Modeling and Prediction of the Species’ Range of Neurobasis chinensis (Linnaeus, 1758) under Climate Change
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
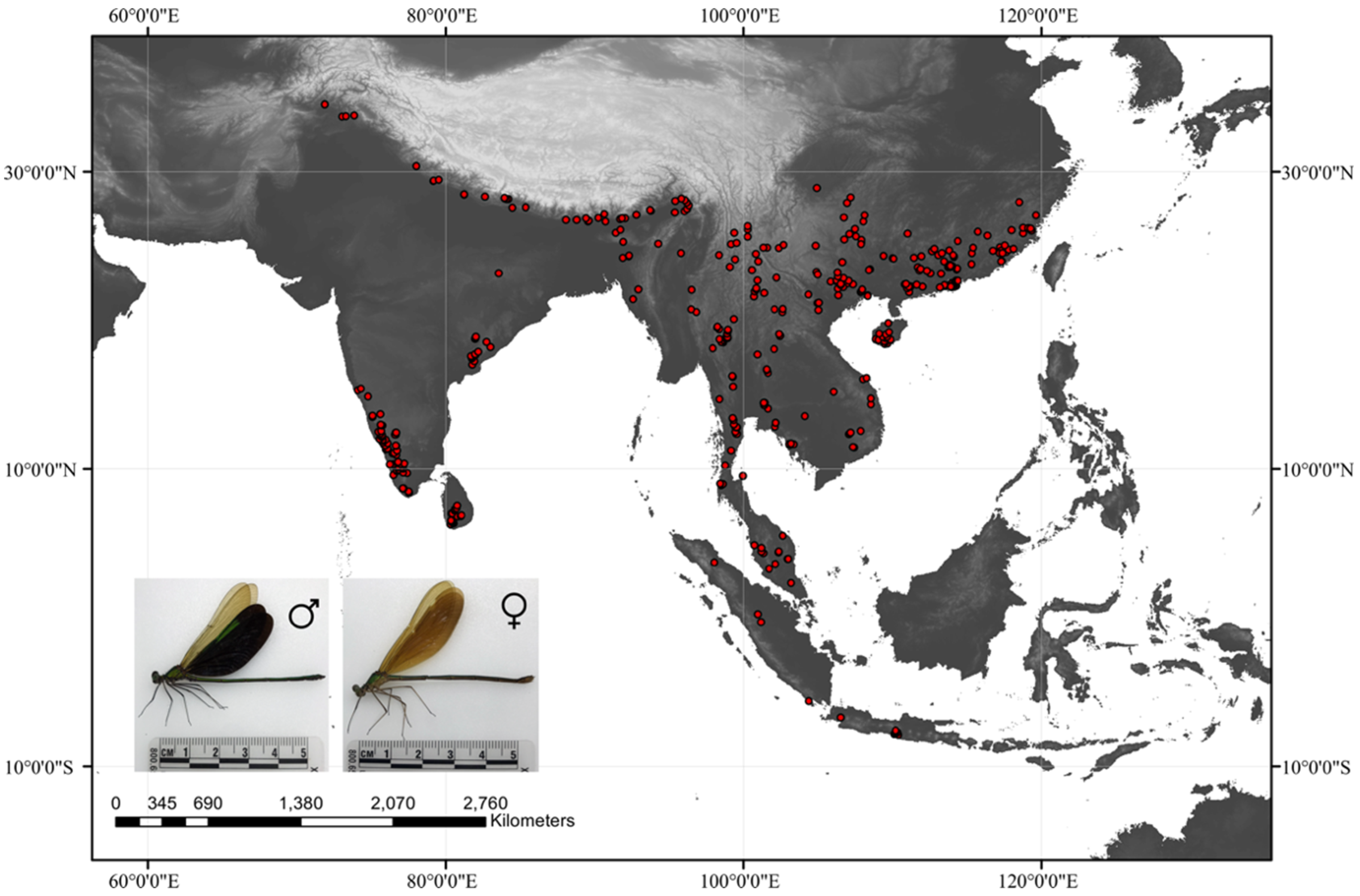
2.1. Data Collection
2.2. Environmental Variables
2.3. Distribution Modeling and Statistical Analysis
3. Results
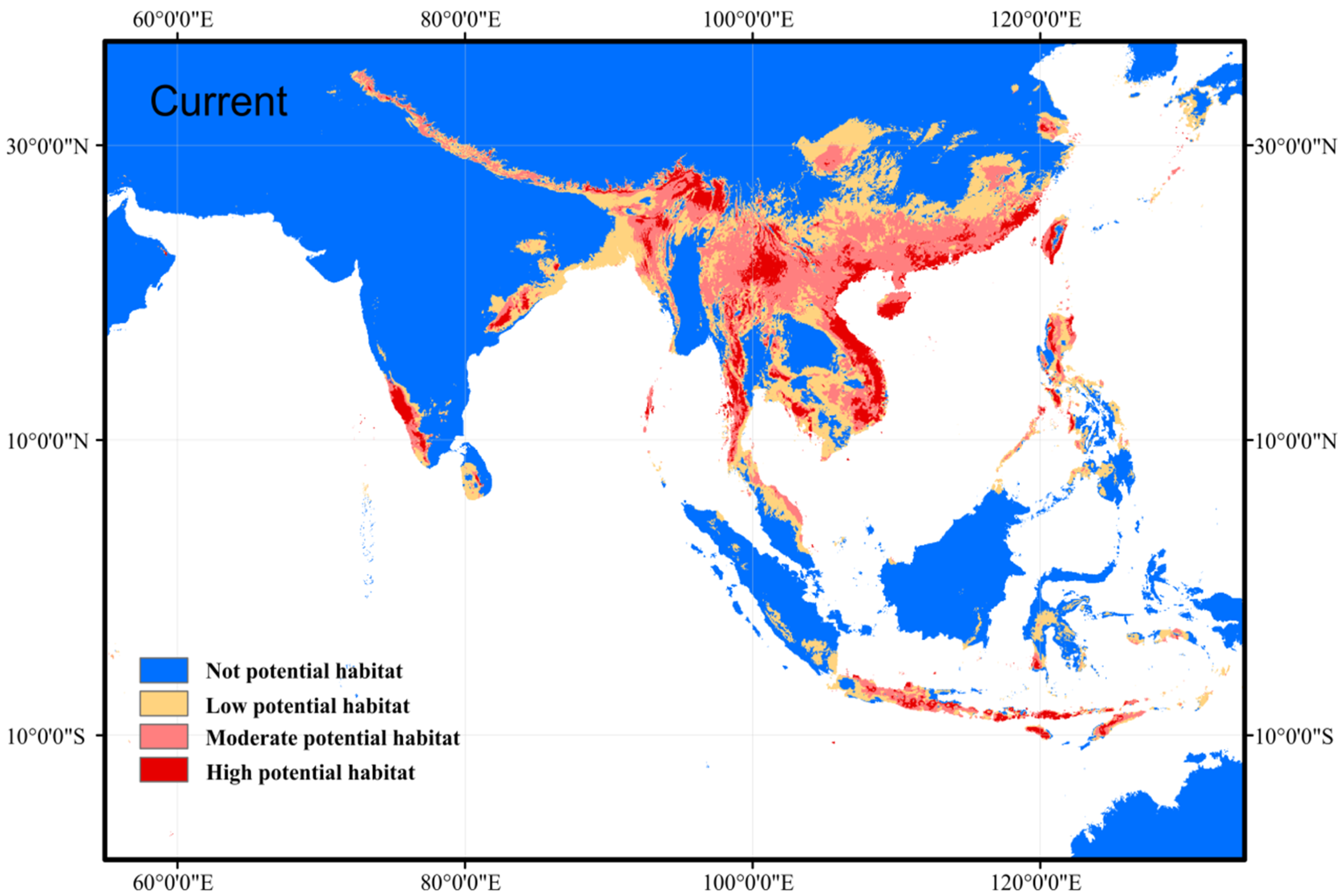
3.1. Prediction of Current Suitable Habitat Areas and Model Accuracy
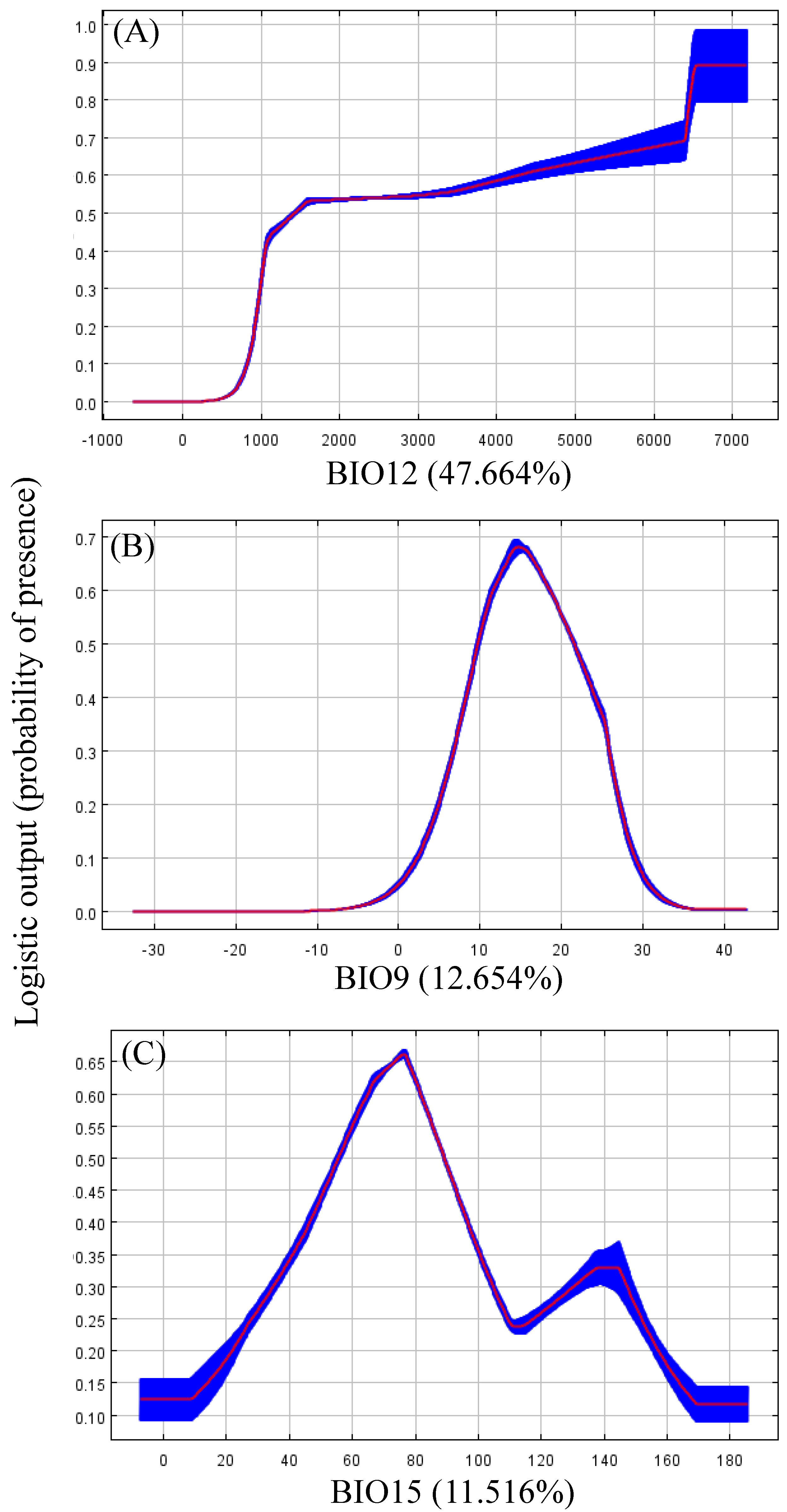
3.2. Important Environmental Variables
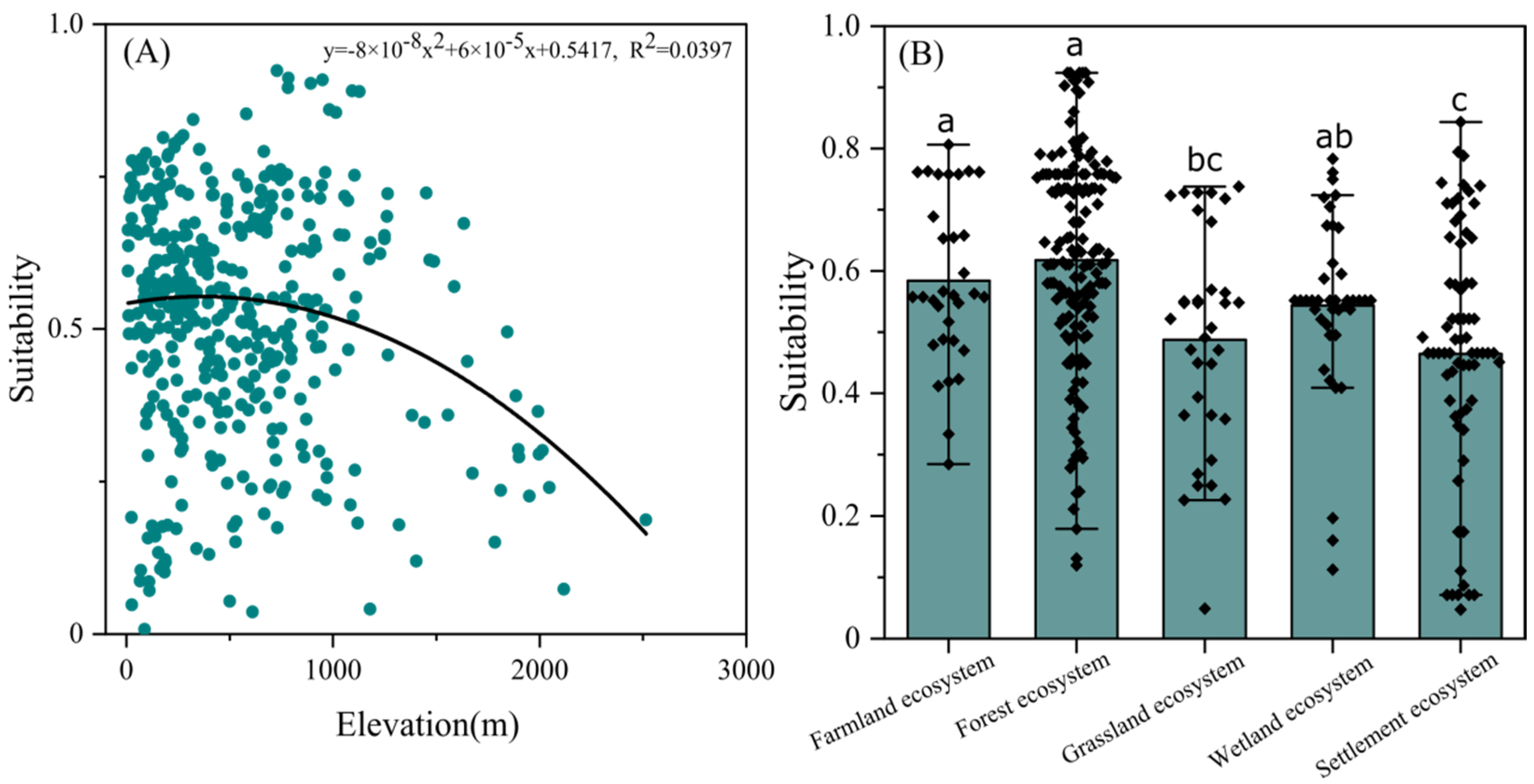
3.3. Habitat Selection
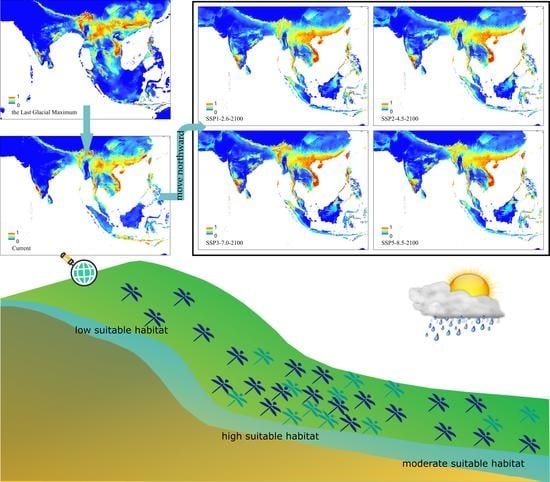
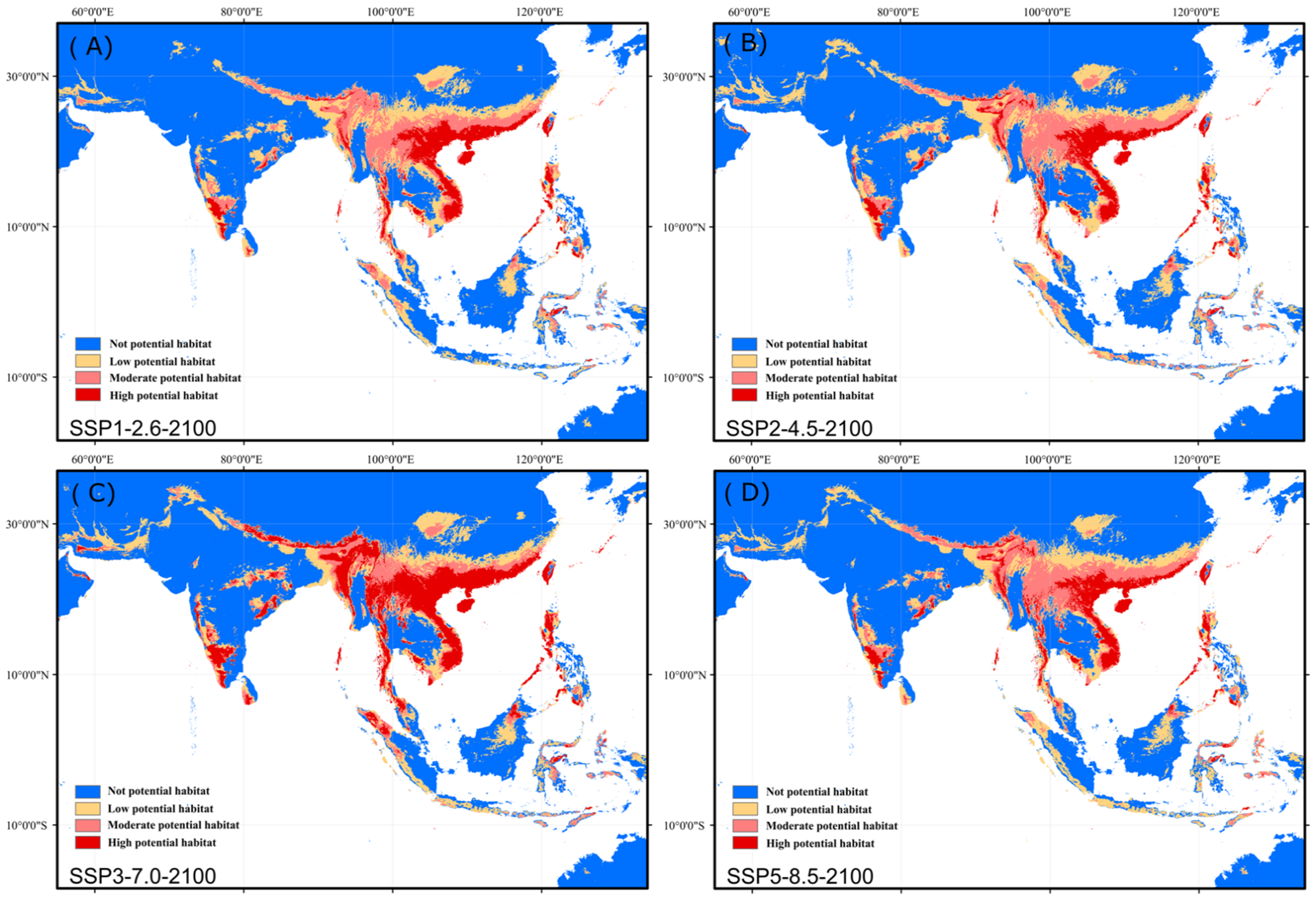
3.4. Future Changes in Suitable Habitat Area
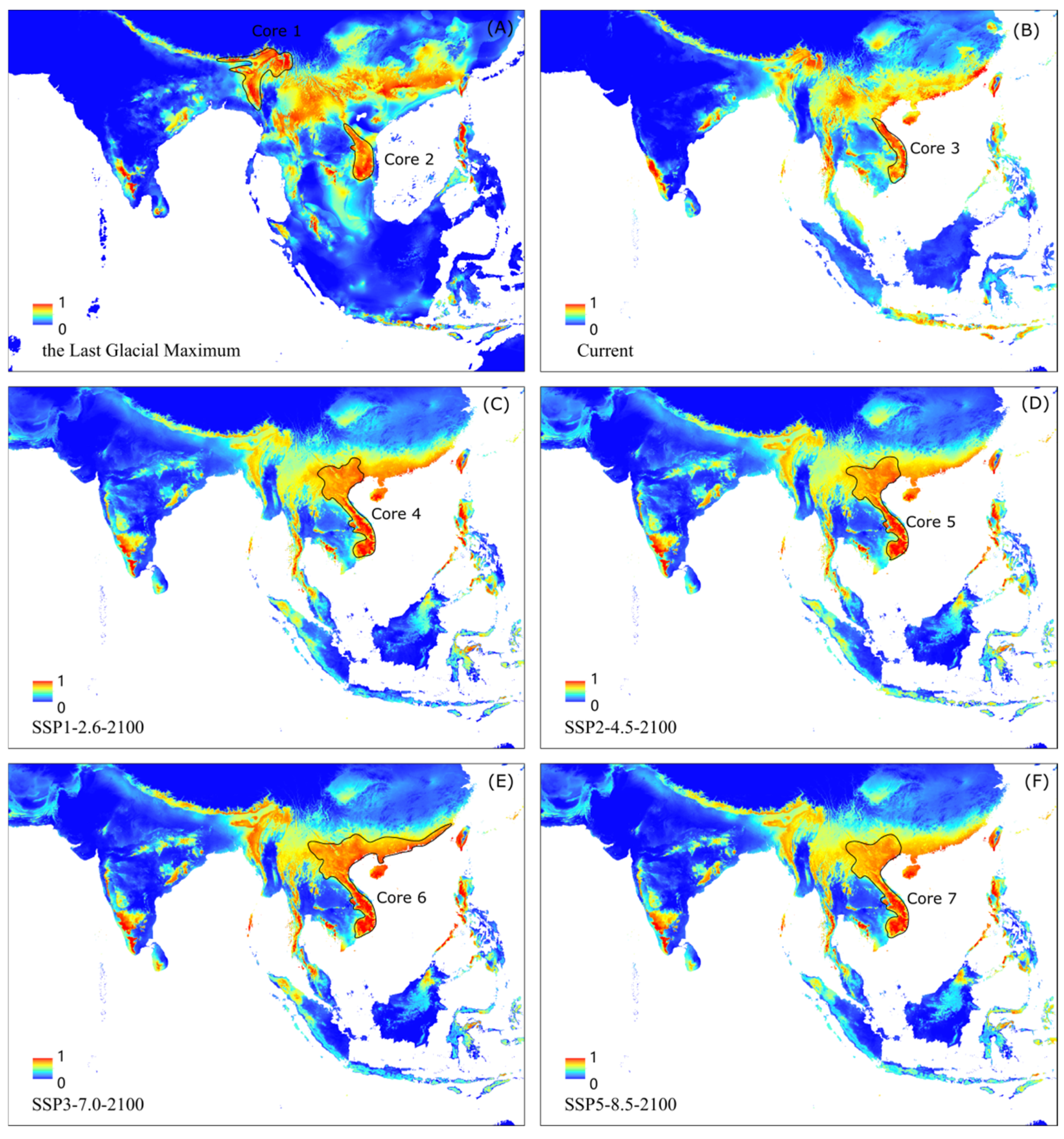
3.5. The Core Distribution Shifts
4. Discussion
4.1. Current and Future Potential Distribution
4.2. Habitat Selection and Critical Climate Factors
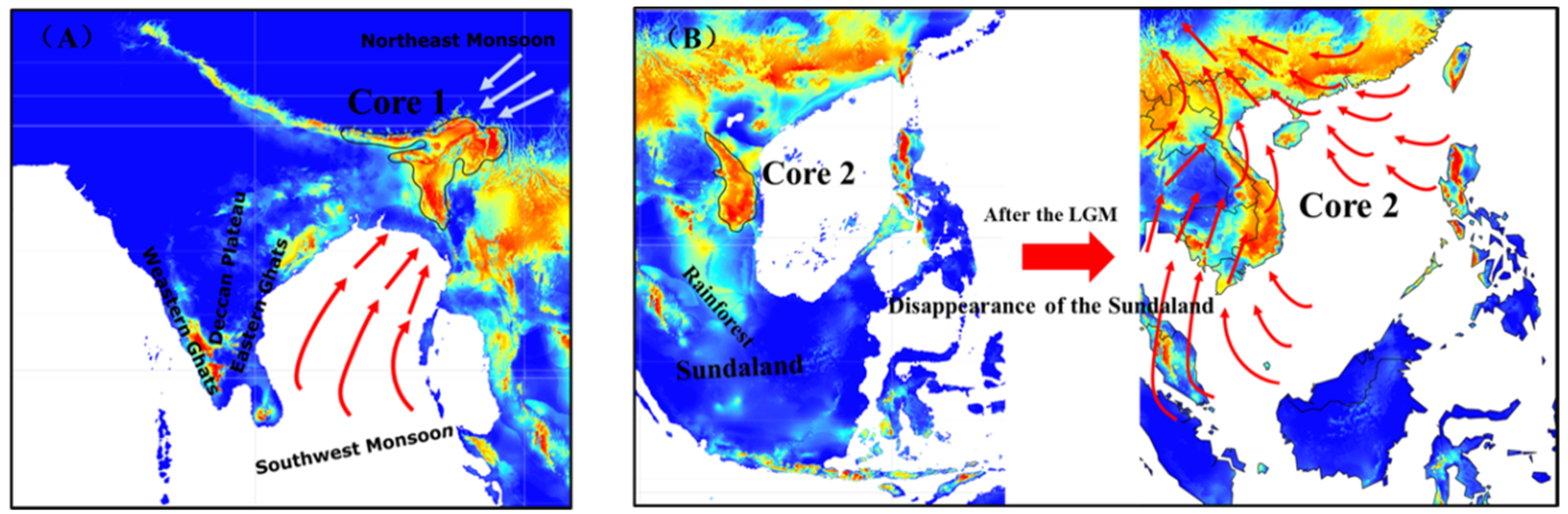
4.3. Historical Core Distribution and Its Future Shift
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.M. Dragonflies and Damselflies of China; Chongqing University Press: Chongqing, China, 2019; pp. 7, 1003–1004. [Google Scholar]
- Orr, A.G.; Hämäläinen, M. The Metalwing Demoiselles of the Eastern Tropics—Their Identification and Biology; Natural History Publications (Borneo): Kota Kinabalu, Malaysia, 2007; pp. 5–25. [Google Scholar]
- Dow, R.A. Neurobasis chinensis. In The IUCN Red List of Threatened Species 2009: E.T163763A5648117; IUCN: Gland, Switzerland, 2009. [Google Scholar] [CrossRef]
- Subramanian, K.; Gadgil, M. Dragonflies of India, a Field Guide; Vigyan Prasar: New Delhi, India, 2009; pp. 156–165.
- Nair, M.V. Dragonflies and Damselflies of Orissa and Eastern India; Wildlife Organisation, Forest and Environment Department, Government of Orissa: Bhubaneswar, India, 2011; pp. 152–154.
- De Paula, A.S.; Barreto, C. Potential Distribution of Nysius simulans (Hemiptera: Lygaeidae) in Soybean Crops in South America Under Current and Future Climate. J. Econ. Èntomol. 2020, 113, 1702–1710. [Google Scholar] [CrossRef]
- Kumari, P.; Wani, I.A.; Khan, S.; Verma, S.; Mushtaq, S.; Gulnaz, A.; Paray, B.A. Modeling of Valeriana wallichii Habitat Suitability and Niche Dynamics in the Himalayan Region under Anticipated Climate Change. Biology 2022, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Allen, S.K.; Plattner, G.K.; Nauels, A.; Xia, Y.; Stocker, T.F. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Abstract for Decision-Makers, Groupe D’experts Intergouvernemental sur L’evolution du Climat/Intergovernmental Panel on Climate Change-IPCC; C/O World Meteorological Organization: Geneva, Switzerland, 2013; p. 3544. [Google Scholar]
- Jepsen, J.U.; Kapari, L.; Hagen, S.B.; Schott, T.; Vindstad, O.P.L.; Nilssen, A.C.; Ims, R.A. Rapid northwards expansion of a forest insect pest attributed to spring phenology matching with sub-Arctic birch. Glob. Chang. Biol. 2011, 17, 2071–2083. [Google Scholar] [CrossRef]
- Li, A.; Wang, J.; Wang, R.; Yang, H.; Yang, W.; Yang, C.; Jin, Z. MaxEnt modeling to predict current and future distributions of Batocera lineolata (Coleoptera: Cerambycidae) under climate change in China. Ecoscience 2020, 27, 23–31. [Google Scholar] [CrossRef]
- Post, E.; Forchhammer, M.C.; Bret-Harte, M.S.; Callaghan, T.V.; Christensen, T.R.; Elberling, B.; Fox, A.D.; Gilg, O.; Hik, D.S.; Høye, T.T.; et al. Ecological dynamics across the Arctic associated with recent climate change. Science 2009, 325, 1355–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, C.; Nelson, T.A.; Jelinski, D.E.; Wulder, M.A.; Boots, B. Spatial–temporal analysis of species range expansion: The case of the mountain pine beetle, Dendroctonus ponderosae. J. Biogeogr. 2009, 36, 1446–1458. [Google Scholar] [CrossRef]
- Xu, D.; Li, X.; Jin, Y.; Zhuo, Z.; Yang, H.; Hu, J.; Wang, R. Influence of climatic factors on the potential distribution of pest Heortia vitessoides Moore in China. Glob. Ecol. Conserv. 2020, 23, e01107. [Google Scholar] [CrossRef]
- Choudhary, J.S.; Mali, S.S.; Fand, B.B.; Das, B. Predicting the invasion potential of indigenous restricted mango fruit borer, Citripestis eutraphera (Lepidoptera: Pyralidae) in India based on MaxEnt modelling. Curr. Sci. 2019, 116, 636–642. [Google Scholar] [CrossRef]
- Santana, P.A., Jr.; Kumar, L.; Da Silva, R.S.; Pereira, J.L.; Picanço, M.C. Assessing the impact of climate change on the worldwide distribution of Dalbulus maidis (DeLong) using MaxEnt. Pest Manag. Sci. 2019, 75, 2706–2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castex, V.; Beniston, M.; Calanca, P.; Fleury, D.; Moreau, J. Pest management under climate change: The importance of understanding tritrophic relations. Sci. Total Environ. 2018, 616, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Sun, J. Predicting the distribution of Stipa purpurea across the Tibetan Plateau via the MaxEnt model. BMC Ecol. 2018, 18, 10. [Google Scholar] [CrossRef] [Green Version]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Sharma, S.; Arunachalam, K.; Bhavsar, D.; Kala, R. Modeling habitat suitability of Perilla frutescens with MaxEnt in Uttarakhand—A conservation approach. J. Appl. Res. Med. Aromat. Plants 2018, 10, 99–105. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Wang, C.; Liu, C.; Li, H. Climate change may alter genetic diversity of Duchesnea indica, a clonal plant species. Biochem. Syst. Ecol. 2016, 66, 114–122. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Meinshausen, M.; Nicholls, Z.R.; Lewis, J.; Gidden, M.J.; Vogel, E.; Freund, M.; Beyerle, U.; Gessner, C.; Nauels, A.; Bauer, N.P.; et al. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci. Model Dev. 2020, 13, 3571–3605. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Anderson, R.P. A framework for using niche models to estimate impacts of climate change on species distributions. Ann. N. Y. Acad. Sci. 2013, 1297, 8–28. [Google Scholar] [CrossRef]
- Dormann, C.F. Promising the future? Global change projections of species distributions. Basic Appl. Ecol. 2007, 8, 387–397. [Google Scholar] [CrossRef]
- Elith, J.H.; Graham, C.P.; Anderson, R.; Dudík, M.; Ferrier, S.; Guisan, A.J.; Hijmans, R.; Huettmann, F.R.; Leathwick, J.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Papeş, M. Ecological niche modelling confirms potential north-east range expansion of the nine-banded armadillo (Dasypus novemcinctus) in the USA. J. Biogeogr. 2015, 42, 803–807. [Google Scholar] [CrossRef]
- Van Zonneveld, M.; Jarvis, A.; Dvorak, W.; Lema, G.; Leibing, C. Climate change impact predictions on Pinus patula and Pinus tecunumanii populations in Mexico and Central America. For. Ecol. Manag. 2009, 257, 1566–1576. [Google Scholar] [CrossRef]
- Pelini, S.L.; Dzurisin, J.D.; Prior, K.M.; Williams, C.M.; Marsico, T.D.; Sinclair, B.J.; Hellmann, J.J. Translocation experiments with butterflies reveal limits to enhancement of poleward populations under climate change. Proc. Natl. Acad. Sci. USA 2009, 106, 11160–11165. [Google Scholar] [CrossRef] [Green Version]
- Nelson, W.A.; Bjørnstad, O.N.; Yamanaka, T. Recurrent insect outbreaks caused by temperature-driven changes in system stability. Science 2013, 341, 796–799. [Google Scholar] [CrossRef] [Green Version]
- Gavin, D.G.; Fitzpatrick, M.C.; Gugger, P.F.; Heath, K.D.; Rodríguez-Sánchez, F.; Dobrowski, S.Z.; Hampe, A.; Hu, F.S.; Ashcroft, M.B.; Bartlein, P.J.; et al. Climate refugia: Joint inference from fossil records, species distribution models and phylogeography. New Phytol. 2014, 204, 37–54. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Fang, N.Q. Records of paleo-monsoon of core bar9427 in northeastern Indian ocean during last glaciation. Earth Sci. J. China Univ. Geosci. 2006, 31, 765–772. [Google Scholar]
- Sun, X.; Li, X.; Luo, Y.; Chen, X. The vegetation and climate at the last glaciation on the emerged continental shelf of the South China Sea. Palaeogeogr. Palaeoclim. Palaeoecol. 2000, 160, 301–316. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Wang, P.; Stattegger, K. Vegetation on the Sunda Shelf, South China Sea, during the last glacial maximum. Palaeogeogr. Palaeoclim. Palaeoecol. 2009, 278, 88–97. [Google Scholar] [CrossRef]
- Gorog, A.J.; Sinaga, M.H.; Engstrom, M.D. Vicariance or dispersal? Historical biogeography of three Sunda shelf murine rodents (Maxomys surifer, Leopoldamys sabanus and Maxomys whiteheadi). Biol. J. Linn. Soc. 2004, 81, 91–109. [Google Scholar] [CrossRef] [Green Version]
- Gathorne-Hardy, F.; Davies, R.G.; Eggleton, P.; Jones, D. Quaternary rainforest refugia in south-east Asia: Using termites (Isoptera) as indicators. Biol. J. Linn. Soc. 2002, 75, 453–466. [Google Scholar] [CrossRef] [Green Version]
- Wurster, C.M.; Bird, M.I.; Bull, I.D.; Creed, F.; Bryant, C.; Dungait, J.A.; Paz, V. Forest contraction in north equatorial Southeast Asia during the Last Glacial Period. Proc. Natl. Acad. Sci. USA 2010, 107, 15508–15511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Ding, Z.; Li, Y.; Wang, X.; Jiang, W.; Huang, X. Warming-induced northwestward migration of the East Asian monsoon rain belt from the Last Glacial Maximum to the mid-Holocene. Proc. Natl. Acad. Sci. USA 2015, 112, 13178–13183. [Google Scholar] [CrossRef] [Green Version]
- Webster, P.J.; Magana, V.O.; Palmer, T.; Shukla, J.; Tomas, R.; Yanai, M.; Yasunari, T. Monsoons: Processes, predictability, and the prospects for prediction. J. Geophys. Res. Ocean. 1998, 103, 14451–14510. [Google Scholar] [CrossRef]
| Abbreviations | Environmental Variables |
|---|---|
| BIO1 | Annual mean temperature |
| BIO2 | Mean diurnal range (mean of monthly max temperature–min temperature) |
| BIO3 | Isothermality ((BIO2/BIO7) × 100) |
| BIO4 | Temperature seasonality (standard deviation × 100) |
| BIO5 | Max temperature of warmest month |
| BIO6 | Min temperature of coldest month |
| BIO7 | Temperature annual range (BIO5–BIO6) |
| BIO8 | Mean temperature of wettest quarter |
| BIO9 | Mean temperature of driest quarter |
| BIO10 | Mean temperature of warmest quarter |
| BIO11 | Mean temperature of coldest quarter |
| BIO12 | Annual precipitation |
| BIO13 | Precipitation of wettest month |
| BIO14 | Precipitation of driest month |
| BIO15 | Precipitation seasonality (coefficient of variation) |
| BIO16 | Precipitation of wettest quarter |
| BIO17 | Precipitation of driest quarter |
| BIO18 | Precipitation of warmest quarter |
| BIO19 | Precipitation of coldest quarter |
| Ele. | Elevation (meter a.s.l.) |
| Scenario | High Suitable Habitat | Moderate Suitable Habitat | Low Suitable Habitat | Core Habitat | Total |
|---|---|---|---|---|---|
| Current | 4.87 × 105 | 1.37 × 106 | 1.73 × 106 | 1.67 × 104 | 3.59 × 106 |
| SSP1-2.6 | 7.30 × 105 | 1.32 × 106 | 2.16 × 106 | 5.34 × 104 | 4.20 × 106 |
| SSP2-4.5 | 7.15 × 105 | 1.45 × 106 | 2.27 × 106 | 5.70 × 104 | 4.43 × 106 |
| SSP3-7.0 | 1.52 × 106 | 1.08 × 106 | 2.02 × 106 | 6.22 × 105 | 4.62 × 106 |
| SSP5-8.5 | 7.37 × 105 | 1.35 × 106 | 2.12 × 106 | 4.22 × 104 | 4.21 × 106 |
| Abbreviation | Variable | Explanation | Importance |
|---|---|---|---|
| BIO2 | Mean diurnal range in temperature | 2.237% | 0.445 |
| BIO5 | Max temperature of warmest month | 8.500% | 7.356 |
| BIO8 | Mean temperature of wettest quarter | 2.195% | 4.198 |
| BIO9 | Mean temperature of driest quarter | 12.654% | 32.538 |
| BIO12 | Annual precipitation | 47.664% | 10.636 |
| BIO14 | Precipitation of driest month | 0.515% | 1.090 |
| BIO15 | Precipitation seasonality | 11.516% | 8.095 |
| BIO18 | Precipitation of warmest quarter | 5.036% | 19.720 |
| BIO19 | Precipitation of coldest quarter | 9.683% | 15.921 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Wang, H.; Xiao, S.; Guan, Z.; Zhang, H.; Dumont, H.J.; Han, B.-P. Modeling and Prediction of the Species’ Range of Neurobasis chinensis (Linnaeus, 1758) under Climate Change. Biology 2022, 11, 868. https://doi.org/10.3390/biology11060868
Liao J, Wang H, Xiao S, Guan Z, Zhang H, Dumont HJ, Han B-P. Modeling and Prediction of the Species’ Range of Neurobasis chinensis (Linnaeus, 1758) under Climate Change. Biology. 2022; 11(6):868. https://doi.org/10.3390/biology11060868
Chicago/Turabian StyleLiao, Jian, Haojie Wang, Shaojun Xiao, Zhaoying Guan, Haomiao Zhang, Henri J. Dumont, and Bo-Ping Han. 2022. "Modeling and Prediction of the Species’ Range of Neurobasis chinensis (Linnaeus, 1758) under Climate Change" Biology 11, no. 6: 868. https://doi.org/10.3390/biology11060868
APA StyleLiao, J., Wang, H., Xiao, S., Guan, Z., Zhang, H., Dumont, H. J., & Han, B.-P. (2022). Modeling and Prediction of the Species’ Range of Neurobasis chinensis (Linnaeus, 1758) under Climate Change. Biology, 11(6), 868. https://doi.org/10.3390/biology11060868