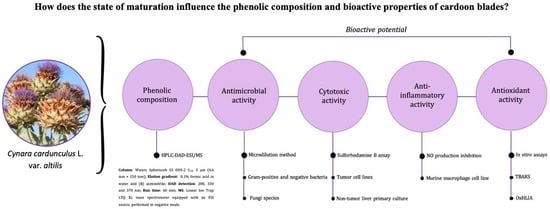
Phenolic Composition and Antioxidant, Anti-Inflammatory, Cytotoxic, and Antimicrobial Activities of Cardoon Blades at Different Growth Stages
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction
2.3. Polyphenolic Composition Analysis
2.4. In Vitro Evaluation of Biological Activities
2.4.1. Antioxidant Activity
2.4.2. Cytotoxic and Hepatotoxic Activities
2.4.3. Anti-Inflammatory Activity
2.4.4. Antimicrobial Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Compounds Composition
3.2. Bioactive Properties
3.2.1. Antioxidant Activity
3.2.2. Cytotoxic Activity
3.2.3. Hepatotoxic Activity
3.2.4. Anti-Inflammatory Activity
3.2.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conceição, C.; Martins, P.; Alvarenga, N.; Dias, J.; Lamy, E.; Garrido, L.; Gomes, S.; Freitas, S.; Belo, A.; Brás, T.; et al. Cynara cardunculus: Use in Cheesemaking and Pharmaceutical Applications. In Technological Approaches for Novel Applications in Dairy Processing; IntechOpen: London, UK, 2012; pp. 73–107. [Google Scholar]
- De Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Zayed, A.; Serag, A.; Farag, M.A. Cynara cardunculus L.: Outgoing and potential trends of phytochemical, industrial, nutritive and medicinal merits. J. Funct. Foods 2020, 69, 103937. [Google Scholar] [CrossRef]
- Gostin, A.I.; Waisundara, V.Y. Edible flowers as functional food: A review on artichoke (Cynara cardunculus L.). Trends Food Sci. Technol. 2019, 86, 381–391. [Google Scholar] [CrossRef]
- Gominho, J.; Curt, M.D.; Lourenço, A.; Fernández, J.; Pereira, H. Cynara cardunculus L. as a biomass and multi-purpose crop: A review of 30 years of research. Biomass Bioenergy 2018, 109, 257–275. [Google Scholar] [CrossRef]
- Ierna, A.; Sortino, O.; Mauromicale, G. Biomass, seed and energy yield of Cynara cardunculus L. as affected by environment and season. Agronomy 2020, 10, 1548. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Barreira, J.C.M.; Alves, M.J.; Barracosa, P.; Ferreira, I.C.F.R. Phenolic profile and bioactivity of cardoon (Cynara cardunculus L.) inflorescence parts: Selecting the best genotype for food applications. Food Chem. 2018, 268, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Mandim, F.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Kostić, M.; Soković, M.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Phenolic composition and biological properties of Cynara cardunculus L. var. altilis petioles: Influence of the maturity stage. Antioxidants 2021, 10, 1907. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Pereira, C.; Tzortzakis, N.; Vaz, J.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Bioactivities, chemical composition and nutritional value of Cynara cardunculus L. seeds. Food Chem. 2019, 289, 404–412. [Google Scholar] [CrossRef]
- Khaldi, S.; Naouari, M.; Ben Jemaa, A. Cardoon (Cynara cardunculus L.) oil from cultivated and wild Tunisian populations and its antimicrobial activity. Ind. Crops Prod. 2021, 171, 113852. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Kostic, M.; Soković, M.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Seasonal variation in bioactive properties and phenolic composition of cardoon (Cynara cardunculus var. altilis) bracts. Food Chem. 2021, 336, 127744. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Giannoulis, K.D.; Dias, M.I.; Fernandes, Â.; Pinela, J.; Kostic, M.; Soković, M.; Barros, L.; Santos-Buelga, C.; et al. Seasonal variation of bioactive properties and phenolic composition of Cynara cardunculus var. altilis. Food Res. Int. 2020, 134, 109281. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.M.; Simões, I. Cardoon-based rennets for cheese production. Appl. Microbiol. Biotechnol. 2018, 102, 4675–4686. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.H.; Andrade, M.A.; Vilarinho, F.; Castanheira, I.; Fernando, A.L.; Loizzo, M.R.; Silva, A.S. A new insight on cardoon: Exploring new uses besides cheese making with a view to zero waste. Foods 2020, 9, 564. [Google Scholar] [CrossRef] [PubMed]
- Chihoub, W.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C.F.R. Valorisation of the green waste parts from turnip, radish and wild cardoon: Nutritional value, phenolic profile and bioactivity evaluation. Food Res. Int. 2019, 126, 108651. [Google Scholar] [CrossRef] [Green Version]
- Cabiddu, A.; Contini, S.; Gallo, A.; Lucini, L.; Bani, P.; Decandia, M.; Molle, G.; Piluzza, G.; Sulas, L. In vitro fermentation of cardoon seed press cake—A valuable byproduct from biorefinery as a novel supplement for small ruminants. Ind. Crops Prod. 2019, 130, 420–427. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Phenolic acids and flavonoids in leaf and floral stem of cultivated and wild Cynara cardunculus L. genotypes. Food Chem. 2011, 126, 417–422. [Google Scholar] [CrossRef]
- Haghani, S.; Hadidi, M.; Pouramin, S.; Adinepour, F.; Hasiri, Z.; Moreno, A.; Munekata, P.E.S.; Lorenzo, J.M. Application of Cornelian Cherry (Cornus mas L.) Peel in Probiotic Ice Cream: Functionality and Viability during Storage. Antioxidants 2021, 10, 1777. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Pinela, J.; Dias, M.I.; Giannoulis, K.D.; Kostić, M.; Soković, M.; Queijo, B.; Santos-Buelga, C.; Ferreira, I.C.F.R.; et al. Chemical composition and biological activity of cardoon (Cynara cardunculus L. var. altilis) seeds harvested at different maturity stages. Food Chem. 2022, 369, 130875. [Google Scholar] [CrossRef]
- Francaviglia, R.; Bruno, A.; Falcucci, M.; Farina, R.; Renzi, G.; Russo, D.E.; Sepe, L.; Neri, U. Yields and quality of Cynara cardunculus L. wild and cultivated cardoon genotypes. A case study from a marginal land in Central Italy. Eur. J. Agron. 2016, 72, 10–19. [Google Scholar] [CrossRef]
- Archontoulis, S.V.; Struik, P.C.; Vos, J.; Danalatos, N.G. Phenological growth stages of Cynara cardunculus: Codification and description according to the BBCH scale. Ann. Appl. B 2013, 156, 253–270. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Mandim, F.; Barros, L.; Calhelha, R.C.; Abreu, R.M.V.; Pinela, J.; Alves, M.J.; Heleno, S.; Santos, P.F.; Ferreira, I.C.F.R. Calluna vulgaris (L.) Hull: Chemical characterization, evaluation of its bioactive properties and effect on the vaginal microbiota. Food Funct. 2019, 10, 78–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roriz, C.L.; Xavier, V.; Heleno, S.A.; Pinela, J.; Dias, M.I.; Calhelha, R.C.; Morales, P.; Ferreira, I.C.F.R.; Barros, L. Chemical and bioactive features of Amaranthus caudatus L. flowers and optimized ultrasound-assisted extraction of betalains. Foods 2021, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Pinela, J.; Dias, M.I.; Calhelha, R.C.; Alves, M.J.; Mocan, A.; García, P.A.; Barros, L.; Ferreira, I.C.F.R. Exploring the phytochemical profile of Cytinus hypocistis (L.) L. as a source of health-promoting biomolecules behind its in vitro bioactive and enzyme inhibitory properties. Food Chem. Toxicol. 2020, 136, 111071. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Â.; Liberal, Â.; Pinela, J.; Finimundy, T.C.; Bancessi, A.; Ćirić, A.; Soković, M.; Catarino, L.; Ferreira, I.C.F.R.; Barros, L. Compositional features and biological activities of wild and commercial Moringa oleifera leaves from Guinea-Bissau. Food Biosci. 2021, 43, 101300. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Santos, S.A.O.; Guerra, Â.R.; Guerreiro, O.; Freire, C.S.R.; Rocha, S.M.; Duarte, M.F.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of different morphological parts of Cynara cardunculus L. var. altilis (DC). Ind. Crops Prod. 2014, 61, 460–471. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Tzortzakis, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional value and bioactive compounds characterization of plant parts from Cynara cardunculus L. (Asteraceae) cultivated in central Greece. Front. Plant Sci. 2018, 9, 459. [Google Scholar] [CrossRef] [Green Version]
- Mandim, F.; Dias, M.I.; Pinela, J.; Barracosa, P.; Ivanov, M.; Ferreira, I.C.F.R. Chemical composition and in vitro biological activities of cardoon (Cynara cardunculus L. var. altilis DC.) seeds as influenced by viability. Food Chem. 2020, 323, 126838. [Google Scholar] [CrossRef]
- Shallan, M.A.; Ali, M.A.; Meshrf, W.A.; Marrez, D.A. In vitro antimicrobial, antioxidant and anticancer activities of globe artichoke (Cynara cardunculus var. scolymus L.) bracts and receptacles ethanolic extract. Biocatal. Agric. Biotechnol. 2020, 29, 101774. [Google Scholar] [CrossRef]
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) byproducts as a potential source of health-promoting antioxidant phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Guerra, Â.R.; Guerreiro, O.; Santos, S.A.O.; Oliveira, H.; Freire, C.S.R.; Silvestre, A.J.D.; Duarte, M.F. Antiproliferative effects of Cynara cardunculus L. var. altilis (DC) lipophilic extracts. Int. J. Mol. Sci. 2017, 18, 63. [Google Scholar] [CrossRef] [PubMed]

| Sampling Date | Principal Growth Stage |
|---|---|
| 10 September | PGS 1 |
| 10 October | |
| 10 November | |
| 30 November | PGS 2 |
| 9 January | PGS 3 |
| 8 February | PGS 3/4 |
| 8 March | PGS 4 |
| 7 April | PGS 4/5 |
| 26 April | PGS 5 |
| 10 May | PGS 5/6 |
| 24 May | PGS 6 |
| 12 June | PGS 6/7 |
| 4 July | PGS 7/8 |
| 18 July | |
| 9 August | PGS 8 |
| 29 August | PGS 9 |
| Peak | Rt (min) | λmax (nm) | [M-H]− (m/z) | MS2 (m/z) | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 4.50 | 327 | 353 | 191 (100), 179 (5), 161 (5), 135 (5) | 3-O-Caffeoylquinic acid |
| 2 | 6.69 | 266 | 153 | 109(100) | Protocatechuic acid |
| 3 | 6.80 | 326 | 353 | 173 (100), 179 (11), 191 (10), 161 (5), 135 (5) | 4-O-Caffeoylquinic acid |
| 4 | 7.01 | 326 | 353 | 191 (100), 179 (10), 161 (5), 135 (5) | cis 5-O-Caffeoylquinic acid |
| 5 | 7.11 | 326 | 353 | 191 (100), 179 (7), 173 (5), 135 (5) | trans 5-O-Caffeoylquinic acid |
| 6 | 10.43 | 326 | 353 | 191 (100), 179 (11), 161 (5), 135 (5) | Caffeoylquinic acid derivate |
| 7 | 16.13 | 285/sh324 | 463 | 287 (100) | Eriodictyol-O-hexuronoside |
| 8 | 16.83 | 322 | 515 | 353 (100), 335 (26), 191 (64), 17 9(10) | cis 1,3-Di-O-caffeoylquinic acid |
| 9 | 18.49 | 345 | 461 | 285 (100) | Luteolin-O-hexuronoside derivative I |
| 10 | 18.70 | 334 | 515 | 353 (100), 179 (35), 173 (29), 353 (10), 191 (10), 135 (8), 161 (5) | trans 1,3-Di-O-caffeyolquinic acid |
| 11 | 18.78 | 345 | 461 | 285 (100) | Luteolin-O-hexuronoside derivative II |
| 12 | 18.93 | 340 | 447 | 285 (100) | Luteolin-O-hexoside |
| 13 | 20.41 | 344 | 515 | 353 (100), 191 (12), 335 (10) | cis 1,5-Di-O-cafffeoylquinic acid |
| 14 | 20.50 | 328 | 515 | 353 (100), 191 (5), 335 (12) | trans 1,5-Di-O-cafffeoylquinic acid |
| 15 | 22.66 | 329 | 515 | 353 (100), 335 (5), 229 (3), 255 (5), 203 (6), 191 (69), 179 (12), 173 (4, MS3 base peak) | cis 3,4-Di-O-cafffeoylquinic acid |
| 16 | 22.70 | 329 | 515 | 353 (100), 335 (5), 229 (3), 255 (6), 203 (3), 191 (76), 179 (11), 173 (5, MS3 base peak) | trans 3,4-Di-O-cafffeoylquinic acid |
| 17 | 22.89 | 329 | 515 | 353 (100), 335 (32), 191 (20), 179 (12) | cis 3,5-Di-O-cafffeoylquinic acid |
| 18 | 23.65 | 332 | 533 | 489 (100), 285 (20) | Luteolin-O-malonyl hexoside derivative I |
| 19 | 23.67 | 346 | 533 | 489 (50), 447 (5), 285 (100) | Luteolin-O-malonyl hexoside derivative II |
| 20 | 25.60 | 330 | 515 | 353 (100), 191 (13, MS3 base peak) | trans 3,5-Di-O-caffeolyquinic acid |
| Sample | Compound 1 | Compound 2 | Compound 3 | Compound 4 | Compound 5 | Compound 6 | Compound 7 | Compound 8 | Compound 9 | Compound 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| B1 | n.d. | n.d. | 3.5 ± 0.1 d | n.d. | 25.5 ± 0.6 a | n.d. | n.d. | n.d. | n.d. | 3.08 ± 0.05 d |
| B2 | n.d. | n.d. | 3.81 ± 0.02 c | n.d. | 3.33 ± 0.03 e | n.d. | n.d. | n.d. | n.d. | 3.4 ± 0.1 c |
| B3 | n.d. | n.d. | 5.4 ± 0.2 b | n.d. | 19.5 ± 0.3 c | n.d. | n.d. | n.d. | n.d. | 3.6 ± 0.1 b |
| B4 | 1.530 ± 0.002 e | 1.68 ± 0.04 b | n.d. | 42.6 ± 0.1 a | n.d. | 2.6 ± 0.1 c | 0.174 ± 0.001 h | 1.241 ± 0.003 c | 2.988 ± 0.003 d | n.d. |
| B5 | 1.212 ± 0.005 f | 1.383 ± 0.002 d | n.d. | 36.63 ± 0.02 b | n.d. | 2.124 ± 0.005 ef | 0.8220 ± 0.0003 e | 0.66 ± 0.02 j | 2.7 ± 0.1 e | n.d. |
| B6 | 2.90 ± 0.01 d | 2.53 ± 0.03 a | n.d. | 35.47 ± 0.02 c | n.d. | 4.06 ± 0.02 a | 0.83 ± 0.01 e | 0.86 ± 0.01 g | 0.417 ± 0.005 k | n.d. |
| B7 | 0.584 ± 0.003 i | 1.18 ± 0.02 e | n.d. | 31.05 ± 0.05 d | n.d. | 3.120 ± 0.003 b | 0.934 ± 0.001 c | 1.04 ± 0.01 e | 2.23 ± 0.02 f | n.d. |
| B8 | 1.206 ± 0.001 f | 0.825 ± 0.003 h | n.d. | 26.1 ± 0.2 f | n.d. | 2.01 ± 0.04 g | 0.979 ± 0.002 b | 0.97 ± 0.01 f | 3.19 ± 0.02 c | n.d. |
| B9 | 0.88 ± 0.01 h | 1.53 ± 0.04 c | n.d. | 21.21 ± 0.04 h | n.d. | 2.171 ± 0.004 e | 1.05 ± 0.01 a | 1.05 ± 0.01 e | 7.3 ± 0.1 a | n.d. |
| B10 | 3.15 ± 0.03 c | 1.38 ± 0.01 d | n.d. | 29.8 ± 0.3 e | n.d. | 2.52 ± 0.05 d | 0.86 ± 0.01 d | 0.707 ± 0.005 i | 1.946 ± 0.001 h | n.d. |
| B11 | 5.56 ± 0.01 b | 1.15 ± 0.02 e | n.d. | 24.6 ± 0.1 g | n.d. | 1.33 ± 0.02 h | 0.636 ± 0.002 g | 0.761 ± 0.003 h | 1.45 ± 0.01 j | n.d. |
| B12 | 6.6 ± 0.1 a | 1.061 ± 0.003 f | n.d. | 20.5 ± 0.3 j | n.d. | 1.31 ± 0.01 h | 0.82 ± 0.02 e | 1.193 ± 0.003 d | 1.68 ± 0.03 i | n.d. |
| B13 | 1.1623 ± 0.0003 g | 1.416 ± 0.003 d | n.d. | 20.83 ± 0.03 i | n.d. | 1.98 ± 0.01 g | 0.93 ± 0.01 c | 1.37 ± 0.02 a | 3.93 ± 0.01 b | n.d. |
| B14 | 0.91 ± 0.02 h | 0.89 ± 0.01 g | n.d. | 13.3 ± 0.3 k | n.d. | 2.09 ± 0.03 f | 0.78 ± 0.01 f | 1.28 ± 0.01 b | 2.1615 ± 0.0004 g | n.d. |
| B15 | n.d. | n.d. | 3.6 ± 0.1 d | n.d. | 11.63 ± 0.02 d | n.d. | n.d. | n.d. | n.d. | 1.9 ± 0.1 e |
| B16 | n.d. | n.d. | 8.2 ± 0.1 a | n.d. | 23.3 ± 0.03 b | n.d. | n.d. | n.d. | n.d. | 9.03 ± 0.02 a |
| Compound 11 | Compound 12 | Compound 13 | Compound 14 | Compound 15 | Compound 16 | Compound 17 | Compound 18 | Compound 19 | Compound 20 | |
| B1 | n.d. | 24.2 ± 0.1 e | n.d. | 33.5 ± 0.5 c | n.d. | 3.82 ± 0.01 d | n.d. | 6.70 ± 0.01 e | n.d. | n.d. |
| B2 | n.d. | 101 ± 1 a | n.d. | 36 ± 2 b | n.d. | 6.0 ± 0.3 b | n.d. | 18.6 ± 0.3 b | n.d. | n.d. |
| B3 | n.d. | 56.5 ± 2.5 b | n.d. | 31.1 ± 0.2 d | n.d. | 7.4 ± 0.2 a | n.d. | 54 ± 1 a | n.d. | n.d. |
| B4 | 3.335 ± 0.003 j | n.d. | 25.9 ± 0.1 b | n.d. | 3.8 ± 0.1 f | n.d. | 1.70 ± 0.01 g | n.d. | 9.4 ± 0.1 c | 0.80 ± 0.02 e |
| B5 | 10.6 ± 0.1 f | n.d. | 18.4 ± 0.5 e | n.d. | 2.6 ± 0.1 g | n.d. | 1.60 ± 0.01 h | n.d. | 9.07 ± 0.02 d | 0.84 ± 0.01 d |
| B6 | 13.9 ± 0.1 d | n.d. | 1.982 ± 0.001 h | n.d. | 7.34 ± 0.03 a | n.d. | 2.174 ± 0.002 b | n.d. | 15.9 ± 0. 2 b | 1.442 ± 0.003 a |
| B7 | 18.0 ± 0.3 b | n.d. | 19.0 ± 0.2 d | n.d. | 5.27 ± 0.01 b | n.d. | 1.67 ± 0.02 g | n.d. | 16.0 ± 0.1 b | 1.43 ± 0.03 a |
| B8 | 16.36 ± 0.04 c | n.d. | 31.73 ± 0.04 a | n.d. | 2.502 ± 0.002 h | n.d. | 1.49 ± 0.01 i | n.d. | 19.6 ± 0.1 a | 1.29 ± 0.01 b |
| B9 | 20.06 ± 0.03 a | n.d. | 7.14 ± 0.04 g | n.d. | 2.38 ± 0.02 i | n.d. | 1.99 ± 0.01 d | n.d. | 9.27 ± 0.03 c | 0.90 ± 0.01 c |
| B10 | 12.1 ± 0.1 e | n.d. | 17.291 ± 0.002 f | n.d. | 3.98 ± 0.002 e | n.d. | 2.0 ± 0.1 c | n.d. | 5.17 ± 0.02 f | 1.29 ± 0.02 b |
| B11 | 10.686 ± 0.003 f | n.d. | 20.8 ± 0.1 c | n.d. | 4.13 ± 0.02 d | n.d. | 1.83 ± 0.04 f | n.d. | 5.5 ± 0.1 e | 0.84 ± 0.02 d |
| B12 | 8.3 ± 0.1 h | n.d. | 1.697 ± 0.001 h | n.d. | 1.81 ± 0.04 k | n.d. | 1.88 ± 0.04 e | n.d. | 1.89 ± 0.01 h | 0.63 ± 0.01 h |
| B13 | 9.09 ± 0.04 g | n.d. | 19.12 ± 0.01 d | n.d. | 1.974 ± 0.002 j | n.d. | 2.020 ± 0.002 cd | n.d. | 1.640 ± 0.003 i | 0.719 ± 0.002 g |
| B14 | 7.8 ± 0.3 i | n.d. | 17.5 ± 0.1 f | n.d. | 4.66 ± 0.01 c | n.d. | 2.28 ± 0.03 a | n.d. | 2.2 ± 0.1 g | 0.76 ± 0.02 f |
| B15 | n.d. | 41 ± 1 c | n.d. | 18.17 ± 0.05 e | n.d. | 2.95 ± 0.03 e | n.d. | 9.1 ± 0.5 d | n.d. | n.d. |
| B16 | n.d. | 39.7 ± 0.4 d | n.d. | 39.6 ± 0.5 a | n.d. | 5.23 ± 0.01 c | n.d. | 9.1 ± 0.3 d | n.d. | n.d. |
| Sample | TBARS (IC50, µg/mL) | OxHLIA (IC50, µg/mL) | |
|---|---|---|---|
| Δt 60 min | Δt 120 min | ||
| B1 | 5.2 ± 0.1 l | 92 ± 2 b | 126 ± 3 bc |
| B2 | 3.0 ± 0.1 m | 96 ± 1 b | 137 ± 2 b |
| B3 | 1.61 ± 0.03 m | 99 ± 7 b | 173 ± 8 a |
| B4 | 46.7 ± 0.2 g | 26 ± 1 hi | 45 ± 1 i |
| B5 | 54 ± 2 e | 52 ± 1 defg | 95 ± 2 fg |
| B6 | 49.4 ± 0.3 f | 59 ± 2 cd | 111 ± 1 de |
| B7 | 43.0 ± 0.3 h | 44 ± 1 g | 78 ± 1 h |
| B8 | 11.6 ± 0.1 j | 25 ± 1 i | 47.4 ± 0.5 i |
| B9 | 85 ± 1 c | 58 ± 2 cde | 101 ± 3 efg |
| B10 | 38.4 ± 0.1 ij | 33 ± 1 h | 58 ± 1 i |
| B11 | 81.4 ± 0.1 d | 60 ± 3 cd | 116 ± 6 cd |
| B12 | 198 ± 1 a | 49.4 ± 0.3 fg | 88 ± 6 gh |
| B13 | 81.9 ± 0.2 d | 50 ± 3 efg | 95 ± 7 fg |
| B14 | 126 ± 3 b | 54 ± 2 cdef | 103 ± 3 def |
| B15 | 6.01 ± 0.04 l | 62 ± 2 c | 114 ± 2 cde |
| B16 | 8.7 ± 0.2 k | 112 ± 2 a | 183 ± 6 a |
| Trolox | 9.1 ± 0.3 | 21.2 ± 0.7 | 41.1 ± 0.8 |
| Sample | Cytotoxic Activity (GI50, µg/mL) | ||||
|---|---|---|---|---|---|
| MCF-7 | NCI-H460 | HeLa | HepG2 | PLP2 | |
| B1 | 30 ± 1 d | 27 ± 2 g | 24 ± 1 c | 21 ± 2 de | 61 ± 2 c |
| B2 | 24 ± 1 e | 20.5 ± 0.9 h | 23 ± 1 cd | 25 ± 2 c | 41 ± 1 e |
| B3 | 58 ± 4 b | 53 ± 2 c | 44.2 ± 0.4 b | 36 ± 1 b | 80 ± 3 b |
| B4 | 38 ± 3 c | 47 ± 1 d | 20 ± 2 de | 23 ± 1 cd | 51 ± 1 d |
| B5 | 42 ± 3 c | 40.7 ± 1.5 e | 16 ± 1 g | 45 ± 4 a | 52 ± 1 d |
| B6 | 24.5 ± 1.2 e | 33.5 ± 1.0 f | 20 ± 1 ef | 15 ± 1 ef | 41 ± 4 e |
| B7 | 87 ± 4 a | 89 ± 8 a | 9.3 ± 0.5 h | 18 ± 2 e | 95 ± 2 a |
| B8 | 8 ± 1 h | 8.7 ± 0.4 j | 10.0 ± 0.3 h | 13 ± 1 fg | 17.9 ± 1.5 h |
| B9 | 10.1 ± 0.3 h | 40 ± 1 e | 9 ± 1 h | 11.1 ± 0.4 gh | 42 ± 2 e |
| B10 | 16 ± 1 g | 16 ± 1 hi | 10 ± 1 h | 16 ± 1 ef | 24.1 ± 2.5 g |
| B11 | 7.1 ± 0.5 h | 15.0 ± 0.4 i | 11 ± 1 h | 13 ± 1 fg | 17.1 ± 1.5 h |
| B12 | 10 ± 1 h | 10 ± 1 j | 18 ± 2 fg | 9.1 ± 0.4 h | 21 ± 2 gh |
| B13 | 9.0 ± 0.8 h | 12 ± 1 ij | 9.0 ± 0.4 h | 9 ± 1 h | 17 ± 2 h |
| B14 | 19 ± 1 fg | 15 ± 1 i | 16 ± 1 g | 16 ± 1 ef | 30.5 ± 1.1 f |
| B15 | 22 ± 1 ef | 26 ± 1 g | 20.5 ± 1.5 de | 17 ± 1 e | 48 ± 2 d |
| B16 | 62 ± 1 b | 76 ± 1 b | 51 ± 3 a | 38 ± 3 b | 95 ± 2 a |
| Ellipticine | 1.21 ± 0.02 | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.10 ± 0.09 | 2.3 ± 0.2 |
| Sample | NO Production Inhibition (IC50, µg/mL) |
|---|---|
| B1 | 30 ± 3 ef |
| B2 | 32 ± 2 e |
| B3 | 53 ± 5 b |
| B4 | 48 ± 2 c |
| B5 | 39 ± 2 d |
| B6 | 27 ± 1 f |
| B7 | 72 ± 3 a |
| B8 | 24.6 ± 0.5 g |
| B9 | 32 ± 1 e |
| B10 | 16.2 ± 0.4 g |
| B11 | 13.5 ± 0.9 gh |
| B12 | 12 ± 1 gh |
| B13 | 10 ± 1 h |
| B14 | 16 ± 1 g |
| B15 | 30 ± 3 ef |
| B16 | 56 ± 2 b |
| Dexamethasone | 16 ± 1 |
| Antibacterial Activity (mg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | B. cereus | S. aureus | L. monocytogenes | E. cloacae | E. coli | S. typhimurium | ||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| B1 | 0.58 | 1.17 | 1.17 | 2.33 | 1.17 | 2.33 | 1.17 | 2.33 | 1.17 | 2.33 | 1.17 | 2.33 |
| B2 | 0.58 | 1.15 | 2.31 | 4.61 | 1.15 | 2.31 | 1.15 | 2.31 | 1.15 | 2.31 | 1.15 | 2.31 |
| B3 | 1.17 | 2.33 | 2.33 | 4.66 | 0.58 | 1.17 | 2.33 | 4.66 | 1.17 | 2.33 | 2.33 | 4.66 |
| B4 | 0.91 | 1.81 | 1.81 | 3.63 | 3.63 | 7.26 | 3.63 | 7.26 | 0.91 | 1.81 | 1.81 | 3.63 |
| B5 | 0.86 | 1.72 | 1.72 | 3.43 | 1.72 | 3.43 | 1.72 | 3.43 | 0.86 | 1.72 | 1.72 | 3.43 |
| B6 | 3.07 | 3.07 | 3.07 | 3.07 | 3.07 | 6.15 | 1.54 | 3.07 | 1.54 | 3.07 | 1.54 | 3.07 |
| B7 | 3.57 | 3.57 | 0.89 | 0.89 | 1.78 | 3.57 | 1.78 | 3.57 | 1.78 | 3.57 | 1.78 | 3.57 |
| B8 | 0.80 | 1.60 | 0.80 | 11.60 | 1.60 | 3.21 | 1.60 | 3.21 | 0.80 | 1.60 | 1.60 | 3.21 |
| B9 | 1.61 | 1.61 | 0.81 | 1.61 | 0.81 | 3.22 | 0.81 | 1.61 | 0.81 | 1.61 | 0.81 | 1.61 |
| B10 | 0.89 | 1.78 | 1.78 | 3.55 | 1.78 | 3.55 | 3.55 | 7.11 | 1.78 | 3.55 | 3.55 | 7.11 |
| B11 | 0.78 | 1.55 | 1.55 | 3.10 | 1.55 | 3.10 | 1.55 | 3.10 | 1.55 | 3.10 | 1.55 | 3.10 |
| B12 | 0.43 | 0.87 | 1.74 | 3.48 | 1.74 | 3.48 | 1.74 | 3.48 | 3.48 | 6.96 | 3.48 | 6.96 |
| B13 | 0.77 | 1.54 | 0.77 | 1.54 | 0.77 | 3.07 | 0.77 | 3.07 | 0.77 | 3.07 | 0.77 | 3.07 |
| B14 | 0.82 | 1.63 | 3.27 | 6.54 | 1.63 | 3.27 | 1.63 | 3.27 | 1.63 | 3.27 | 3.27 | 6.54 |
| B15 | 0.58 | 1.16 | 2.32 | 4.64 | 2.32 | 4.64 | 2.32 | 4.64 | 2.32 | 4.64 | 1.16 | 2.32 |
| B16 | 0.58 | 1.16 | 1.16 | 2.32 | 1.16 | 2.32 | 1.16 | 2.32 | 1.16 | 2.32 | 1.16 | 2.32 |
| Streptomycin | 0.10 | 0.20 | 0.04 | 0.10 | 0.20 | 0.30 | 0.20 | 0.30 | 0.20 | 0.30 | 0.20 | 0.30 |
| Ampicillin | 0.25 | 0.40 | 0.25 | 0.45 | 0.40 | 0.50 | 0.25 | 0.50 | 0.40 | 0.50 | 0.75 | 1.20 |
| Antifungal Activity (mg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | A. fumigatus | A. versicolor | A. niger | P. funiculosum | P. ochrochloron | P. verrucosum var. cyclopium | ||||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| B1 | 1.83 | 3.66 | 1.83 | 3.66 | 0.92 | 1.83 | 1.83 | 3.66 | 1.83 | 3.66 | 0.92 | 1.83 |
| B2 | 0.92 | 1.84 | 3.69 | 7.37 | 1.84 | 3.69 | 0.92 | 1.84 | 1.84 | 3.69 | 1.84 | 3.69 |
| B3 | 1.86 | 3.71 | 1.86 | 3.71 | 0.93 | 1.86 | 1.86 | 3.71 | 0.93 | 1.86 | 1.86 | 3.71 |
| B4 | 4.84 | 9.68 | 0.60 | 1.21 | >9.68 | >9.68 | 0.60 | 1.21 | 0.30 | 0.60 | 0.30 | 0.60 |
| B5 | 4.58 | 9.16 | 1.14 | 2.29 | >9.16 | >9.16 | 1.14 | 2.29 | 0.57 | 1.14 | 0.57 | 1.14 |
| B6 | 4.1 | 8.2 | 0.51 | 1.02 | >8.2 | >8.2 | 0.51 | 1.02 | 0.51 | 1.02 | 0.51 | 1.02 |
| B7 | 1.19 | 2.38 | 1.19 | 2.38 | 1.19 | 2.38 | 0.59 | 1.19 | 0.59 | 1.19 | 1.19 | 2.38 |
| B8 | 2.14 | 4.28 | 1.07 | 2.14 | >8.56 | >8.56 | 1.07 | 2.14 | 1.07 | 2.14 | 0.53 | 1.07 |
| B9 | 1.07 | 2.15 | 1.07 | 2.15 | 2.15 | 4.3 | 1.07 | 2.15 | 1.07 | 2.15 | 1.07 | 2.15 |
| B10 | 4.74 | 9.48 | 2.37 | 4.74 | >9.48 | >9.48 | 1.18 | 2.37 | 1.18 | 2.37 | 1.18 | 2.37 |
| B11 | 0.78 | 1.55 | 0.39 | 0.78 | 0.78 | 1.55 | 0.39 | 0.78 | 0.78 | 1.55 | 0.78 | 1.55 |
| B12 | 0.58 | 1.16 | 0.58 | 1.16 | 0.58 | 1.16 | 1.16 | 2.32 | 1.16 | 2.32 | 1.16 | 2.32 |
| B13 | 2.05 | 4.1 | 1.02 | 2.05 | 1.02 | 2.05 | 1.02 | 2.05 | 0.51 | 1.02 | 1.02 | 2.05 |
| B14 | 0.54 | 1.09 | 0.54 | 1.09 | 1.09 | 2.18 | 0.54 | 1.09 | 1.09 | 2.18 | 0.54 | 1.09 |
| B15 | 3.64 | 7.28 | 0.91 | 1.82 | 0.91 | 1.82 | 1.82 | 3.64 | 1.82 | 3.64 | 0.91 | 1.82 |
| B16 | 3.69 | 7.37 | 0.92 | 1.84 | 1.84 | 3.69 | 0.92 | 1.84 | 0.92 | 1.84 | 1.84 | 3.69 |
| Ketoconazole | 0.25 | 0.50 | 0.2 | 0.5 | 0.2 | 0.5 | 0.2 | 0.5 | 1.0 | 1.5 | 0.2 | 0.3 |
| Bifonazole | 0.15 | 0.20 | 0.1 | 0.2 | 0.15 | 0.2 | 0.2 | 0.25 | 0.2 | 0.25 | 0.1 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandim, F.; Petropoulos, S.A.; Pinela, J.; Dias, M.I.; Kostic, M.; Soković, M.; Ferreira, I.C.F.R.; Santos-Buelga, C.; Barros, L. Phenolic Composition and Antioxidant, Anti-Inflammatory, Cytotoxic, and Antimicrobial Activities of Cardoon Blades at Different Growth Stages. Biology 2022, 11, 699. https://doi.org/10.3390/biology11050699
Mandim F, Petropoulos SA, Pinela J, Dias MI, Kostic M, Soković M, Ferreira ICFR, Santos-Buelga C, Barros L. Phenolic Composition and Antioxidant, Anti-Inflammatory, Cytotoxic, and Antimicrobial Activities of Cardoon Blades at Different Growth Stages. Biology. 2022; 11(5):699. https://doi.org/10.3390/biology11050699
Chicago/Turabian StyleMandim, Filipa, Spyridon A. Petropoulos, José Pinela, Maria Inês Dias, Marina Kostic, Marina Soković, Isabel C. F. R. Ferreira, Celestino Santos-Buelga, and Lillian Barros. 2022. "Phenolic Composition and Antioxidant, Anti-Inflammatory, Cytotoxic, and Antimicrobial Activities of Cardoon Blades at Different Growth Stages" Biology 11, no. 5: 699. https://doi.org/10.3390/biology11050699
APA StyleMandim, F., Petropoulos, S. A., Pinela, J., Dias, M. I., Kostic, M., Soković, M., Ferreira, I. C. F. R., Santos-Buelga, C., & Barros, L. (2022). Phenolic Composition and Antioxidant, Anti-Inflammatory, Cytotoxic, and Antimicrobial Activities of Cardoon Blades at Different Growth Stages. Biology, 11(5), 699. https://doi.org/10.3390/biology11050699