Whole-Transcriptome Analysis Identifies Gender Dimorphic Expressions of Mrnas and Non-Coding Rnas in Chinese Soft-Shell Turtle (Pelodiscus sinensis)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. P. sinensis Collection and Total RNA Isolation
2.2. Library Construction and Sequencing
2.3. Quality Control and Transcriptome Assembly
2.4. Differential Expression Analysis and Functional Annotation
2.5. Validation of Candidate mRNAs and ncRNAs by qRT-PCR
2.6. Construction of the ceRNA Interaction Network
3. Results
3.1. Overview of Transcriptome Sequencing
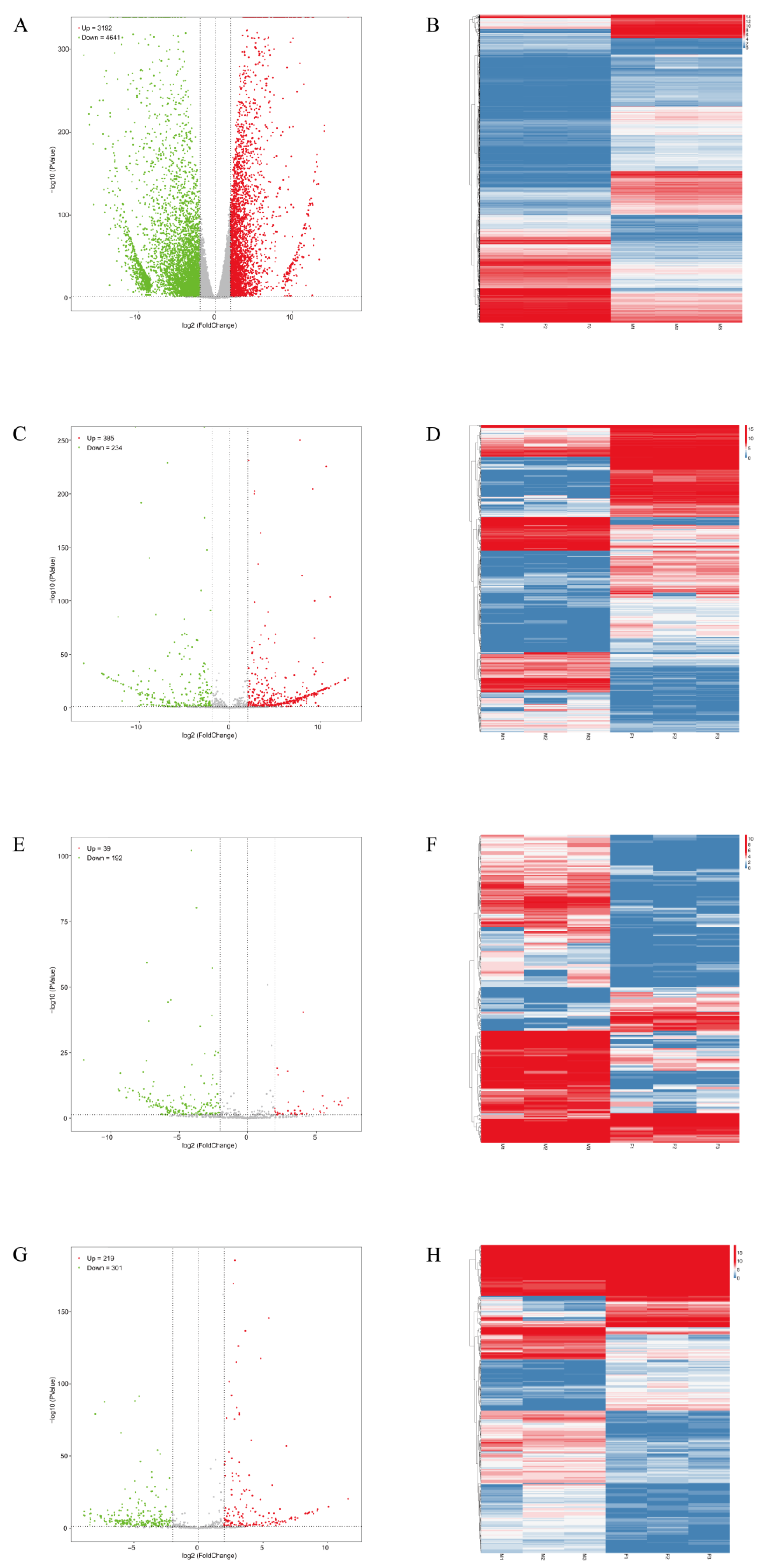
3.2. Identification of DE mRNAs and ncRNAs
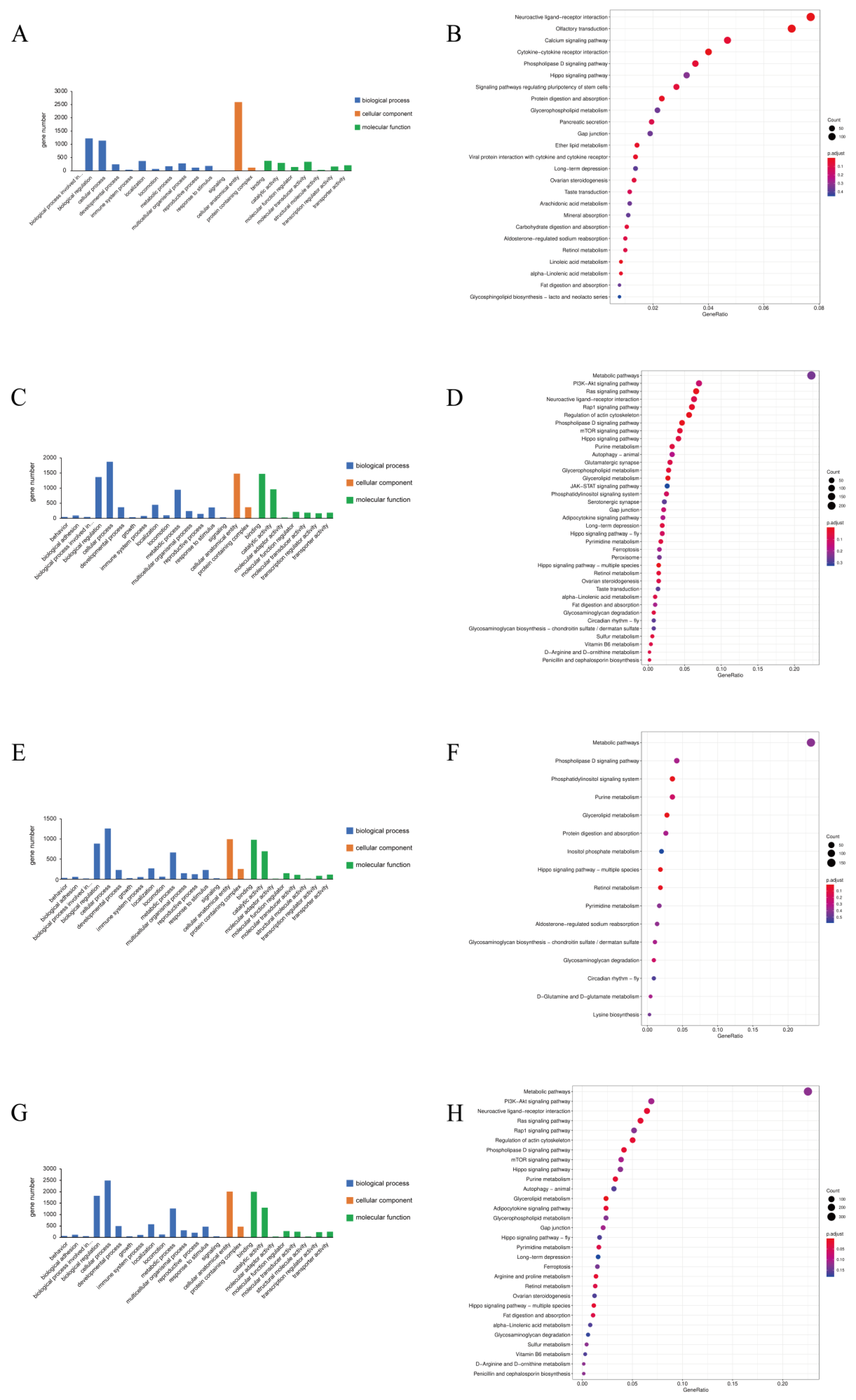
3.3. GO and KEGG Analysis of DE mRNAs and ncRNAs
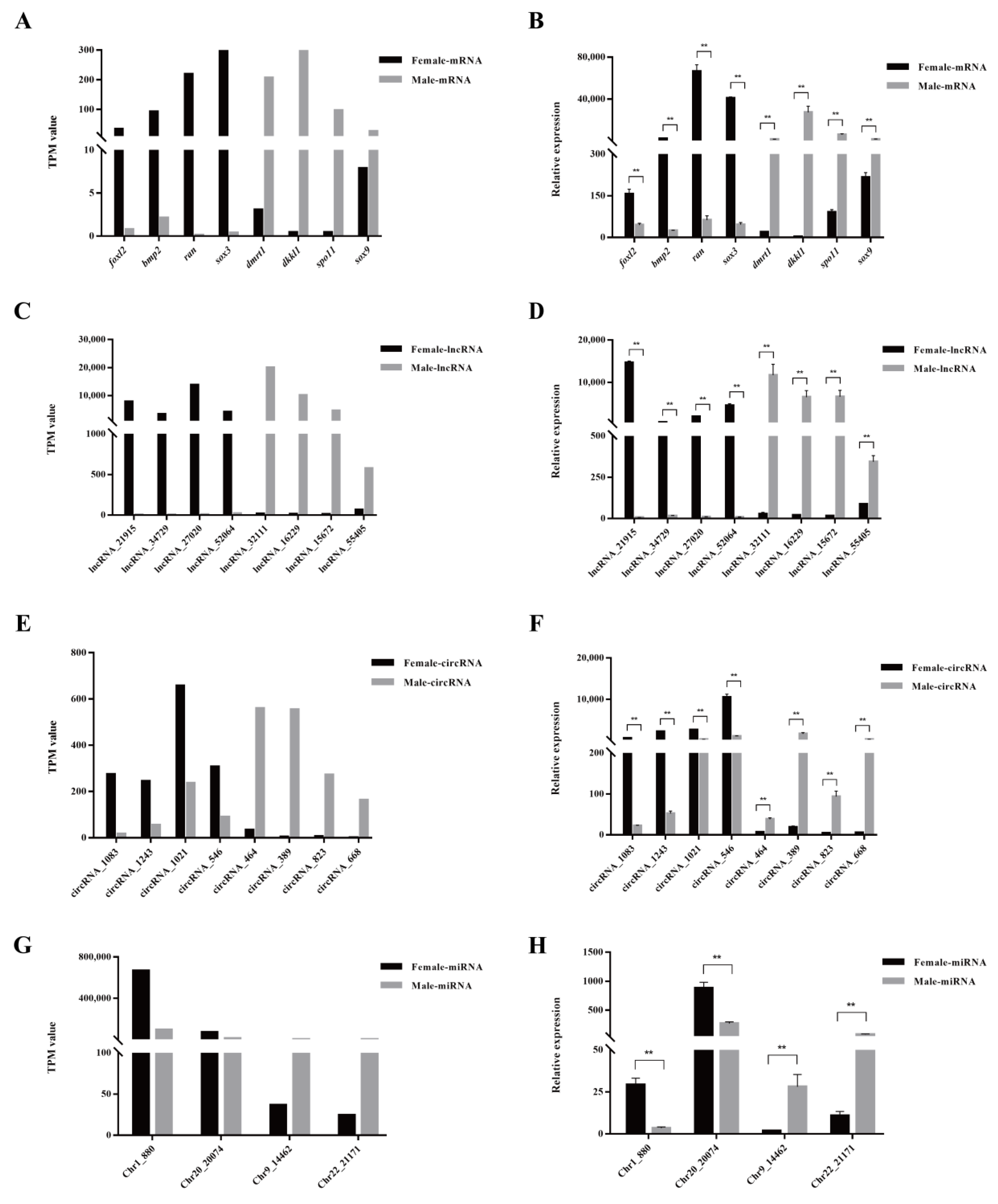
3.4. Validation of DE mRNAs and ncRNAs
3.5. Construction of the lncRNA/circRNA–miRNA–mRNA Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mei, J.; Gui, J.F. Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Sci. China Life Sci. 2015, 58, 124–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidir, A.; Aaqillah-Amr, M.A.; Azra, M.N.; Shahreza, M.S.; Abualreesh, M.H.; Peng, T.H.; Ma, H.Y.; Waiho, K.; Fazhan, H.; Ikhwanuddin, M. Sexual dimorphism of mud crab, genus Scylla between sexes based on morphological and physiological characteristics. Aquac. Res. 2021, 52, 5943–5961. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Wang, Y.; Li, W.; Hong, X.; Zhu, X.; Xu, H. Comparative transcriptome analysis reveals the sexual dimorphic expression profiles of mRNAs and non-coding RNAs in the Asian yellow pond turtle (Meauremys mutica). Gene 2020, 750, 144756. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.; Chen, K.; Gao, D.; Wu, Y.; Chen, Z.; Luo, Q.; Liu, H.; Zhao, J. Comparative transcriptome analysis on four types of gonadal tissues of blotched snakehead (Channa maculata). Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 35, 100708. [Google Scholar] [CrossRef]
- Liu, H.; Guan, B.; Xu, J.; Hou, C.; Tian, H.; Chen, H. Genetic manipulation of sex ratio for the large-scale breeding of YY super-male and XY all-male yellow catfish (Pelteobagrus fulvidraco (Richardson)). Mar. Biotechnol. 2013, 15, 321–328. [Google Scholar] [CrossRef]
- Angienda, P.O.; Aketch, B.O.; Waindi, E.N. Development of all-male fingerlings by heat treatment and the genetic mechanism of heat induced sex determination in nile tilapia (Oreochromis niloticus L.). Adv. Mater. 2013, 21, 3727–3729. [Google Scholar]
- Zhao, J.; Ou, M.; Wang, Y.; Liu, H.; Chen, K. Breeding of YY super-male of blotched snakehead (Channa maculata) and production of all-male hybrid (Channa argus ♀ × C. maculata ♂). Aquaculture 2021, 538, 736450. [Google Scholar] [CrossRef]
- Bureau of Fisheries, Ministry of Agriculture and Rural Affairs of PR China. China Fisheries Statistics Yearbook; China Agriculture Press: Beijing, China, 2021; p. 35. [Google Scholar]
- Zhou, X.; Zhu, Y. Sex-specific growth characteristics of Chinese soft-shelled turtle, Pelodiscus sinensis. Chin. Aquac. 2011, 32, 11–13. [Google Scholar]
- Nie, L.; Guo, C.; Wang, M.A.; Wang, Q. Sex determination mechanism of Trionyx Sinensis. Chin. J. Appl. Environ. Biol. 2001, 7, 258–261. [Google Scholar]
- Zheng, J.; Zhu, M. Isolation and sequence analysis of the Sox-1, -2, -3 homologs in Trionyx sinensis and Alligator sinensis having temperature-dependent sex determination. Biochem. Genet. 2006, 44, 101–112. [Google Scholar] [CrossRef]
- Zhu, D.; Sun, X. Sex determination in Trionyx sinensis. Chin. J. Zool. 2000, 6, 37–38. [Google Scholar]
- Kawagoshi, T.; Uno, Y.; Matsubara, K.; Matsuda, Y.; Nishida, C. The ZW micro-sex chromosomes of the Chinese soft-shelled turtle (Pelodiscus sinensis, Trionychidae, Testudines) have the same origin as chicken chromosome 15. Cytogenet. Genome Res. 2009, 125, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, D.; Stanyon, R.; Engstrom, T.; Valenzuela, N. A ZZ/ZW microchromosome system in the spiny softshell turtle, Apalone spinifera, reveals an intriguing sex chromosome conservation in Trionychidae. Chromosome Res. 2013, 21, 137–147. [Google Scholar] [CrossRef]
- Mu, Y.; Zhao, B.; Tang, W.Q.; Sun, B.J.; Zeng, Z.G.; Valenzuela, N.; Du, W.G. Temperature-dependent sex determination ruled out in the Chinese soft-shelled turtle (Pelodiscus sinensis) via molecular cytogenetics and incubation experiments across populations. Sex. Dev. 2015, 9, 111–117. [Google Scholar] [CrossRef]
- Sun, W.; Cai, H.; Zhang, G.; Zhang, H.; Bao, H.; Wang, L.; Ye, J.; Qian, G.; Ge, C. Dmrt1 is required for primary male sexual differentiation in Chinese soft-shelled turtle Pelodiscus sinensis. Sci. Rep. 2017, 7, 4433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xiao, L.; Sun, W.; Li, P.; Zhou, Y.; Qian, G.; Ge, C. Knockdown of R-spondin1 leads to partial sex reversal in genetic female Chinese soft-shelled turtle Pelodiscus sinensis. Gen. Comp. Endocrinol. 2021, 309, 113788. [Google Scholar] [CrossRef] [PubMed]
- Capel, B. Vertebrate sex determination: Evolutionary plasticity of a fundamental switch. Nat. Rev. Genet. 2017, 18, 675–689. [Google Scholar] [CrossRef]
- Hiramatsu, R.; Matoba, S.; Kanai-Azuma, M.; Tsunekawa, N.; Katoh-Fukui, Y.; Kurohmaru, M.; Morohashi, K.; Wilhelm, D.; Koopman, P.; Kanai, Y. A critical time window of Sry action in gonadal sex determination in mice. Development 2009, 136, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, S.; Okada, E.; Umemoto, H.; Tamura, K.; Uno, Y.; Nishida-Umehara, C.; Matsuda, Y.; Takamatsu, N.; Shiba, T.; Ito, M. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc. Natl. Acad. Sci. USA 2008, 105, 2469–2474. [Google Scholar] [CrossRef] [Green Version]
- Nagaoka, S.I.; Nakaki, F.; Miyauchi, H.; Nosaka, Y.; Ohta, H.; Yabuta, Y.; Kurimoto, K.; Hayashi, K.; Nakamura, T.; Yamamoto, T.; et al. ZGLP1 is a determinant for the oogenic fate in mice. Science 2020, 367, 6482. [Google Scholar] [CrossRef]
- Vidal, V.P.; Chaboissier, M.C.; de Rooij, D.G.; Schedl, A. Sox9 induces testis development in XX transgenic mice. Nat. Genet. 2001, 28, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Nagahama, Y.; Shinomiya, A.; Sato, T.; Matsuda, C.; Kobayashi, T.; Morrey, C.E.; Shibata, N.; Asakawa, S.; Shimizu, N.; et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 2002, 417, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Sato, T.; Yamamoto, Y.; Watakabe, I.; Ohkawa, Y.; Suyama, M.; Kobayashi, S.; Tanaka, M. Foxl3 is a germ cell-intrinsic factor involved in sperm-egg fate decision in medaka. Science 2015, 349, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Roeszler, K.N.; Ohnesorg, T.; Cummins, D.M.; Farlie, P.G.; Doran, T.J.; Sinclair, A.H. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 2009, 461, 267–271. [Google Scholar] [CrossRef]
- Ioannidis, J.; Taylor, G.; Zhao, D.; Liu, L.; Idoko-Akoh, A.; Gong, D.; Lovell-Badge, R.; Guioli, S.; McGrew, M.J.; Clinton, M. Primary sex determination in birds depends on DMRT1 dosage, but gonadal sex does not determine adult secondary sex characteristics. Proc. Natl. Acad. Sci. USA 2021, 118, e2020909118. [Google Scholar] [CrossRef]
- Ge, C.; Ye, J.; Weber, C.; Sun, W.; Zhang, H.; Zhou, Y.; Cai, C.; Qian, G.; Capel, B. The histone demethylase KDM6B regulates temperature-dependent sex determination in a turtle species. Science 2018, 360, 645–648. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Sun, W.; Cai, H.; Bao, H.; Zhang, Y.; Qian, G.; Ge, C. The role of anti-mullerian hormone in testis differentiation reveals the significance of the TGF-beta pathway in reptilian sex determination. Genetics 2019, 213, 1317–1327. [Google Scholar] [CrossRef]
- Rastetter, R.H.; Smith, C.A.; Wilhelm, D. The role of non-coding RNAs in male sex determination and differentiation. Reproduction 2015, 150, R93–R107. [Google Scholar] [CrossRef] [Green Version]
- Burgos, M.; Hurtado, A.; Jiménez, R.; Barrionuevo, F.J. Non-Coding RNAs: lncRNAs, miRNAs, and piRNAs in sexual development. Sex. Dev. 2021, 15, 335–350. [Google Scholar] [CrossRef]
- Real, F.M.; Sekido, R.; Lupiáñez, D.G.; Lovell-Badge, R.; Jiménez, R.; Burgos, M. A microRNA (mmu-miR-124) prevents Sox9 expression in developing mouse ovarian cells. Biol. Reprod. 2013, 89, 78. [Google Scholar] [CrossRef]
- Roeszler, K.N.; Itman, C.; Sinclair, A.H.; Smith, C.A. The long non-coding RNA, MHM, plays a role in chicken embryonic development, including gonadogenesis. Dev. Biol. 2012, 366, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Hacker, A.; Capel, B.; Goodfellow, P.; Lovell-Badge, R. Expression of Sry, the mouse sex determining gene. Development 1995, 121, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Jeske, Y.W.; Bowles, J.; Greenfield, A.; Koopman, P. Expression of a linear Sry transcript in the mouse genital ridge. Nat. Genet. 1995, 10, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Siniscalchi, C.; Di Palo, A.; Russo, A.; Potenza, N. The lncRNAs at X chromosome inactivation center: Not just a matter of sex dosage compensation. Int. J. Mol. Sci. 2022, 23, 611. [Google Scholar] [CrossRef] [PubMed]
- Kartha, R.V.; Subramanian, S. Competing endogenous RNAs (ceRNAs): New entrants to the intricacies of gene regulation. Front. Genet. 2014, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, P.; Wu, X.; Zhu, X.; Xu, H. A novel dynamic expression of vasa in male germ cells during spermatogenesis in the Chinese soft-shell turtle (Pelidiscus sinensis). J. Exp. Zool. B Mol. Dev. Evol. 2017, 328, 230–239. [Google Scholar] [CrossRef]
- Farhadi, A.; Lv, L.; Song, J.; Zhang, Y.; Ye, S.; Zhang, N.; Zheng, H.; Li, S.; Zhang, Y.; Ikhwanuddin, M.; et al. Whole-transcriptome RNA sequencing revealed the roles of chitin-related genes in the eyestalk abnormality of a novel mud crab hybrid (Scylla serrata ♀ × S. paramamosain ♂). Int. J. Biol. Macromol. 2022, 208, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Ye, Z.; Stanton, R. Misuse of RPKM or TPM normalization when comparing across samples and sequencing protocols. RNA 2020, 26, 903–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef] [Green Version]
- Bishop, C.E.; Whitworth, D.J.; Qin, Y.; Agoulnik, A.I.; Agoulnik, I.U.; Harrison, W.R.; Behringer, R.R.; Overbeek, P.A. A transgenic insertion upstream of sox9 is associated with dominant XX sex reversal in the mouse. Nat. Genet. 2000, 26, 490–494. [Google Scholar] [CrossRef]
- Koopman, P.; Gubbay, J.; Vivian, N.; Goodfellow, P.; Lovell-Badge, R. Male development of chromosomally female mice transgenic for Sry. Nature 1991, 351, 117–121. [Google Scholar] [CrossRef]
- Hui, H.B.; Xiao, L.; Sun, W.; Zhou, Y.J.; Zhang, H.Y.; Ge, C.T. Sox9 is indispensable for testis differentiation in the red-eared slider turtle, a reptile with temperature-dependent sex determination. Zool. Res. 2021, 42, 721–725. [Google Scholar] [CrossRef]
- Warr, N.; Siggers, P.; May, J.; Chalon, N.; Pope, M.; Wells, S.; Chaboissier, M.C.; Greenfield, A. Gadd45g is required for timely Sry expression independently of RSPO1 activity. Reproduction 2022, 163, 333–340. [Google Scholar] [CrossRef]
- Uhlenhaut, N.H.; Jakob, S.; Anlag, K.; Eisenberger, T.; Sekido, R.; Kress, J.; Treier, A.C.; Klugmann, C.; Klasen, C.; Holter, N.I.; et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 2009, 139, 1130–1142. [Google Scholar] [CrossRef] [Green Version]
- Miyauchi, H.; Ohta, H.; Nagaoka, S.; Nakaki, F.; Sasaki, K.; Hayashi, K.; Yabuta, Y.; Nakamura, T.; Yamamoto, T.; Saitou, M. Bone morphogenetic protein and retinoic acid synergistically specify female germ-cell fate in mice. EMBO J. 2017, 36, 3100–3119. [Google Scholar] [CrossRef]
- Kashimada, K.; Pelosi, E.; Chen, H.; Schlessinger, D.; Wilhelm, D.; Koopman, P. FOXL2 and BMP2 act cooperatively to regulate follistatin gene expression during ovarian development. Endocrinology 2011, 152, 272–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, P.; Ryan, J.; Sim, H.; Czech, D.P.; Sinclair, A.H.; Koopman, P.; Harley, V.R. Wnt signaling in ovarian development inhibits Sf1 activation of Sox9 via the Tesco enhancer. Endocrinology 2012, 153, 901–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaboissier, M.C.; Kobayashi, A.; Vidal, V.I.; Lützkendorf, S.; Van de Kant, H.J.; Wegner, M.; De Rooij, D.G.; Behringer, R.R.; Schedl, A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 2004, 131, 1891–1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carré, G.A.; Couty, I.; Hennequet-Antier, C.; Govoroun, M.S. Gene expression profiling reveals new potential players of gonad differentiation in the chicken embryo. PLoS ONE 2011, 6, e23959. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, X.; Wang, X.; Sun, C.; Zheng, J.; Li, J.; Yi, G.; Yang, N. The m6A methylation regulates gonadal sex differentiation in chicken embryo. J. Anim. Sci. Biotechnol. 2022, 13, 52. [Google Scholar] [CrossRef]
- Bergstrom, D.E.; Young, M.; Albrecht, K.H.; Eicher, E.M. Related function of mouse SOX3, SOX9, and SRY HMG domains assayed by male sex determination. Genesis 2000, 28, 111–124. [Google Scholar] [CrossRef]
- Takehana, Y.; Matsuda, M.; Myosho, T.; Suster, M.L.; Kawakami, K.; Shin, I.T.; Kohara, Y.; Kuroki, Y.; Toyoda, A.; Fujiyama, A.; et al. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat. Commun. 2014, 5, 4157. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Gui, J.F. Molecular mechanisms underlying sex change in hermaphroditic groupers. Fish. Physiol. Biochem. 2010, 36, 181–193. [Google Scholar] [CrossRef]
- Oshima, Y.; Naruse, K.; Nakamura, Y.; Nakamura, M. Sox3: A transcription factor for Cyp19 expression in the frog Rana rugosa. Gene 2009, 445, 38–48. [Google Scholar] [CrossRef]
- Yan, Q.; Wu, X.; Chen, C.; Diao, R.; Lai, Y.; Huang, J.; Chen, J.; Yu, Z.; Gui, Y.; Tang, A.; et al. Developmental expression and function of DKKL1/Dkkl1 in humans and mice. Reprod. Biol. Endocrinol. 2012, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, K.J.; DePamphilis, M.L. Soggy, a spermatocyte-specific gene, lies 3.8 kb upstream of and antipodal to TEAD-2, a transcription factor expressed at the beginning of mouse development. Nucleic Acids Res. 2000, 28, 3982–3990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohn, M.J.; Kaneko, K.J.; DePamphilis, M.L. DkkL1 (Soggy), a Dickkopf family member, localizes to the acrosome during mammalian spermatogenesis. Mol. Reprod. Dev. 2005, 71, 516–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekido, R.; Lovell-Badge, R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 2008, 453, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Dakhova, O.; O’Day, D.; Kinet, N.; Yucer, N.; Wiese, M.; Shetty, G.; Ducy, P. Dickkopf-like1 regulates postpubertal spermatocyte apoptosis and testosterone production. Endocrinology 2009, 150, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Smirnova, N.A.; Romanienko, P.J.; Khil, P.P.; Camerini-Otero, R.D. Gene expression profiles of Spo11−/− mouse testes with spermatocytes arrested in meiotic prophase I. Reproduction 2006, 132, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Baudat, F.; Manova, K.; Yuen, J.P.; Jasin, M.; Keeney, S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol. Cell. 2000, 6, 989–998. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Zhiping, L.; Yuan, N.; Guixiu, O.; Changzhi, C.; Shangye, C.; Liangguo, L.; Pinhong, Y. Sexually dimorphic reproductive defects in zebrafish with spo11 mutation. Aquac. Res. 2020, 51, 4916–4924. [Google Scholar]
- Ozaki, Y.; Miura, C.; Miura, T. Molecular cloning and gene expression of Spo11 during spermatogenesis in the Japanese eel, Anguilla japonica. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 143, 309–314. [Google Scholar] [CrossRef]
- Hsiao, P.W.; Lin, D.L.; Nakao, R.; Chang, C. The linkage of Kennedy’s neuron disease to ARA24, the first identified androgen receptor polyglutamine region-associated coactivator. J. Biol. Chem. 1999, 274, 20229–20234. [Google Scholar] [CrossRef] [Green Version]
- Harada, N.; Ohmori, Y.; Yamaji, R.; Higashimura, Y.; Okamoto, K.; Isohashi, F.; Nakano, Y.; Inui, H. ARA24/Ran enhances the androgen-dependent NH2- and COOH-terminal interaction of the androgen receptor. Biochem. Biophys. Res. Commun. 2008, 373, 373–377. [Google Scholar] [CrossRef]
- Kaur, G.; Jans, D.A. Dual nuclear import mechanisms of sex determining factor SRY: Intracellular Ca2+ as a switch. FASEB J. 2011, 25, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Hanover, J.A.; Love, D.C.; Prinz, W.A. Calmodulin-driven nuclear entry: Trigger for sex determination and terminal differentiation. J. Biol. Chem. 2009, 284, 12593–12597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyota, K.; Masuda, S.; Sugita, S.; Miyaoku, K.; Yamagishi, G.; Akashi, H.; Miyagawa, S. Estrogen receptor 1 (ESR1) agonist induces ovarian differentiation and aberrant müllerian duct development in the Chinese soft-shelled turtle, Pelodiscus sinensis. Zool. Stud. 2020, 59, e54. [Google Scholar] [PubMed]
- Wu, P.F.; Wang, X.F.; Gao, F.; Du, W.G. Role of Cyp19a1 in the female pathway of a freshwater turtle species (Mauremys reevesii) with temperature-dependent sex determination. Zool. Res. 2022, 43, 4. [Google Scholar] [CrossRef]
- Levasseur, A.; Paquet, M.; Boerboom, D.; Boyer, A. Yes-associated protein and WW-containing transcription regulator 1 regulate the expression of sex-determining genes in Sertoli cells, but their inactivation does not cause sex reversal. Biol. Reprod. 2017, 97, 162–175. [Google Scholar] [CrossRef]
- Shah, K.; Seeley, S.; Schulz, C.; Fisher, J.; Gururaja Rao, S. Calcium channels in the heart: Disease states and drugs. Cells 2022, 11, 943. [Google Scholar] [CrossRef]
- Weber, C.; Zhou, Y.; Lee, J.G.; Looger, L.L.; Qian, G.; Ge, C.; Capel, B. Temperature-dependent sex determination is mediated by pSTAT3 repression of Kdm6b. Science 2020, 368, 303–306. [Google Scholar] [CrossRef]
- Li, Y.H.; Chen, T.M.; Huang, B.M.; Yang, S.H.; Wu, C.C.; Lin, Y.M.; Chuang, J.I.; Tsai, S.J.; Sun, H.S. FGF9 is a downstream target of SRY and sufficient to determine male sex fate in ex vivo XX gonad culture. Biol. Reprod. 2020, 103, 1300–1313. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, M.; An, S.; Liang, Y.; Yang, H.; Pang, J.; Liu, Z.; Zhang, G.; Wang, F. Long non-coding RNA LOC105611671 modulates fibroblast growth factor 9 (FGF9) expression by targeting oar-miR-26a to promote testosterone biosynthesis in Hu sheep. Reprod. Fertil. Dev. 2020, 32, 373–382. [Google Scholar] [CrossRef]
- Hu, S.; Li, L.; Yeh, S.; Cui, Y.; Li, X.; Chang, H.C.; Jin, J.; Chang, C. Infiltrating T cells promote prostate cancer metastasis via modulation of FGF11→miRNA-541→androgen receptor (AR)→MMP9 signaling. Mol. Oncol. 2015, 9, 44–57. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Q.; Xu, W.; Wu, Z.; Li, D. Integrated analysis of miR-430 on steroidogenesis-related gene expression of larval rice field eel Monopterus albus. Int. J. Mol. Sci. 2021, 22, 6994. [Google Scholar] [CrossRef] [PubMed]
- Viñas, A.; Taboada, X.; Vale, L.; Robledo, D.; Hermida, M.; Vera, M.; Martínez, P. Mapping of DNA sex-specific markers and genes related to sex differentiation in turbot (Scophthalmus maximus). Mar. Biotechnol. 2012, 14, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Li, S.; Wang, Q.; Tang, L.; Huang, F.; Zhang, Z.; Mahboobe, S.; Shao, C. lncRNA DMRT2-AS acts as a transcriptional regulator of dmrt2 involving in sex differentiation in the Chinese tongue sole (Cynoglossus semilaevis). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 253, 110542. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Lei, L.; Chen, C.; Wang, Y.; Liu, X.; Geng, L.; Li, R.; Chen, H.; Hong, X.; Yu, L.; et al. Whole-Transcriptome Analysis Identifies Gender Dimorphic Expressions of Mrnas and Non-Coding Rnas in Chinese Soft-Shell Turtle (Pelodiscus sinensis). Biology 2022, 11, 834. https://doi.org/10.3390/biology11060834
Zhu J, Lei L, Chen C, Wang Y, Liu X, Geng L, Li R, Chen H, Hong X, Yu L, et al. Whole-Transcriptome Analysis Identifies Gender Dimorphic Expressions of Mrnas and Non-Coding Rnas in Chinese Soft-Shell Turtle (Pelodiscus sinensis). Biology. 2022; 11(6):834. https://doi.org/10.3390/biology11060834
Chicago/Turabian StyleZhu, Junxian, Luo Lei, Chen Chen, Yakun Wang, Xiaoli Liu, Lulu Geng, Ruiyang Li, Haigang Chen, Xiaoyou Hong, Lingyun Yu, and et al. 2022. "Whole-Transcriptome Analysis Identifies Gender Dimorphic Expressions of Mrnas and Non-Coding Rnas in Chinese Soft-Shell Turtle (Pelodiscus sinensis)" Biology 11, no. 6: 834. https://doi.org/10.3390/biology11060834
APA StyleZhu, J., Lei, L., Chen, C., Wang, Y., Liu, X., Geng, L., Li, R., Chen, H., Hong, X., Yu, L., Wei, C., Li, W., & Zhu, X. (2022). Whole-Transcriptome Analysis Identifies Gender Dimorphic Expressions of Mrnas and Non-Coding Rnas in Chinese Soft-Shell Turtle (Pelodiscus sinensis). Biology, 11(6), 834. https://doi.org/10.3390/biology11060834




