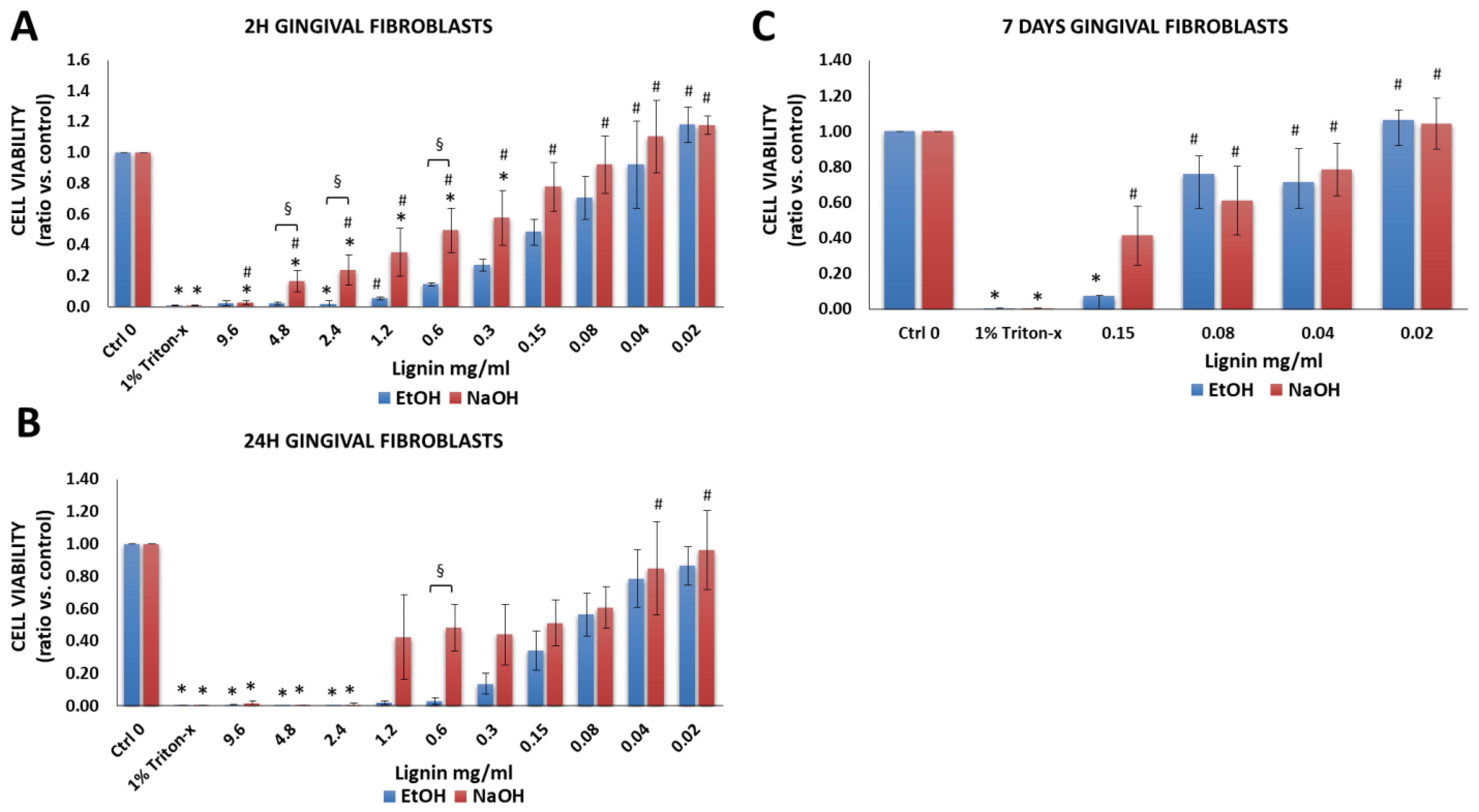
3.2.5. Gingival Fibroblasts
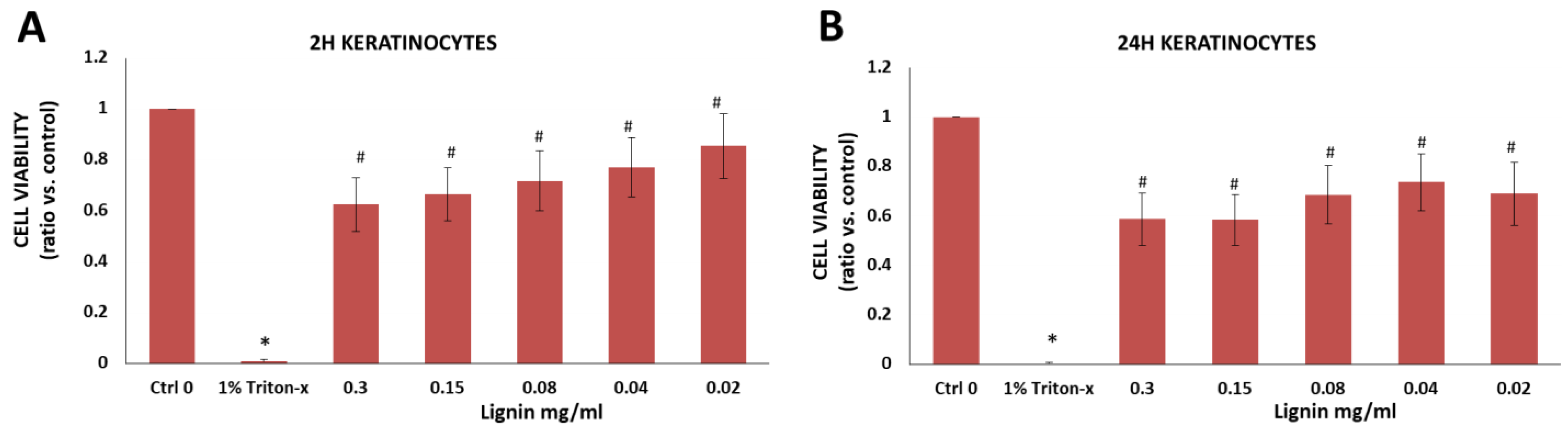
3.2.6. Immortalized Human Keratinocytes
3.3. Using Low MW Organosolv Lignin with Another Biomaterial to Fabricate Hydrogels That May Be Potentially Useful for Tissue Engineering Applications
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bajwaa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwac, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crop. Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Melro, E.; Filipe, A.; Sousa, D.; Medronho, B.; Romano, A. Revisiting lignin: A tour through its structural features, characterization methods and applications. New J. Chem. 2021, 45, 6986–7013. [Google Scholar] [CrossRef]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How far is lignin from being a biomedical material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Witzler, M.; Alzagameem, A.; Bergs, M.; Khaldi-Hansen, B.E.; Klein, S.E.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-derived biomaterials for drug release and tissue engineering. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef] [Green Version]
- Dornish, M.; Kaplan, D.; Skaugrud, O. Standards and guidelines for biopolymers in tissue-engineered medical products: Astm alginate and chitosan standard guides. American society for testing and materials. Ann. N. Y. Acad. Sci. 2001, 944, 388–397. [Google Scholar] [CrossRef]
- Domínguez-Roblesa, J.; Cárcamo-Martínez, A.; Stewart, S.A.; Donnelly, R.F.; Larrañeta, E.; Borrega, M. Lignin for pharmaceutical and biomedical applications—Could this become a reality? Sustain. Chem. Pharm. 2020, 18, 100320. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Mitjans, M. Lignins and their derivatives with beneficial effects on human health. Int. J. Mol. Sci. 2017, 18, 1219. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Dai, L.; Xu, C.; Wang, K.; Zheng, C.; Si, C. Lignin-based micro- and nanomaterials and their composites in biomedical applications. ChemSusChem 2020, 13, 4266–4283. [Google Scholar] [CrossRef]
- Erakovic, S.; Jankovic, A.; Tsui, G.C.; Tang, C.Y.; Miskovic-Stankovic, V.; Stevanovic, T. Novel bioactive antimicrobial lignin containing coatings on titanium obtained by electrophoretic deposition. Int. J. Mol. Sci. 2014, 15, 12294–12322. [Google Scholar] [CrossRef] [Green Version]
- Jankovic, A.; Erakovic, S.; Ristoscu, C.; Mihailescu Serban, N.; Duta, L.; Visan, A.; Stan, G.E.; Popa, A.C.; Husanu, M.A.; Luculescu, C.R.; et al. Structural and biological evaluation of lignin addition to simple and silver-doped hydroxyapatite thin films synthesized by matrix-assisted pulsed laser evaporation. J. Mater. Sci. Mater. Med. 2015, 26, 5333. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Alqahtani, A.; Al-Thabit, A.; Roni, M.; Syed, R. Novel lignin nanoparticles for oral drug delivery. J. Mater. Chem. B 2019, 7, 4461–4473. [Google Scholar] [CrossRef]
- Freitas, F.M.C.; Cerqueira, M.A.; Goncalves, C.; Azinheiro, S.; Garrido-Maestu, A.; Vicente, A.A.; Pastrana, L.M.; Teixeira, J.A.; Michelin, M. Green synthesis of lignin nano- and micro-particles: Physicochemical characterization, bioactive properties and cytotoxicity assessment. Int. J. Biol. Macromol. 2020, 163, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, I.; Tanase, C.E. Design, characterization and preliminary biological evaluation of new lignin-pla biocomposites. Int. J. Biol. Macromol. 2018, 114, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Gleuwitz, F.R.; Sivasankarapillai, G.; Chen, Y.; Friedrich, C.; Laborie, M.G. Lignin-assisted stabilization of an oriented liquid crystalline cellulosic mesophase, part b: Toward the molecular origin and mechanism. Biomacromolecules 2020, 21, 2276–2284. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.; Wang, G.; Sun, H.; Su, W.; Si, C. Lignin fractionation: Effective strategy to reduce molecule weight dependent heterogeneity. Ind. Crop. Prod. 2021, 165, 113442. [Google Scholar] [CrossRef]
- Izaguirre, N.; Robles, E.; Llano-Ponte, R.; Labidi, J.; Erdociac, X. Fine-tune of lignin properties by its fractionation with a sequential organic solvent extraction. Ind. Crop. Prod. 2022, 175, 114251. [Google Scholar] [CrossRef]
- Saluja, B.; Thakkar, J.N.; Li, H.; Desai, U.R.; Sakagami, M. Novel low molecular weight lignins as potential anti-emphysema agents: In vitro triple inhibitory activity against elastase, oxidation and inflammation. Pulm. Pharmacol. Ther. 2013, 26, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Kubo, S.; Uraki, Y.; Sano, Y. Preparation of carbon fibers from softwood lignin by atmospheric acetic acid pulping. Carbon 1998, 36, 1119–1124. [Google Scholar] [CrossRef]
- Meng, X.; Crestini, C.; Ben, H.; Hao, N.; Pu, Y.; Ragauskas, A.J.; Argyropoulos, D.S. Determination of hydroxyl groups in biorefinery resources via quantitative (31)p nmr spectroscopy. Nat. Protoc. 2019, 14, 2627–2647. [Google Scholar] [CrossRef]
- Hansen, C.M. The Three Dimensional Solubility Parameter; Danish Technical Press: Copenhagen, Denmark, 1967. [Google Scholar]
- Brun, J.; Abruzzese, T.; Rolauffs, B.; Aicher, W.K.; Hart, M.L. Choice of xenogenic-free expansion media significantly influences the myogenic differentiation potential of human bone marrow-derived mesenchymal stromal cells. Cytotherapy 2016, 18, 344–359. [Google Scholar] [CrossRef]
- Brun, J.; Lutz, K.A.; Neumayer, K.M.; Klein, G.; Seeger, T.; Uynuk-Ool, T.; Worgotter, K.; Schmid, S.; Kraushaar, U.; Guenther, E.; et al. Smooth muscle-like cells generated from human mesenchymal stromal cells display marker gene expression and electrophysiological competence comparable to bladder smooth muscle cells. PLoS ONE 2015, 10, e0145153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, B.; Uynuk-Ool, T.; Rothdiener, M.; Palm, J.; Hart, M.L.; Stegemann, J.P.; Rolauffs, B. Engineering the geometrical shape of mesenchymal stromal cells through defined cyclic stretch regimens. Sci. Rep. 2017, 7, 6640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uynuk-Ool, T.; Rothdiener, M.; Walters, B.; Hegemann, M.; Palm, J.; Nguyen, P.; Seeger, T.; Stockle, U.; Stegemann, J.P.; Aicher, W.K.; et al. The geometrical shape of mesenchymal stromal cells measured by quantitative shape descriptors is determined by the stiffness of the biomaterial and by cyclic tensile forces. J. Tissue Eng. Regen. Med. 2017, 11, 3508–3522. [Google Scholar] [CrossRef] [PubMed]
- Seeger, T.; Hart, M.; Patarroyo, M.; Rolauffs, B.; Aicher, W.K.; Klein, G. Mesenchymal stromal cells for sphincter regeneration: Role of laminin isoforms upon myogenic differentiation. PLoS ONE 2015, 10, e0137419. [Google Scholar] [CrossRef]
- Rothdiener, M.; Hegemann, M.; Uynuk-Ool, T.; Walters, B.; Papugy, P.; Nguyen, P.; Claus, V.; Seeger, T.; Stoeckle, U.; Boehme, K.A.; et al. Stretching human mesenchymal stromal cells on stiffness-customized collagen type i generates a smooth muscle marker profile without growth factor addition. Sci. Rep. 2016, 6, 35840. [Google Scholar] [CrossRef]
- Felka, T.; Rothdiener, M.; Bast, S.; Uynuk-Ool, T.; Zouhair, S.; Ochs, B.G.; De Zwart, P.; Stoeckle, U.; Aicher, W.K.; Hart, M.L.; et al. Loss of spatial organization and destruction of the pericellular matrix in early osteoarthritis in vivo and in a novel in vitro methodology. Osteoarthr. Cartil. 2016, 24, 1200–1209. [Google Scholar] [CrossRef] [Green Version]
- Rolauffs, B.; Williams, J.M.; Aurich, M.; Grodzinsky, A.J.; Kuettner, K.E.; Cole, A.A. Proliferative remodeling of the spatial organization of human superficial chondrocytes distant from focal early osteoarthritis. Arthritis Rheum. 2010, 62, 489–498. [Google Scholar]
- Aurich, M.; Hofmann, G.O.; Best, N.; Rolauffs, B. Induced redifferentiation of human chondrocytes from articular cartilage lesion in alginate bead culture after monolayer dedifferentiation: An alternative cell source for cell-based therapies? Tissue Eng. Part A 2018, 24, 275–286. [Google Scholar] [CrossRef]
- Altmann, B.; Lochner, A.; Swain, M.; Kohal, R.J.; Giselbrecht, S.; Gottwald, E.; Steinberg, T.; Tomakidi, P. Differences in morphogenesis of 3d cultured primary human osteoblasts under static and microfluidic growth conditions. Biomaterials 2014, 35, 3208–3219. [Google Scholar] [CrossRef]
- Rabel, K.; Kohal, R.J.; Steinberg, T.; Tomakidi, P.; Rolauffs, B.; Adolfsson, E.; Palmero, P.; Furderer, T.; Altmann, B. Controlling osteoblast morphology and proliferation via surface micro-topographies of implant biomaterials. Sci. Rep. 2020, 10, 12810. [Google Scholar] [CrossRef]
- Altmann, B.; Rabel, K.; Kohal, R.J.; Proksch, S.; Tomakidi, P.; Adolfsson, E.; Bernsmann, F.; Palmero, P.; Furderer, T.; Steinberg, T. Cellular transcriptional response to zirconia-based implant materials. Dent. Mater. 2017, 33, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Proksch, S.; Steinberg, T.; Vach, K.; Hellwig, E.; Tomakidi, P. Shaping oral cell plasticity to osteogenic differentiation by human mesenchymal stem cell coculture. Cell Tissue Res. 2014, 356, 159–170. [Google Scholar] [CrossRef]
- Proksch, S.; Steinberg, T.; Stampf, S.; Schwarz, U.; Hellwig, E.; Tomakidi, P. Crosstalk on cell behavior in interactive cocultures of hmscs with various oral cell types. Tissue Eng. Part A 2012, 18, 2601–2610. [Google Scholar] [CrossRef]
- Proksch, S.; Kirsch, K.; Vach, K.; Hellwig, E.; Tomakidi, P. Comparative differentiation analysis of distinct oral tissue-derived cells in response to osteogenic stimulation. Clin. Oral Investig. 2019, 23, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Roesch-Ely, M.; Steinberg, T.; Bosch, F.X.; Mussig, E.; Whitaker, N.; Wiest, T.; Kohl, A.; Komposch, G.; Tomakidi, P. Organotypic co-cultures allow for immortalized human gingival keratinocytes to reconstitute a gingival epithelial phenotype in vitro. Differentiation 2006, 74, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Buskermolen, J.K.; Reijnders, C.M.; Spiekstra, S.W.; Steinberg, T.; Kleverlaan, C.J.; Feilzer, A.J.; Bakker, A.D.; Gibbs, S. Development of a full-thickness human gingiva equivalent constructed from immortalized keratinocytes and fibroblasts. Tissue Eng. Part C Methods 2016, 22, 781–791. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Standard Organization: Geneva, Switzerland, 2009.
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-based biomaterials: Opportunities and challenges in cartilage tissue engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Aicher, W.K.; Hart, M.L.; Stallkamp, J.; Klünder, M.; Ederer, M.; Sawodny, O.; Vaegler, M.; Sievert, K.D.; Stenzl, A. Towards a treatment of urinary incontinence: Application of mesenchymal stromal cells for regeneration of the sphincter muscle. J. Clin. Med. 2013, 3, 197–215. [Google Scholar] [CrossRef] [Green Version]
- Aicher, W.K.; Buhring, H.J.; Hart, M.; Rolauffs, B.; Badke, A.; Klein, G. Regeneration of cartilage and bone by defined subsets of mesenchymal stromal cells—Potential and pitfalls. Adv. Drug Deliv. Rev. 2011, 63, 342–351. [Google Scholar] [CrossRef]
- Hart, M.L.; Neumayer, K.M.; Vaegler, M.; Daum, L.; Amend, B.; Sievert, K.D.; Di Giovanni, S.; Kraushaar, U.; Guenther, E.; Stenzl, A.; et al. Cell-based therapy for the deficient urinary sphincter. Curr. Urol. Rep. 2013, 14, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.L.; Izeta, A.; Herrera-Imbroda, B.; Amend, B.; Brinchmann, J.E. Cell therapy for stress urinary incontinence. Tissue Eng. Part B Rev. 2015, 21, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K. Recent trends in multipotent human mesenchymal stem/stromal cells: Learning from history and advancing clinical applications. OMICS 2021, 25, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int. 2018, 2018, 8031718. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Zylinska, B.; Silmanowicz, P.; Sobczynska-Rak, A.; Jarosz, L.; Szponder, T. Treatment of articular cartilage defects: Focus on tissue engineering. Vivo 2018, 32, 1289–1300. [Google Scholar] [CrossRef] [Green Version]
- Aurich, M.; Hofmann, G.O.; Rolauffs, B. Tissue engineering-relevant characteristics of ex vivo and monolayer-expanded chondrocytes from the notch versus trochlea of human knee joints. Int. Orthop. 2017, 41, 2327–2335. [Google Scholar] [CrossRef]
- Selig, M.; Lauer, J.C.; Hart, M.L.; Rolauffs, B. Mechanotransduction and stiffness-sensing: Mechanisms and opportunities to control multiple molecular aspects of cell phenotype as a design cornerstone of cell-instructive biomaterials for articular cartilage repair. Int. J. Mol. Sci. 2020, 21, 5399. [Google Scholar] [CrossRef]
- Rothdiener, M.; Uynuk-Ool, T.; Sudkamp, N.; Aurich, M.; Grodzinsky, A.J.; Kurz, B.; Rolauffs, B. Human osteoarthritic chondrons outnumber patient- and joint-matched chondrocytes in hydrogel culture-future application in autologous cell-based oa cartilage repair? J. Tissue Eng. Regen. Med. 2018, 12, e1206–e1220. [Google Scholar] [CrossRef]
- Niemeyer, P.; Albrecht, D.; Andereya, S.; Angele, P.; Ateschrang, A.; Aurich, M.; Baumann, M.; Bosch, U.; Erggelet, C.; Fickert, S.; et al. Autologous chondrocyte implantation (aci) for cartilage defects of the knee: A guideline by the working group “clinical tissue regeneration” of the german society of orthopaedics and trauma (dgou). Knee 2016, 23, 426–435. [Google Scholar] [CrossRef] [Green Version]
- Shanbhag, S.; Pandis, N.; Mustafa, K.; Nyengaard, J.R.; Stavropoulos, A. Alveolar bone tissue engineering in critical-size defects of experimental animal models: A systematic review and meta-analysis. J. Tissue Eng. Regen. Med. 2017, 11, 2935–2949. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Ponche, A.; Bigerelle, M. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 2: Biological aspects. Proc. Inst. Mech. Eng. H 2010, 224, 1487–1507. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, H.; Ninomiya, T.; Hiraga, T.; Yoshiba, K.; Yoshiba, N.; Kasahara, E.; Ozawa, H.; Nakamura, H. Potential of periodontal ligament cells to regenerate alveolar bone. J. Oral Biosci. 2010, 52, 72–80. [Google Scholar] [CrossRef]
- Hakkinen, L.; Larjava, H.; Fournier, B.P. Distinct phenotype and therapeutic potential of gingival fibroblasts. Cytotherapy 2014, 16, 1171–1186. [Google Scholar] [CrossRef]
- Rikken, G.; Niehues, H.; van den Bogaard, E.H. Organotypic 3d skin models: Human epidermal equivalent cultures from primary keratinocytes and immortalized keratinocyte cell lines. Methods Mol. Biol. 2020, 2154, 45–61. [Google Scholar]
- Smits, J.P.H.; Niehues, H.; Rikken, G.; van Vlijmen-Willems, I.; van de Zande, G.; Zeeuwen, P.; Schalkwijk, J.; van den Bogaard, E.H. Immortalized n/tert keratinocytes as an alternative cell source in 3d human epidermal models. Sci. Rep. 2017, 7, 11838. [Google Scholar] [CrossRef]
- Olsson, A.M.; Salmén, L. The effect of lignin composition on the viscoelastic properties of wood. Nord. Pulp Pap. Res. J. 1997, 12, 140–144. [Google Scholar] [CrossRef]
- Freudenberg, K.; Neish, A.C. Constitution and Biosynthesis of Lignin; Springer: Berlin/Heidelberg, Germany, 1968. [Google Scholar]
- Freudenberg, K. Biosynthesis and constitution of lignin. Nature 1959, 183, 1152–1155. [Google Scholar] [CrossRef]
- Sun, S.; Huang, Y.; Sun, R.; Tu, M. The strong association of condensed phenolic moieties in isolated lignins with their inhibition of enzymatic hydrolysis. Green Chem. 2016, 18, 4276–4286. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Comparative antioxidant and cytotoxic effects of lignins from different sources. Bioresour. Technol. 2008, 99, 6683–6687. [Google Scholar] [CrossRef]
- Morganti, P.; Fusco, A.; Paoletti, I.; Perfetto, B.; Del Ciotto, P.; Palombo, M.; Chianese, A.; Baroni, A.; Donnarumma, G. Anti-inflammatory, immunomodulatory, and tissue repair activity on human keratinocytes by green innovative nanocomposites. Materials 2017, 10, 843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kai, D.; Wei, R.; Lingling, T.; Chee, P.; Liu, Y.; Ramakrishna, S.; Loh, X. Engineering poly(lactide)–lignin nanofibers with antioxidant activity for biomedical application. ACS Sustain. Chem. Eng. 2016, 4, 5268–5276. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Qasem, A.A.; Alshatwi, A.A. Borassus flabellifer biomass lignin: Isolation and characterization of its antioxidant and cytotoxic properties. Sustain. Chem. Pharm. 2018, 10, 89–96. [Google Scholar] [CrossRef]
- Cigan, A.D.; Roach, B.L.; Nims, R.J.; Tan, A.R.; Albro, M.B.; Stoker, A.M.; Cook, J.L.; Vunjak-Novakovic, G.; Hung, C.T.; Ateshian, G.A. High seeding density of human chondrocytes in agarose produces tissue-engineered cartilage approaching native mechanical and biochemical properties. J. Biomech. 2016, 49, 1909–1917. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.K.; Ogando, C.R.; Barabino, G.A. In vitro evaluation of the influence of substrate mechanics on matrix-assisted human chondrocyte transplantation. J. Funct. Biomater. 2020, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Buschmann, M.D.; Gluzband, Y.A.; Grodzinsky, A.J.; Kimura, J.H.; Hunziker, E.B. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J. Orthop. Res. 1992, 10, 745–758. [Google Scholar] [CrossRef]
- Quinn, T.M.; Schmid, P.; Hunziker, E.B.; Grodzinsky, A.J. Proteoglycan deposition around chondrocytes in agarose culture: Construction of a physical and biological interface for mechanotransduction in cartilage. Biorheology 2002, 39, 27–37. [Google Scholar]
- Nguyen, B.V.; Wang, Q.G.; Kuiper, N.J.; El Haj, A.J.; Thomas, C.R.; Zhang, Z. Biomechanical properties of single chondrocytes and chondrons determined by micromanipulation and finite-element modelling. J. R. Soc. Interface 2010, 7, 1723–1733. [Google Scholar] [CrossRef]
- Mahata, D.; Jana, M.; Jana, A.; Mukherjee, A.; Mondal, N.; Saha, T.; Sen, S.; Nando, G.B.; Mukhopadhyay, C.K.; Chakraborty, R.; et al. Lignin-graft-polyoxazoline conjugated triazole a novel anti-infective ointment to control persistent inflammation. Sci. Rep. 2017, 7, 46412. [Google Scholar] [CrossRef] [Green Version]
- Semel, G.; Wolff, A.; Shilo, D.; Akrish, S.; Emodi, O.; Rachmiel, A. Mandibular osteomyelitis associated with dental implants. A case series. Eur. J. Oral Implantol. 2016, 9, 435–442. [Google Scholar]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| SEC Data | 31P NMR Data | DSC Data | Hansen Solubility Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fraction | MW (g/mol) | Polydispersity Index (Mw/Mn) | Aliphatic OH (mmol/g) | Phenolic OH (mmol/g) | S/G Ratio | Tg/°C | δD(MPa1/2) | δP(MPa1/2) | δH(MPa1/2) |
| Raw lignin | 3723 | 3.2 | 2.43 * | 2.20 * | 2.0 * | ||||
| Low MW fraction | 2543 | 2.2 | 2.72 | 1.91 | 2.62 | 123 | 21.0 | 15.4 | 17.7 |
| High MW fraction | 6025 | 3.1 | 1.95 | 1.87 | 2.10 | 174 | 19.1 | 12.9 | 12.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menima-Medzogo, J.A.; Walz, K.; Lauer, J.C.; Sivasankarapillai, G.; Gleuwitz, F.R.; Rolauffs, B.; Laborie, M.-P.; Hart, M.L. Characterization and In Vitro Cytotoxicity Safety Screening of Fractionated Organosolv Lignin on Diverse Primary Human Cell Types Commonly Used in Tissue Engineering. Biology 2022, 11, 696. https://doi.org/10.3390/biology11050696
Menima-Medzogo JA, Walz K, Lauer JC, Sivasankarapillai G, Gleuwitz FR, Rolauffs B, Laborie M-P, Hart ML. Characterization and In Vitro Cytotoxicity Safety Screening of Fractionated Organosolv Lignin on Diverse Primary Human Cell Types Commonly Used in Tissue Engineering. Biology. 2022; 11(5):696. https://doi.org/10.3390/biology11050696
Chicago/Turabian StyleMenima-Medzogo, Jules A., Kathrin Walz, Jasmin C. Lauer, Gopakumar Sivasankarapillai, F. Robert Gleuwitz, Bernd Rolauffs, Marie-Pierre Laborie, and Melanie L. Hart. 2022. "Characterization and In Vitro Cytotoxicity Safety Screening of Fractionated Organosolv Lignin on Diverse Primary Human Cell Types Commonly Used in Tissue Engineering" Biology 11, no. 5: 696. https://doi.org/10.3390/biology11050696
APA StyleMenima-Medzogo, J. A., Walz, K., Lauer, J. C., Sivasankarapillai, G., Gleuwitz, F. R., Rolauffs, B., Laborie, M.-P., & Hart, M. L. (2022). Characterization and In Vitro Cytotoxicity Safety Screening of Fractionated Organosolv Lignin on Diverse Primary Human Cell Types Commonly Used in Tissue Engineering. Biology, 11(5), 696. https://doi.org/10.3390/biology11050696










