Lympho-Hematopoietic Microenvironments and Fish Immune System
Abstract
:Simple Summary
Abstract
1. Introduction
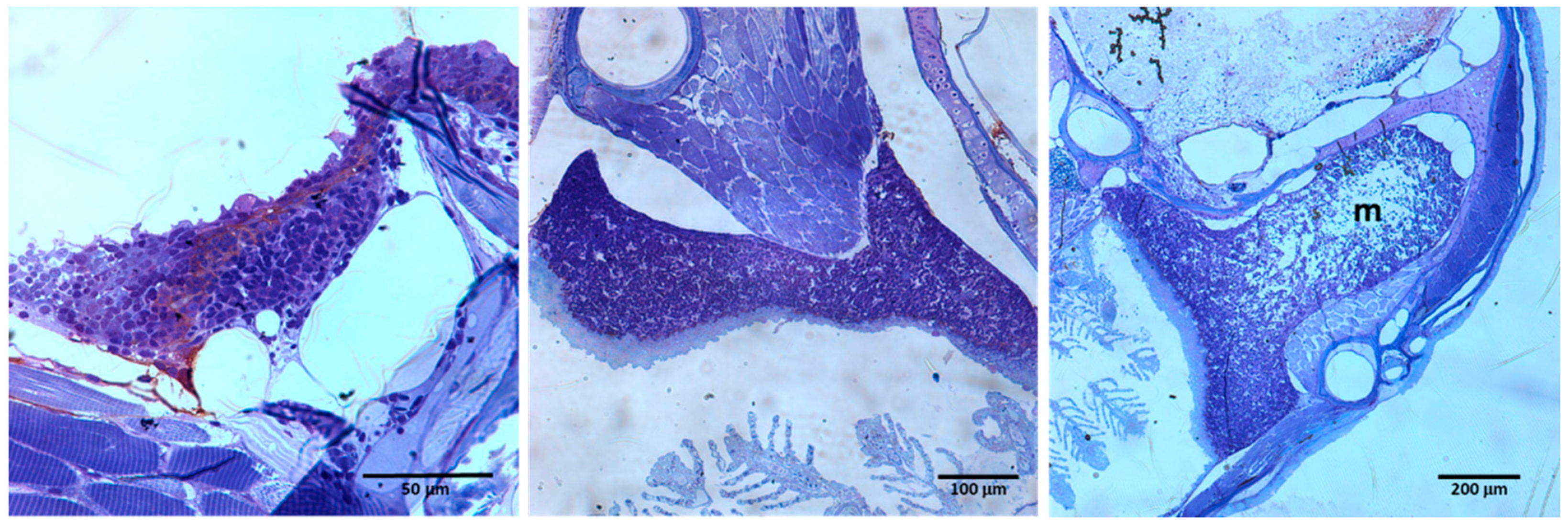
2. The Lympho-Hematopoietic Sites of Embryonic Teleost Fish and the Appearance of Their Primary and Secondary Lymphoid Organs
3. The Teleost Adult Lymphoid Organs
3.1. The Thymus: Histological Organization and Non-Lymphoid Cell Components
3.2. The Thymus: The Lymphoid Cell Components
3.3. The Thymus: Mechanisms That Govern Both Phenotypical T Cell Differentiation and Thymocyte Education
3.4. The Evolution of Foxn1/Foxn4 Family of Transcription Factors and the Thymic Epithelial Microenvironments
3.5. The Dual Condition of Teleost Kidney as a Primary Lymphoid Organ and Active Participant in the Immune Responses Resemble That of the Bone Marrow of Higher Vertebrates
3.6. The Spleen, the Immunological Memory and the Absence of Germinal Centers in Teleosts
3.7. The Mucosal-Associated Lymphoid Tissues (MALT) in Teleost Fish
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Du Pasquier, L. Meeting the demand for innate and adaptive immunities during evolution. Scand. J. Immunol. 2005, 62, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Boehm, T.; Hess, I.; Swann, J.B. Evolution of lymphoid tissues. Trends Immunol. 2012, 33, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Van de Peer, Y.; Maere, S.; Meyer, A. The evolutionary significance of ancient genome duplications. Nat. Rev. Genet. 2009, 10, 725–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.B. MHC and adaptive immunity in teleost fishes. Immunogenetics 2017, 69, 521–528. [Google Scholar] [CrossRef]
- Berthelot, C.; Brunet, F.; Chalopin, D.; Juanchich, A.; Bernard, M.; Noel, B.; Bento, P.; Da Silva, C.; Labadie, K.; Alberti, A.; et al. The rainbow trout genome provides novel insights into evolution after whole-genome duplication in vertebrates. Nat. Commun. 2014, 5, 3657. [Google Scholar] [CrossRef] [Green Version]
- Willett, C.E.; Cortes, A.; Zuasti, A.; Zapata, A.G. Early hematopoiesis and developing lymphoid organs in the zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1999, 214, 323–336. [Google Scholar] [CrossRef]
- Wattrus, S.J.; Zon, L.I. Stem cell safe harbor: The hematopoietic stem cell niche in zebrafish. Blood Adv. 2018, 2, 3063–3069. [Google Scholar] [CrossRef] [Green Version]
- Hartenstein, V. Blood cells and blood cell development in the animal kingdom. Annu. Rev. Cell Dev. Biol. 2006, 22, 677–712. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, J.Y.; Kim, A.D.; Violette, E.P.; Stachura, D.L.; Cisson, J.L.; Traver, D. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development 2007, 134, 4147–4156. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, J.Y.; Chi, N.C.; Santoso, B.; Teng, S.; Stainier, D.Y.; Traver, D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 2010, 464, 108–111. [Google Scholar] [CrossRef] [Green Version]
- Kissa, K.; Herbomel, P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 2010, 464, 112–115. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, J.; Feng, S.; He, S.; Zhao, S.; Zhu, L.; Jin, W.; Dai, Y.; Luo, L.; Qu, J.Y.; et al. The first wave of T lymphopoiesis in zebrafish arises from aorta endothelium independent of hematopoietic stem cells. J. Exp. Med. 2017, 214, 3347–3360. [Google Scholar] [CrossRef] [Green Version]
- Murayama, E.; Kissa, K.; Zapata, A.; Mordelet, E.; Briolat, V.; Lin, H.F.; Handin, R.I.; Herbomel, P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 2006, 25, 963–975. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Xu, J.; Wen, Z. Migratory path of definitive hematopoietic stem/progenitor cells during zebrafish development. Blood 2007, 109, 5208–5214. [Google Scholar] [CrossRef]
- Jin, H.; Sood, R.; Xu, J.; Zhen, F.; English, M.A.; Liu, P.P.; Wen, Z. Definitive hematopoietic stem/progenitor cells manifest distinct differentiation output in the zebrafish VDA and PBI. Development 2009, 136, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Tamplin, O.J.; Durand, E.M.; Carr, L.A.; Childs, S.J.; Hagedorn, E.J.; Li, P.; Yzaguirre, A.D.; Speck, N.A.; Zon, L.I. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 2015, 160, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Mahony, C.B.; Fish, R.J.; Pasche, C.; Bertrand, J.Y. tfec controls the hematopoietic stem cell vascular niche during zebrafish embryogenesis. Blood 2016, 128, 1336–1345. [Google Scholar] [CrossRef] [Green Version]
- Mahony, C.B.; Pasche, C.; Bertrand, J.Y. Oncostatin M and Kit-Ligand Control Hematopoietic Stem Cell Fate during Zebrafish Embryogenesis. Stem Cell Rep. 2018, 10, 1920–1934. [Google Scholar] [CrossRef]
- Blaser, B.W.; Moore, J.L.; Hagedorn, E.J.; Li, B.; Riquelme, R.; Lichtig, A.; Yang, S.; Zhou, Y.; Tamplin, O.J.; Binder, V.; et al. CXCR1 remodels the vascular niche to promote hematopoietic stem and progenitor cell engraftment. J. Exp. Med. 2017, 214, 1011–1027. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, Y.; Liu, F.; Wang, L. Rac2 Regulates the Migration of T Lymphoid Progenitors to the Thymus during Zebrafish Embryogenesis. J. Immunol. 2020, 204, 2447–2454. [Google Scholar] [CrossRef]
- Mold, J.E.; Venkatasubrahmanyam, S.; Burt, T.D.; Michaelsson, J.; Rivera, J.M.; Galkina, S.A.; Weinberg, K.; Stoddart, C.A.; McCune, J.M. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 2010, 330, 1695–1699. [Google Scholar] [CrossRef] [Green Version]
- Michaelsson, J.; Mold, J.E.; McCune, J.M.; Nixon, D.F. Regulation of T cell responses in the developing human fetus. J. Immunol. 2006, 176, 5741–5748. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.W.; Kim, M.Y.; Leibbrandt, A.; Parnell, S.M.; Jenkinson, W.E.; Glanville, S.H.; McConnell, F.M.; Scott, H.S.; Penninger, J.M.; Jenkinson, E.J.; et al. RANK signals from CD4+3− inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J. Exp. Med. 2007, 204, 1267–1272. [Google Scholar] [CrossRef]
- Danilova, N.; Steiner, L.A. B cells develop in the zebrafish pancreas. Proc. Natl. Acad. Sci. USA 2002, 99, 13711–13716. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y.M. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef]
- Zapata, A.; Diez, B.; Cejalvo, T.; Gutierrez-de Frias, C.; Cortes, A. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 2006, 20, 126–136. [Google Scholar] [CrossRef]
- Comazzetto, S.; Shen, B.; Morrison, S.J. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev. Cell 2021, 56, 1848–1860. [Google Scholar] [CrossRef]
- Shen, B.; Tasdogan, A.; Ubellacker, J.M.; Zhang, J.; Nosyreva, E.D.; Du, L.; Murphy, M.M.; Hu, S.; Yi, Y.; Kara, N.; et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature 2021, 591, 438–444. [Google Scholar] [CrossRef]
- Stosik, M.P.; Tokarz-Deptula, B.; Deptula, W. Specific humoral immunity in Osteichthyes. Cent. Eur. J. Immunol. 2018, 43, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, T.; Hu, Y.; Tian, G.; Secombes, C.J.; Wang, T. Expansion of fish CCL20_like chemokines by genome and local gene duplication: Characterisation and expression analysis of 10 CCL20_like chemokines in rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 2020, 103, 103502. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Wang, Z.L.; Choi, T.I.; Huang, W.T.; Wang, H.T.; Han, Y.Y.; Zhu, L.Y.; Kim, H.T.; Choi, J.H.; Lee, J.S.; et al. Chd7 Is Critical for Early T-Cell Development and Thymus Organogenesis in Zebrafish. Am. J. Pathol. 2018, 188, 1043–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Ning, G.; Yang, S.; Yan, Y.; Cao, Y.; Wang, Q. BMP signaling is required for nkx2.3-positive pharyngeal pouch progenitor specification in zebrafish. PLoS Genet. 2019, 15, e1007996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Wei, Y.; Liu, F. Regulatory mechanisms of thymus and T cell development. Dev. Comp. Immunol. 2013, 39, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Bjørgen, H.; Koppang, E.O. Anatomy of teleost fish immune structures and organs. Immunogenetics 2021, 73, 53–63. [Google Scholar] [CrossRef]
- Barraza, F.; Montero, R.; Wong-Benito, V.; Valenzuela, H.; Godoy-Guzman, C.; Guzman, F.; Kollner, B.; Wang, T.; Secombes, C.J.; Maisey, K.; et al. Revisiting the Teleost Thymus: Current Knowledge and Future Perspectives. Biology 2020, 10, 8. [Google Scholar] [CrossRef]
- Zapata, A.G.; Chibá, A.; Varas, A. Cells and Tissues of the Immune System of Fish. In The Fish Immune System. Organism, Pathogen, and Environment; Iwama, G., Nakanishi, T., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 1–62. [Google Scholar]
- Grevellec, A.; Tucker, A.S. The pharyngeal pouches and clefts: Development, evolution, structure and derivatives. Semin. Cell Biol. 2010, 21, 325–332. [Google Scholar] [CrossRef]
- Picchietti, S.; Abelli, L.; Guerra, L.; Randelli, E.; Proietti Serafini, F.; Belardinelli, M.C.; Buonocore, F.; Bernini, C.; Fausto, A.M.; Scapigliati, G. MHC II-beta chain gene expression studies define the regional organization of the thymus in the developing bony fish Dicentrarchus labrax (L.). Fish Shellfish Immunol. 2015, 42, 483–493. [Google Scholar] [CrossRef]
- Heinecke, R.D.; Chettri, J.K.; Buchmann, K. Adaptive and innate immune molecules in developing rainbow trout, Oncorhynchus mykiss eggs and larvae: Expression of genes and occurrence of effector molecules. Fish Shellfish Immunol. 2014, 38, 25–33. [Google Scholar] [CrossRef]
- Tatner, M.F.; Manning, M.J. The morphology of the trout, Salmo gairdneri Richardson, thymus: Some practical and theoretical considerations. J. Fish Biol. 1982, 21, 27–32. [Google Scholar] [CrossRef]
- Castillo, A.; Razquin, B.; Villena, A.; Zapata, A.G.; López-Fierro, P. Thymic barriers to antigen entry during the posthatching development of the thymus of rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immunol. 1998, 8, 157–170. [Google Scholar] [CrossRef]
- Chilmonczyk, S. The thymus in fish: Development and possible function in the immune response. Annu. Rev. Fish Dis. 1992, 2, 181–200. [Google Scholar] [CrossRef]
- Munoz, J.J.; Zapata, A.G. Thymus ontogeny and development. In Thymus Transcriptome and Cell Biology; Passos, G.A., Ed.; Springer: Cham, Switzerland, 2019; pp. 19–34. [Google Scholar]
- Romano, N.; Fanelli, M.; Maria Del Papa, G.; Scapigliati, G.; Mastrolia, L. Histological and cytological studies on the developing thymus of sharpsnout seabream, Diplodus puntazzo. J. Anat. 1999, 194, 39–50. [Google Scholar] [CrossRef]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Wen, Z.; Lam, T.J.; Sin, Y.M. Morphologic transformation of the thymus in developing zebrafish. Dev. Dyn. 2002, 225, 87–94. [Google Scholar] [CrossRef]
- Castillo, A.; Razquin, B.E.; Lopez-Fierro, P.; Alvarez, F.; Zapata, A.; Villena, A.J. Enzyme-and immuno-histochemical study of the thymic stroma in the rainbow trout, Salmo gairdneri, Richardson. Thymus 1990, 15, 153–166. [Google Scholar]
- Ronza, P.; Robledo, D.; Losada, A.P.; Bermudez, R.; Pardo, B.G.; Martinez, P.; Quiroga, M.I. The Teleost Thymus in Health and Disease: New Insights from Transcriptomic and Histopathological Analyses of Turbot, Scophthalmus maximus. Biology 2020, 9, 221. [Google Scholar] [CrossRef]
- Bajoghli, B.; Kuri, P.; Inoue, D.; Aghaallaei, N.; Hanelt, M.; Thumberger, T.; Rauzi, M.; Wittbrodt, J.; Leptin, M. Noninvasive In Toto Imaging of the Thymus Reveals Heterogeneous Migratory Behavior of Developing T Cells. J. Immunol. 2015, 195, 2177–2186. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Iwanami, N.; Hoa, V.Q.; Furutani-Seiki, M.; Takahama, Y. Noninvasive intravital imaging of thymocyte dynamics in medaka. J. Immunol. 2007, 179, 1605–1615. [Google Scholar] [CrossRef] [Green Version]
- Zapata, A. Lymphoid organs of teleost fish. I. Ultrastructure of the thymus of Rutilus rutilus. Dev. Comp. Immunol. 1981, 5, 427–436. [Google Scholar] [CrossRef]
- Gorgollon, P. Fine structure of the thymus in the adult cling fish Sicyases sanguineus (Pisces, Gobiesocidae). J. Morphol. 1983, 177, 25–40. [Google Scholar] [CrossRef]
- Fournier-Betz, V.; Quentel, C.; Lamour, F.; LeVen, A. Immunocytochemical detection of Ig-positive cells in blood, lymphoid organs and the gut associated lymphoid tissue of the turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2000, 10, 187–202. [Google Scholar] [CrossRef]
- Vigliano, F.A.; Losada, A.P.; Castello, M.; Bermudez, R.; Quiroga, M.I. Morphological and immunohistochemical characterisation of the thymus in juvenile turbot (Psetta maxima, L.). Cell Tissue Res. 2011, 346, 407–416. [Google Scholar] [CrossRef]
- Zapata, A. Lymphoid organs of teleost fist. II. Ultrastructure of renal lymphoid tissue of Rutilus rutilus and Gobio gobio. Dev. Comp. Immunol. 1981, 5, 685–690. [Google Scholar] [CrossRef]
- Törö, I.; Oláh, I. Electron microscopic study of guinea-pig thymus. Acta Morphol. Acad. Sci. Hung. 1966, 14, 275–290. [Google Scholar]
- Bornstein, C.; Nevo, S.; Giladi, A.; Kadouri, N.; Pouzolles, M.; Gerbe, F.; David, E.; Machado, A.; Chuprin, A.; Toth, B.; et al. Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature 2018, 559, 622–626. [Google Scholar] [CrossRef]
- Miller, C.N.; Proekt, I.; Von Moltke, J.; Wells, K.L.; Rajpurkar, A.R.; Wang, H.; Rattay, K.; Khan, I.S.; Metzger, T.C.; Pollack, J.L.; et al. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature 2018, 559, 627–631. [Google Scholar] [CrossRef]
- Cejalvo, T.; Munoz, J.J.; Tobajas, E.; Alfaro, D.; Garcia-Ceca, J.; Zapata, A. Conditioned deletion of ephrinB1 and/or ephrinB2 in either thymocytes or thymic epithelial cells alters the organization of thymic medulla and favors the appearance of thymic epithelial cysts. Histochem. Cell Biol. 2015, 143, 517–529. [Google Scholar] [CrossRef]
- Zapata, A.; Amemiya, C.T. Phylogeny of lower vertebrates and their immunological structures. Curr. Top. Microbiol. Immunol. 2000, 248, 67–107. [Google Scholar] [CrossRef]
- Nakamura, H.; Nakano, K.; Yasuda, M. The Ontogeny of Thymic Myoid Cells in the Chicken. Development 1986, 28, 185–190. [Google Scholar]
- Savino, W.; Santa-Rosa, G.L. The thymus gland in the loricariidean catfish Harttia sp. Dev. Comp. Immunol. 1982, 6, 375–380. [Google Scholar] [CrossRef]
- Yocum, D.; Cuchens, M.; Clem, L.W. The hapten-carrier effect in teleost fish. J. Immunol. 1975, 114, 925–927. [Google Scholar]
- Nakanishi, T. Effects of X-irradiation and thymectomy on the immune response of the marine teleost, Sebastiscus marmoratus. Dev. Comp. Immunol. 1986, 10, 519–527. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takizawa, F.; Fischer, U.; Dijkstra, J.M. Along the Axis between Type 1 and Type 2 Immunity; Principles Conserved in Evolution from Fish to Mammals. Biology 2015, 4, 814–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, T.; Takizawa, F.; Furihata, M.; Soto-Lampe, V.; Dijkstra, J.M.; Fischer, U. Teleost cytotoxic T cells. Fish Shellfish Immunol. 2019, 95, 422–439. [Google Scholar] [CrossRef] [PubMed]
- Laing, K.J.; Zou, J.J.; Purcell, M.K.; Phillips, R.; Secombes, C.J.; Hansen, J.D. Evolution of the CD4 family: Teleost fish possess two divergent forms of CD4 in addition to lymphocyte activation gene-3. J. Immunol. 2006, 177, 3939–3951. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.; Mitra, S.; Wyse, C.; Alnabulsi, A.; Zou, J.; Weerdenburg, E.M.; Van der Sar, A.M.; Wang, D.; Secombes, C.J.; Bird, S. First Demonstration of Antigen Induced Cytokine Expression by CD4-1+ Lymphocytes in a Poikilotherm: Studies in Zebrafish (Danio rerio). PLoS ONE 2015, 10, e0126378. [Google Scholar] [CrossRef] [Green Version]
- Kono, T.; Korenaga, H. Cytokine Gene Expression in CD4 Positive Cells of the Japanese Pufferfish, Takifugu rubripes. PLoS ONE 2013, 8, e66364. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Fang, W.; Xiang, L.X.; Pan, R.L.; Shao, J.Z. Identification of Treg-like cells in Tetraodon: Insight into the origin of regulatory T subsets during early vertebrate evolution. Cell. Mol. Life Sci. 2011, 68, 2615–2626. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Katakura, F.; Someya, K.; Dijkstra, J.M.; Moritomo, T.; Nakanishi, T. Clonal growth of carp (Cyprinus carpio) T cells in vitro: Long-term proliferation of Th2-like cells. Fish Shellfish Immunol. 2013, 34, 433–442. [Google Scholar] [CrossRef]
- Toda, H.; Saito, Y.; Koike, T.; Takizawa, F.; Araki, K.; Yabu, T.; Somamoto, T.; Suetake, H.; Suzuki, Y.; Ototake, M.; et al. Conservation of characteristics and functions of CD4 positive lymphocytes in a teleost fish. Dev. Comp. Immunol. 2011, 35, 650–660. [Google Scholar] [CrossRef]
- Takizawa, F.; Magadan, S.; Parra, D.; Xu, Z.; Korytář, T.; Boudinot, P.; Sunyer, J.O. Novel Teleost CD4-Bearing Cell Populations Provide Insights into the Evolutionary Origins and Primordial Roles of CD4+ Lymphocytes and CD4+ Macrophages. J. Immunol. 2016, 196, 4522–4535. [Google Scholar] [CrossRef] [Green Version]
- Aghaallaei, N.; Inoue, D.; Hasel de Carvalho, E.; Dick, A.M.; Wittbrodt, J.; Leptin, M.; Bajoghli, B. Notch1 deficiency alters the migratory behavior of developing T cells and calcium signaling in the thymus of medaka. Eur. J. Immunol. 2022, 52, 261–269. [Google Scholar] [CrossRef]
- Smelty, P.; Marchal, C.; Renard, R.; Sinzelle, L.; Pollet, N.; Dunon, D.; Jaffredo, T.; Sire, J.Y.; Fellah, J.S. Identification of the pre-T-cell receptor alpha chain in nonmammalian vertebrates challenges the structure-function of the molecule. Proc. Natl. Acad. Sci. USA 2010, 107, 19991–19996. [Google Scholar] [CrossRef] [Green Version]
- Hess, I.; Boehm, T. Intravital imaging of thymopoiesis reveals dynamic lympho-epithelial interactions. Immunity 2012, 36, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Bajoghli, B.; Aghaallaei, N.; Hess, I.; Rode, I.; Netuschil, N.; Tay, B.H.; Venkatesh, B.; Yu, J.K.; Kaltenbach, S.L.; Holland, N.D.; et al. Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates. Cell 2009, 138, 186–197. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Lu, Y.; He, Y.; Feng, Z.; Zhan, Y.; Huang, X.; Liu, Q.; Zhang, J.; Li, H.; Huang, H.; et al. Ikzf1 regulates embryonic T lymphopoiesis via Ccr9 and Irf4 in zebrafish. J. Biol. Chem. 2019, 294, 16152–16163. [Google Scholar] [CrossRef]
- Aghaallaei, N.; Dick, A.M.; Tsingos, E.; Inoue, D.; Hasel, E.; Thumberger, T.; Toyoda, A.; Leptin, M.; Wittbrodt, J.; Bajoghli, B. αβ/γδ T cell lineage outcome is regulated by intrathymic cell localization and environmental signals. Sci. Adv. 2021, 7, eabg3613. [Google Scholar] [CrossRef]
- Bajoghli, B.; Dick, A.M.; Claasen, A.; Doll, L.; Aghaallaei, N. Zebrafish and Medaka: Two Teleost Models of T-Cell and Thymic Development. Int. J. Mol. Sci. 2019, 20, 4179. [Google Scholar] [CrossRef] [Green Version]
- Laing, K.J.; Hansen, J.D. Fish T cells: Recent advances through genomics. Dev. Comp. Immunol. 2011, 35, 1282–1295. [Google Scholar] [CrossRef]
- Dijkstra, J.M.; Takizawa, F.; Fischer, U.; Friedrich, M.; Soto-Lampe, V.; Lefevre, C.; Lenk, M.; Karger, A.; Matsui, T.; Hashimoto, K. Identification of a gene for an ancient cytokine, interleukin 15-like, in mammals; interleukins 2 and 15 co-evolved with this third family member, all sharing binding motifs for IL-15Ralpha. Immunogenetics 2014, 66, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Lawir, D.F.; Hess, I.; Sikora, K.; Iwanami, N.; Siamishi, I.; Schorpp, M.; Boehm, T. Evolutionary transition from degenerate to nonredundant cytokine signaling networks supporting intrathymic T cell development. Proc. Natl. Acad. Sci. USA 2019, 116, 26759–26767. [Google Scholar] [CrossRef] [Green Version]
- Moore, T.A.; Von Freeden-Jeffry, U.; Murray, R.; Zlotnik, A. Inhibition of gamma delta T cell development and early thymocyte maturation in IL-7-/- mice. J. Immunol. 1996, 157, 2366–2373. [Google Scholar]
- Iwanami, N.; Sikora, K.; Richter, A.S.; Monnich, M.; Guerri, L.; Soza-Ried, C.; Lawir, D.F.; Mateos, F.; Hess, I.; O’Meara, C.P.; et al. Forward Genetic Screens in Zebrafish Identify Pre-mRNA-Processing Pathways Regulating Early T Cell Development. Cell Rep. 2016, 17, 2259–2270. [Google Scholar] [CrossRef] [Green Version]
- Fischer, U.; Dijkstra, J.M.; Kollner, B.; Kiryu, I.; Koppang, E.O.; Hordvik, I.; Sawamoto, Y.; Ototake, M. The ontogeny of MHC class I expression in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2005, 18, 49–60. [Google Scholar] [CrossRef]
- Koppang, E.O.; Hordvik, I.; Bjerkas, I.; Torvund, J.; Aune, L.; Thevarajan, J.; Endresen, C. Production of rabbit antisera against recombinant MHC class II beta chain and identification of immunoreactive cells in Atlantic salmon (Salmo salar). Fish Shellfish Immunol. 2003, 14, 115–132. [Google Scholar] [CrossRef]
- Bassity, E.; Clark, T.G. Functional identification of dendritic cells in the teleost model, rainbow trout (Oncorhynchus mykiss). PLoS ONE 2012, 7, e33196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, T. The adaptive phenotype of cortical thymic epithelial cells. Eur. J. Immunol. 2009, 39, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Grimholt, U. Whole genome duplications have provided teleosts with many roads to peptide loaded MHC class I molecules. BMC Evol. Biol. 2018, 18, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltis, M.; Criscitiello, M.F.; Ohta, Y.; Keefe, M.; Trede, N.S.; Goitsuka, R.; Flajnik, M.F. Evolutionarily conserved and divergent regions of the autoimmune regulator (Aire) gene: A comparative analysis. Immunogenetics 2008, 60, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Sarder, M.R.; Fischer, U.; Dijkstra, J.M.; Kiryu, I.; Yoshiura, Y.; Azuma, T.; Kollner, B.; Ototake, M. The MHC class I linkage group is a major determinant in the in vivo rejection of allogeneic erythrocytes in rainbow trout (Oncorhynchus mykiss). Immunogenetics 2003, 55, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Quiniou, S.M.; Wilson, M.; Bengten, E.; Waldbieser, G.C.; Clem, L.W.; Miller, N.W. MHC RFLP analyses in channel catfish full-sibling families: Identification of the role of MHC molecules in spontaneous allogeneic cytotoxic responses. Dev. Comp. Immunol. 2005, 29, 457–467. [Google Scholar] [CrossRef]
- Nakanishi, T.; Fischer, U.; Dijkstra, J.M.; Hasegawa, S.; Somamoto, T.; Okamoto, N.; Ototake, M. Cytotoxic T cell function in fish. Dev. Comp. Immunol. 2002, 26, 131–139. [Google Scholar] [CrossRef]
- Ott, J.A.; Castro, C.D.; Deiss, T.C.; Ohta, Y.; Flajnik, M.F.; Criscitiello, M.F. Somatic hypermutation of T cell receptor alpha chain contributes to selection in nurse shark thymus. eLife 2018, 7, e28477. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ohigashi, I.; Nitta, T.; Sakata, M.; Tanaka, K.; Murata, S.; Kanagawa, O.; Takahama, Y. Thymic nurse cells provide microenvironment for secondary T cell receptor alpha rearrangement in cortical thymocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 20572–20577. [Google Scholar] [CrossRef] [Green Version]
- Flano, E.; Alvarez, F.; Lopez-Fierro, P.; Razquin, B.E.; Villena, A.J.; Zapata, A.G. In vitro and in situ characterization of fish thymic nurse cells. Dev. Immunol. 1996, 5, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, K. New function of zebrafish regulatory T cells in organ regeneration. Curr. Opin. Immunol. 2020, 63, 7–13. [Google Scholar] [CrossRef]
- Quintana, F.J.; Iglesias, A.H.; Farez, M.F.; Caccamo, M.; Burns, E.J.; Kassam, N.; Oukka, M.; Weiner, H.L. Adaptive autoimmunity and Foxp3-based immunoregulation in zebrafish. PLoS ONE 2010, 5, e9478. [Google Scholar] [CrossRef] [Green Version]
- Kasheta, M.; Painter, C.A.; Moore, F.E.; Lobbardi, R.; Bryll, A.; Freiman, E.; Stachura, D.; Rogers, A.B.; Houvras, Y.; Langenau, D.M.; et al. Identification and characterization of T reg-like cells in zebrafish. J. Exp. Med. 2017, 214, 3519–3530. [Google Scholar] [CrossRef]
- Sugimoto, K.; Hui, S.P.; Sheng, D.Z.; Nakayama, M.; Kikuchi, K. Zebrafish FOXP3 is required for the maintenance of immune tolerance. Dev. Comp. Immunol. 2017, 73, 156–162. [Google Scholar] [CrossRef]
- Anderson, G.; Baik, S. The primordial thymus: Everything you need under one roof. Immunity 2014, 41, 178–180. [Google Scholar] [CrossRef] [Green Version]
- Swann, J.B.; Weyn, A.; Nagakubo, D.; Bleul, C.C.; Toyoda, A.; Happe, C.; Netuschil, N.; Hess, I.; Haas-Assenbaum, A.; Taniguchi, Y.; et al. Conversion of the thymus into a bipotent lymphoid organ by replacement of FOXN1 with its paralog, FOXN4. Cell Rep. 2014, 8, 1184–1197. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, R.; Swann, J.; Nusser, A.; Trancoso, I.; Schorpp, M.; Boehm, T. Evolution of thymopoietic microenvironments. Open Biol. 2021, 11, 200383. [Google Scholar] [CrossRef]
- Swann, J.B.; Nusser, A.; Morimoto, R.; Nagakubo, D.; Boehm, T. Retracing the evolutionary emergence of thymopoiesis. Sci. Adv. 2020, 6, eabd9585. [Google Scholar] [CrossRef]
- Miracle, A.L.; Anderson, M.K.; Litman, R.T.; Walsh, C.J.; Luer, C.A.; Rothenberg, E.V.; Litman, G.W. Complex expression patterns of lymphocyte-specific genes during the development of cartilaginous fish implicate unique lymphoid tissues in generating an immune repertoire. Int. Immunol. 2001, 13, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Sorhus, E.; Fiksdal, I.U.; Espedal, P.G.; Bergh, O.; Rodseth, O.M.; Morton, H.C.; Nerland, A.H. Ontogeny of lymphoid organs and development of IgM-bearing cells in Atlantic halibut (Hippoglossus hippoglossus L.). Fish Shellfish Immunol. 2009, 26, 385–395. [Google Scholar] [CrossRef]
- Nitta, T.; Tsutsumi, M.; Nitta, S.; Muro, R.; Suzuki, E.C.; Nakano, K.; Tomofuji, Y.; Sawa, S.; Okamura, T.; Penninger, J.M.; et al. Fibroblasts as a source of self-antigens for central immune tolerance. Nat. Immunol. 2020, 21, 1172–1180. [Google Scholar] [CrossRef]
- Baryawno, N.; Przybylski, D.; Kowalczyk, M.S.; Kfoury, Y.; Severe, N.; Gustafsson, K.; Kokkaliaris, K.D.; Mercier, F.; Tabaka, M.; Hofree, M.; et al. A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell 2019, 177, 1915–1932. [Google Scholar] [CrossRef] [PubMed]
- Bajoghli, B.; Guo, P.; Aghaallaei, N.; Hirano, M.; Strohmeier, C.; McCurley, N.; Bockman, D.E.; Schorpp, M.; Cooper, M.D.; Boehm, T. A thymus candidate in lampreys. Nature 2011, 470, 90–94. [Google Scholar] [CrossRef]
- Takaba, H.; Imai, T.; Miki, S.; Morishita, Y.; Miyashita, A.; Ishikawa, N.; Nishizumi, H.; Sakano, H. A major allogenic leukocyte antigen in the agnathan hagfish. Sci. Rep. 2013, 3, 1716. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Hirano, M.; Aghaallaei, N.; Bajoghli, B.; Boehm, T.; Cooper, M.D. Organization of lamprey variable lymphocyte receptor C locus and repertoire development. Proc. Natl. Acad. Sci. USA 2013, 110, 6043–6048. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, N.; Uinuk-ool, T.S.; Sato, A.; Samonte, I.E.; Figueroa, F.; Mayer, W.E.; Klein, J. Identification of chemokines and a chemokine receptor in cichlid fish, shark, and lamprey. Immunogenetics 2003, 54, 884–895. [Google Scholar] [CrossRef]
- Sun, Z.; Qin, Y.; Liu, D.; Wang, B.; Jia, Z.; Wang, J.; Gao, Q.; Zou, J.; Pang, Y. The evolution and functional characterization of CXC chemokines and receptors in lamprey. Dev. Comp. Immunol. 2021, 116, 103905. [Google Scholar] [CrossRef] [PubMed]
- Shevyrev, D.; Tereshchenko, V.; Kozlov, V.; Sennikov, S. Phylogeny, Structure, Functions, and Role of AIRE in the Formation of T-Cell Subsets. Cells 2022, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.J.; Gao, M.; Hirano, M.; Iyer, L.M.; Luo, M.; Schorpp, M.; Cooper, M.D.; Aravind, L.; Mariuzza, R.A.; Boehm, T. Selection of the lamprey VLRC antigen receptor repertoire. Proc. Natl. Acad. Sci. USA 2014, 111, 14834–14839. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Velikovsky, C.A.; Xu, G.; Iyer, L.M.; Tasumi, S.; Kerzic, M.C.; Flajnik, M.F.; Aravind, L.; Pancer, Z.; Mariuzza, R.A. A structural basis for antigen recognition by the T cell-like lymphocytes of sea lamprey. Proc. Natl. Acad. Sci. USA 2010, 107, 13408–13413. [Google Scholar] [CrossRef] [Green Version]
- Zapata, A.G.; Cooper, E.L. The Immune System: Comparative Histophysiology; John Wiley and Sons: Chichester, UK, 1990; p. 335. [Google Scholar]
- Ellis, A.E.; De Sousa, M. Phylogeny of the lymphoid system. I. A study of the fate of circulating lymphocytes in plaice. Eur. J. Immunol. 1974, 4, 338–343. [Google Scholar] [CrossRef]
- Zapata, A. Ultrastructural study of the teleost fish kidney. Dev. Comp. Immunol. 1979, 3, 55–65. [Google Scholar] [CrossRef]
- Patel, B.; Banerjee, R.; Samanta, M.; Das, S. Diversity of Immunoglobulin (Ig) Isotypes and the Role of Activation-Induced Cytidine Deaminase (AID) in Fish. Mol. Biotechnol. 2018, 60, 435–453. [Google Scholar] [CrossRef]
- Al-Adhami, M.A.; Kunz, Y.W. Haemopoietic centres in the developing angelfish, Pterophyllum scalare (cuvier and valenciennes). Wilhelm Roux’s Arch. Dev. Biol. 1976, 179, 393–401. [Google Scholar] [CrossRef]
- Hansen, J.D.; Zapata, A.G. Lymphocyte development in fish and amphibians. Immunol. Rev. 1998, 166, 199–220. [Google Scholar] [CrossRef]
- Edholm, E.S.; Stafford, J.L.; Quiniou, S.M.; Waldbieser, G.; Miller, N.W.; Bengten, E.; Wilson, M. Channel catfish, Ictalurus punctatus, CD4-like molecules. Dev. Comp. Immunol. 2007, 31, 172–187. [Google Scholar] [CrossRef]
- Kato, G.; Goto, K.; Akune, I.; Aoka, S.; Kondo, H.; Hirono, I. CD4 and CD8 homologues in Japanese flounder, Paralichthys olivaceus: Differences in the expressions and localizations of CD4-1, CD4-2, CD8alpha and CD8beta. Dev. Comp. Immunol. 2013, 39, 293–301. [Google Scholar] [CrossRef]
- Somamoto, T.; Yoshiura, Y.; Nakanishi, T.; Ototake, M. Molecular cloning and characterization of two types of CD8α from ginbuna crucian carp, Carassius auratus langsdorfii. Dev. Comp. Immunol. 2005, 29, 693–702. [Google Scholar] [CrossRef]
- Fischer, U.; Utke, K.; Ototake, M.; Dijkstra, J.M.; Köllner, B. Adaptive cell-mediated cytotoxicity against allogeneic targets by CD8-positive lymphocytes of rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 2003, 27, 323–337. [Google Scholar] [CrossRef]
- Stosik, M.; Tokarz-Deptula, B.; Deptula, W. Immunological memory in teleost fish. Fish Shellfish Immunol. 2021, 115, 95–103. [Google Scholar] [CrossRef]
- Fillatreau, S.; Six, A.; Magadan, S.; Castro, R.; Sunyer, J.O.; Boudinot, P. The astonishing diversity of Ig classes and B cell repertoires in teleost fish. Front. Immunol. 2013, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Bromage, E.S.; Kaattari, I.M.; Zwollo, P.; Kaattari, S.L. Plasmablast and plasma cell production and distribution in trout immune tissues. J. Immunol. 2004, 173, 7317–7323. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Findly, R.C.; Dickerson, H.W. Cutaneous antibody-secreting cells and B cells in a teleost fish. Dev. Comp. Immunol. 2008, 32, 500–508. [Google Scholar] [CrossRef]
- Findly, R.C.; Zhao, X.; Noe, J.; Camus, A.C.; Dickerson, H.W. B cell memory following infection and challenge of channel catfish with Ichthyophthirius multifiliis. Dev. Comp. Immunol. 2013, 39, 302–311. [Google Scholar] [CrossRef]
- Ma, C.; Ye, J.; Kaattari, S.L. Differential compartmentalization of memory B cells versus plasma cells in salmonid fish. Eur. J. Immunol. 2013, 43, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Fu, S.; Yin, X.; Guo, Z.; Wang, A.; Ye, J. Long-Lived Plasma Cells Secrete High-Affinity Antibodies Responding to a T-Dependent Immunization in a Teleost Fish. Front. Immunol. 2019, 10, 2324. [Google Scholar] [CrossRef]
- Rombout, J.H.W.M.; Koumans-van Diepen, J.C.E.; Emmer, P.M.; Taverne-Thiele, J.J.; Taverne, N. Characterization of carp thrombocytes with specific monoclonal antibodies. J. Fish Biol. 1996, 49, 521–531. [Google Scholar] [CrossRef]
- Jaros, J.; Korytar, T.; Huong, D.T.; Weiss, M.; Köllner, B. Rainbow trout (Oncorhynchus mykiss) thrombocytes are involved in MHC II dependent antigen presentation. Fish Shellfish Immunol. 2013, 34, 1657. [Google Scholar] [CrossRef]
- Fink, I.R.; Ribeiro, C.M.; Forlenza, M.; Taverne-Thiele, A.; Rombout, J.H.; Savelkoul, H.F.; Wiegertjes, G.F. Immune-relevant thrombocytes of common carp undergo parasite-induced nitric oxide-mediated apoptosis. Dev. Comp. Immunol. 2015, 50, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Katakura, F.; Sugie, Y.; Hayashi, K.; Nishiya, K.; Miyamae, J.; Okano, M.; Nakanishi, T.; Moritomo, T. Thrombopoietin (TPO) induces thrombocytic colony formation of kidney cells synergistically with kit ligand A and a non-secretory TPO variant exists in common carp. Dev. Comp. Immunol. 2018, 84, 327–336. [Google Scholar] [CrossRef]
- Stosik, M.; Tokarz-Deptuła, B.; Deptuła, W. Characterisation of Thrombocytes in Osteichthyes. J. Vet. Res. 2019, 63, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Nagasawa, T.; Nakayasu, C.; Rieger, A.M.; Barreda, D.R.; Somamoto, T.; Nakao, M. Phagocytosis by Thrombocytes is a Conserved Innate Immune Mechanism in Lower Vertebrates. Front. Immunol. 2014, 5, 445. [Google Scholar] [CrossRef] [Green Version]
- Nagasawa, T.; Somamoto, T.; Nakao, M. Carp thrombocyte phagocytosis requires activation factors secreted from other leukocytes. Dev. Comp. Immunol. 2015, 52, 107–111. [Google Scholar] [CrossRef]
- Köllner, B.; Fischer, U.; Rombout, J.H.; Taverne-Thiele, J.J.; Hansen, J.D. Potential involvement of rainbow trout thrombocytes in immune functions: A study using a panel of monoclonal antibodies and RT-PCR. Dev. Comp. Immunol. 2004, 28, 1049–1062. [Google Scholar] [CrossRef]
- Tischendorf, F. On the evolution of the spleen. Experientia 1985, 41, 145–152. [Google Scholar] [CrossRef]
- Zapata, A. Lymphoid organs of teleost fish. III. Splenic lymphoid tissue of Rutilus rutilus and Gobio gobio. Dev. Comp. Immunol. 1982, 6, 87–94. [Google Scholar] [CrossRef]
- Ferren, F.A., Jr. Role of the spleen in the immune response of teleosts and elasmobranchs. J. Fla. Med. Assoc. 1967, 54, 434–437. [Google Scholar]
- Roberts, R.J. Melanin-containing cells of teleost fish and their relation to disease. In The Pathology of Fishes; Ribelin, W.E., Migali, G., Eds.; University of Wisconsin: Madison, WI, USA, 1975; pp. 339–428. [Google Scholar]
- Agius, C.; Agbede, S.A. An electron microscopical study on the genesis of lipofucsin, melanin and haemosiderin in the haemopoietic tissues of fish. J. Fish Biol. 1984, 24, 471–488. [Google Scholar] [CrossRef]
- Herráez, M.P.; Zapata, A.G. Structure and function of the melano-macrophage centres of the goldfish Carassius auratus. Vet. Immunol. Immunopathol. 1986, 12, 117–126. [Google Scholar] [CrossRef]
- Ellis, A.E.; Munroe, A.L.S.; Roberts, R.J. A study of the phagocytic system and the fate of intraperitoneally injected particulate material in the plaice (Pleuronectes platessa L.). J. Fish Biol. 1976, 8, 67–68. [Google Scholar] [CrossRef]
- Lamers, C.H.; De Haas, M.J. Antigen localization in the lymphoid organs of carp (Cyprinus carpio). Cell Tissue Res. 1985, 242, 491–498. [Google Scholar] [CrossRef]
- Steinel, N.C.; Bolnick, D.I. Melanomacrophage Centers As a Histological Indicator of Immune Function in Fish and Other Poikilotherms. Front. Immunol. 2017, 8, 827. [Google Scholar] [CrossRef] [Green Version]
- Saunders, H.L.; Oko, A.L.; Scott, A.N.; Fan, C.W.; Magor, B.G. The cellular context of AID expressing cells in fish lymphoid tissues. Dev. Comp. Immunol. 2010, 34, 669–676. [Google Scholar] [CrossRef]
- Magor, B.G. Antibody Affinity Maturation in Fishes-Our Current Understanding. Biology 2015, 4, 512–524. [Google Scholar] [CrossRef] [Green Version]
- Muthupandian, A.; Waly, D.; Magor, B.G. Do ectothermic vertebrates have a home in which to affinity mature their antibody responses? Dev. Comp. Immunol. 2021, 119, 104021. [Google Scholar] [CrossRef]
- Zwollo, P.; Cole, S.; Bromage, E.; Kaattari, S. B cell heterogeneity in the teleost kidney: Evidence for a maturation gradient from anterior to posterior kidney. J. Immunol. 2005, 174, 6608–6616. [Google Scholar] [CrossRef] [Green Version]
- Wiens, G.D.; Glenney, G.W. Origin and evolution of TNF and TNF receptor superfamilies. Dev. Comp. Immunol. 2011, 35, 1324–1335. [Google Scholar] [CrossRef]
- Bird, S.; Zou, J.; Savan, R.; Kono, T.; Sakai, M.; Woo, J.; Secombes, C. Characterisation and expression analysis of an interleukin 6 homologue in the Japanese pufferfish, Fugu rubripes. Dev. Comp. Immunol. 2005, 29, 775–789. [Google Scholar] [CrossRef]
- Ohtani, M.; Miyadai, T.; Hiroishi, S. Molecular cloning of the BCL-6 gene, a transcriptional repressor for B-cell differentiation, in torafugu (Takifugu rubripes). Mol. Immunol. 2006, 43, 1047–1053. [Google Scholar] [CrossRef]
- Dooley, H.; Flajnik, M.F. Shark immunity bites back: Affinity maturation and memory response in the nurse shark, Ginglymostoma cirratum. Eur. J. Immunol. 2005, 35, 936–945. [Google Scholar] [CrossRef]
- Salinas, I.; Zhang, Y.A.; Sunyer, J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011, 35, 1346–1365. [Google Scholar] [CrossRef] [Green Version]
- Salinas, I. The Mucosal Immune System of Teleost Fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef] [Green Version]
- Haugarvoll, E.; Bjerkas, I.; Nowak, B.F.; Hordvik, I.; Koppang, E.O. Identification and characterization of a novel intraepithelial lymphoid tissue in the gills of Atlantic salmon. J. Anat. 2008, 213, 202–209. [Google Scholar] [CrossRef]
- Bjorgen, H.; Loken, O.M.; Aas, I.B.; Fjelldal, P.G.; Hansen, T.; Austbo, L.; Koppang, E.O. Visualization of CCL19-like transcripts in the ILT, thymus and head kidney of Atlantic salmon (Salmo salar L.). Fish Shellfish Immunol. 2019, 93, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Kong, W.; Yin, Y.X.; Dong, F.; Huang, Z.Y.; Yin, G.M.; Dong, S.; Salinas, I.; Zhang, Y.A.; Xu, Z. Mucosal immunoglobulins protect the olfactory organ of teleost fish against parasitic infection. PLoS Pathog. 2018, 14, e1007251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loken, O.M.; Bjorgen, H.; Hordvik, I.; Koppang, E.O. A teleost structural analogue to the avian bursa of Fabricius. J. Anat. 2020, 236, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Takizawa, F.; Parra, D.; Gomez, D.; Von Gersdorff Jorgensen, L.; LaPatra, S.E.; Sunyer, J.O. Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods. Nat. Commun. 2016, 7, 10728. [Google Scholar] [CrossRef] [Green Version]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef] [Green Version]
- Lovy, J.; Wright, G.M.; Speare, D.J. Morphological presentation of a dendritic-like cell within the gills of chinook salmon infected with Loma salmonae. Dev. Comp. Immunol. 2006, 30, 259–263. [Google Scholar] [CrossRef]
- Lovy, J.; Wright, G.M.; Speare, D.J. Comparative cellular morphology suggesting the existence of resident dendritic cells within immune organs of salmonids. Anat. Rec. 2008, 291, 456–462. [Google Scholar] [CrossRef]
- Alesci, A.; Lauriano, E.R.; Aragona, M.; Capillo, G.; Pergolizzi, S. Marking vertebrates langerhans cells, from fish to mammals. Acta Histochem. 2020, 122, 151622. [Google Scholar] [CrossRef]
- Granja, A.G.; Leal, E.; Pignatelli, J.; Castro, R.; Abos, B.; Kato, G.; Fischer, U.; Tafalla, C. Identification of Teleost Skin CD8alpha+ Dendritic-like Cells, Representing a Potential Common Ancestor for Mammalian Cross-Presenting Dendritic Cells. J. Immunol. 2015, 195, 1825–1837. [Google Scholar] [CrossRef] [Green Version]
- Dee, C.T.; Nagaraju, R.T.; Athanasiadis, E.I.; Gray, C.; Fernandez Del Ama, L.; Johnston, S.A.; Secombes, C.J.; Cvejic, A.; Hurlstone, A.F. CD4-Transgenic Zebrafish Reveal Tissue-Resident Th2- and Regulatory T Cell-like Populations and Diverse Mononuclear Phagocytes. J. Immunol. 2016, 197, 3520–3530. [Google Scholar] [CrossRef]
- Boschi, I.; Randelli, E.; Buonocore, F.; Casani, D.; Bernini, C.; Fausto, A.M.; Scapigliati, G. Transcription of T cell-related genes in teleost fish, and the European sea bass (Dicentrarchus labrax) as a model. Fish Shellfish Immunol. 2011, 31, 655–662. [Google Scholar] [CrossRef]
- Picchietti, S.; Guerra, L.; Bertoni, F.; Randelli, E.; Belardinelli, M.C.; Buonocore, F.; Fausto, A.M.; Rombout, J.H.; Scapigliati, G.; Abelli, L. Intestinal T cells of Dicentrarchus labrax (L.): Gene expression and functional studies. Fish Shellfish Immunol. 2011, 30, 609–617. [Google Scholar] [CrossRef]
- Bernard, D.; Six, A.; Rigottier-Gois, L.; Messiaen, S.; Chilmonczyk, S.; Quillet, E.; Boudinot, P.; Benmansour, A. Phenotypic and functional similarity of gut intraepithelial and systemic T cells in a teleost fish. J. Immunol. 2006, 176, 3942–3949. [Google Scholar] [CrossRef]
- Buonocore, F.; Castro, R.; Randelli, E.; Lefranc, M.P.; Six, A.; Kuhl, H.; Reinhardt, R.; Facchiano, A.; Boudinot, P.; Scapigliati, G. Diversity, molecular characterization and expression of T cell receptor gamma in a teleost fish, the sea bass (Dicentrarchus labrax, L). PLoS ONE 2012, 7, e47957. [Google Scholar] [CrossRef]
- Rombout, J.H.W.M.; Van den Berg, A.A. Immunological importance of the second gut segment of carp. I. Uptake and processing of antigens by epithelial cells and macrophages. J. Fish Biol. 1989, 35, 13–22. [Google Scholar] [CrossRef]
- Rombout, J.H.W.M.; Van den Berg, A.A.; Van den Berg, C.T.G.A.; Witte, P.; Egberts, E. Immunological importance of the second gut segment of carp. III. Systemic and/or mucosal immune responses after immunization with soluble or particulate antigen. J. Fish Biol. 1989, 35, 179–186. [Google Scholar] [CrossRef]
- Rombout, J.H.; Yang, G.; Kiron, V. Adaptive immune responses at mucosal surfaces of teleost fish. Fish Shellfish Immunol. 2014, 40, 634–643. [Google Scholar] [CrossRef] [Green Version]
- Resseguier, J.; Dalum, A.S.; Pasquier, L.D.; Zhang, Y.; Koppang, E.O.; Boudinot, P.; Wiegertjes, G.F. Lymphoid Tissue in Teleost Gills: Variations on a Theme. Biology 2020, 9, 127. [Google Scholar] [CrossRef]
- Kato, G.; Miyazawa, H.; Nakayama, Y.; Ikari, Y.; Kondo, H.; Yamaguchi, T.; Sano, M.; Fischer, U. A Novel Antigen-Sampling Cell in the Teleost Gill Epithelium With the Potential for Direct Antigen Presentation in Mucosal Tissue. Front. Immunol. 2018, 9, 2116. [Google Scholar] [CrossRef]
- Lovmo, S.D.; Speth, M.T.; Repnik, U.; Koppang, E.O.; Griffiths, G.W.; Hildahl, J.P. Translocation of nanoparticles and Mycobacterium marinum across the intestinal epithelium in zebrafish and the role of the mucosal immune system. Dev. Comp. Immunol. 2017, 67, 508–518. [Google Scholar] [CrossRef]
- Koppang, E.O.; Fischer, U.; Moore, L.; Tranulis, M.A.; Dijkstra, J.M.; Kollner, B.; Aune, L.; Jirillo, E.; Hordvik, I. Salmonid T cells assemble in the thymus, spleen and in novel interbranchial lymphoid tissue. J. Anat. 2010, 217, 728–739. [Google Scholar] [CrossRef]
- Aas, I.B.; Austbo, L.; Konig, M.; Syed, M.; Falk, K.; Hordvik, I.; Koppang, E.O. Transcriptional characterization of the T cell population within the salmonid interbranchial lymphoid tissue. J. Immunol. 2014, 193, 3463–3469. [Google Scholar] [CrossRef] [Green Version]
- Norte dos Santos, C.C.; Adams, M.B.; Leef, M.J.; Nowak, B.F. Changes in the interbranchial lymphoid tissue of Atlantic salmon (Salmo salar) affected by amoebic gill disease. Fish Shellfish Immunol. 2014, 41, 600–607. [Google Scholar] [CrossRef]
- Sepahi, A.; Salinas, I. The evolution of nasal immune systems in vertebrates. Mol. Immunol. 2016, 69, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Martín, A.; Simón, R.; Abós, B.; Díaz-Rosales, P.; Tafalla, C. Rainbow trout mount a robust specific immune response upon anal administration of thymus-independent antigens. Dev. Comp. Immunol. 2020, 109, 103715. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, T.; Hammarstrom, L.; Zhao, Y. The Immunoglobulins: New Insights, Implications, and Applications. Annu. Rev. Anim. Biosci. 2020, 8, 145–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinas, I.; Fernandez-Montero, A.; Ding, Y.; Sunyer, J.O. Mucosal immunoglobulins of teleost fish: A decade of advances. Dev. Comp. Immunol. 2021, 121, 104079. [Google Scholar] [CrossRef]
- Mirete-Bachiller, S.; Olivieri, D.N.; Gambon-Deza, F. Immunoglobulin T genes in Actinopterygii. Fish Shellfish Immunol. 2021, 108, 86–93. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef]
- Ji, J.F.; Hu, C.B.; Shao, T.; Fan, D.D.; Zhang, N.; Lin, A.F.; Xiang, L.X.; Shao, J.Z. Differential immune responses of immunoglobulin Z subclass members in antibacterial immunity in a zebrafish model. Immunology 2021, 162, 105–120. [Google Scholar] [CrossRef]
- Kong, W.G.; Yu, Y.Y.; Dong, S.; Huang, Z.Y.; Ding, L.G.; Cao, J.F.; Dong, F.; Zhang, X.T.; Liu, X.; Xu, H.Y.; et al. Pharyngeal Immunity in Early Vertebrates Provides Functional and Evolutionary Insight into Mucosal Homeostasis. J. Immunol. 2019, 203, 3054–3067. [Google Scholar] [CrossRef]
- Du, Y.; Tang, X.; Zhan, W.; Xing, J.; Sheng, X. Immunoglobulin Tau Heavy Chain (IgT) in Flounder, Paralichthys olivaceus: Molecular Cloning, Characterization, and Expression Analyses. Int. J. Mol. Sci. 2016, 17, 1571. [Google Scholar] [CrossRef] [Green Version]
- Evenhuis, J.P.; Cleveland, B.M. Modulation of rainbow trout (Oncorhynchus mykiss) intestinal immune gene expression following bacterial challenge. Vet. Immunol. Immunopathol. 2012, 146, 8–17. [Google Scholar] [CrossRef]
- Ballesteros, N.A.; Rodriguez Saint-Jean, S.; Perez-Prieto, S.I.; Aquilino, C.; Tafalla, C. Modulation of genes related to the recruitment of immune cells in the digestive tract of trout experimentally infected with infectious pancreatic necrosis virus (IPNV) or orally vaccinated. Dev. Comp. Immunol. 2014, 44, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Takizawa, F.; Casadei, E.; Shibasaki, Y.; Ding, Y.; Sauters, T.J.C.; Yu, Y.; Salinas, I.; Sunyer, J.O. Specialization of mucosal immunoglobulins in pathogen control and microbiota homeostasis occurred early in vertebrate evolution. Sci. Immunol. 2020, 5, eaay3254. [Google Scholar] [CrossRef]
- Tongsri, P.; Meng, K.; Liu, X.; Wu, Z.; Yin, G.; Wang, Q.; Liu, M.; Xu, Z. The predominant role of mucosal immunoglobulin IgT in the gills of rainbow trout (Oncorhynchus mykiss) after infection with Flavobacterium columnare. Fish Shellfish Immunol. 2020, 99, 654–662. [Google Scholar] [CrossRef]
- Austbo, L.; Aas, I.B.; Konig, M.; Weli, S.C.; Syed, M.; Falk, K.; Koppang, E.O. Transcriptional response of immune genes in gills and the interbranchial lymphoid tissue of Atlantic salmon challenged with infectious salmon anaemia virus. Dev. Comp. Immunol. 2014, 45, 107–114. [Google Scholar] [CrossRef]
- Xu, Z.; Parra, D.; Gomez, D.; Salinas, I.; Zhang, Y.A.; Von Gersdorff Jorgensen, L.; Heinecke, R.D.; Buchmann, K.; LaPatra, S.; Sunyer, J.O. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc. Natl. Acad. Sci. USA 2013, 110, 13097–13102. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Liu, X.; Sato, H.; Zhang, Q.; Li, A.; Zhang, J. RNA-seq analysis of local tissue of Carassius auratus gibelio with pharyngeal myxobolosis: Insights into the pharyngeal mucosal immune response in a fish-parasite dialogue. Fish Shellfish Immunol. 2019, 94, 99–112. [Google Scholar] [CrossRef]
- Tadiso, T.M.; Krasnov, A.; Skugor, S.; Afanasyev, S.; Hordvik, I.; Nilsen, F. Gene expression analyses of immune responses in Atlantic salmon during early stages of infection by salmon louse (Lepeophtheirus salmonis) revealed bi-phasic responses coinciding with the copepod-chalimus transition. BMC Genom. 2011, 12, 141. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yu, Y.; Huang, Z.; Dong, S.; Luo, Y.; Yu, W.; Yin, Y.; Li, H.; Liu, Y.; Zhou, X.; et al. Immunoglobulin (Ig) heavy chain gene locus and immune responses upon parasitic, bacterial and fungal infection in loach, Misgurnus anguillicaudatus. Fish Shellfish Immunol. 2019, 86, 1139–1150. [Google Scholar] [CrossRef]
- Velazquez, J.; Acosta, J.; Lugo, J.M.; Reyes, E.; Herrera, F.; Gonzalez, O.; Morales, A.; Carpio, Y.; Estrada, M.P. Discovery of immunoglobulin T in Nile tilapia (Oreochromis niloticus): A potential molecular marker to understand mucosal immunity in this species. Dev. Comp. Immunol. 2018, 88, 124–136. [Google Scholar] [CrossRef]
- Zapata, A.G.; Torroba, M.; Vicente, A.; Varas, A.; Sacedón, R.; Jiménez, E. The relevance of cell microenvironments for the appearance of lympho-haemopoietic tissues in primitive vertebrates. Histol. Histopathol. 1995, 10, 761–778. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapata, A.G. Lympho-Hematopoietic Microenvironments and Fish Immune System. Biology 2022, 11, 747. https://doi.org/10.3390/biology11050747
Zapata AG. Lympho-Hematopoietic Microenvironments and Fish Immune System. Biology. 2022; 11(5):747. https://doi.org/10.3390/biology11050747
Chicago/Turabian StyleZapata, Agustín G. 2022. "Lympho-Hematopoietic Microenvironments and Fish Immune System" Biology 11, no. 5: 747. https://doi.org/10.3390/biology11050747
APA StyleZapata, A. G. (2022). Lympho-Hematopoietic Microenvironments and Fish Immune System. Biology, 11(5), 747. https://doi.org/10.3390/biology11050747




